Abstract
Introduction
Evidence of variations in bladder filling effecting prostate stability and therefore treatment and side‐effects is well established with intensity modulated radiation therapy (IMRT). This study aimed to increase bladder volume reproducibility for prostate radiation therapy by implementing a bladder scanning (BS) protocol that could assist patients' bladder filling at computed tomography (CT) simulation and treatment.
Methods
Based on a retrospective review of 524 prostate cancer patients, a bladder volume of 250–350 mL was adopted as ‘ideal’ for achieving planning dose constraints. A prospective cohort study was conducted to assess the clinical utility of measuring patients' bladder volumes at CT simulation using an ultrasound bladder scanner (Verathon 9400 BladderScan®). A revised bladder preparation protocol was utilised by a bladder scan group (BS) and a non‐BS group followed the standard departmental bladder preparation protocol. Time and volume data for the BS group (n = 17) were compared with the non‐BS group (n = 17).
Results
The BS cohort had a CT bladder volume range of 221–588 mL; mean 379 mL, SD 125 mL. The non‐BS group had a larger range: 184–757 mL; mean 373 mL, SD 160 mL (P = 0.9171). There was a positive correlation between CT volume and BS volume in the BS group (r = 0.797; P = 0.0002) although BS volumes were smaller: range 160–420 mL; mean 251 mL; SD 91 mL; P < 0.0001). The maximum bladder volume receiving 50 Gy (V50) from the BS group was 46.4%, mean 24.5%. The maximum bladder V50 from the non‐BS group was 50.9%, mean 27.3% (P = 0.5178). Treatment data from weekly cone beam CT scans were also compared over 6 weeks. They were assessed as being a pass if bladder and bowel requirements were acceptable. The BS group proceeded to treatment on the basis of a pass 92.7% of the time, whereas the pass rate for non‐BS group was 75%; difference 17.7% (P < 0.0001).
Conclusion
The BS is a useful tool for achieving consistent, appropriately sized bladder volumes in prostate cancer patients.
Keywords: Diagnostic imaging, prostate, radiotherapy, ultrasonography, urinary bladder
Introduction
Evidence of variations in bladder filling effecting prostate stability and therefore treatment and side‐effects is well established with intensity modulated radiation therapy (IMRT).1, 2 In a pilot study, Dearnaley et al. showed that prostate cancer dose escalation achieved better tumour control; however, dose escalation increased toxicity to normal tissues.3 The principal dose‐limiting structures in prostate radiotherapy are the bladder and rectum.2 Bladder filling can help control the motion of internal organs and decrease toxicities by reducing the volume of bladder being irradiated and, importantly, by moving the small bowel superiorly out of the treatment fields.1 Hence, there is a need to standardise bladder volumes for both planning and treatment to moderate this influence on prostate movement and risk of normal tissue toxicities.2
Prostate radiation therapy patients attend treatment every weekday for up to 9 weeks and make up ~20% of all new cancer diagnosis each year.4 These patients are typically treated to a dose of 81 Gy/45# fractions for high and intermediate risk prostates and 66 Gy/33# for post‐prostatectomies, with a 7 or 9 field dynamic multi leaf collimator (DMLC) Monaco IMRT plan. Compliance with required bladder preparation is very difficult for many patients and non‐compliance has a negative impact on treatment delivery in our radiation therapy departments.
Bladder scanning (BS) with ultrasound is a strategy that has been considered for increasing consistency with bladder volumes prior to radiotherapy for prostate cancer. Although BS provides an effective means of assessing bladder volume prior to treatment,5 studies have shown that improvements in bladder volume consistency are more difficult to attain1, 5, 6, 7 Nevertheless, a small number of articles have supported the use of the BS in a radiation therapy setting.2, 6, 7
Based on clinical observations from staff, several patients regularly struggled to achieve and maintain appropriately full bladder volumes which caused a level of distress for them. It was decided to embark on a quality improvement project to see if the BS could assist in these cases. In turn, we anticipated this would reduce radiation exposure through repeated cone beam computed tomography (CBCT) imaging and also avoid the frequent unplanned treatment delays and linear accelerator rescheduling that would occur.
The aim of this prospective cohort study was to increase bladder volume reproducibility for prostate IMRT radiation therapy patients. To this end, we developed a BS protocol to assists patients' bladder filling at computed tomography (CT) simulation and treatment using a Verathon 9400 Bladder Scanner. Filling techniques and time delay variations were explored in order to establish a procedure that would increase consistency and compliance from patients. We hypothesised that BS during CT simulation would result in less patients being taken off the bed at the time of treatment to correct for bladder filling anomalies.
Materials and Methods
Study approval
The study was approved and supported by the North Coast Area Health Service, New South Wales Human Research Ethics Committee as a Quality Assurance program. (Approval No: QA073).
Equipment: BladderScan®
The Verathon 9400 BladderScan®, Verathon Medical (Australia) is a battery‐operated ultrasound instrument that provides a non‐invasive measurement of urinary bladder volume. The hand‐held probe is accompanied by a portable console that provides aiming guides to ensure the operator places the probe in the optimal position for an accurate measurement.
Patients are positioned supine in the treatment position and ultrasound gel is applied one inch above the patient's pubic symphysis. The operator stands on the patients' right side, placing the probe on the gel and aiming it slightly towards the patient's coccyx. When the scan button is pressed, the illustrated guide shows if the scan is ‘off target’ and in what direction to reposition the probe if necessary. The calculated bladder volume is displayed on the screen.
Identifying an appropriately full bladder
In order to determine an appropriate bladder volume range that would satisfy planning constraints, 524 bladder volumes were retrospectively analysed from prostate planning assessment data in our electronic medical record system (Mosaiq™, Elekta Pty Ltd., North Sydney, Australia) collected across three departments between November 2008 and November 2011. Bladder volumes ranged from 41 mL to 1526 mL, with a mean volume of 321 mL (SD 190 mL). An analysis of the bladder volume versus the number of patients exceeding the V50 < 50 Gy bladder planning constraint8 used in our institute showed an inverse relationship with larger bladder volumes increasingly meeting treatment criteria (Fig. 1). With a target bladder volume of 250–350 mL, the chances of exceeding the V50 < 50 Gy bladder constraint was <5%. Based on these observations this was selected as the target bladder volume range for subsequent work in evaluating and developing the protocol for use of the bladder scanner.
Figure 1.
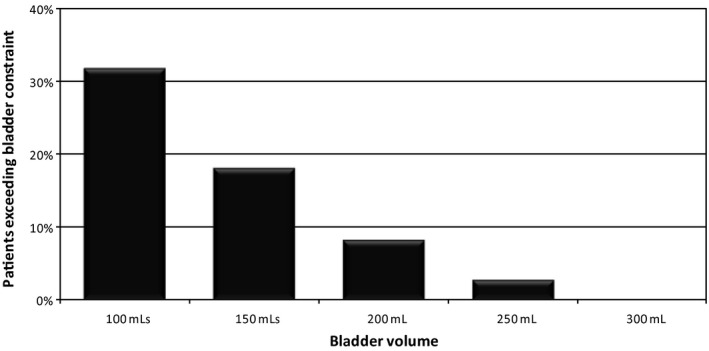
Bladder volumes outlined in the focal system exceeding the V50 < 50 Gy planning dose constraint.7 (V50 < 50 Gy: 50% of bladder volume to receive < 50 Gy).
Feasibility study
A feasibility study was conducted at one department with five patients who were receiving prostate cancer IMRT alone, prostate + seminal vesicle or post‐prostatectomy IMRT. The aim was to determine the feasibility of using a BS strategy to identify any practical or logistic difficulties which might affect a larger prospective study.
Each patient was asked to follow the department protocol for bowel preparation in the 2 weeks prior to their CT simulation appointment. Patients were asked to arrive at the department 1 h before their appointment time without filling their bladder. Upon arrival, patients were instructed to empty their bladder and bowels and were given three cups (600 mL) of water to drink. The patient was to hold their bladder until the CT simulation was complete. A BS was performed 30 min post‐drinking and every 15 min thereafter until the desired bladder volume of ≥250 mL was achieved. If the bladder volume had not reached ≥250 mL after 30 min, a further cup of water was given and the process repeated until the desired bladder volume of ≥250 mL was achieved. Once the bladder volume was achieved, the simulation procedure continued onto setup and CT scanning (Fig. 2).
Figure 2.
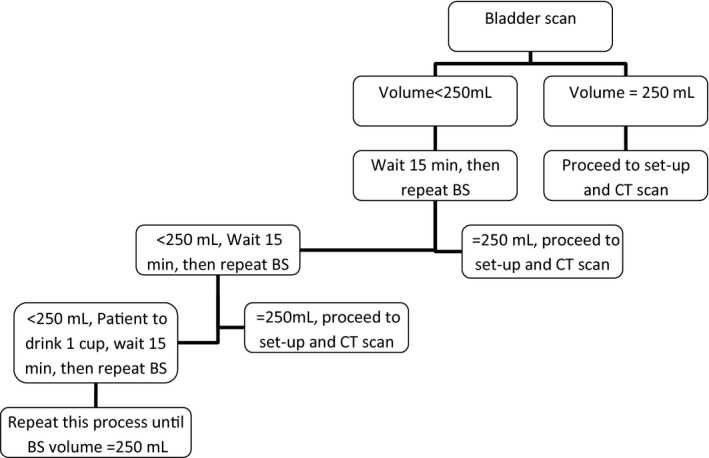
Protocol for bladder filling using the BS at simulation during the feasibility study. BS, bladder scanner; CT, computed tomography.
Using an Excel spreadsheet (Microsoft Corp) we documented the BS volumes at each required time period, the approximate time between the last BS and the CT, the volume of bladder as outlined in the Focal™ system and a comment relating to the patient's general hydration. Once the patient began treatment, fractions 1–3 and weekly thereafter (CBCT) results were also collected. Patients routinely had a minimum of nine CBCT data sets collected. These results recorded the patient's bladder and rectal size.
Records from the feasibility study showed three patients were CT scanned within the 1 h of drinking 600 mL with their final BS completed at 45 mins. The two other patients were scanned within 1 h 15 min with their last BS occurring 1 h after drinking. All patients were instructed how much water they are required to drink daily for treatment and how long prior to treatment they are to drink it. It was recorded on their appointment card for day 1 of treatment for an easy reference.
Results from the feasibility study were used to inform the prospective study. A systematic difference was found between bladder volumes measured with the bladder scanner and those measured through contouring the outer wall of the bladder on CT. The difference in volume measured between the two modalities in our setting was approximately 119 mL (range 70–180 mL) with the BS being consistently lower than the Focal™ system. This result is not unexpected given that CT is a spatially accurate modality whereas the portable instrument has known variance across a wider range. We therefore changed the minimum bladder volume threshold for treatment when using BS to 150 mL, on the basis that this would approximate 250 mL when outlined in the Focal™ system.
Prospective study
A prospective study was conducted with 34 IMRT prostate patients, prostate plus seminal vesicles and post‐prostatectomy patients from one department. Patient demographics are provided in Table 1. Patients deemed ineligible for this study included post‐prostatectomy patients requiring contrast who had not had their bloods tested prior to the day of simulation. (Testing bloods on the day of simulation made the time‐delayed scanning impossible due to the time uncertainty before blood results were available). Patients with restricted fluid intake, such as certain cardiac and renal patients, were also deemed ineligible.
Table 1.
Patient demographics
| Parameters | BS = 17 | Non‐BS = 17 |
|---|---|---|
| Age | ||
| Median (range) | 69 (60–77) | 66 (55–78) |
| <69 years (n) | 9 | 11 |
| ≥69 years (n) | 8 | 6 |
| PSA (ng/mL) | ||
| Median (range) | 10.6 (1.7–32) | 38.8 (0.08–410) |
| ≤10.5 (n) | 11 | 13 |
| ≥10.6 (n) | 6 | 4 |
| T stage | ||
| T1 | 5 (29.3%) | 5 (29.4%) |
| T2 | 10 (58.9%) | 5 (29.4%) |
| T3 | 2 (11.8%) | 5 (29.4%) |
| T4 | 0 (0%) | 1 (5.9%) |
| TX | 0 (0%) | 1 (5.9%) |
| Gleason score | ||
| 6 | 2 (11.7%) | 1 (5.9%) |
| 7 | 6 (35.4%) | 12 (70.6%) |
| 8 | 4 (23.5%) | 1 (5.9%) |
| 9 | 4 (23.5%) | 2 (11.7%) |
| 10 | 1 (5.9%) | 1 (5.9%) |
| Grade | ||
| High risk | 8 (47.1%) | 2 (11.7%) |
| Intermediate risk | 8 (47.1%) | 10 (58.9%) |
| Post‐prostatectomy | 1 (5.9%) | 5 (29.4%) |
Gleason score, gleason grading system; grade, tumour grade; PSA, prostate specific antigen; T stage, tumour stage.
All patients were treated with curative intent and standard dose of 81 Gy in 45# for high and intermediate prostates and 66 Gy/33# for post‐prostatectomies. Sequentially allocated, seventeen cases, referred to as the non‐BS group followed the standard departmental bladder and bowel preparation. Patients were asked to empty their bladder and bowels one hour prior to their simulation appointment and to drink 600 mL (3 cups) of water immediately. Patients were CT scanned at their appointment time if they verbally confirmed their bladder felt full. The seventeen cases in the BS group followed the same protocol as the feasible study. The first BS was performed 30 min post‐drinking and repeated as per Figure 3. The same volume and time data points collected during the feasibility study were recorded for the prospective study in an Excel spread sheet. Based on experiences from the feasibility study, it was deemed necessary to generalise the treatment comments to render them useful for analysis. Staff were re‐educated in the required CBCT review process and then asked to consistently format the CBCT comments according to the following template: ‘Bladder = (1/4, 1/2, ¾, more than or =) planned, rectum = (good; too much gas; too much matter), move = (>, < or =) 3 mm, (+/− Patient. re‐educated)’. The CBCT comments were categorised into either a ‘pass’ or ‘fail’ status based on treatment acceptability as referred to in the results section. The CBCT review process had already been established as a quick subjective assessment of key parameters of isocentre position, rectum status and bladder status. The requirements of the BS study added further formalisation of the CBCT review process and provided a more rigorous documentation and categorisation which became normal clinical practice. The image assessment is still a subjective interpretation and is not a hard measurement based process nor is one needed, as it is not critical to the BS study outcomes. Comments were then categorised into five groups (Table 2) with three groups proceeding to treatment (a ‘pass’) and two groups classified as a ‘fail’.
Figure 3.
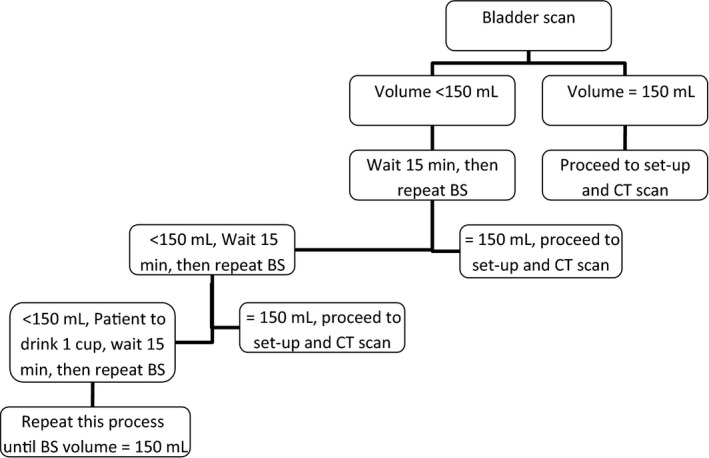
Protocol for bladder filling using the BS at simulation during the prospective study. BS, bladder scanner; CT, computed tomography.
Table 2.
Categorisation of suitability for radiotherapy based on bladder scanner results
| Category | Description | Outcome |
|---|---|---|
| Bladder=Planned | Volume as planned | Pass |
| Bladder>Plan | Volume greater than planned | Pass |
| Bladder≥½ | Volume greater than 50% of planned volume | Pass |
| Bladder<½ or too large | Volume less than 50% of planned volume or too large | Fail |
| Rectum issues | Excessive gas or faecal matter | Fail |
Statistical analysis
Data were assessed for normality using the Anderson–Darling test; normally distributed data were analysed using parametric statistics. Differences between groups were sought using Student's t‐test, paired or unpaired, as appropriate. The relationship between CT and BS volume measurements were further assessed using a Pearson correlation. Proportions were compared using a 2‐sample z‐test (2‐tailed). Statistical analysis was performed using Excel statistical functions (Microsoft) and MaxStat (Jever, Germany). A probability value P < 0.05 was considered significant.
Results
In the prospective study, CT scan data from the seventeen patients in the BS group were compared to data from seventeen patients in the non‐BS group (Table 3). In the BS group, the time and volume comparisons resulted in eight patients achieving the required bladder volume 45 min post‐drinking 600 mL of water, three achieving it at 30 min and six patients at 60 min.
Table 3.
Effect of BS use on bladder volume and treatment delivery assessed against the V50 < 50 Gy bladder constraint
| Parameter | Non‐BS group (n = 17) | BS group (n = 17) | Significance (P) |
|---|---|---|---|
| Bladder volume (mL)a | |||
| Mean (SD) | 373 (160) | 379 (125) | 0.9171 |
| Minimum–Maximum | 184–757 | 221–588 | |
| V50 (%)b | |||
| Mean (SD) | 27.3 (14.1) | 24.5 (10.5) | 0.5178 |
| Minimum–Maximum | 8.7–50.9 | 9.0–46.4 | |
| Overall CBCT ‘pass’ rate (%) | |||
| Average | 75.0 | 92.7 | <0.0001 |
Bladder volumes (outer wall) outlined in the focal system.
Bladder volume of 50% of dose.
The BS cohort had a bladder volume range of 221–588 mL; mean 379 mL; SD 125 mL. Although the non‐BS group had a larger range (184–757 mL), the mean volume was not significantly different (mean 373 mL; SD 160 mL; P = 0.9171).
Bladder volumes determined by BS were significantly smaller than those determined by CT: range 160–420 mL; mean 251 mL; SD 91 mL; P < 0.0001). The difference between the means was 128 mL. However, there was a positive correlation between CT volume (inner wall) and BS in the BS group (r = 0.797; P = 0.002, n = 17).
Delivery of treatment was assessed against the V50 < 50 Gy bladder constraint (Table 3).8 The maximum V50 from the BS group was 46.4%, with an average of 24.5%. The maximum V50 from the non‐BS group was marginally higher at 50.9%, with an average of 27.3%. The difference between the groups was not significant (P = 0.5178).
The clinical utility of the BS was further explored by comparing data from the weekly CBCT scans of the two groups over 6 weeks of treatment, as shown in Figure 4. Overall, the BS group achieved a ‘pass’ 92.7% of the time, whereas the non‐BS group was able to proceed to treatment on the basis of a ‘pass’ 75% of the time, a difference in compliance of 17.7%. The difference was highly significant (P < 0.0001). This indicated that patients going through the BS process at time of simulation resulted in practical improvements in the CBCT observed outcomes. It should be noted, that patients were not considered as having failed on rectal issues alone as this group were excluded from the above figures therefore they represent true bladder filling issues that were effectively avoided by the BS group.
Figure 4.
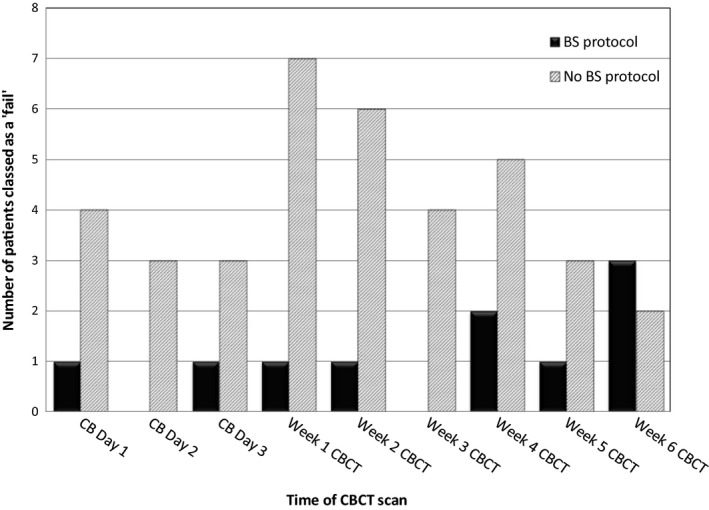
Comparison of ‘fails’ (patients taken off the bed to resolve bladder issues) between BS (n = 17) and non‐BS groups (n = 17) over 6 weeks of radiotherapy. BS Protocol, bladder scanner group; No BS Protocol, non‐bladder scanner group; CB, cone beam; CBCT, cone beam computed tomography.
Discussion
Our feasibility study confirmed that the routine use of the BS before CT simulation planning was feasible in our departments. During prostate cancer treatment, patients are often taken off the treatment couch to resolve bladder size issues before treatment can be delivered, resulting in delayed patient throughput. Prior to this study, our clinical observation was that patients often struggled to fill their bladder to the required volumes, increasing potential radiation‐induced toxicities or in some cases increasing the bladder volume to uncomfortable levels. This resulted in daily time delays for patients receiving treatment, inefficiencies in service delivery, and bladder status becoming stressful for many patients.
As mentioned earlier, bladder volumes measured with the bladder scanner were approximately 100 mL lower than the Focal™ system contoured volume (outer wall) on the CT dataset in the region of interest. We therefore established a minimum bladder volume threshold for treatment when using BS of 150 mL, on the basis that this would approximate 250 mL in practice. Possible factors contributing to the difference in measured volumes are as follows: Firstly, the time between BS and CT: the delay averaged 10 min, however this time was not considered to be significant, as the difference was still noted when a phantom was assessed. Secondly, there are known limitations to the precision of BS measurements: according to the manufacturer of the bladder scanner (Verathon), a discrepancy of approximately ±15 mL or ±15% may be expected. These limitations to the absolute accuracy do not appear to diminish the utility of the device; they simply require wider latitude and tolerances in the interpretation of values reported in this setting. To minimise discrepancies, the inner wall of the bladder could be contoured by using a negative 3 mm expansion as the smaller volumes would tend to reduce the variation. Thirdly, inter‐operator variability: due to staff rostering, it was not practical to have one dedicated person perform the BS. For quality control, the Verathon product representative recalibrated each BS at all three cancer departments. Bladder scanners were not exchanged between sites so all patients were scanned on the same scanner to avoid the introduction of any systematic errors. As there were many new staff working in the departments, there was also a ‘hands on’ retraining in‐service held by the product representative. We documented that this qualitatively decreased variation from inter‐operator variability.
Previous studies1, 2, 6 found strong correlations between the measured BS volume and CT contoured volume in prostate radiotherapy patients. Correlation coefficients (r) varied between 0.856 and 0.95.1 While the statistical correlation between the two measurements was highly significant in our study (r = 0.797; P = 0.002), the association was somewhat weaker than the quoted studies. To rule out the possible effect of a small sample size causing the discrepancy we retrospectively compared the BS volume to the contoured Focal volume for a further 20 patients and found similar results (results not shown).
From the feasibility study it was also recognised that a systematic nomenclature of the treatment CBCT comments was necessary in order to render them useful for further analysis. The CBCT comments collected from treatment were categorised into two main groups, either a pass group or a fail group. The ‘pass’ group comprised comments stating: bladder = planned, bladder > planned and bladder ≥ ½. Bladder = planned was selected when the bladder on CBCT matched the contour on the planning dataset. Bladder > plan was selected when the bladder on CBCT was greater than the planning contour and did not distort the prostate/PTV. Bladder ≥ ½ was used when the CBCT bladder was ≥ ½ the planned bladder contour and small bowel did not fall into the treatment field. These subgroups meant the patient was able to continue onto treatment without being taken off the bed to correct for any bladder volume concerns. The ‘fail’ group was established with comments stating: bladder < ½ or too large or rectum issues described. Bladder < ½ was used when the CBCT bladder was < ½ the planned bladder as this may cause the V50 < 50 Gy to go into minor violation. A bladder too large or a rectum issue was used when the bladder/rectum distorted the prostate/PTV. These subgroups resulted in the patient being taken off the bed to either fill/empty their bladder or resolve rectum issues.
The mean bladder volume difference between the BS and non‐BS group was only 6 mL, which would be considered an insignificant variation. Regardless of this, the use of the bladder scan device was beneficial as it reduced the large variation in the range of bladder volumes achieved at the time of CT simulation. Based on the very small difference in the volume mean, it can also be hypothesised that the significant difference in the ‘pass’ rate once on treatment can be associated with the bladder scan device allowing individualised patient education in regards to bladder filling preparation.
Our results demonstrate that the BS is a useful tool in prostate cancer radiotherapy. Not only can it help avoid unnecessary radiation exposure at the time of CT simulation by ensuring a target bladder volume is achieved prior to CT scanning but the process of establishing an initial starting volume prior to the CT simulation is in and of itself useful. The additional bladder filling information at set time points reveals more about the patient hydration status and helps staff understand the individual patients filling habit. It also allows for the opportunity to further educate the patient and get more detailed hydration history from the patient.
All the information discovered using a BS protocol can provide useful insight that can be fed back to the patient to further improve their compliance and assistance with hydration and this leads to greater consistency over the course of treatment. Having the staff and patient understand the initial state and bladder‐filling behaviour better allows for all subsequent treatments that follow to be more consistent.
Our findings led to the revision of our patient bladder preparation letters and bladder filling protocols. The study also resulted in improved interactions with our cancer care coordinator, ensuring patients were generally hydrated as this was an important factor in attempting to achieve optimal bladder volumes and this issue was discussed upon first patient contact. Our dieticians also refined our bowel preparation protocols and introduced the use of daily magnesium supplements in an attempt to decrease bowel issues.
This study changed our practice in other ways. Along with supporting evidence from Mullaney et al.,9 at CT simulation, patients are now asked to arrive 45 min early where they are instructed to empty their bladder and bowel, then drink 600 mL of water. The BS is then used to assess their bladder volume after 45 min and the criterion of reaching ≥150 mL is used. We have also implemented an upper volume limit of 300 mL as measured on the BS, to prevent patients over filling during their CT simulation and therefore assisting them to consistently reach requisite bladder volumes on treatment. Overfilling had been a problem for some patients causing stress especially those who may have been struggling with incontinence and urgency. At completion of the CT simulation appointment patients have their drinking volume and time reinforced, further education is provided if patients do not convey a thorough understanding.
Bloods for intravenous contrast are now being taken and tested on the day before CT simulation to avoid delays associated with bladder filling requirements. Therefore, the only ineligible patients in our current clinical practice are those with restricted fluid intake.
A bladder and bowel assessment has also been developed in our electronic medical record to document the final bladder volume as found at the time of simulation. The full assessment will be completed during simulation, however if the patient's drinking volume, time delay or recorded bladder volume on the BS changes during planning or treatment, the necessary components will be re‐entered. Our assessment will allow information to be easily recorded and accessible when bladder volumes or bowel concerns are being addressed. It also allows data items to be assessed individually. This data may help us further refine the bladder filling process in the future.
A review of 20 patients (620 images) 2 years after implementing our bladder filling protocol for all pelvis bladder filling showed that patients were only getting off the couch 4% of the time (2% bladder issue and 2% rectal issues). This has increased our ‘pass’ rate to 96.2% compared to 92.7% at the early introduction stage. We have not enforced a BS standard routine for treatment; the radiation therapist decides whether a BS is required after discussion has taken place with the patient about their hydration and bladder comfort. Comments that would indicate a BS is required may include: ‘I do not have the urge to urinate yet’; ‘I have been to the toilet since I drank 600 mL’; ‘I do not feel as full as other days’ and ‘I have been doing physical activity today’.
The study provided a platform for the optimisation of bladder volumes, allowing its use to be extended to all patients requiring monitoring of their bladder filling status, including cases with other pelvic malignancies such as rectal, endometrial and cervical cancer.
Conclusion
The routine use of the BS has increased scheduled treatment efficiency and potentially improved patient care in prostate cancer IMRT patients. These improvements were facilitated by revised protocols concerning bladder filling and improved communication with patients.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
We thank Nicholas Hutchinson for his contribution to the Bladder Scanner Workgroup.
J Med Radiat Sci 63 (2016) 179–185
References
- 1. Stam MR, van Lin EN, van der Vight LP, Kaanders JH, Visser AG. Bladder filling variation during radiation treatment of prostate cancer: Can the use of a bladder ultrasound scanner and biofeedback optimize bladder filling? Int J Radiat Oncol Biol Phys 2006; 65: 371–7. [DOI] [PubMed] [Google Scholar]
- 2. O'Doherty UM, McNair HA, Norman AR, et al. Variability of bladder filling in patients receiving radical radiotherapy to the prostate. Radiother Oncol 2006; 79: 335–40. [DOI] [PubMed] [Google Scholar]
- 3. Dearnaley DP, Hall E, Lawrence D, et al. Pilot study of dose escalation using conformal radiotherapy in prostate cancer: PSA control and side effects. Br J Cancer 2005; 92: 488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Currow D, Thomson W. Cancer in NSW: Incidence Report 2009. Cancer Institute NSW, Sydney, 2014. [Google Scholar]
- 5. Hynds S, McGarry CK, Mitchell DM, et al. Assessing the daily consistency of bladder filling using an ultrasonic Bladderscan device in men receiving radical conformal radiotherapy for prostate cancer. Br J Radiol 2011; 84: 813–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gawthrop J, Oates R. Measured bladder volume for radiotherapy of the prostate using the hand‐held BladderScan BVI 3000. Radiographers 2012; 59: 8–12. [Google Scholar]
- 7. O'Shea E, Armstrong A, O'Hara T, O'Neill L, Thirion P. Validation of an external ultrasound device for bladder volume measurements in prostate conformal radiotherapy. Radiography 2008; 14: 178–83. [Google Scholar]
- 8. EviQ Cancer Treatments Online . Radiation oncology, prostate, intermediate risk, EBRT, definitive. Dated 21 March 2011.
- 9. Mullaney LM, O'Shea E, Dunne MT, et al. 2014. A randomized trial comparing bladder volume consistency during fractionated prostate radiation therapy. Prac Radiat Oncol, 4: e203–12. [DOI] [PubMed] [Google Scholar]


