Abstract
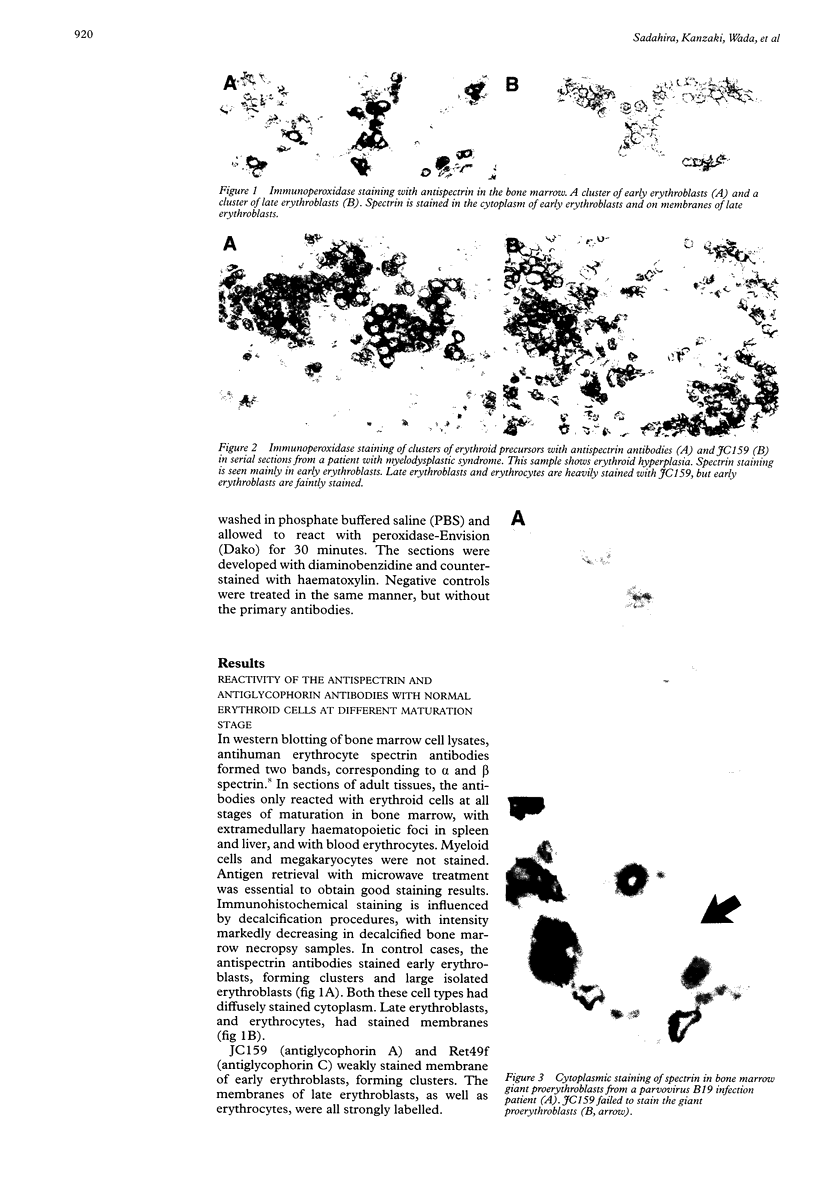
AIM: To investigate whether spectrin can be used as an immunohistochemical marker for erythroid precursors in routinely processed paraffin embedded bone marrow sections. METHODS: Bone marrow biopsies and clot sections were stained with rabbit antihuman erythrocyte spectrin antibodies, specific for erythroid cells as shown by western blotting and bone marrow smears, and compared to sections stained with antiglycophorin monoclonal antibodies (JC159 and Ret49f). RESULTS: Antispectrin antibodies resulted in diffuse cytoplasmic staining of early erythroblasts and membranous staining of late erythroblasts as well as erythrocytes. In haematopathological samples, immature erythroid cell clusters were clearly identified. In contrast, antiglycophorin monoclonal antibodies resulted in only membranous staining of late erythroblasts, and faint staining of early erythroblasts. CONCLUSIONS: Spectrin may be a superior marker to glycophorin for the identification of erythroid precursors in paraffin embedded sections.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battifora H. The multitumor (sausage) tissue block: novel method for immunohistochemical antibody testing. Lab Invest. 1986 Aug;55(2):244–248. [PubMed] [Google Scholar]
- Kurec A. S., Cruz V. E., Barrett D., Mason D. Y., Davey F. R. Immunophenotyping of acute leukemias using paraffin-embedded tissue sections. Am J Clin Pathol. 1990 Apr;93(4):502–509. doi: 10.1093/ajcp/93.4.502. [DOI] [PubMed] [Google Scholar]
- Loken M. R., Shah V. O., Dattilio K. L., Civin C. I. Flow cytometric analysis of human bone marrow: I. Normal erythroid development. Blood. 1987 Jan;69(1):255–263. [PubMed] [Google Scholar]
- Morey A. L., Fleming K. A. Immunophenotyping of fetal haemopoietic cells permissive for human parvovirus B19 replication in vitro. Br J Haematol. 1992 Oct;82(2):302–309. doi: 10.1111/j.1365-2141.1992.tb06422.x. [DOI] [PubMed] [Google Scholar]
- Morrow J. S., Marchesi V. T. Self-assembly of spectrin oligomers in vitro: a basis for a dynamic cytoskeleton. J Cell Biol. 1981 Feb;88(2):463–468. doi: 10.1083/jcb.88.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehls V., Zeitler-Zapf P., Drenckhahn D. Different sequences of expression of band 3, spectrin, and ankyrin during normal erythropoiesis and erythroleukemia. Am J Pathol. 1993 May;142(5):1565–1573. [PMC free article] [PubMed] [Google Scholar]
- Pinkus G. S., Said J. W. Intracellular hemoglobin-a specific marker for erythroid cells in paraffin sections. An immunoperoxidase study of normal, megaloblastic, and dysplastic erythropoiesis, including erythroleukemia and other myeloproliferative disorders. Am J Pathol. 1981 Mar;102(3):308–313. [PMC free article] [PubMed] [Google Scholar]
- Tricot G., De Wolf-Peeters C., Vlietinck R., Verwilghen R. L. Bone marrow histology in myelodysplastic syndromes. II. Prognostic value of abnormal localization of immature precursors in MDS. Br J Haematol. 1984 Oct;58(2):217–225. doi: 10.1111/j.1365-2141.1984.tb06079.x. [DOI] [PubMed] [Google Scholar]
- Wada H., Kanzaki A., Yawata A., Inoue T., Kaku M., Takezono M., Sugihara T., Yamada O., Yawata Y. Late expression of red cell membrane protein 4.2 in normal human erythroid maturation with seven isoforms of the protein 4.2 gene. Exp Hematol. 1999 Jan;27(1):54–62. doi: 10.1016/s0301-472x(98)00014-9. [DOI] [PubMed] [Google Scholar]
- Wickrema A., Koury S. T., Dai C. H., Krantz S. B. Changes in cytoskeletal proteins and their mRNAs during maturation of human erythroid progenitor cells. J Cell Physiol. 1994 Sep;160(3):417–426. doi: 10.1002/jcp.1041600304. [DOI] [PubMed] [Google Scholar]