Abstract
Painful peripheral neuropathy is a severe adverse effect of chemotherapeutic drugs such as paclitaxel (Taxol). The glutamate N-methyl-d-aspartate receptors (NMDARs) are critically involved in the synaptic plasticity associated with neuropathic pain. However, paclitaxel treatment does not alter the postsynaptic NMDAR activity of spinal dorsal horn neurons. In this study, we determined whether paclitaxel affects presynaptic NMDAR activity by recording excitatory postsynaptic currents (EPSCs) of dorsal horn neurons in spinal cord slices. In paclitaxel-treated rats, the baseline frequency of miniature EPSCs (mEPSCs) was significantly increased; the NMDAR antagonist 2-amino-5-phosphonopentanoic acid (AP5) completely normalized this frequency. Also, AP5 significantly reduced the amplitude of monosynaptic EPSCs evoked by dorsal root stimulation and reversed the reduction in the paired-pulse ratio of evoked EPSCs in paclitaxel-treated rats. Blocking GluN2A-containing, but not GluN2B-containing, NMDARs largely decreased the frequency of mEPSCs and the amplitude of evoked EPSCs of dorsal horn neurons in paclitaxel-treated rats. Furthermore, inhibition of protein kinase C fully reversed the increased frequency of mEPSCs and the amplitude of evoked EPSCs in paclitaxel-treated rats. Paclitaxel treatment significantly increased the protein level of GluN2A and phosphorylated GluN1 in the dorsal root ganglion. In addition, intrathecal injection of AP5 or systemic administration of memantine profoundly attenuated pain hypersensitivity induced by paclitaxel. Our findings indicate that paclitaxel treatment induces tonic activation of presynaptic NMDARs in the spinal cord through protein kinase C to potentiate nociceptive input from primary afferent nerves. Targeting presynaptic NMDARs at the spinal cord level may be an effective strategy for treating chemotherapy-induced neuropathic pain.
Keywords: electrophysiology, ion channel, neuron, pain, synaptic plasticity, toxicity
Introduction
The severe neuropathic pain caused by many effective chemotherapeutic agents, including paclitaxel (Taxol), bortezomib, oxaliplatin, and vincristine, is often the reason for discontinuation of what is otherwise life-saving therapy (1). Paclitaxel, which acts by stabilizing the microtubule cytoskeleton against depolymerization, is widely used to treat patients with breast, lung, ovarian, and other types of solid cancers. The peripheral neuropathy induced by paclitaxel treatment is frequently associated with burning pain, tingling, and numbness in the feet and hands (2–4). Such conditions are chronic and respond poorly to standard analgesics such as opioids, anticonvulsants, and antidepressants (1). Paclitaxel treatment can also damage primary afferent nerves and neurons in the dorsal root ganglion (DRG) 3 (5–7). However, the molecular mechanisms responsible for the increased nociceptive input in paclitaxel-induced neuropathic pain are not fully known.
The glutamate N-methyl-d-aspartate receptors (NMDARs) are tetramers that contain two essential GluN1 subunits co-assembled with two GluN2 and/or GluN3 subunits, and GluN2 subunits are required for glutamate-dependent NMDAR activation (8). NMDARs are commonly present at the postsynaptic densities of excitatory synapses, where they mediate the slow excitatory postsynaptic potential. Although they are not involved in normal nociception, NMDARs at the spinal cord level play a critical role in central sensitization and in the development of neuropathic pain after traumatic nerve injury (9–12). Increased postsynaptic NMDAR activity in spinal dorsal horn neurons contributes to neuropathic pain by increasing neuronal excitability and diminishing synaptic inhibition via K+-Cl− cotransporter-2 proteolysis (10, 11). Nevertheless, paclitaxel treatment does not significantly alter the postsynaptic NMDAR activity of spinal dorsal horn neurons (13).
However, NMDARs are also present presynaptically, where they predominantly regulate neurotransmitter release. Presynaptic NMDARs in the brain can enhance spontaneous neurotransmitter release and can be tonically activated by ambient glutamate (14–16). At the spinal cord level, NMDARs are expressed at the central terminals of primary afferent nerves and at second-order dorsal horn neurons (9, 17, 18). Under physiological conditions, presynaptic NMDARs in the spinal dorsal horn are latent and not functionally active in regulating glutamate release (9, 19). However, in opioid-induced hyperalgesia and traumatic nerve injury-induced chronic pain conditions, presynaptic NMDARs in the spinal cord become active and are tonically stimulated by endogenous glutamate (9, 18–20). Whether paclitaxel treatment affects presynaptic NMDAR activity in the spinal cord is unclear.
Therefore, in the present study, we investigated the potential effect of paclitaxel treatment on presynaptic NMDAR activity in the spinal dorsal horn using a rat model. Our findings suggest that paclitaxel treatment induces tonic activation of presynaptic NMDAR activity in the spinal cord through protein kinase C (PKC), which contributes to increased nociceptive input from primary afferent nerves and to the development of neuropathic pain. Our study provides new insight into the subunit composition, signaling, and functional significance of presynaptic NMDARs in the spinal cord in paclitaxel-induced painful neuropathy.
Results
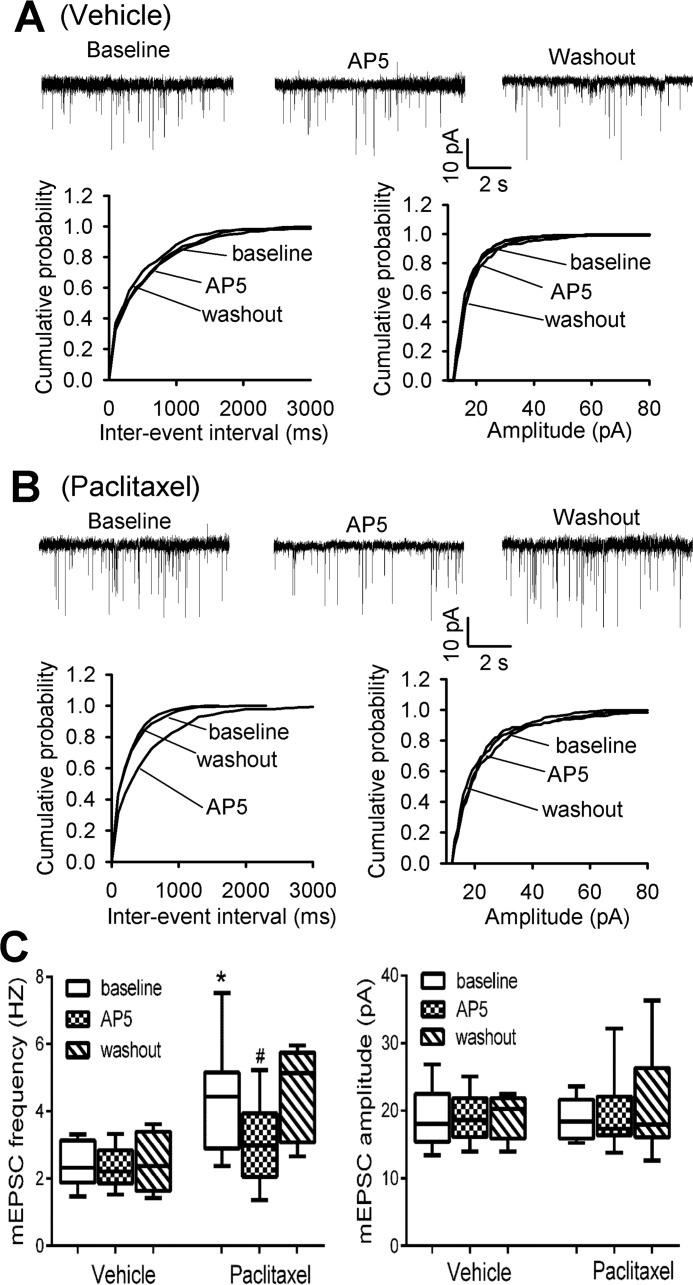
Paclitaxel Increases Presynaptic NMDAR Activity of Dorsal Horn Neurons
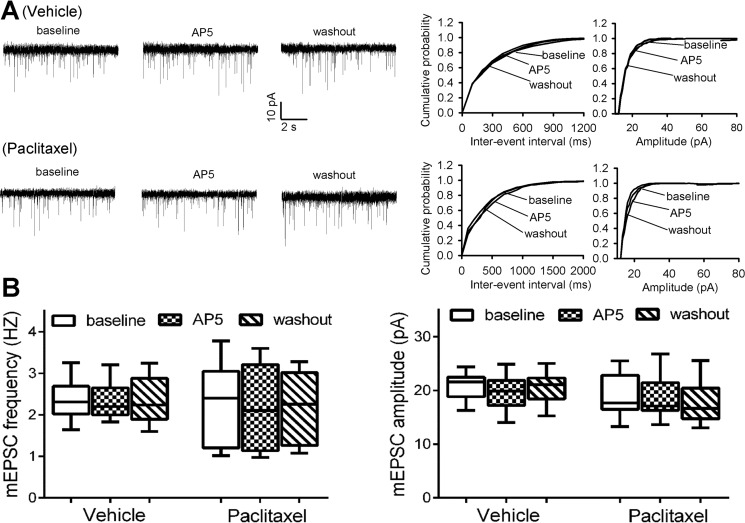
In our previous study, we found that paclitaxel treatment has no effect on the activity of postsynaptic NMDARs in the spinal dorsal horn (13). We thus determined whether paclitaxel alters the activity of presynaptic NMDARs by recording glutamatergic mEPSCs, which reflect spontaneous quantal release of glutamate from presynaptic terminals (11, 18). In lamina II neurons of vehicle-treated rats, the baseline frequency of mEPSCs was 2.44 ± 0.25 Hz (n = 8 neurons). Bath application of AP5 (50 μm), a specific NMDAR antagonist, had no significant effect on the frequency and amplitude of mEPSCs in these neurons (Fig. 1, A and C). In contrast, the baseline frequency of mEPSCs in lamina II neurons of paclitaxel-treated rats (4.36 ± 0.45 Hz, n = 12 neurons) was much higher than that in vehicle-treated rats (Fig. 1, B and C). Also, bath application of 50 μm AP5 completely normalized the frequency of mEPSCs increased by paclitaxel in the neurons of paclitaxel-treated rats. These data suggest that paclitaxel treatment increases the activity of presynaptic NMDARs of spinal dorsal horn neurons.
FIGURE 1.
Paclitaxel increases presynaptic NMDAR activity in the spinal cord. A, representative recording traces and cumulative plots show the baseline and effect of bath application of 50 μm AP5 on the frequency and amplitude of mEPSCs of a lamina II neuron from a vehicle-treated rat. B, original traces and cumulative plots show the baseline and effect of AP5 on the frequency and amplitude of mEPSCs of a lamina II neuron recorded from a paclitaxel-treated rat. C, box/whisker plots show the baseline and effect of AP5 on the frequency and amplitude of mEPSCs in vehicle-treated rats (n = 8 neurons, 4 rats) and paclitaxel-treated (n = 12 neurons, 5 rats). *, p < 0.05 compared with the respective baseline control. #, p < 0.05 compared with baseline in the vehicle-treated group.
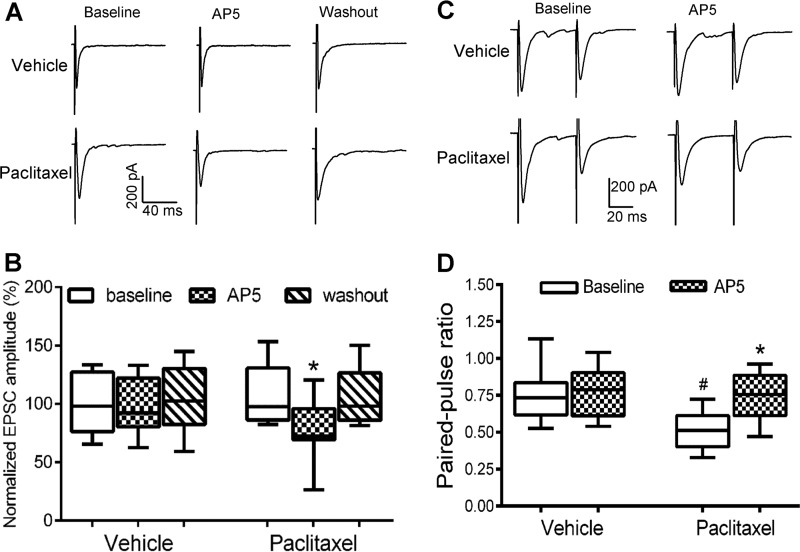
Paclitaxel Increases NMDAR Activity at Primary Afferent Terminals in the Spinal Cord
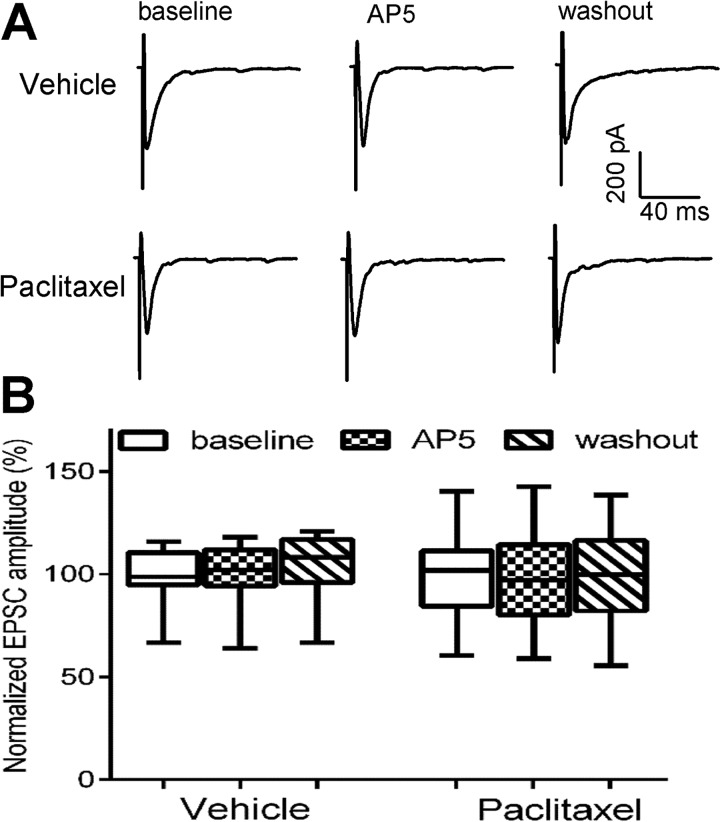
To specifically determine whether paclitaxel treatment increases the activity of NMDARs at primary afferent terminals, we recorded monosynaptic EPSCs of spinal dorsal horn neurons evoked from the dorsal root, which represents induced glutamate released from primary afferent terminals (21, 22). Because the amplitude of evoked EPSCs largely depends on the stimulation intensity, the amplitude of evoked EPSCs was normalized to the baseline for each recorded neuron. Bath application of 50 μm AP5 significantly reduced the amplitude of monosynaptic EPSCs of lamina II neurons in paclitaxel-treated rats (n = 10 neurons, Fig. 2, A and B). However, AP5 had no significant effect on the amplitude of monosynaptic EPSCs of lamina II neurons in vehicle-treated rats (n = 12 neurons).
FIGURE 2.
Paclitaxel increases the activity of NMDARs at primary afferent terminals. A, representative recordings show the effect of 50 μm AP5 on monosynaptic EPSCs of a lamina II neuron evoked from the dorsal root in a vehicle-treated rat and a paclitaxel-treated rat. B, group data show the effect of AP5 on the mean amplitude of evoked EPSCs of lamina II neurons in vehicle-treated rats (n = 11 neurons, 4 rats) and paclitaxel-treated rats (n = 10 neurons, 4 rats). *, p < 0.05 compared with the respective baseline control. C, original current traces show EPSCs of dorsal horn neurons evoked by a pair of pulses before and during AP5 application in a vehicle-treated rat and a paclitaxel-treated rat. D, box/whisker plots show changes in the paired-pulse ratio of evoked EPSCs and the AP5 effect in vehicle-treated rats (n = 14 neurons, 5 rats) and paclitaxel-treated rats (n = 12 neurons, 4 rats). *, p < 0.05 compared with the respective baseline control. #, p < 0.05 compared with baseline in the vehicle-treated group.
Furthermore, we examined the effect of paclitaxel treatment on the paired-pulse ratio (PPR) of monosynaptically evoked EPSCs in spinal dorsal horn neurons. The PPR was markedly reduced in paclitaxel-treated rats, compared with that in vehicle-treated rats (Fig. 2, C and D). Bath application of AP5 had no significant effect on the PPR in vehicle control rats. However, AP5 application significantly inhibited the first evoked EPSCs more than the second evoked EPSCs, resulting in an increase in the PPR of paclitaxel-treated rats (Fig. 2, C and D). These results suggest that presynaptic NMDAR activity at primary afferent terminals is increased by paclitaxel treatment.
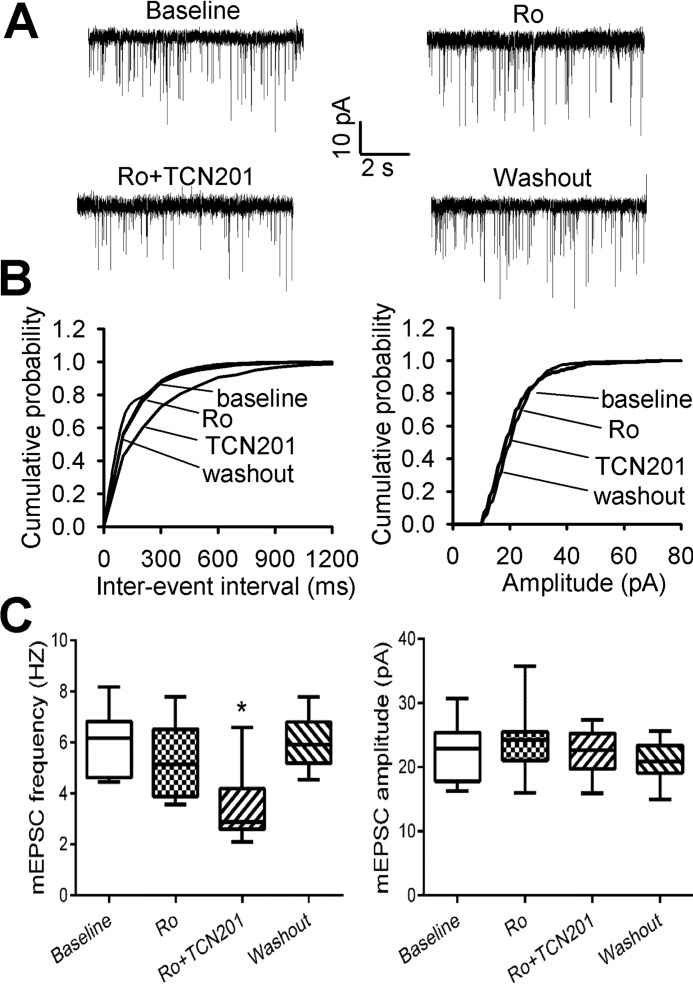
GluN2A Subunit Contributes to Increased Presynaptic NMDAR Activity Induced by Paclitaxel
NMDARs in the spinal dorsal horn mainly contain GluN2A and GluN2B subunits (9, 10). To determine the contribution of GluN2A and GluN2B subunits to the increased presynaptic NMDAR activity of dorsal horn neurons induced by paclitaxel, we used Ro 25-6981, a highly specific GluN2B-containing NMDAR antagonist (23), and TCN201, a GluN2A-selective antagonist (24, 25). Previous studies have shown that bath application of 0.6 μm Ro 25-6981 maximally blocks GluN2B-containing NMDAR-EPSCs (9, 18) and that 10 μm TCN201 inhibits GluN2A-containing NMDAR but has no effect on NMDARs containing GluN2B, GluN2C, or GluN2D (24). Bath application of Ro 25–6981 did not significantly alter the frequency or amplitude of mEPSCs of lamina II neurons in paclitaxel-treated rats. In these neurons, further application with 10 μm TCN201 significantly reduced the frequency, but not the amplitude, of mEPSCs (n = 9 neurons, Fig. 3, A–C).
FIGURE 3.
Paclitaxel increases GluN2A-containing presynaptic NMDARs of spinal dorsal horn neurons. A and B, representative traces and cumulative plots show the effect of bath application of 0.6 μm Ro 25–6981 (Ro) and 10 μm TCN201 (Ro+TCN201) on the frequency and amplitude of mEPSCs of a lamina II neuron from a paclitaxel-treated rat. C, group data of the effects of Ro 25–6981 (Ro) and TCN201 (Ro+TCN201) on the frequency and amplitude of mEPSCs in paclitaxel-treated rats (n = 9 neurons, 4 rats). *, p < 0.05 compared with the baseline control.
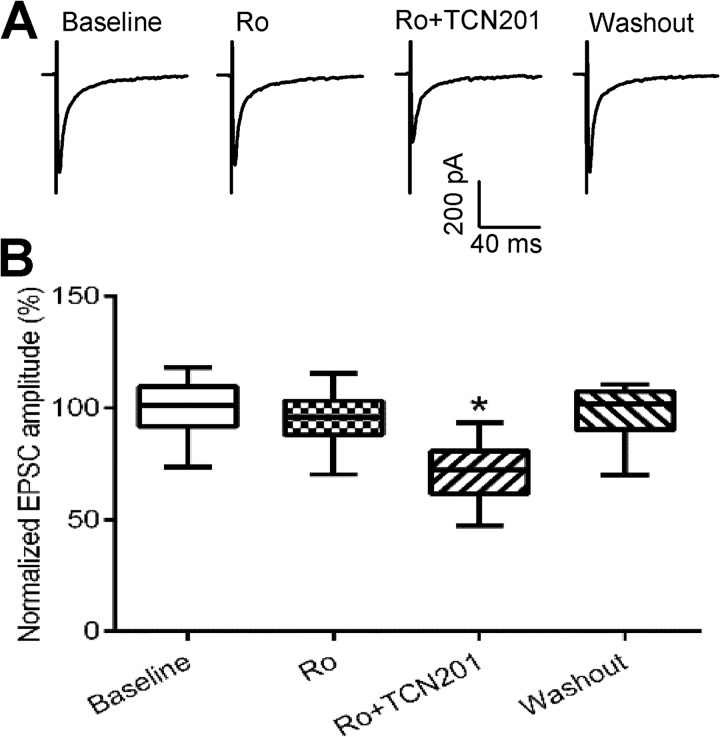
We also determined the relative contribution of GluN2A and GluN2B subunits to the increased NMDAR activity at primary afferent terminals in paclitaxel-treated rats. Bath application of 0.6 μm Ro 25-6981 did not significantly reduce the amplitude of monosynaptic EPSCs of 12 lamina II neurons in paclitaxel-treated rats. Nevertheless, further application of 10 μm TCN201 significantly decreased the amplitude of EPSCs of these neurons (Fig. 4). These results suggest that paclitaxel treatment primarily increases the activity of GluN2A-containing presynaptic NMDARs in the spinal cord.
FIGURE 4.
Paclitaxel increases the activity of GluN2A-containing NMDARs at primary afferent terminals. A, original recordings show the effect of 0.6 μm Ro 25–6981 (Ro) and 10 μm TCN201 (Ro+TCN201) on the evoked monosynaptic EPSCs of a lamina II neuron from a paclitaxel-treated rat. B, summary data show the effect of Ro 25–6981 (Ro) and TCN201 (Ro+TCN201) on the mean amplitude of evoked EPSCs of lamina II neurons in paclitaxel-treated rats (n = 12 neurons, 4 rats). *, p < 0.05 compared with the baseline control.
PKC Mediates the Paclitaxel-induced Increase in Presynaptic NMDAR Activity in the Spinal Cord
Paclitaxel increases PKC activity in the DRG, and PKC inhibition attenuates paclitaxel-induced pain hypersensitivity (26). However, the way in which increased PKC activation leads to neuropathic pain after paclitaxel injections is unclear. Increased PKC activity can promote NMDAR surface expression and regulate the channel gating (27, 28). We thus determined whether PKC plays a role in the paclitaxel-induced increase in presynaptic NMDAR activity in the spinal cord. Chelerythrine is a specific membrane-permeable inhibitor of the catalytic site of PKC (29). It has been shown that 10 μm chelerythrine inhibits PKC activity in spinal cord slices (18). In vehicle-treated rats, bath application of 50 μm AP5 had no effect on the baseline frequency of mEPSCs or the amplitude of evoked monosynaptic EPSCs in the spinal cord slices incubated with 10 μm chelerythrine for 1–2 h (Figs. 5 and 6). Chelerythrine normalized the baseline frequency of mEPSCs from paclitaxel-treated rats (n = 11 neurons, Fig. 5, A and B). In these neurons, application of 50 μm AP5 had no effect on the frequency of mEPSCs. Also, AP5 had no effect on the amplitude of EPSCs monosynaptically evoked from the dorsal root in paclitaxel-treated rats (n = 10 neurons, Fig. 6, A and B). These findings suggest that PKC plays a critical role in the paclitaxel-induced increase in presynaptic NMDAR activity in the spinal cord.
FIGURE 5.
PKC mediates the paclitaxel-induced potentiation in presynaptic NMDAR activity of spinal dorsal horn neurons. A, representative recordings and cumulative plots show the lack of effect of 50 μm AP5 on the frequency and amplitude of mEPSCs of a lamina II neuron and cumulative plots from a spinal cord slice pretreated with 10 μm chelerythrine in a vehicle-treated rat and a paclitaxel-treated rat. B, box/whisker plots show the effect of AP5 on the mean frequency and amplitude of mEPSCs in spinal cord slices pretreated with chelerythrine in vehicle-treated (n = 10 neurons, 4 rats) and paclitaxel-treated rats (n = 11 neurons, 5 rats).
FIGURE 6.
PKC mediates the paclitaxel-induced activation of presynaptic NMDARs at primary afferent terminals. A, original current traces show the lack of effect of 50 μm AP5 on the amplitude of monosynaptic EPSCs of a lamina II neuron from a spinal cord slice pretreated with 10 μm chelerythrine in a vehicle-treated rat and a paclitaxel-treated rat. B, group data show the effect of AP5 on the amplitude of evoked EPSCs in spinal cord slices pretreated with chelerythrine in vehicle-treated (n = 11 neurons, 4 rats) and paclitaxel-treated rats (n = 10 neurons, 3 rats).
Paclitaxel Treatment Increases the Protein Levels of GluA2 and Phosphorylated GluN1 in the DRG
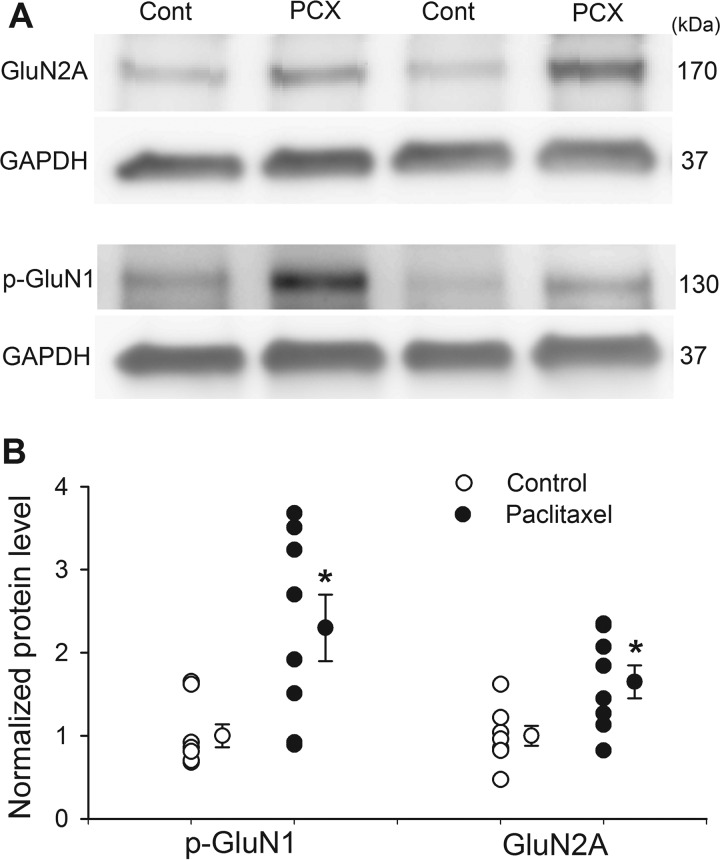
The above electrophysiological data suggest that paclitaxel-induced increases in NMDAR activity at primary afferent terminals are mediated by GluN2A and PKC-dependent phosphorylation. We conducted additional biochemical experiments to determine whether the protein levels of GluN2A and PKC-phosphorylated GluN1 in the DRG is increased by paclitaxel treatment. Western immunoblotting analysis showed the protein level of GluN2A in the DRG tissue was significantly greater in paclitaxel-treated than in vehicle-treated rats (Fig. 7).
FIGURE 7.
Paclitaxel increases the protein levels of GluN2A and PKC-dependent NMDAR phosphorylation in the DRG. A, representative gel images show protein levels of GluN2A and Ser896-phosphorylated GluN1 (p-GluN1) in the DRG tissues obtained from a paclitaxel (PCX)-treated rat and a vehicle-treated control (Cont) rat. B, scatter plots and mean data (means ± S.E.) show changes of the amount of GluN2A and Ser896 phosphorylated GluN1 (p-GluN1) proteins in the DRG of paclitaxel-treated (n = 8) and vehicle-treated control (n = 8) rats. GAPDH was used as a loading control. *, p < 0.05 compared with vehicle-treated control rats.
Because the serine residue 896 of the GluN1 subunit is selectively phosphorylated by PKC (30), we used a phospho-GluN1 (Ser896) antibody to measure paclitaxel-induced changes in PKC-dependent NMDAR phosphorylation. Immunoblotting showed that the amount of Ser896-phosphorylated GluN1 proteins in the DRG was much higher in paclitaxel-treated than in vehicle-treated rats (Fig. 7).
Spinal NMDARs Contribute to Pain Hypersensitivity Induced by Paclitaxel Treatment
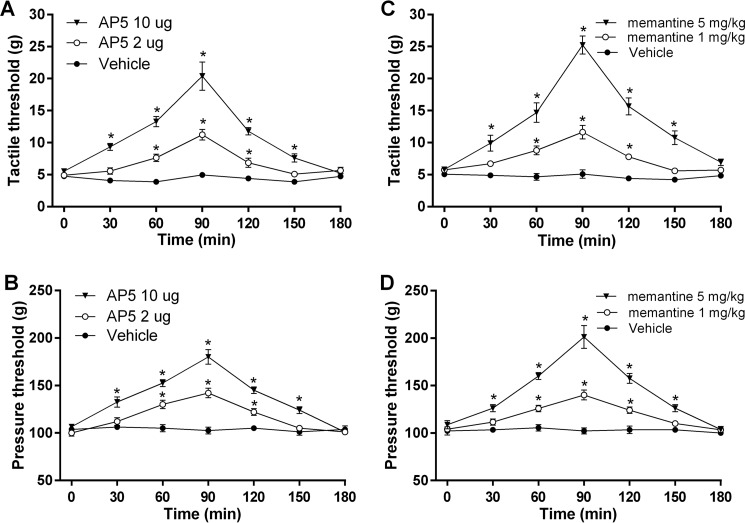
Having shown that the presynaptic NMDAR activity in the spinal cord is increased in rats treated with paclitaxel, we next determined whether increased NMDAR activity contributes to pain hypersensitivity caused by paclitaxel. The baseline tactile and pressure withdrawal thresholds of the hindpaw before paclitaxel injections were 23.76 ± 3.98 g and 161.3 ± 3.98 g, respectively. Paclitaxel treatment caused a large reduction in tactile (allodynia) and pressure (mechanical hyperalgesia) withdrawal thresholds (Fig. 8). We tested the effect of intrathecal injection of AP5 via lumbar puncture on tactile allodynia and mechanical hyperalgesia 2–3 weeks after treatment with paclitaxel. We have shown that intrathecal injection of 0.1–10 μg of AP5 attenuates pain hypersensitivity induced by spinal nerve injury or a calcineurin inhibitor in rats (9, 11). AP5 was dissolved in saline and administered in a volume of 20 μl. Intrathecal injection of the vehicle had no significant effect on tactile allodynia or hyperalgesia. In contrast, intrathecal injection of both 2 and 10 μg of AP5 produced a significant reversal of tactile allodynia and mechanical hyperalgesia in paclitaxel-treated rats (n = 10–12 rats per group, Fig. 8, A and B). The effect of a single injection of 10 μg of AP5 was maximal at 90 min and lasted for about 2.5 h.
FIGURE 8.
Blocking NMDARs reduces the pain hypersensitivity of rats treated with paclitaxel. A and B, time course of the effect of intrathecal injection of vehicle (n = 10 rats) or 2 μg of AP5 (n = 10 rats) and 10 μg of AP5 (n = 12 rats) on the paw withdrawal threshold measured with von Frey filaments (A) and a pressure stimulus (B) in rats treated with paclitaxel. C and D, time course of the effect of a single intraperitoneal injection of vehicle (n = 9 rats) or memantine (1 mg/kg, n = 12 rats; 5 mg/kg, n = 8 rats) on the paw withdrawal threshold tested with von Frey filaments (C) and a noxious pressure stimulus (D) in rats treated with paclitaxel. Data are expressed as means ± S.E. *, p ≤ 0.05 compared with the baseline (time 0).
Systemic Administration of Memantine Reverses Pain Hypersensitivity Caused by Paclitaxel
Memantine, an orally active NMDAR antagonist, is used clinically to treat patients with Alzheimer disease and has relatively few adverse effects (31). We thus determined whether systemic administration of memantine could reduce the mechanical hypersensitivity caused by paclitaxel. We have shown that intraperitoneal injection of 1–10 mg/kg of memantine attenuates pain hypersensitivity caused by FK506, a calcineurin inhibitor (9). In paclitaxel-treated rats, intraperitoneal injection of the vehicle produced no effect on tactile allodynia or mechanical hyperalgesia. Intraperitoneal administration of memantine (1 and 5 mg/kg) significantly reduced tactile allodynia and mechanical hyperalgesia in paclitaxel-treated rats (n = 8–12 rats per group; Fig. 8, C and D). At 10 mg/kg, memantine's effect was maximal at 90 min and subsided 2.5 h after administration. These data suggest that the increased NMDAR activity at the spinal cord level contributes to the pain hypersensitivity induced by paclitaxel treatment.
Discussion
Our findings indicate that paclitaxel treatment induces tonic activation of presynaptic NMDARs in the spinal cord through PKC to potentiate nociceptive input from primary afferent nerves. In the present study, we used the spinal cord slice preparation because it preserves the intrinsic connection between primary afferents and second-order sensory neurons in the spinal cord and express native NMDARs. Changes in presynaptic NMDAR activity can be measured by recording mEPSCs and NMDAR-mediated monosynaptic EPSCs evoked directly from stimulation of the dorsal root in spinal cord slices. We found that paclitaxel treatment significantly increased baseline mEPSC frequency of dorsal horn neurons, which was readily normalized to the level seen in vehicle-treated rats by bath application of AP5, a selective NMDAR antagonist that competitively inhibits the glutamate binding site on GluN2 subunits (32). The increased frequency of mEPSCs in paclitaxel-treated rats was recorded in the presence of 1.2 mm extracellular Mg2+ and tetrodotoxin, which blocks voltage-gated sodium channels and action potential generation. Presynaptic NMDARs are more resistant to Mg2+ blockage and action potential suppression than are postsynaptic NMDARs (15, 33). We also found that AP5 significantly reduced the amplitude of EPSCs monosynaptically evoked from primary afferents in paclitaxel-treated rats, which is consistent with previous studies showing that presynaptic NMDARs also regulate evoked (action potential-driven) neurotransmitter release in the cortex and cerebellum (15, 16). In addition, we showed that paclitaxel treatment profoundly decreased the PPR of evoked EPSCs, which was significantly reversed by AP5. These findings indicate that presynaptic NMDARs are tonically activated by ambient glutamate in paclitaxel-treated rats.
Activation of NMDARs and the resulting Ca2+ influx through these channels are crucial for synaptic plasticity in physiological and pathological conditions, including memory and neuropathic pain (8). Presynaptic NMDARs, which have been recorded at many cortical synapses (14, 34, 35), can regulate neurotransmitter release by causing Ca2+ influx to trigger vesicle exocytosis. The sources of glutamate for the paclitaxel-induced tonic activation of presynaptic NMDARs probably originate from the same primary afferent terminals on which the NMDARs are expressed (autoreceptor) or from excitatory interneurons in the dorsal horn owing to the diminished synaptic inhibition caused by paclitaxel treatment (13), resulting in direct synaptic excitation of presynaptic boutons (axo-axonic). Alternatively, the paclitaxel-induced tonic activation of presynaptic NMDARs in the spinal cord may result from glutamate diffusion from an adjacent active terminal (spillover), activity-dependent release of glutamate from the dendrites of a postsynaptic cell (retrograde), reduced glutamate reuptake due to reduced glutamate transporter activity, and/or glutamate release from surrounding glial cells (paracrine).
The findings of the present study help elucidate the molecular composition of presynaptic NMDARs affected by paclitaxel treatment. In cortical neurons, presynaptic NMDARs mainly contain the GluN2B subunit (15, 33). NMDARs in the spinal cord are mainly composed of GluN1, GluN2A, and GluN2B subunits (36, 37). We have shown that both GluN2A- and GluN2B-containing NMDARs contribute to increased activity of presynaptic NMDARs in the spinal cord induced by repeated treatments with morphine or FK506 (9, 18). In the present study, we found that TCN201, a highly selective GluN2A antagonist (24, 25), significantly reduced the frequency of mEPSCs of dorsal horn neurons in paclitaxel-treated rats. Similarly, TCN201 significantly reduced the amplitude of the monosynaptic EPSCs of dorsal horn neurons evoked from the dorsal root in paclitaxel-treated rats. Interestingly, Ro 25–6981, which displays a 3,300-fold greater selectivity for the GluN2B than other NMDAR subunits (23), had no significant effect on the mEPSC frequency or the amplitude of evoked monosynaptic EPSCs of dorsal horn neurons in paclitaxel-treated rats. We also showed that paclitaxel significantly increased the protein level of GluN2A in the DRG. Therefore, our findings suggest that GluN2A-containing NMDARs on primary afferent terminals predominantly mediate increased synaptic glutamate release to spinal dorsal horn neurons induced by paclitaxel.
Our findings also provide new insight into the signaling mechanisms responsible for increased NMDAR activity at primary afferents after paclitaxel treatment. The way in which presynaptic NMDAR activity promotes tonic transmitter release is unclear, although presynaptic NMDARs in the cortex can function with minimal extracellular or intracellular Ca2+ requirement (38, 39) and in a PKC-dependent manner (38). The balance between phosphorylation and dephosphorylation, which is mediated by the kinases and phosphatases, respectively, is essential to maintaining NMDAR activity. Dysregulation of this balance in favor of phosphorylation is likely to be a pivotal mechanism for potentiating NMDAR activity in pathological pain conditions. Inhibition of calcineurin (i.e. protein phosphatase 2B) increases presynaptic NMDAR activity of the spinal dorsal horn neuron, which supports the notion that increased phosphorylation can potentiate presynaptic NMDAR activity. An increase in NMDAR phosphorylation in the spinal cord has been reported at a PKC-dependent site, Ser896 (40). PKC is critically involved in the increased presynaptic NMDAR activity at primary afferent terminals in opioid-induced hyperalgesia (18). Paclitaxel seems to predominantly increase the activity of PKCβII, PKCδ, and PKCϵ in the DRG (26). Increased PKC activity may enhance NMDAR function by reducing the Mg2+ block of NMDARs and/or promoting NMDAR trafficking to the plasma membrane (27, 28). In this study, we showed that inhibition of PKC abolished the increase in the frequency of mEPSCs and the amplitude of evoked EPSCs mediated by NMDARs in paclitaxel-treated rats, suggesting that PKC plays an essential role in paclitaxel-induced increases in presynaptic NMDAR activity in the spinal cord. Thus, presynaptic NMDARs probably serve as a downstream signaling mechanism of PKC to potentiate nociceptive input in paclitaxel-induced neuropathic pain. Our finding that paclitaxel caused a large increase in the protein amount of Ser896 phosphorylated GluN1 in the DRG further supports this conclusion. Nevertheless, whether PKC directly phosphorylates NMDARs or indirectly phosphorylates NMDAR-interacting proteins remains uncertain.
We also found that blocking NMDARs at the spinal cord level by intrathecal injection of AP5 profoundly reversed pain hypersensitivity induced by paclitaxel, which is in agreement with other studies showing that NMDAR antagonists are effective for treating patients with certain neuropathic pain conditions (41–43). In addition, we showed that systemic administration of memantine, a clinically used and orally active NMDAR antagonist, effectively reduced tactile allodynia and mechanical hyperalgesia in paclitaxel-treated rats. These findings provide further evidence about the causal relationship between augmented NMDAR activity at the spinal cord level and pain hypersensitivity induced by paclitaxel. Our findings have important therapeutic implications because memantine could conceivably be an effective treatment for patients with paclitaxel-induced neuropathic pain.
In conclusion, our study provides substantial new evidence that paclitaxel treatment increases the activity of presynaptic GluN2A-containing NMDARs in the spinal cord. The PKC-mediated tonic activation of presynaptic NMDARs contributes to increased glutamatergic input from primary afferents and pain hypersensitivity induced by paclitaxel. This new information is important for understanding the mechanisms of chemotherapy-induced synaptic plasticity and suggests new strategies for treating this neuropathic pain condition.
Experimental Procedures
Animal Model and Paclitaxel Treatment
Experiments were carried out on adult male Sprague-Dawley rats (220–250 g; Harlan, Indianapolis, IN). All procedures and protocols were approved by The University of Texas MD Anderson Cancer Center and were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. To induce peripheral neuropathy, We injected rats intraperitoneally with paclitaxel (2 mg/kg; TEVA Pharmaceuticals, North Wales, PA) on four alternate days (days 1, 3, 5, and 7; total cumulative dose of 8 mg/kg) as described previously (44). Rats in the control group received intraperitoneal injection of the vehicle (Cremophor EL/ethanol, 1:1) on the same four alternate days. We confirmed the presence of mechanical hyperalgesia and tactile allodynia in the hindpaw of rats 10–12 days after the completion of paclitaxel treatment. All final electrophysiological recordings and behavioral tests were done 2–3 weeks after the final paclitaxel and vehicle injections.
Nociceptive Behavioral Tests
Rats were individually placed on a mesh floor within suspended chambers and allowed to acclimate for at least 30 min. To determine their tactile sensitivity, we applied a series of calibrated von Frey filaments perpendicularly to the plantar surface of both hindpaws with sufficient force to bend the filament for 6 s. Brisk withdrawal or flinching of the paw was considered a positive response. In the absence of a response, we applied the filament of the next greater force. After a response, we applied the filament of the next lower force. The tactile stimulus producing a 50% likelihood of withdrawal response was calculated using the “up-down” method (45).
To quantify the mechanical nociceptive threshold, we conducted the paw pressure (Randall-Selitto) test on the left hindpaw using an analgesiometer (Ugo Basil, Varese, Italy). To activate the device, we pressed a foot pedal to activate a motor that applied a constantly increasing force on a linear scale. When the animal displayed pain by either withdrawing of the paw or vocalizing, the pedal was immediately released, and the animal's nociceptive threshold on the scale was recorded (9, 46).
Spinal Cord Slice Preparation
Rats were anesthetized with 2–3% isoflurane, and the lumbar spinal cord at the L3-L6 level was removed through laminectomy. The spinal tissues were immediately placed in an ice-cold sucrose artificial cerebrospinal fluid presaturated with 95% O2 and 5% CO2. The artificial cerebrospinal fluid contained (in mm) 234 sucrose, 3.6 KCl, 1.2 MgCl2, 2.5 CaCl2, 1.2 NaH2PO4, 12.0 glucose, and 25.0 NaHCO3. The spinal cord tissue in artificial cerebrospinal fluid was then placed onto the stage of a vibratome (Leica, Germany), and the spinal cords were sliced transversely into 400-μm sections that were preincubated in Krebs solution oxygenated with 95% O2 and 5% CO2 at 34 °C for at least 1 h before being transferred to the recording chamber. The Krebs solution contained (in mm) 117.0 NaCl, 3.6 KCl, 1.2 MgCl2, 2.5 CaCl2, 1.2 NaH2PO4, 11.0 glucose, and 25.0 NaHCO3.
Electrophysiological Recordings of Dorsal Horn Neurons
The spinal cord slice was placed in a glass-bottomed chamber and continuously perfused with Krebs solution at 5.0 ml/min at 34 °C maintained by an inline solution heater and a temperature controller. The spinal lamina II was identified on an upright fixed-stage microscope with differential interference contrast/infrared illumination. Lamina II outer zone neurons were visualized and selected for recordings because they predominantly receive nociceptive input from primary afferent nerves (47). Most lamina II neurons are glutamate-releasing excitatory interneurons (48), which form a network that plays a critical role in transmitting nociceptive information from the primary afferents. Excitatory postsynaptic currents (EPSCs) were recorded using whole-cell voltage-clamp techniques. The impedance of the glass electrode was 4–7 MΩ when the pipette was filled with the internal solution containing (in mm) 135 potassium gluconate, 5 KCl, 2.0 MgCl2, 0.5 CaCl2, 5.0 HEPES, 5.0 EGTA, 5.0 ATP-Mg, 0.5 Na-GTP, and 10 QX314 (adjusted to pH 7.25 with 1.0 m KOH, 280–300 mosM).
EPSCs were evoked from the dorsal root using a bipolar tungsten electrode connected to a stimulator (0.2 ms, 0.3–0.6 mA, 0.1 Hz; Grass Instruments, Quincy, MA) (21, 22). Monosynaptic EPSCs were identified on the basis of the constant latency and absence of conduction failure of evoked EPSCs in response to a 20-Hz electrical stimulation, as we described previously (22, 49). To measure the paired-pulse ratio (PPR), two EPSCs were evoked by a pair of stimuli given at 50-ms intervals. The PPR was expressed as the ratio of the amplitude of the second synaptic response to the amplitude of the first synaptic response (22). In addition, miniature EPSCs (mEPSCs) were recorded in the presence of 2 μm strychnine, 10 μm bicuculline, and 1 μm tetrodotoxin at a holding potential of −60 mV (9, 46). The input resistance was monitored, and the recording was abandoned if it changed by more than 15%. EPSCs were recorded using an amplifier (MultiClamp 700A, Axon Instruments, Foster City, CA), filtered at 1–2 kHz, and digitized at 10 kHz. Only one neuron was recorded in each spinal cord slice, and at least three rats were used in each recording protocol.
All drugs were freshly prepared in artificial cerebrospinal fluid before the experiments and delivered via syringe pumps to reach their final concentrations. AP5 was purchased from Abcam (Cambridge, MA), chelerythrine was purchased from Sigma-Aldrich, and memantine was obtained from Tocris Bioscience (Avonmouth, Bristol, UK).
Western Immunoblotting
Two weeks after treatment with paclitaxel and vehicle, the lumbar DRG tissues were rapidly removed from rats anesthetized with 3% isoflurane. The DRG tissues were homogenized in ice-cold radioimmune precipitation assay buffer containing cocktails of protease and phosphatase inhibitors (Sigma). The homogenate was centrifuged at 12,000 × g for 10 min at 4 °C, and the supernatant was collected. The samples were subjected to 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel and transferred to polyvinylidene difluoride membrane. The blots were probed with rabbit polyclonal anti-phospho-GluN1 (Ser896) antibody (Cat. ABN88, EMD Millipore, Billerica, MA) or mouse monoclonal anti-GluN2A antibody (Cat. N327/95; 1:1000; NeuroMab, Davis, CA). An ECL kit (GE Healthcare) was used to detect the protein band, which was visualized and quantified with the Odyssey Fc Imager (LI-COR Biosciences, Lincoln, NE). The amount of phospho-GluN1 and GluN2A proteins was normalized by GAPDH in the same samples. The mean values of phospho-GluN1 and GluN2A proteins in vehicle-treated rats were considered to be 1.
Data Analysis
The amplitude of the evoked EPSCs was quantify by averaging 10 consecutive EPSCs off-line with Clampfit 10.0 software (Axon Instruments). The mEPSCs were analyzed off-line using the peak detection MiniAnalysis program (Synaptosoft, Leonia, NJ). The cumulative probability of the amplitude and the inter-event interval of the mEPSCs were compared using the Kolmogorov-Smirnov test, which estimates the probability that two distributions are similar. To compare the differences in electrophysiological data between groups, we used one-way analysis of variance followed by the Tukey post hoc test. All electrophysiological summary data were presented in box-whisker plots, which show the minimum, 25th percentile, median, 75th percentile, and maximum. To determine the treatment effects on hyperalgesia and allodynia, we used one-way analysis of variance followed by the Dunnett post hoc test. p < 0.05 was considered to be statistically significant.
Author Contributions
J. D. X., S. R. C., and H. C. conducted experiments. J. D. X., S. R. C., W. A. Z., and H. L. P. performed data analysis. H. L. P. and S. R. C. conceived the project and wrote the manuscript with input from the other authors.
This work was supported by Grants DE022015 and NS073935 from the National Institutes of Health and by the N.G. and Helen T. Hawkins Endowment (to H.-L. P.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- DRG
- dorsal root ganglion
- AP5
- 2-amino-5-phosphonopentanoic acid
- EPSCs
- excitatory postsynaptic currents
- mEPSCs
- miniature excitatory postsynaptic currents
- NMDARs
- N-methyl-d-aspartate receptors
- PKC
- protein kinase C.
References
- 1. Sisignano M., Baron R., Scholich K., and Geisslinger G. (2014) Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat. Rev. Neurol. 10, 694–707 [DOI] [PubMed] [Google Scholar]
- 2. Scripture C. D., Figg W. D., and Sparreboom A. (2006) Peripheral neuropathy induced by paclitaxel: recent insights and future perspectives. Curr. Neuropharmacol. 4, 165–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee J. J., and Swain S. M. (2006) Peripheral neuropathy induced by microtubule-stabilizing agents. J. Clin. Oncol. 24, 1633–1642 [DOI] [PubMed] [Google Scholar]
- 4. Grisold W., Cavaletti G., and Windebank A. J. (2012) Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro. Oncol. 14, iv45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hara T., Chiba T., Abe K., Makabe A., Ikeno S., Kawakami K., Utsunomiya I., Hama T., and Taguchi K. (2013) Effect of paclitaxel on transient receptor potential vanilloid 1 in rat dorsal root ganglion. Pain 154, 882–889 [DOI] [PubMed] [Google Scholar]
- 6. Matsumoto M., Inoue M., Hald A., Xie W., and Ueda H. (2006) Inhibition of paclitaxel-induced A-fiber hypersensitization by gabapentin. J. Pharmacol. Exp. Ther 318, 735–740 [DOI] [PubMed] [Google Scholar]
- 7. Scuteri A., Nicolini G., Miloso M., Bossi M., Cavaletti G., Windebank A. J., and Tredici G. (2006) Paclitaxel toxicity in post-mitotic dorsal root ganglion (DRG) cells. Anticancer Res. 26, 1065–1070 [PubMed] [Google Scholar]
- 8. Traynelis S. F., Wollmuth L. P., McBain C. J., Menniti F. S., Vance K. M., Ogden K. K., Hansen K. B., Yuan H., Myers S. J., and Dingledine R. (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 62, 405–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen S. R., Hu Y. M., Chen H., and Pan H. L. (2014) Calcineurin inhibitor induces pain hypersensitivity by potentiating pre- and postsynaptic NMDA receptor activity in spinal cords. J. Physiol. 592, 215–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen S. R., Zhou H. Y., Byun H. S., Chen H., and Pan H. L. (2014) Casein kinase II regulates N-methyl-D-aspartate receptor activity in spinal cords and pain hypersensitivity induced by nerve injury. J. Pharmacol. Exp. Ther 350, 301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou H. Y., Chen S. R., Byun H. S., Chen H., Li L., Han H. D., Lopez-Berestein G., Sood A. K., and Pan H. L. (2012) N-methyl-D-aspartate receptor- and calpain-mediated proteolytic cleavage of K+-Cl- cotransporter-2 impairs spinal chloride homeostasis in neuropathic pain. J. Biol. Chem. 287, 33853–33864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamamoto T., and Yaksh T. L. (1992) Spinal pharmacology of thermal hyperesthesia induced by constriction injury of sciatic nerve. Excitatory amino acid antagonists. Pain 49, 121–128 [DOI] [PubMed] [Google Scholar]
- 13. Chen S. R., Zhu L., Chen H., Wen L., Laumet G., and Pan H. L. (2014) Increased spinal cord Na(+)-K(+)-2Cl(-) cotransporter-1 (NKCC1) activity contributes to impairment of synaptic inhibition in paclitaxel-induced neuropathic pain. J. Biol. Chem. 289, 31111–31120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bouvier G., Bidoret C., Casado M., and Paoletti P. (2015) Presynaptic NMDA receptors: Roles and rules. Neuroscience 311, 322–340 [DOI] [PubMed] [Google Scholar]
- 15. Brasier D. J., and Feldman D. E. (2008) Synapse-specific expression of functional presynaptic NMDA receptors in rat somatosensory cortex. J. Neurosci. 28, 2199–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Glitsch M., and Marty A. (1999) Presynaptic effects of NMDA in cerebellar Purkinje cells and interneurons. J. Neurosci. 19, 511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu H., Wang H., Sheng M., Jan L. Y., Jan Y. N., and Basbaum A. I. (1994) Evidence for presynaptic N-methyl-D-aspartate autoreceptors in the spinal cord dorsal horn. Proc. Natl. Acad. Sci. U.S.A. 91, 8383–8387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao Y. L., Chen S. R., Chen H., and Pan H. L. (2012) Chronic opioid potentiates presynaptic but impairs postsynaptic N-methyl-D-aspartic acid receptor activity in spinal cords: implications for opioid hyperalgesia and tolerance. J. Biol. Chem. 287, 25073–25085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li L., Chen S. R., Chen H., Wen L., Hittelman W. N., Xie J. D., and Pan H. L. (2016) Chloride homeostasis critically regulates synaptic NMDA receptor activity in neuropathic pain. Cell Rep. 15, 1376–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yan X., Jiang E., Gao M., and Weng H. R. (2013) Endogenous activation of presynaptic NMDA receptors enhances glutamate release from the primary afferents in the spinal dorsal horn in a rat model of neuropathic pain. J. Physiol. 591, 2001–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li D. P., Chen S. R., Pan Y. Z., Levey A. I., and Pan H. L. (2002) Role of presynaptic muscarinic and GABA(B) receptors in spinal glutamate release and cholinergic analgesia in rats. J. Physiol. 543, 807–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou H. Y., Chen S. R., Chen H., and Pan H. L. (2010) Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J. Neurosci. 30, 4460–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fischer G., Mutel V., Trube G., Malherbe P., Kew J. N., Mohacsi E., Heitz M. P., and Kemp J. A. (1997) Ro 25–6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitro. J. Pharmacol. Exp. Ther 283, 1285–1292 [PubMed] [Google Scholar]
- 24. Hansen K. B., Ogden K. K., and Traynelis S. F. (2012) Subunit-selective allosteric inhibition of glycine binding to NMDA receptors. J. Neurosci. 32, 6197–6208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Edman S., McKay S., Macdonald L. J., Samadi M., Livesey M. R., Hardingham G. E., and Wyllie D. J. (2012) TCN 201 selectively blocks GluN2A-containing NMDARs in a GluN1 co-agonist dependent but non-competitive manner. Neuropharmacology 63, 441–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. He Y., and Wang Z. J. (2015) Nociceptor β II, δ, and ϵ isoforms of PKC differentially mediate paclitaxel-induced spontaneous and evoked pain. J. Neurosci. 35, 4614–4625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen L., and Huang L. Y. (1992) Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature 356, 521–523 [DOI] [PubMed] [Google Scholar]
- 28. Lan J. Y., Skeberdis V. A., Jover T., Grooms S. Y., Lin Y., Araneda R. C., Zheng X., Bennett M. V., and Zukin R. S. (2001) Protein kinase C modulates NMDA receptor trafficking and gating. Nat. Neurosci. 4, 382–390 [DOI] [PubMed] [Google Scholar]
- 29. Herbert J. M., Augereau J. M., Gleye J., and Maffrand J. P. (1990) Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem. Biophys. Res. Commun. 172, 993–999 [DOI] [PubMed] [Google Scholar]
- 30. Tingley W. G., Ehlers M. D., Kameyama K., Doherty C., Ptak J. B., Riley C. T., and Huganir R. L. (1997) Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J. Biol. Chem. 272, 5157–5166 [DOI] [PubMed] [Google Scholar]
- 31. Farlow M. R., and Cummings J. L. (2007) Effective pharmacologic management of Alzheimer's disease. Am. J. Med. 120, 388–397 [DOI] [PubMed] [Google Scholar]
- 32. Chen P. E., Geballe M. T., Stansfeld P. J., Johnston A. R., Yuan H., Jacob A. L., Snyder J. P., Traynelis S. F., and Wyllie D. J. (2005) Structural features of the glutamate binding site in recombinant NR1/NR2A N-methyl-D-aspartate receptors determined by site-directed mutagenesis and molecular modeling. Mol. Pharmacol. 67, 1470–1484 [DOI] [PubMed] [Google Scholar]
- 33. Woodhall G., Evans D. I., Cunningham M. O., and Jones R. S. (2001) NR2B-containing NMDA autoreceptors at synapses on entorhinal cortical neurons. J. Neurophysiol. 86, 1644–1651 [DOI] [PubMed] [Google Scholar]
- 34. Corlew R., Brasier D. J., Feldman D. E., and Philpot B. D. (2008) Presynaptic NMDA receptors: newly appreciated roles in cortical synaptic function and plasticity. Neuroscientist 14, 609–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bardoni R., Torsney C., Tong C. K., Prandini M., and MacDermott A. B. (2004) Presynaptic NMDA receptors modulate glutamate release from primary sensory neurons in rat spinal cord dorsal horn. J. Neurosci. 24, 2774–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Momiyama A. (2000) Distinct synaptic and extrasynaptic NMDA receptors identified in dorsal horn neurones of the adult rat spinal cord. J. Physiol. 523, 621–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nagy G. G., Watanabe M., Fukaya M., and Todd A. J. (2004) Synaptic distribution of the NR1, NR2A and NR2B subunits of the N-methyl-D-aspartate receptor in the rat lumbar spinal cord revealed with an antigen-unmasking technique. Eur. J. Neurosci. 20, 3301–3312 [DOI] [PubMed] [Google Scholar]
- 38. Kunz P. A., Roberts A. C., and Philpot B. D. (2013) Presynaptic NMDA receptor mechanisms for enhancing spontaneous neurotransmitter release. J. Neurosci. 33, 7762–7769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Berretta N., and Jones R. S. (1996) Tonic facilitation of glutamate release by presynaptic N-methyl-D-aspartate autoreceptors in the entorhinal cortex. Neuroscience 75, 339–344 [DOI] [PubMed] [Google Scholar]
- 40. Brenner G. J., Ji R. R., Shaffer S., and Woolf C. J. (2004) Peripheral noxious stimulation induces phosphorylation of the NMDA receptor NR1 subunit at the PKC-dependent site, serine-896, in spinal cord dorsal horn neurons. Eur. J. Neurosci. 20, 375–384 [DOI] [PubMed] [Google Scholar]
- 41. Sinis N., Birbaumer N., Gustin S., Schwarz A., Bredanger S., Becker S. T., Unertl K., Schaller H. E., and Haerle M. (2007) Memantine treatment of complex regional pain syndrome - A preliminary report of six cases. Clin. J. Pain 23, 237–243 [DOI] [PubMed] [Google Scholar]
- 42. Schwartzman R. J., Alexander G. M., Grothusen J. R., Paylor T., Reichenberger E., and Perreault M. (2009) Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: A double-blind placebo controlled study. Pain 147, 107–115 [DOI] [PubMed] [Google Scholar]
- 43. Sigtermans M. J., van Hilten J. J., Bauer M. C. R., Arbous M. S., Marinus J., Sarton E. Y., and Dahan A. (2009) Ketamine produces effective and long-term pain relief in patients with Complex Regional Pain Syndrome Type 1. Pain 145, 304–311 [DOI] [PubMed] [Google Scholar]
- 44. Polomano R. C., Mannes A. J., Clark U. S., and Bennett G. J. (2001) A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain 94, 293–304 [DOI] [PubMed] [Google Scholar]
- 45. Chaplan S. R., Bach F. W., Pogrel J. W., Chung J. M., and Yaksh T. L. (1994) Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 [DOI] [PubMed] [Google Scholar]
- 46. Chen S. R., Chen H., Yuan W. X., and Pan H. L. (2011) Increased presynaptic and postsynaptic α2-adrenoceptor activity in the spinal dorsal horn in painful diabetic neuropathy. J. Pharmacol. Exp. Ther 337, 285–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pan Y. Z., and Pan H. L. (2004) Primary afferent stimulation differentially potentiates excitatory and inhibitory inputs to spinal lamina II outer and inner neurons. J. Neurophysiol. 91, 2413–2421 [DOI] [PubMed] [Google Scholar]
- 48. Santos S. F., Rebelo S., Derkach V. A., and Safronov B. V. (2007) Excitatory interneurons dominate sensory processing in the spinal substantia gelatinosa of rat. J. Physiol. 581, 241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou H. Y., Chen S. R., Chen H., and Pan H. L. (2008) Sustained inhibition of neurotransmitter release from nontransient receptor potential vanilloid type 1-expressing primary afferents by mu-opioid receptor activation-enkephalin in the spinal cord. J. Pharmacol. Exp. Ther. 327, 375–382 [DOI] [PubMed] [Google Scholar]