Abstract
Cerebral malaria is a severe and often fatal complication of Plasmodium falciparum infection. It is characterized by parasite sequestration, a breakdown of the blood-brain barrier, and a strong inflammation in the brain. We investigated the role of the cannabinoid receptor 2 (CB2), an important modulator of neuroinflammatory responses, in experimental cerebral malaria (ECM). Strikingly, mice with a deletion of the CB2-encoding gene (Cnr2−/−) inoculated with Plasmodium berghei ANKA erythrocytes exhibited enhanced survival and a diminished blood-brain barrier disruption. Therapeutic application of a specific CB2 antagonist also conferred increased ECM resistance in wild type mice. Hematopoietic derived immune cells were responsible for the enhanced protection in bone marrow (BM) chimeric Cnr2−/− mice. Mixed BM chimeras further revealed that CB2-expressing cells contributed to ECM development. A heterogeneous CD11b+ cell population, containing macrophages and neutrophils, expanded in the Cnr2−/− spleen after infection and expressed macrophage mannose receptors, arginase-1 activity, and IL-10. Also in the Cnr2−/− brain, CD11b+ cells that expressed selected anti-inflammatory markers accumulated, and expression of inflammatory mediators IFN-γ and TNF-α was reduced. Finally, the M2 macrophage chemokine CCL17 was identified as an essential factor for enhanced survival in the absence of CB2, because CCL17 × Cnr2 double-deficient mice were fully susceptible to ECM. Thus, targeting CB2 may be promising for the development of alternative treatment regimes of ECM.
Keywords: chemokine, endocannabinoid, macrophage, malaria, neuroinflammation
Introduction
Cerebral malaria (CM) 6 is a life-threatening complication of Plasmodium falciparum infection and one of the leading causes of mortality worldwide (1–3). CM pathology is associated with a sequestration of parasitized red blood cells within the CNS microvasculature, which triggers an excessive inflammatory CNS response and an increase in blood-brain barrier permeability (1, 2). CM can be modeled in mice through inoculation with Plasmodium berghei ANKA (PbA)-infected red blood cells (1–8). Those mice develop severe neurological symptoms of ECM within 5–8 days after infection that lead to coma and death by days 7–9 (4). Disease pathogenesis is associated with a widespread activation of microglial cells, the production of inflammatory cytokines, and endothelial cell damage (1, 6). It was suggested that macrophages support pathogenic T cell responses in the CNS leading to the death of mice with experimental CM (ECM). Conversely, macrophages are also capable of mediating anti-parasite responses by clearance of parasites or production of IL-10, a cytokine shown to limit disease progression (9–11).
The endocannabinoid system plays a key role in immune modulation of the CNS by signaling via CB2 (12–19). It may therefore hold a therapeutic potential for the treatment of infectious CNS disorders. CB2 signaling affects various macrophage functions, such as antigen uptake, antigen presentation, and chemokine/cytokine production. For example, macrophages isolated from Cnr2−/− mice presented reduced T cell stimulatory potential in vitro compared with WT controls, indicating CB2-mediated alterations in antigen processing (20, 21). In addition, it was demonstrated that activation of CB2 negatively regulated IL-12p40 and NO production but enhanced IL-10 release in LPS-activated macrophages (22, 23).
In this study, we investigated the role of CB2 in ECM by using Cnr2−/− mice, mixed bone marrow (BM) chimeras, and a pharmacological approach. We could demonstrate that CB2 modulates susceptibility to ECM associated with anti-inflammatory macrophage effector responses. Our findings and the fact that the therapeutic application of the CB2 antagonist SR144528 conferred enhanced CM resistance in C57BL/6 mice provide the basis for the development of novel therapeutic strategies targeting CB2 for the treatment of ECM.
Results
Enhanced Protection of Cnr2−/− Mice against ECM Is Mediated by Immune Cells
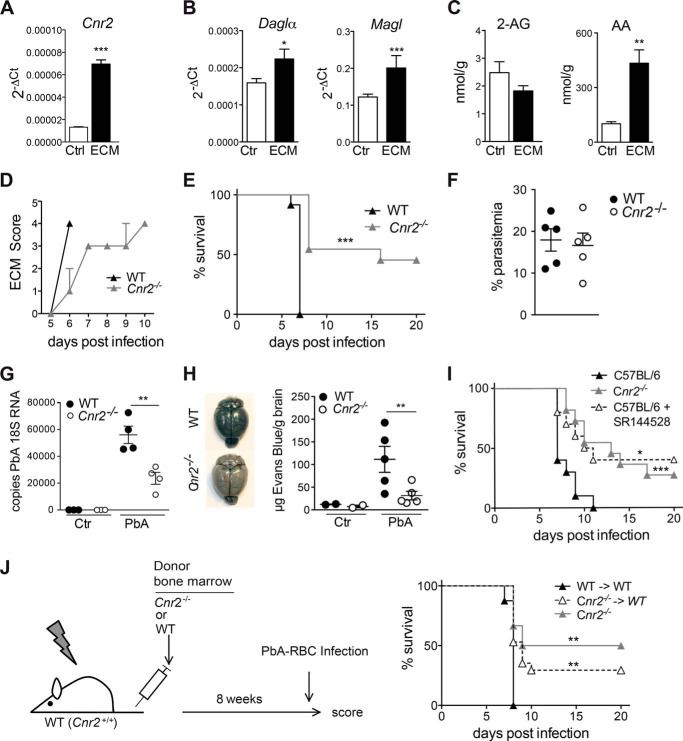
We studied different components of the endocannabinoid system after PbA infection of C57BL/6 mice (20, 24). Expression of the cannabinoid receptor 2 (Cnr2) mRNA was markedly enhanced in the brain at day 6 after infection (Fig. 1A), whereas cannabinoid receptor 1 (Cnr1) expression remained unchanged (2−ΔCt, control: 0.009 ± 0.00065; ECM: 0.009 ± 0.0003). Fatty acid amide hydrolase and diacylglycerol lipase β brain mRNA levels were not altered (2−ΔCt, control: 0.0054 ± 0.0008; ECM WT: 0.0052 ± 0.0015). However, diacylglycerol lipase α, an enzyme involved in the biosynthesis of the endocannabinoid 2-arachidonylglycerol (2-AG), and monoacylglycerol lipase, an enzyme metabolizing 2-AG, were significantly increased (Fig. 1B). The enhanced expression of these enzymes suggested an increased turnover of 2-AG. We determined indeed similar 2-AG levels in PbA-infected and control mice, whereas arachidonic acid (AA) levels were higher, potentially reflecting 2-AG hydrolysis (Fig. 1C). Thus, brain 2-AG signaling via CB2 seems to be affected by PbA infection.
FIGURE 1.
Enhanced protection of Cnr2−/− mice from ECM is mediated by immune cells. A, Cnr2 mRNA expression in the brains of control and day 6 PbA-infected C57BL/6 mice. B, diacylglycerol lipase α (Daglα) and monoacylglycerol lipase mRNA expression in the brains of control and day 6 infected C57BL/6 mice (mean ± S.E. (error bars), n = 3–5 mice/group). C, levels of 2-AG and arachidonic acid (AA) in the brains of control or day 6 infected C57BL/6 mice (mean ± S.E. of nmol/g of total extracted brain, n = 10 mice/group). D, clinical score of infected mice. Clinical symptoms were scored daily as follows: 0, no clinical symptoms; 1, ruffled fur; 2, hunching; 3, wobbly gait; 4, limb paralysis; 5, convulsion; 6, coma. Representative data of one of at least five independent experiments are shown (n = 10–12 mice/group). E, survival of WT and Cnr2−/− mice after infection with PbA (n = 10–12 mice/group). F, peripheral parasitemia in WT and Cnr2−/− mice at day 6 after PbA infection as determined by Giemsa-stained thin blood smears. Representative data of one of three independent experiments are shown (n = 5 mice/group). G, parasite-specific 18S RNA expression in the brains of day 6 infected WT and Cnr2−/− mice, naive Cnr2−/− mice, and WT controls. H, reduced cerebral vascular leakage in PbA-infected Cnr2−/− mice, as assessed by blue staining of the brains of injected mice with Evans Blue on day 6 after PbA infection. The diagram shows mean ± S.E. μg of Evans Blue/g of brain tissue. I, survival of PbA-infected C57BL/6 mice treated daily with the CB2 antagonist SR144528 or vehicle and infected Cnr2−/− controls. Data from one of two representative experiments are shown (n = 8–12 mice/group). J, survival of PbA-infected BM chimeras and Cnr2−/− controls. Lethally irradiated WT mice were reconstituted with WT or Cnr2−/− BM cells and PbA-infected after successful reconstitution. One representative experiment of two independent experiments is shown (n = 7–12 mice/group). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
We found that PbA-infected WT mice developed severe ECM starting at day 5 and succumbed to ECM 24–48 h later (Fig. 1, D and E). In contrast, infected Cnr2−/− mice exhibited a diminished clinical score and an enhanced survival (p < 0.0001). Approximately 50% of the infected Cnr2−/− mice were protected from development of ECM and finally succumbed to hyperparasitemia and severe anemia after >20 days. This variability in survival points toward threshold effects influencing cerebral symptoms and survival of Cnr2−/− mice. Parasitemia in Cnr2−/− mice was similar to controls, indicating that a reduced parasite burden in the blood did not account for enhanced survival (Fig. 1F). However, attenuated ECM in Cnr2−/− mice was accompanied by a reduction of parasite-specific 18S RNA levels in the brain (Fig. 1G). The reduced 18S RNA in the presence of a comparable parasitemia in Cnr2−/− versus WT mice indicates a reduction in parasite sequestration in the brain (25). We therefore investigated the integrity of the blood-brain-barrier (BBB) by assessing the diffusion of Evans Blue into the brain (1, 2). In contrast to WT mice, infected Cnr2−/− brains displayed a reduced Evans Blue staining, indicative of an attenuated BBB disruption (Fig. 1H). Thus, the severity of PbA infection and incidence of ECM were significantly reduced in Cnr2−/− mice, which could account for the higher survival rate of these mice.
We next asked whether severity of ECM could also be modulated by pharmacological blockade of CB2 (26). PbA-infected C57BL/6 mice were treated daily with the CB2 antagonist SR144528. Most strikingly, these mice also showed an enhanced survival rate (p = 0.0116; Fig. 1I). These data demonstrate that the reduced ECM incidence in Cnr2−/− mice is due to a disrupted CB2 signaling rather than being a consequence of compensatory developmental changes. They also indicate a therapeutic potential of CB2 antagonists/inverse agonists.
To evaluate whether expression of CB2 on hematopoietically derived immune cells affects disease pathology, we reconstituted lethally irradiated WT mice with BM cells from Cnr2−/− (Cnr2−/− → WT) or WT (WT → WT) mice (Fig. 1J). Cnr2−/− → WT mice showed enhanced survival after PbA infection (p < 0.01), whereas all WT → WT chimeras succumbed to ECM. Thus, CB2 deficiency on immune cells, but not radioresistant CNS cells, protects against ECM.
Cnr2−/− Myeloid-derived Cells Mediate Enhanced ECM Resistance in BM Chimeras
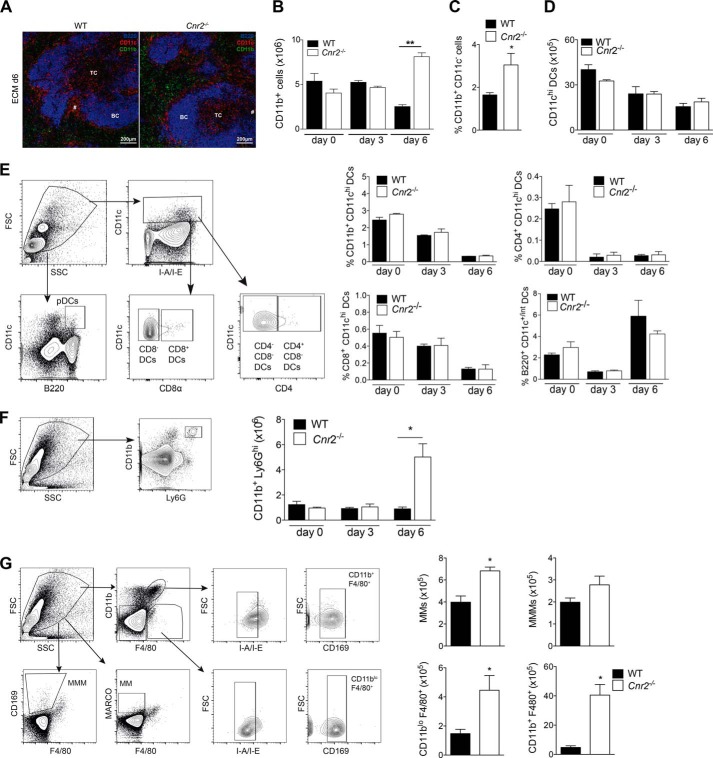
Recruitment of immune cells to the brain is an important step in ECM pathology (7, 8). The absolute number of mononuclear brain-infiltrating cells was markedly reduced in Cnr2−/− mice at day 6 after PbA infection (Fig. 2A). Within this population, we observed increased percentages of CD11b+ cells; >31% of cells that immigrated into the Cnr2−/− brain expressed CD11b, whereas <14% of CD11b+ cells were found in WT brains (Fig. 2B). Equivalent percentages of CD4+ T cells were found in the WT and Cnr2−/− brains (data not shown), whereas percentages of CD8+ T cells were reduced in the brains of Cnr2−/− mice (Fig. 2C). We also found a reduced number of CD4+ CD25+ FoxP3+ T cells in the Cnr2−/− brain, indicating that a Treg-mediated mechanism does not contribute to disease protection in the absence of CB2 signaling (WT: 0.02 × 104 ± 0.01; Cnr2−/−: 0.01 × 104 ± 0.005) (27).
FIGURE 2.
CB2-deficient myeloid-derived cells mediate enhanced ECM resistance in BM chimeras. A, reduced numbers of brain-infiltrating mononuclear cells in Cnr2−/− and WT mice at day 6 after PbA infection. Shown are mean numbers ± S.E. (error bars) of mononuclear cells isolated from the brains of the indicated mice at days 0, 3, and 6 after infection (n = 7–9 mice/group). B, enhanced percentages of brain-infiltrating CD11b+ myeloid cells in day 6 infected Cnr2−/− mice as determined by flow cytometry. Shown are mean percentages ± S.E. of CD45hi CD11b+ cells in brain infiltrates isolated from the indicated mice at days 0, 3, and 6 after PbA infection (n = 7–9 mice/group). C, reduced percentages of brain-infiltrating CD8+ TCR+ cells in day 6 PbA-infected Cnr2−/− mice as determined by flow cytometry. Shown are mean percentages ± S.E. of CD8+ TCR+ cells (pregated for CD45hi expression) in brain infiltrates isolated from the indicated mice at days 0, 3, and 6 after PbA infection (n = 7–9 mice/group). D, survival of mixed BM chimeric mice after PbA infection. BM chimeras were generated by reconstitution of lethally irradiated WT mice with BM cells from WT mice, Cnr2−/− mice, or mixed BM from Cnr2−/− and RAG-2−/− mice. One representative experiment of two independent experiments is shown (n = 8–10 mice/group). E, equivalent IFNγ and GrzB production by MACS-purified CD8+ T cells from day 6 PbTg OVA-infected WT or Cnr2−/− mice (mean ± S.E., n = 5–6 mice/group). CD8+ T cells were restimulated with 1 μm SIINFEKL peptide-loaded antigen-presenting cells from day 6 PbTg OVA-infected RAG-2−/− mice. Supernatants were analyzed for IFNγ and GrzB with ELISA. F, weight of spleens from WT or Cnr2−/− controls or at day 6 after PbA infection (mean ± S.E., n = 5–8 mice/group). G, number of spleen cells in WT or Cnr2−/− control mice or at day 6 after PbA infection (mean ± S.E., n = 8–10 mice/group). H, percentages of GrzB+ NK1.1+ cells in WT or Cnr2−/− spleens at day 6 after infection as determined by flow cytometry (mean ± S.E., n = 6–8 mice/group). I, IFN-γ and GrzB production by whole spleen cells from WT and Cnr2−/− mice 6 days after PbTg OVA infection. Spleen cells were restimulated with 1 μm SIINFEKL peptide overnight, and supernatants were analyzed by ELISA (mean ± S.E., n = 6 mice/group). J, percentages of GrzB+ CD8+ TCR+ T cells in WT or Cnr2−/− spleens at day 6 after infection with PbTg OVA as determined by flow cytometry. Diagram shows percentages of cells (mean ± S.E., n = 6 mice/group). K, IFN-γ and GrzB production by MACS-purified spleen-isolated CD8+ T cells from day 6 PbTg OVA-infected WT or Cnr2−/− mice. CD8+ T cells were restimulated with 1 μm SIINFEKL peptide-loaded C57BL/6 dendritic cells, and supernatants were analyzed for IFN-γ and GrzB production (mean ± S.E., n = 4 mice/group).*, p < 0.05; **, p < 0.01; ***, p < 0.001.
We therefore focused on the reduced brain recruitment of CD8 T lymphocytes and the increased percentage of CD11b+ myeloid-derived cells in the brains of infected Cnr2−/− mice, because both cell types are essential for acquisition and regulation of immunity in ECM (8, 28, 29). To determine whether CB2 expression in myeloid or lymphoid cells was required for susceptibility to ECM, we generated mixed BM chimeras by reconstitution of lethally irradiated WT mice with mixed BM from Cnr2−/− and RAG-2−/− mice (Cnr2−/− + RAG-2−/− → WT). In these mice, hematopoietically derived immune cells were CB2-deficient, with the exception of myeloid cells originating from RAG-2−/− BM cells (Fig. 2D). As controls, irradiated WT mice were reconstituted with Cnr2−/− (Cnr2−/− → WT) or WT BM cells (WT → WT). Again, infectedCnr2−/− → WT mice demonstrated enhanced survival in comparison with WT → WT chimeric mice (p = 0.0089). However, mixed BM chimeras (Cnr2−/− + RAG-2−/− → WT) showed 100% mortality (Fig. 2D). These data point toward a contribution of CB2-expressing myeloid cells to the enhanced susceptibility to ECM. To analyze a possible role of CB2 in modulating CD8 T cell responses in this model, we investigated Th1 responses and cytotoxicity in Cnr2−/− and WT T cells (Fig. 2E). IFN-γ and granzyme B (GrzB) production was equivalent in Cnr2−/− and WT CD8+ T cells when stimulated with cognate peptide-loaded spleen-derived DCs from infected RAG-2−/− mice, indicating that CB2 may not have a major CD8+ T cell intrinsic function under these conditions.
Impact of CB2 Deficiency on the T, B, and NK Cell Compartment in the Spleen after PbA Infection
Next, we investigated immune responses in the spleens of infected Cnr2−/− and WT mice. Cnr2−/− spleens exhibited increased weight and total splenic cell numbers at day 6, indicating an altered cellular composition (Fig. 2, F and G). Flow cytometry determined that CD4+ and CD8+ T cells, as well as B220+ CD11c− B cells, were present in equivalent numbers in the spleens of infected WT and Cnr2−/− mice (CD4+ T cells in WT: 17.65 × 106 ± 5.3 versus Cnr2−/−: 18.79 × 106 ± 4.0; CD8+ T cells in WT: 10.94 × 106 ± 7.18 versus Cnr2−/−: 9.65 × 106 ± 4.8; B220+ CD11c− B cells in WT: 51.8 × 106 ± 39.9 versus Cnr2−/−: 53.5 × 106 ± 33.5). No differences in NK cell numbers and percentages were found in the spleens from day 6 infected WT and Cnr2−/− mice (percentage of NK1.1 cells in WT: 0.97 ± 0.2 versus Cnr2−/−: 0.75 ± 0.3; number of NK1.1 cells in WT: 1.6 × 106 ± 0.3 versus Cnr2−/−: 2.1 × 106 ± 1.0). Equivalent percentages of GrzB-producing NK cells in infected mice further suggested that NK cells may not play a central role in immune protection against ECM in Cnr2−/− mice (Fig. 2H). We next investigated whether parasite-specific CD8+ T cell responses, which are required in ECM induction (30, 31), were altered in Cnr2−/− mice. Splenocytes isolated from Cnr2−/− and WT mice were isolated at day 6 after infection with an OVA-transgenic parasite strain. Cnr2−/− splenocytes produced lower levels of IFN-γ and GrzB after restimulation with the SIINFEKL peptide when compared with WT splenocytes (Fig. 2I). Also, reduced percentages of GrzB+ CD8+ T cells in spleen cultures of Cnr2−/− mice indicated diminished splenic anti-parasitic T cell responses in the infected Cnr2−/− mice (Fig. 2J). In contrast, MACS-purified CD8+ T cells from the spleens of infected Cnr2−/− mice produced these factors equivalently to WT CD8+ T cells when restimulated with peptide presented by CB2-competent antigen-presenting cells (Fig. 2K). Thus, CB2 does not affect CD8 T cell function in ECM in a cell-intrinsic way.
CB2 Does Not Affect Antigen Presentation Capacities of BM DCs but Polarization of BM Macrophages in Vitro
To characterize CD11b+ myeloid cells in Cnr2−/− mice in more detail, we first analyzed dendritic cells and macrophages generated from the BM of Cnr2−/− and WT mice. GM-CSF-generated Cnr2−/− BM DCs induced equivalent OT-II T cell responses when compared with WT BM DCs, as represented by proliferation, IFN-γ release, and GrzB production (Fig. 3, A and B). These data indicate that CB2 does not affect basic T cell priming properties of BM DCs. M-CSF-generated CD11b+ BM macrophages from Cnr2−/− mice expressed enhanced Ym1 and arginase-1 (Arg-1) mRNA levels under IL-4-stimulatory conditions (Fig. 3C). They also exhibited enhanced expression of mannose macrophage receptors (MMRs) and secreted reduced levels of the pro-inflammatory cytokines TNF and IL-6 (Fig. 3, D and E), pointing toward anti-inflammatory properties of these cells (32).
FIGURE 3.
CB2 does not affect antigen presentation capacities of BM DCs but phenotype and function of CD11b+ BM macrophages. A, equal T cell stimulatory capacity of WT or Cnr2−/− BM DCs. The diagram shows percentages of divided OT-II transgenic T cells after coculture with peptide-pulsed WT or Cnr2−/− BM DCs (mean ± S.E. (error bars), n = 3 mice/group). The diagram shows mean percentage ± S.E. of CFSE-positive CD4+ T cells that had undergone at least one division as determined after 72 h by flow cytometry. B, comparable IFN-γ and GrzB production in OT-II transgenic T cells after coculture with peptide-pulsed WT or Cnr2−/− BM DCs. Shown is the amount of IFN-γ and GrzB in supernatants as determined by ELISA after 24 h of coculture (mean ± S.E., n = 3 mice/group). C, Ym1 and Arg-1 mRNA expression in WT or Cnr2−/− BM macrophages (mean ± S.E., n = 5–6 mice/group). D, enhanced percentages of MMR+ CD11b+ cells in LPS-stimulated Cnr2−/− BM macrophages (mean ± S.E., n = 5 mice/group). E, reduced TNF and IL-6 secretion in LPS-stimulated Cnr2−/− BM macrophages as determined by ELISA (mean ± S.E., n = 5–6 mice/group). F, numbers of CD11b+ CD11c− cells, Ly6Ghi neutrophils, and CD11chi MHC-II+ DCs in the blood of infected WT or Cnr2−/− mice. Shown are mean numbers ± S.D. (error bars) of cells of the indicated mice at days 0, 3, and 6 after infection (n = 3–4 mice/group). G, percentages of conventional DCs and plasmacytoid DCs in the blood of infected mice as determined by flow cytometry. Diagrams show mean percentages ± S.D. of DC subsets in the blood of the indicated mice at days 0, 3, and 6 after PbA infection. *, p < 0.05; ***, p < 0.001.
Enhanced Numbers of Macrophages and Neutrophils in the Spleens of Cnr2−/− Mice after PbA Infection
In a next step, we investigated myeloid cell responses in vivo in infected Cnr2−/− and WT mice. In the blood, numbers of CD11b+ CD11c− cells and Ly6Ghi neutrophils increased equivalently in Cnr2−/− and WT mice (Fig. 3F). In addition, comparable numbers of CD11chi MHC II+ DCs were found at all time points after infection with equal proportions of conventional DCs (CD11b+ CD11c+, CD4+ CD11c+ DCs, and CD8+ CD11c+ DCs) as well as B220+ plasmacytoid DCs (Fig. 3G).
However, in the splenic red pulp of infected Cnr2−/− mice, CD11b expression was markedly increased at day 6 after infection when compared with WT controls (Fig. 4A). Flow cytometry further verified enhanced percentages of CD11b+ cells in the spleens of Cnr2−/− mice at this time point (Fig. 4B). Because CD11b is expressed on several myeloid cell types comprising macrophages, neutrophils, and DCs, we further characterized CD11b-expressing cells in Cnr2−/− mice (33–35). Higher percentages of CD11b+ cells that were negative for CD11c accumulated in the spleens of infected Cnr2−/− mice, indicating that these cells do not exclusively represent CD11b-expressing DC subsets (Fig. 4C). Rather, numbers of CD11chi DCs decreased equally in the spleens of both mouse strains after infection (Fig. 4D). Furthermore, we identified no differences in the percentages of conventional and plasmacytoid DCs in WT versus Cnr2−/− mice at day 6 after infection (Fig. 4E). However, counts of CD11b+ Ly6Ghi neutrophils markedly increased at this time point of infection in Cnr2−/− mice but not in WT mice (Fig. 4F). We further investigated whether increased numbers of CD11b+ cells in the Cnr2−/− spleen also comprise macrophages by utilizing previously published gating strategies (36) (Fig. 4G). Red pulp macrophages (CD11blo F4/80+ CD169lo MHC IIlo), marginal zone macrophages (F4/80− CD11b− MARCO+; MMs) and a subset of CD11b+ F4/80hi CD169lo MHC-IIlo cells accumulated in the spleens of infected Cnr2−/− mice, with the exception of metallophilic marginal zone macrophages (F4/80− CD11b− CD169+; MMMs) (Fig. 4G). These data suggest that accumulated CD11b+ cells in Cnr2−/− versus WT spleens contain macrophages as well as neutrophils.
FIGURE 4.
Macrophages and neutrophils accumulate in the spleens of infected Cnr2−/− mice at day 6 when compared with WT controls. A, representative spleen sections of day 6 infected WT (left) and Cnr2−/− (right) mice using immunofluorescence for B220 (blue), CD11c (red), and CD11b (green). TC, T cell zone; BC, B cell zone. B, enhanced numbers of CD11b+ cells in the spleens of day 6 infected Cnr2−/− mice as determined by flow cytometry (mean ± S.E. (error bars), n = 8–10 mice/group). **, p < 0.01. C, percentages of CD11b+ CD11c− cells in the spleens of WT and Cnr2−/− mice at day 6 after PbA infection as determined by flow cytometry (mean ± S.E., n = 4 mice/group; *, p < 0.05). D, numbers of CD11chi DCs in the spleens of WT and Cnr2−/− mice after infection as determined by flow cytometry (mean ± S.D. (error bars), n = 3–4 mice/group). E, DC subsets in the spleens of WT and Cnr2−/− mice after PbA infection. Representative dot plots show gating strategies of splenic DC subsets. The diagrams show the percentages of DC subsets from the spleens of indicated mice at days 0, 3, and 6 after PbA infection (mean ± S.D., n = 3–4 mice/group). F, representative dot plot shows gating strategies for neutrophils in the spleen. The diagram shows numbers of CD11b+ Ly6Ghi cells in the spleen at days 0, 3, and 6 of WT and Cnr2−/− 6 days after PbA infection as determined by flow cytometry (n = 3–4 mice/group; mean ± S.E.; *, p < 0.05). G, representative dot plots showing gating strategies for macrophage subsets in the spleen. The diagram shows the numbers of macrophage subsets in the spleens of WT and Cnr2−/− at day 6 after PbA infection (n = 4 mice/group; mean ± S.E.; *, p < 0.05).
CD11b+ Cells in the Spleens of Infected Cnr2−/− Mice Exhibit an Anti-inflammatory Phenotype and Function
We next investigated the phenotypic and functional profiles of splenic CD11b+ cells in PbA-infected Cnr2−/− and WT mice. At day 6 after infection, CD11b+ cells in the Cnr2−/− spleen exhibited enhanced expression of MMRs and Arg-1 activity (Fig. 5, A and B). Furthermore, isolated Cnr2−/− CD11b+ cells secreted higher amounts of the anti-inflammatory cytokine IL-10 at this time point, suggesting that these cells exerted protective functions in Cnr2−/− mice after PbA infection (Fig. 5C).
FIGURE 5.
Enhanced expression of MMR, arginase-1 activity, and IL-10 in CD11b+ cells and higher IL-10 production by macrophages and neutrophils in the spleens of infected Cnr2−/− mice. A, percentages of MMR+ CD11b+ cells in the spleens of day 6 infected WT and Cnr2−/− mice as determined by flow cytometry (mean ± S.E. (error bars), n = 5–6 mice/group). B, measurement of functional arginase-1 activity in spleen-isolated CD11b+ cells of control or day 6 infected mice. One representative experiment of two independent experiments is shown in each section (mean ± S.E., n = 5–10 mice/group). C, IL-10 secretion in spleen-isolated CD11b+ cells from WT and Cnr2−/− mice at day 3 and day 6 after PbA infection after rechallenge with LPS for 15 h (mean ± S.E., n = 5–6 mice/group). D, diagrams show mean percentages of cytokine-secreting CD11chi MHC II+ DCs from the spleens of WT and Cnr2−/− mice 15 h after stimulation with iRBCs or unstimulated as determined by flow cytometry (mean ± S.E., n = 4 mice/group). E, TNF and IL-10 production by MACS-purified Ly6G+ neutrophils isolated from the spleens of WT and Cnr2−/− mice at day 3 or 6 after PbA infection as determined after rechallenge with LPS for 15 h by cytokine bead assays (mean ± S.E., n = 3–5 mice/group). F, diagrams show percentages of cytokine-secreting Ly6Ghi neutrophils from the spleens of day 6 infected Cnr2−/− and WT mice 15 h after stimulation with iRBCs or unstimulated as determined by flow cytometry (mean ± S.E., n = 4 mice/group). G, diagrams show mean percentages of cytokine-secreting macrophage subsets from day 6 infected WT and Cnr2−/− mice 15 h after stimulation with iRBCs or unstimulated as determined by flow cytometry (mean ± S.E., n = 4 mice/group). H, cytokine concentrations in the blood from WT and Cnr2−/− mice at days 0, 3, and 6 after PbA infection as determined by cytokine bead assays (mean ± S.E., n = 3–5 mice/group). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
To clarify the identity of IL-10-producing CD11b+ cells in Cnr2−/− mice, we measured cytokine release in DCs, neutrophils, and macrophages isolated from the spleens of PbA-infected mice. We found no difference in the percentages of TNF- and IL-10-producing CD11chi MHC-II+ DCs in the spleens of day 6 infected Cnr2−/− and WT mice in vitro and an equivalent increase of TNF+ DCs after LPS restimulation of these cells (Fig. 5D). However, Ly6G+ cells bona fide neutrophils purified from the Cnr2−/− spleen at day 3 after infection exhibited reduced TNF release and markedly enhanced amounts of IL-10 at a later time point as compared with WT controls (Fig. 5E). In accordance, higher percentages of IL-10-producing CD11b+ Ly6Ghi neutrophils from the spleens of day 6 infected Cnr2−/− mice were detected by flow cytometry after iRBC restimulation when compared with WT control cells, whereas no differences were observed between the genotypes with regard to frequencies of TNF-producing cells (Fig. 5F). Proportions of TNF-producing MMMs were lower in the spleens of Cnr2−/− when compared with WT mice, whereas TNF-producing cells among the other macrophage subsets were equivalent in both mouse strains (Fig. 5G). All macrophage subsets in the spleens of day 6 infected Cnr2−/− mice exhibited higher percentages of IL-10-producing cells when compared with WT mice after iRBC stimulation, with the exception of CD11b+ F4/80+ cells (Fig. 5G). Taken together, these findings indicate that in the spleens of PbA-infected Cnr2−/− mice, higher frequencies as well as absolute numbers of IL-10-producing neutrophils and macrophages were present as compared with WT mice.
In the serum of WT and Cnr2−/− mice, no differences were observed in TNF and CCL2 levels during infection (Fig. 5H). However, reduced levels of IFN-γ, IL-1α, and IL-12 at day 3 as well as higher levels of IL-10 at day 6 hint toward reduced Th1 responses and an anti-inflammatory cytokine milieu in infected Cnr2−/− mice. These data indicate that CB2 also has an impact on systemic cytokine levels after PbA infection.
Protection against ECM in Cnr2−/− Mice Depends on CCL17
Brain infiltrates of Cnr2−/− mice contained an increased percentage of immigrating CD11b+ cells that expressed MMRs+ and elevated amounts of Arg-1 mRNA, whereas the fraction of MHC-II-expressing CD11b+ cells was markedly reduced (Fig. 6, A–C). These cells exhibited high levels of Ly6C, indicating a monocytic origin (Fig. 6D). We detected no differences in the percentages of brain-infiltrating Ly6G+ neutrophils or CD4+ and CD8+ MHC II+ DCs in WT and Cnr2−/− mice (Fig. 6, D and E). No difference in MHC-II expression was observed in CD11c+ DCs in WT and Cnr2−/− brains (Fig. 6F). Thus, in the absence of CB2, a CD11b+ cell subset was recruited to the brain that expresses selected signature molecules with regulatory or recovery macrophage functions rather than effector killing functions. IFN-γ and TNF mRNA expression were also reduced, and inducible nitric-oxide synthase expression was equivalent in the brains of infected Cnr2−/− mice, underscoring a reduced local inflammatory response (Fig. 6G). Reduced percentages of GrzB+ CD8+ T cells in the brains of infected Cnr2−/− mice further indicated diminished anti-parasitic T cell responses (Fig. 6H). To delineate factors contributing to the protective effects of CD11b+ myeloid cells in Cnr2−/− mice, we focused on the CC-chemokine CCL17, because its expression is strongly enhanced in alternatively activated macrophages (32, 37) and was markedly up-regulated by IL-4 stimulation (Fig. 6I) (32, 38). We bred mice lacking CB2 receptors with CCL17-deficient animals (20, 39, 40) and compared the development of ECM in Cnr2−/− mice that also lacked CCL17 on one (Cnr2−/−/CCL17+/−) or both alleles (Cnr2−/−/CCL17−/−). Cnr2−/−/CCL17+/− mice were protected against ECM, as shown before. In sharp contrast, PbA-infected Cnr2−/−/CCL17−/− animals were no longer protected against ECM (Fig. 6J). In addition, the clinical score was reduced in Cnr2−/−/CCL17+/− animals when compared with Cnr2−/−/CCL17−/− mice (Fig. 6K). These data show that CCL17 is an essential mediator for the protective effects of CB2 deletion.
FIGURE 6.
CCL17 mediates protection against ECM in Cnr2−/− mice. A, percentages of MMR+ brain-infiltrating cells (pregated for CD45hi and CD11b expression) in WT and Cnr2−/− mice at day 6 after PbA infection as determined by flow cytometry (mean ± S.E. (error bars), n = 4 mice/group). B, Arg-1 mRNA expression in mononuclear cells isolated from the brains of WT and Cnr2−/− mice at day 6 after PbA infection. mRNA levels are normalized to GAPDH expression (mean ± S.E., n = 4 mice/group). C, percentages of MHC-II+ CD45hi CD11b+ brain-infiltrating cells in WT and Cnr2−/− mice at day 6 after PbA infection (mean ± S.E., n = 5–7 mice/group). D, percentages of Ly6Chi (open bars) and Ly6Ghi (filled bars) brain-infiltrating cells (pregated for CD45hi and CD11b expression) in WT and Cnr2−/− mice at day 6 after PbA infection as determined by flow cytometry (mean ± S.E., n = 4–5 mice/group). E, percentages of CD4+ CD11c+ DCs and CD8+ CD11c+ DCs (pregated for CD45hi MHC IIhi cells) immigrating into the brains of day 6 infected WT and Cnr2−/− mice as determined by flow cytometry (mean ± S.E., n = 4 mice/group). F, percentages of MHC-II+ CD45hi CD11c+ brain-infiltrating cells in WT and Cnr2−/− mice at day 6 after PbA infection (mean ± S.E., n = 4 mice/group). G, Ifng, Tnf, and inos mRNA expression in the brains of control or day 6 PbA-infected WT or Cnr2−/− mice. mRNA levels are normalized to β-actin expression, and results are presented as the mean ± S.E. (n = 3–5 mice/group). H, percentages of GrzB+ CD8+ TCR+ T cells infiltrating into the brains of WT or Cnr2−/− mice at day 6 after infection with 5 × 104 PbTg OVA. The diagram shows percentages of cells (pregated for CD45hi expression) (mean ± S.E., n = 6 mice/group). I, CCL17 mRNA expression in M2 polarized BM macrophages from C57BL/6 mice after differentiation with M-CSF and IL-4 (mean ± S.E., n = 5–6 mice/group). J, survival of Cnr2−/− × CCL17+/− and Cnr2−/− × CCL17−/− mice after PbA infection. One representative experiment of two independent experiments is shown (n = 5–10 mice/group). K, clinical score of PbA-infected Cnr2−/− × CCL17+/− and Cnr2−/− × CCL17−/− mice (n = 10–12 mice/group). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Discussion
In this study, we demonstrate a role of CB2 in the pathophysiology of experimental CM. Cnr2−/− mice exhibit enhanced survival, a reduced parasite load in the brain, and a diminished BBB disruption after PbA infection in comparison with WT mice. Importantly, we demonstrate that therapeutic application of a CB2 antagonist conferred increased CM resistance in C57BL/6 mice. These results indicate a therapeutic potential of targeting CB2 signaling in ECM and may also facilitate the design of human intervention trials for the treatment of parasite-induced encephalitis.
It was suggested that CB2 could act as a chemokine receptor and is therefore functionally involved in immune cell trafficking (41–44). Here we showed that CB2 controls the recruitment of peripheral immune cells into the brain during ECM. The degree of mononuclear cells infiltrating the brain was markedly reduced in PbA-infected Cnr2−/− mice. The most striking finding was the nature of the immune cells invading the brain in these animals. Brain infiltrates in infected Cnr2−/− mice showed reduced numbers of lymphoid CD8+ T cells. In contrast, we found that CD11b+ cells markedly expanded in the spleen, and higher proportions of these cells were determined in brain infiltrates of infected Cnr2−/− mice.
Our findings suggest that increased numbers of CD11b+ cells in Cnr2−/− versus WT spleens represent myeloid macrophages as well as neutrophils. Among those, the specific subset of CD11b+ F4/80hi CD169lo MHC-IIlo cells increased in the spleens of infected Cnr2−/− animals. A corresponding population of F4/80+ CD11b+ cells has recently been described as a macrophage subset that accumulated after IL-33 treatment in the spleens of PbA-infected C57BL/6 mice and mediated resistance to CM due to anti-inflammatory properties (45). However, CD11b has also been described on subsets of adaptive immune cells among those CD8+ T cells (46). Although our findings exclude a differential increase of the major adaptive immune cell subsets in infected Cnr2−/− mice, we cannot fully exclude the possibility that minor populations of non-myeloid CD11b-expressing cells also accumulate in the Cnr2−/− spleen.
We identified CB2-expressing myeloid cells as a critical cell type contributing to enhanced susceptibility to ECM in mixed BM chimeras. It is well established that myeloid-derived macrophages express pattern recognition receptors and phagocytose blood pathogens for subsequent transfer to local DCs for cross-presentation to specific T cells (47). Alternatively activated macrophages are specialized in down-regulating immune responses by a specific anti-inflammatory response pattern (32). In vitro, we found a polarization of Cnr2−/− BM macrophages toward M2. In the spleens of infected Cnr2−/− mice, expanded CD11b+ cells expressed higher levels of selected M2 markers, such as CD206 (macrophage mannose receptor), arginase-1 activity, and IL-10, an anti-inflammatory cytokine known to confer protection against ECM (48, 49). Macrophage subsets and neutrophils expanded in the spleens of Cnr2−/− mice after infection and further exhibited an enhanced capacity to produce IL-10. Also, in the brains of infected Cnr2−/− mice, immigrating CD11b+ cells expressed higher CD206 and reduced MHC-II levels, associated with enhanced arginase-1 production. Furthermore, expression of M1-associated cytokines was reduced in the Cnr2−/− brain when compared with WT. In contrast, we found a concomitant and equivalent expression of the M1-associated cytokine TNF in the majority of splenic macrophages in both genotypes. Thus, rather then representing canonical M2 macrophages, expanded CD11b+ cells in infected Cnr2−/− mice share only selected factors with anti-inflammatory myeloid cells after PbA infection. In fact, multiple reports defined CD11b+ cells as a highly heterogeneous mixture of cells that also include a subset of myeloid-derived suppressor cells (MDSCs) (50). MDSCs have been shown to stain positive for Gr-1, an antibody detecting both Ly6C and Ly6G. A major subset of CD11b+ cells in infected Cnr2−/− mice also expressed Ly6Chi and Ly6Ghi surface molecules, thus reflecting the phenotype of certain MDSC subsets phenotypically identical to neutrophils or inflammatory monocytes (50). MDSCs exhibit potent immunosuppressive activity on T cell functions ex vivo via mechanisms that depend on nitric oxide, arginase, IFN-γ, and cell-cell contact and have functionally been involved in parasitic infections (51, 52). Previous studies demonstrated that CB1 as well as CB2 are involved in the induction of MDSC in mice following THC treatment (53). We found that CD11b+ cells in infected Cnr2−/− mice shared markers with MDSC, such as low levels of MHC class II and enhanced arginase-1 activity. Future studies will precisely define the origin and function of expanded CD11b+ cells in infected Cnr2−/− mice.
We further determined that splenic Cnr2−/− neutrophils markedly increased in the spleens of infected Cnr2−/− mice and shifted toward an anti-inflammatory cytokine profile. Also, in the blood, reduced Th1 responses and a bias toward anti-inflammatory cytokines were detected in infected Cnr2−/− mice. These findings were unexpected, because earlier data indicated that CB2 activation inhibited alternative but also classical marker expression in brain-derived microglia/macrophages in an ischemic stroke model or even favored IL-10 production in LPS/IFN-γ stimulated murine peritoneal macrophage and the transition to an M2 phenotype in liver macrophages (Kupffer cells) (22, 54, 55). These data, in comparison with our findings, may reflect cell-specific differences of CB2 functions that depend on culture conditions, the characteristics of the pathogen, or the respective CB2-expressing myeloid cell subset in vivo.
Parasite-specific cytotoxic T cells in the spleen induced by antigen-presenting cells are critical mediators of ECM-associated pathology (8, 56–63). We demonstrate that anti-parasitic T cell responses were reduced in the brains and spleens of infected Cnr2−/− mice. We also identified a reduced vascular leakage in the brains of infected Cnr2−/− mice. It is possible that the maintenance of the BBB in Cnr2−/− mice is related to the observed reduction in brain-immigrating CD8+ T lymphocytes, which have been implicated as cellular mediators of BBB breakdown via perforin-dependent mechanisms (61, 62). However, production of cytolytic factors was only reduced in whole spleen cultures but not in isolated splenic CD8 T lymphocytes of infected Cnr2−/− mice. This suggests that suppressive effects in the Cnr2−/− spleens affected anti-parasitic T cell responses required for ECM induction but did not operate in a T cell intrinsic way. Generation of mixed BM chimeras further demonstrated that the expression of CB2 on myeloid cells was associated with mortality after PbA infection. Corroborating our in vivo data, we found comparable GrzB and IFN-γ production by Cnr2−/− and WT CD8+ T cells upon stimulation by peptide-loaded CB2-expressing RAG-2−/− DCs in vitro, indicating that CB2 modulates myeloid responses but does not directly affect T cell functions under these conditions. Nevertheless, we cannot fully exclude the possibility that CD8+ T cells contributed to enhanced protection in PbA-infected Cnr2−/− mice by using alternative effector functions, such as perforin.
The M2 macrophage chemokine CCL17 is an essential factor that mediates enhanced protection against ECM in the absence of CB2 as demonstrated by the full susceptibility of CCL17 × Cnr2 double-deficient mice to ECM. We demonstrated before that the CCL17/CCR4 axis affects the function of CCR4+ myeloid cells, but not T lymphocytes, recruited into the brain during CNS autoimmunity (64). It is thus possible that CCL17 favors brain recruitment or function of myeloid cells rather than CD8+ T cells in ECM with beneficial outcome in disease in Cnr2−/− mice. Here we propose a model whereby CB2 is critical for the development of potent anti-parasitic immune responses early during PbA infection in vivo, thereby causing disease pathology. Pharmacological intervention with a CB2 antagonist induced enhanced disease protection, pointing toward its potential for the treatment of fatal malaria in humans. Clinical studies will help to move toward the development of applications using antagonists/inverse agonists acting on CB2 receptors in fatal malaria.
Experimental Procedures
Mice
C57BL/6 (H-2b) and RAG-2 knock-out female mice were purchased from Janvier (France) and/or bred locally. CB2-deficient (Cnr2−/−, N10 backcross to C57BL/6) and CCL17−/−mice (39) and wild type mice were bred locally. All animals were bred and housed according to German guidelines for animal care and the European Union animal welfare guidelines. Ethical approval for the use of all mice in this study was obtained from the Landesamt für Natur, Umwelt und Verbraucherschutz (LANUV), Cologne, Germany (AZ 8.87–50.10.31.08.271).
Endocannabinoid Measurements
Brain endocannabinoid and arachidonic acid levels were measured by GC/MS as reported previously (65). In short, immediately frozen brains were cut along their longitudinal axis and weighed. Chloroform was added (1 ml/100 mg), and the half-brains were homogenized with a Polytron homogenizer (15,000 rpm for 1 min) and sonicated twice for 10 s. The homogenate was added to 10 ml of ice-cold chloroform containing the internal standards (328 pmol of AA-d8, 529 pmol of 2-AG-d5). Folch extraction was performed by adding 5 ml of methanol and 2.5 ml of PBS. The mixture was vigorously vortexed and sonicated for 5 min at 4 °C, followed by centrifugation for 5 min at 800 × g. The organic phase was recovered into a glass vial and dried under N2. The dried organic phase was reconstituted into 1 ml of EtOH and vortexed, and 9 ml of water were added. Solid phase extraction was performed with Sep-Pak cartridges (Waters). Columns were conditioned with 3 ml of methanol (99.8%) and 3 ml of 10% ethanol, and then the samples were applied and washed with 3 ml of 10% ethanol. The arachidonic acid and 2-AG were eluted with 3 ml of ethyl acetate/acetonitrile (8:2). Eluates were dried under N2 and reconstituted in 50 μl of acetonitrile. 20 μl of N,N-diisopropylethylamine (Sigma) and 20 μl of pentafluorobenzyl bromide (Sigma) (1 g in 3 ml of acetonitrile) were added and incubated for 25 min at 45 °C to derivatize arachidonic acid. Excess reagent was evaporated, the sample was reconstituted in 25 μl of dimethylisopropylsilylimidazole (TCI Europe), and 2-AG was derivatized with it for 1 h at room temperature. Samples were stored at −20 °C until GC/MS analysis. Samples were injected in splitless mode into an Agilent 6890N GC (HP-5MS column, 30 m). The oven temperature program was as follows: initial temperature 150 °C for 1 min, followed by an 8 °C/min increase up to 280 °C, which was held for 10 min. Helium was used as a carrier gas at a flow rate of 1.5 ml/min. Coupled to the GC was an Agilent 5975C MSD system. The following ions were used for selected ion monitoring analysis: 2-AG/1-AG, m/z 535; 2-AG-d5/1-AG-d5, m/z 540; AA, m/z 386, 484; AA-d8, m/z 392. Because of a significant amount of spontaneous acyl group migration (isomerization) of 2-AG during extraction, the peak areas of 1(3)-AG and 2-AG were combined for quantitative analysis.
Infection, Treatment, and Vascular Leakage
PbA or OVA-expressing P. berghei (PbTg) parasitized red blood cells (RBCs) (kindly provided by Rachel Lundie and Andrew Waters) were used (59). 8–12-week-old mice were infected intravenously with 5 × 104 PbA-RBCs obtained from donor mice infected intraperitoneally with 1 × 107 PbA-RBCs. Parasitemia was assessed daily after day 4 in Giemsa-stained tail blood smears. The course of disease was monitored twice daily using the following score according to Amante et al. (27): 0, without symptoms; 1, ruffled fur; 2, hunching; 3, wobbly gait; 4, limb paralysis; 5, convulsions; 6, coma. Each sign was given a score of 1. Animals with severe ECM (cumulative score of 5) and severe anemia were sacrificed according to ethics guidelines. For pharmacological treatment, C57BL/6 mice were daily intraperitoneally injected with 25 μg of CB2 antagonist SR144528 (RTI International) in DMSO and PBS. Control groups were injected with DMSO and PBS. The dose of SR144528 was chosen in accordance with earlier studies (66–68). For assessment of vascular leakage, mice were intravenously injected with 200 μl of 2% Evans Blue (Sigma-Aldrich) at day 6 post-infection, and 1 h later, brains were analyzed for dye leakage. After photodocumentation, brains were weighed and incubated in 2 ml of formamide for 48 h at 37 °C. 100 μl of solution were analyzed in triplicate. Absorbance was measured at 620 nm in an ELISA reader. Concentration of Evans Blue was calculated with a standard curve starting at 200 μg/ml and is expressed as μg of Evans Blue/g of brain tissue.
Isolation of Mouse Parasites
Preparation of iRBCs was performed based on earlier published protocols (36). In brief, blood from PbA-infected mice was diluted with PBS (pH 7.4) and centrifuged on Lymphoprep cushions at room temperature for 15 min. After removal of the buffy coat, cell pellets were resuspended in PBS before usage.
Generation of BM Chimeric Mice
Recipient mice were irradiated with 9.5 Grays and reconstituted with 0.8–1.2 × 107 BM cells (or 0.4–0.6 × 107 cells mixed with 0.4–0.6 × 107 BM cells from the indicated donors in mixed chimeras) via tail vein injection ∼7–8 h after irradiation. Mice were treated with antibiotics (trimethoprim and sulfamethoxazole) for 9 days after reconstitution. The level of chimerism was determined by assessing the percentage of CD45.2 donor cells present in the blood of CD45.1 recipients by flow cytometry 7–8 weeks after BM cell transfer. Mice exhibiting >95% CD45.2+ donor cells in peripheral blood cells were PbA-infected as described above.
Cell Isolation
Brain tissue samples were collected from perfused animals, homogenized by digestion with collagenase/dispase (Roche Applied Science) and DNase I (Roche Applied Science, Mannheim, Germany) at 37 °C for 45 min, and gently pressed through a sieve to obtain single cell suspensions. For enrichment of brain mononuclear cells, 30 and 70% Percoll gradients (GE Healthcare UK Ltd., Buckinghamshire, England) were performed and centrifuged at 922 × g for 25 min without brake at room temperature. Cells within the interphase were collected. Splenocytes were isolated by digestion with collagenase type VIII and DNase I (Sigma-Aldrich). CD11b+ cells from spleens were purified in two steps. First, CD11c+ cells were purified from spleens via bead-coupled antibodies (CD11c MicroBeads, Miltenyi Biotec, Bergisch Gladbach, Germany), and the flow-through was subsequently purified for CD11b+ cells (CD11b MicroBeads, Miltenyi Biotec). To isolate splenic neutrophils, the Anti-Ly-6G MicroBead Kit (Miltenyi Biotec) was used. Purified cell populations were cultured in 1 × 106 cells/ml in DMEM supplemented with 10% (v/v) heat-inactivated FCS, 1% MEM, 1% penicillin-streptomycin, 0.1% 2-mercaptoethanol (all from Gibco) and stimulated with 100 ng/ml Escherichia coli LPS serotype 0127:B8 (Sigma-Aldrich) for 16 h. For isolation of lymphoid cells, spleens were gently pressed through a sieve to obtain single lymphocyte suspensions. Lymphocyte isolation from the blood of mice was performed by pipetting freshly drawn blood into ice-cold PBS containing 5 mm EDTA (150 μl of blood, 2 ml of PBS-EDTA). Samples were inverted immediately to prevent clotting. Red blood cells were digested using ACK lysing buffer (Gibco) as described by the manufacturer. Erythrocytes from the blood were isolated via Pancoll gradient isolation (Pan-Biotech) with centrifugation at 1400 rpm/room temperature without brake for 20 min.
Coculture and Proliferation Assays
1 × 105 BM DCs were incubated for 3 h at 37 °C, 5% CO2 in a 100-μl total volume of BM DC medium with or without 1 μm I-Ab OVA(323–339) peptide. Splenocytes were incubated with CFSE (1 μm in PBS) for 20 min at 37 °C in the dark. 1 × 105 CFSE-labeled CD4+ OT-II cells were cocultured with peptide-pulsed or control BM DCs for 3 days. Proliferation was determined on day 3 via flow cytometry.
Flow Cytometry
After FC receptor blockade with anti-CD16/32 mAb (Biozol, Eching, Germany), cells were stained with phycoerythrin-, allophycocyanin-, Alexa Fluor 488-, Alexa Fluor 647-, BV421-, BV510-, or biotin-conjugated mAbs in combination with cyanine 5.5/streptavidin/peridininchlorophyll. mAbs against TCR β-chain, CD4, CD8α, CD45, CD40, CD80, CD86, I-A/I-E, CD11b, CD11c, GrzB, Ly6C, Ly6G, Nk1.1, B220, CD169, F4/80, TNF, and IL-10 (eBiosciences or Biolegend (San Diego, CA)) and MARCO (ABD Serotec) were used. Intracellular TNF and IL-10 staining was performed according to the manufacturer's protocol (Biolegend). Intracellular FoxP3 staining with the anti-mouse/rat FoxP3 staining kit was performed according to the manufacturer's protocol (eBiosciences). Acquisition and analysis were performed with FACSCanto (BD Biosciences) and FlowJoTM software (Tree Star, Ashland, OR).
Generation and in Vitro Stimulation of BM Macrophages and Dendritic Cells
BM cells for generation of BM DCs were isolated from the hind legs of mice, and 5 × 105 cells/ml were cultured in DMEM supplemented with 10% (v/v) heat-inactivated FCS, l-glutamine, MEM, penicillin/streptomycin, 2-mercaptoethanol (all from Gibco), and 10% GM-CSF, and medium was changed at day 3 of culture. BM cells for generation of macrophages were cultured in 1.5 × 105 cells/ml in RPMI supplemented with 10% (v/v) heat-inactivated FCS, l-glutamine, MEM, penicillin/streptomycin, 2-mercaptoethanol (all from Gibco), and 15% conditioned medium of L292 cells. On day 3, adherent cells were recultured in complete medium and harvested on day 6. BM-derived macrophages were stimulated in vitro for the indicated times with 100 ng/ml E. coli LPS serotype 0127:B8 (Sigma-Aldrich). For the generation of M2 macrophages, M-CSF-differentiated BM macrophages from WT and Cnr2−/− mice were stimulated with IL-4 at a concentration of 100 units/ml for 48 h (eBiosciences; biological activity 8 × 105 units/mg). After stimulation, cells were used for flow cytometry or frozen in TRIzol for further quantitative real-time PCR analyses. BM macrophage cells or the indicated splenic cells were stimulated on day 5 or 6, respectively, for 15 h with 5 × 104 iRBCs/well.
Measurement of Cytokines
Cytokines in culture supernatants were determined by a specific sandwich enzyme-linked immunosorbent assay. TNF, IL-6, IFN-γ, IL-10, and GrzB (ELISA Duo-Set, R&D Systems) were used according to the manufacturer's instructions. Plates were read at 450 nm using a SpectraMax 340 microtiter reader (Molecular Devices, Sunnyvale, CA). To determine cytokine levels in the serum or supernatants of CD11b+ and Ly6G+ splenocytes, the LEGENDplexTM multi-analyte flow assay kits (Biolegend) were used according to the manufacturer's recommendations. Serum samples were diluted 2-fold with assay buffer, whereas cell culture supernatants were assayed without dilution. Data analysis was performed using the LEGENDplexTM data analysis software (Biolegend).
Serum Preparation
To collect serum samples, blood was drawn and transferred into clotting activator containing 1.3-ml microtubes (Sarstedt, Nuembrecht, Germany). Coagulation occurred for 30 min at room temperature.
Measurement of Arginase Activity
Splenocytes were prepared as described above. The homogenate was purified for CD11b+ cells via bead-coupled antibodies (CD11b MicroBeads, human and mouse, Miltenyi Biotec) using the autoMACS Pro Separator (Miltenyi Biotec). The measurement of arginase from supernatant of lysed cells was performed according to the manufacturer's protocol (Arginase Assay Kit, Abnova, Jhongli, Taiwan).
Immunofluorescence
For histological analysis, brain tissues were fixed in 4% buffered formalin and embedded in paraffin. Tissue was then sliced (4–6 μm) and stained with hematoxylin. Spleens were fixed in 4% paraformaldehyde at 4 °C for 30–120 min, washed twice in PBS, saturated in 20% sucrose for 8 h, embedded in tissue-freezing medium (Leica), and snap-frozen in 2-methylbutane (Merck) prechilled with liquid nitrogen. Samples were then sliced into 10–12-μm slices, fixed in acetone (Merck), and stained with biotinylated monoclonal rat anti-mouse Abs for CD11b, CD11c, B220, TCR, CD4, and CD8 (all purchased from eBiosciences). Bound antibody was detected using Streptavidin Alexa 647 (eBiosciences), and samples were mounted with Fluoromount-G (Southern Biotechnology Associates, Inc.) for imaging with confocal microscopy. Adobe Photoshop was used for final image processing.
Total RNA Preparation and Taqman Analysis
Mouse tissue or cells were rapidly dissected, snap-frozen in isopentane, and stored at −80 °C. Total RNA was prepared according to the TRIzol method (Invitrogen). Up to 5 μg of RNA and 0.5 μg of oligo(dT)20 primer (Invitrogen) were heated at 70 °C for 4 min, chilled on ice, and then reverse transcribed at 42 °C for 50 min. A total volume of 20 μl included 4 μl of first strand buffer (Invitrogen), 2 μl of 0.1 m DTT, 1 μl of 10 mm dNTPs, 0.5 μl of RNase OUT (Invitrogen), and 200 units of Superscript II reverse transcriptase (Invitrogen). Quantitative RT-PCR of cDNA samples was performed using an ABI 7900 sequence detector (PerkinElmer Life Sciences) and Universal PCR Master Mix (PerkinElmer Life Sciences). After incubation of the samples at 50 °C for 2 min and 95 °C for 10 min, 40 cycles of 95 °C for 15 s and 60 °C for 1 min were applied. Taqman primer and probe sets were ordered from Applied Biosystems as follows: Cnr1 Mm01212171_s1, Cnr2 Mm00438286_m1, diacylglycerol lipase α Mm00813830_m1, diacylglycerol lipase β Mm00523381_m1, fatty acid amide hydrolase Mm00515684_m1, monoacylglycerol lipase Mm00449274_m1, ARG Mm00475988_m1. Results are expressed as -fold change relative to the control samples normalized to GAPDH. RT-PCRs for Ifng, Tnf, and inos were performed as described before (69). RT-PCR was performed in accordance for YM1 (ym-1 primer, gcccttattgagaggagcttta (forward) and tacagcagacaagacatcc (reverse); plasmodial 18S RNA, ctaacatggctttgacgggta (forward) and tgtcactaccctcttattt (reverse)).
Statistical Analysis
Analysis of variance and Bonferroni post hoc tests or two-tailed Student's t tests were used to determine differences between groups unless stated otherwise. Mann-Whitney U tests were used as indicated. Data are given as mean ± S.E. The Mantel-Cox log-rank test was used to compare differences in survival and clinical score between groups. All tests were performed with GraphPad Prism version 5 (GraphPad Software, San Diego, CA). p ≤ 0.05 was considered significant, and asterisks indicate significance: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Author Contributions
J. A., S. Specht, W. M., S. Scheu, A. H., and A. Z. conceived and coordinated the study. J. A., S. Scheu, H. A., B. S., K. S., C. R., R. L., A. K., A. D., J. M. K., K. P., M. F., Ö. A., and D.-M. O. performed the experiments and analyzed the data; J. M. and J. G. performed endocannabinoid measurements. J. A., S. Specht, J. G., I. F., S. Scheu, A. H., and A. Z. wrote the paper. All authors read and approved the manuscript.
Acknowledgments
We thank A. Wojtalla for support with mouse breeding; A. Fattahi-Mehr, Ö. Yilmaz, B. Dubben, Patricia Jebett Korir, and A.-L. Leumann for excellent technical assistance; and M. Hübner and A. Limmer for critical discussion.
This study was supported by German Research Foundation (Deutsche Forschungsgemeinschaft (DFG)) Grant FOR926 (to J. A. and A. Z.); DFG EXC 1003, Grant FF-2014-01 Cells in Motion Cluster of Excellence, Münster, Germany (to J. A.); DFG Grants SCHE692/3-1 and SCHE692/4-1 (to S. Scheu); Strategic Research Fund of Heinrich Heine University Düsseldorf (to S. Scheu); DFG Grant SFB 704 (to I. F.); BONFOR intramural funding scheme of the Medical Faculty, Bonn University, Grants 2012-1-22 and 2013-1-29 (to J. K. and B. S.); intramural Münster IMF funding Grant I-DL121204 (to J. A. and A. D.); and intramural Münster IMF funding Grant I-ST111423 (to J. A.). The authors declare that they have no conflicts of interest with the contents of this article.
- CM
- cerebral malaria
- CB1 and CB2
- cannabinoid receptor 1 and 2, respectively
- ECM
- experimental CM
- BBB
- blood-brain barrier
- RBC
- red blood cell
- iRBC
- infected RBC
- CFSE
- carboxyfluorescein diacetate succinimidyl ester
- PbA
- P. berghei ANKA
- BM
- bone marrow
- 2-AG
- 2-arachidonylglycerol
- AA
- arachidonic acid
- GrzB
- granzyme B
- MMR
- mannose macrophage receptor
- MDSC
- myeloid-derived suppressor cell
- OVA
- ovalbumin.
References
- 1. Hunt N. H., Golenser J., Chan-Ling T., Parekh S., Rae C., Potter S., Medana I. M., Miu J., and Ball H. J. (2006) Immunopathogenesis of cerebral malaria. Int. J. Parasitol. 36, 569–582 [DOI] [PubMed] [Google Scholar]
- 2. Belnoue E., Potter S. M., Rosa D. S., Mauduit M., Grüner A. C., Kayibanda M., Mitchell A. J., Hunt N. H., and Rénia L. (2008) Control of pathogenic CD8+ T cell migration to the brain by IFN-γ during experimental cerebral malaria. Parasite Immunol. 30, 544–553 [DOI] [PubMed] [Google Scholar]
- 3. Snow R. W., Guerra C. A., Noor A. M., Myint H. Y., and Hay S. I. (2005) The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434, 214–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Souza J. B., Hafalla J. C., Riley E. M., and Couper K. N. (2010) Cerebral malaria: why experimental murine models are required to understand the pathogenesis of disease. Parasitology 137, 755–772 [DOI] [PubMed] [Google Scholar]
- 5. Riley E. M., Couper K. N., Helmby H., Hafalla J. C., de Souza J. B., Langhorne J., Jarra W. B., and Zavala F. (2010) Neuropathogenesis of human and murine malaria. Trends Parasitol. 26, 277–278 [DOI] [PubMed] [Google Scholar]
- 6. Medana I. M., Hunt N. H., and Chan-Ling T. (1997) Early activation of microglia in the pathogenesis of fatal murine cerebral malaria. Glia 19, 91–103 [DOI] [PubMed] [Google Scholar]
- 7. Rénia L., Potter S. M., Mauduit M., Rosa D. S., Kayibanda M., Deschemin J. C., Snounou G., and Grüner A. C. (2006) Pathogenic T cells in cerebral malaria. Int. J. Parasitol 36, 547–554 [DOI] [PubMed] [Google Scholar]
- 8. Amante F. H., Haque A., Stanley A. C., Rivera Fde L., Randall L. M., Wilson Y. A., Yeo G., Pieper C., Crabb B. S., de Koning-Ward T. F., Lundie R. J., Good M. F., Pinzon-Charry A., Pearson M. S., Duke M. G., McManus D. P., Loukas A., Hill G. R., and Engwerda C. R. (2010) Immune-mediated mechanisms of parasite tissue sequestration during experimental cerebral malaria. J. Immunol. 185, 3632–3642 [DOI] [PubMed] [Google Scholar]
- 9. Patel S. N., Serghides L., Smith T. G., Febbraio M., Silverstein R. L., Kurtz T. W., Pravenec M., and Kain K. C. (2004) CD36 mediates the phagocytosis of Plasmodium falciparum-infected erythrocytes by rodent macrophages. J. Infect. Dis. 189, 204–213 [DOI] [PubMed] [Google Scholar]
- 10. Ayi K., Patel S. N., Serghides L., Smith T. G., and Kain K. C. (2005) Nonopsonic phagocytosis of erythrocytes infected with ring-stage Plasmodium falciparum. Infect. Immun. 73, 2559–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gazzinelli R. T., Wysocka M., Hieny S., Scharton-Kersten T., Cheever A., Kühn R., Müller W., Trinchieri G., and Sher A. (1996) In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ and TNF-α. J. Immunol. 157, 798–805 [PubMed] [Google Scholar]
- 12. Wolf S. A., and Ullrich O. (2008) Endocannabinoids and the brain immune system: new neurones at the horizon? J. Neuroendocrinol. 20, 15–19 [DOI] [PubMed] [Google Scholar]
- 13. Cabral G. A., and Griffin-Thomas L. (2009) Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Expert Rev. Mol. Med. 11, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centonze D., Rossi S., Finazzi-Agrò A., Bernardi G., and Maccarrone M. (2007) The (endo)cannabinoid system in multiple sclerosis and amyotrophic lateral sclerosis. Int. Rev. Neurobiol. 82, 171–186 [DOI] [PubMed] [Google Scholar]
- 15. Ashton J. C., Rahman R. M., Nair S. M., Sutherland B. A., Glass M., and Appleton I. (2007) Cerebral hypoxia-ischemia and middle cerebral artery occlusion induce expression of the cannabinoid CB2 receptor in the brain. Neurosci. Lett. 412, 114–117 [DOI] [PubMed] [Google Scholar]
- 16. Atwood B. K., and Mackie K. (2010) CB2: a cannabinoid receptor with an identity crisis. Br. J. Pharmacol. 160, 467–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stella N. (2010) Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 58, 1017–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Palazuelos J., Aguado T., Pazos M. R., Julien B., Carrasco C., Resel E., Sagredo O., Benito C., Romero J., Azcoitia I., Fernández-Ruiz J., Guzmán M., and Galve-Roperh I. (2009) Microglial CB2 cannabinoid receptors are neuroprotective in Huntington's disease excitotoxicity. Brain 132, 3152–3164 [DOI] [PubMed] [Google Scholar]
- 19. Racz I., Nadal X., Alferink J., Baños J. E., Rehnelt J., Martín M., Pintado B., Gutierrez-Adan A., Sanguino E., Bellora N., Manzanares J., Zimmer A., and Maldonado R. (2008) Interferon-γ is a critical modulator of CB(2) cannabinoid receptor signaling during neuropathic pain. J. Neurosci. 28, 12136–12145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buckley N. E., McCoy K. L., Mezey E., Bonner T., Zimmer A., Felder C. C., Glass M., and Zimmer A. (2000) Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur. J. Pharmacol. 396, 141–149 [DOI] [PubMed] [Google Scholar]
- 21. Chuchawankul S., Shima M., Buckley N. E., Hartmann C. B., and McCoy K. L. (2004) Role of cannabinoid receptors in inhibiting macrophage costimulatory activity. Int. Immunopharmacol. 4, 265–278 [DOI] [PubMed] [Google Scholar]
- 22. Correa F., Mestre L., Docagne F., and Guaza C. (2005) Activation of cannabinoid CB2 receptor negatively regulates IL-12p40 production in murine macrophages: role of IL-10 and ERK1/2 kinase signaling. Br. J. Pharmacol. 145, 441–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ross R. A., Brockie H. C., and Pertwee R. G. (2000) Inhibition of nitric oxide production in RAW264.7 macrophages by cannabinoids and palmitoylethanolamide. Eur. J. Pharmacol. 401, 121–130 [DOI] [PubMed] [Google Scholar]
- 24. Kogan N. M., and Mechoulam R. (2007) Cannabinoids in health and disease. Dialogues Clin. Neurosci. 9, 413–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baptista F. G., Pamplona A., Pena A. C., Mota M. M., Pied S., and Vigário A. M. (2010) Accumulation of Plasmodium berghei-infected red blood cells in the brain is crucial for the development of cerebral malaria in mice. Infect. Immun. 78, 4033–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pertwee R. G. (2008) Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict. Biol. 13, 147–159 [DOI] [PubMed] [Google Scholar]
- 27. Amante F. H., Stanley A. C., Randall L. M., Zhou Y., Haque A., McSweeney K., Waters A. P., Janse C. J., Good M. F., Hill G. R., and Engwerda C. R. (2007) A role for natural regulatory T cells in the pathogenesis of experimental cerebral malaria. Am. J. Pathol. 171, 548–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McQuillan J. A., Mitchell A. J., Ho Y. F., Combes V., Ball H. J., Golenser J., Grau G. E., and Hunt N. H. (2011) Coincident parasite and CD8 T cell sequestration is required for development of experimental cerebral malaria. Int. J. Parasitol. 41, 155–163 [DOI] [PubMed] [Google Scholar]
- 29. Riley E. M., and Stewart V. A. (2013) Immune mechanisms in malaria: new insights in vaccine development. Nat. Med. 19, 168–178 [DOI] [PubMed] [Google Scholar]
- 30. Haque A., Best S. E., Unosson K., Amante F. H., de Labastida F., Anstey N. M., Karupiah G., Smyth M. J., Heath W. R., and Engwerda C. R. (2011) Granzyme B expression by CD8+ T cells is required for the development of experimental cerebral malaria. J. Immunol. 186, 6148–6156 [DOI] [PubMed] [Google Scholar]
- 31. Hunt N. H., and Grau G. E. (2003) Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 24, 491–499 [DOI] [PubMed] [Google Scholar]
- 32. Gordon S., and Martinez F. O. (2010) Alternative activation of macrophages: mechanism and functions. Immunity 32, 593–604 [DOI] [PubMed] [Google Scholar]
- 33. Mildner A., and Jung S. (2014) Development and function of dendritic cell subsets. Immunity 40, 642–656 [DOI] [PubMed] [Google Scholar]
- 34. Hey Y. Y., and O'Neill H. C. (2012) Murine spleen contains a diversity of myeloid and dendritic cells distinct in antigen presenting function. J. Cell. Mol. Med. 16, 2611–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kastenmüller W., Torabi-Parizi P., Subramanian N., Lämmermann T., and Germain R. N. (2012) A spatially organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell 150, 1235–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu X., Gowda N. M., and Gowda D. C. (2015) Phagosomal acidification prevents macrophage inflammatory cytokine production to malaria, and dendritic cells are the major source at the early stages of infection: implication for malaria protective immunity development. J. Biol. Chem. 290, 23135–23147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beyer M., Mallmann M. R., Xue J., Staratschek-Jox A., Vorholt D., Krebs W., Sommer D., Sander J., Mertens C., Nino-Castro A., Schmidt S. V., and Schultze J. L. (2012) High-resolution transcriptome of human macrophages. PLoS One 7, e45466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martinez F. O., Helming L., and Gordon S. (2009) Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 27, 451–483 [DOI] [PubMed] [Google Scholar]
- 39. Alferink J., Lieberam I., Reindl W., Behrens A., Weiss S., Hüser N., Gerauer K., Ross R., Reske-Kunz A. B., Ahmad-Nejad P., Wagner H., and Förster I. (2003) Compartmentalized production of CCL17 in vivo: strong inducibility in peripheral dendritic cells contrasts selective absence from the spleen. J. Exp. Med. 197, 585–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Specht S., Frank J. K., Alferink J., Dubben B., Layland L. E., Denece G., Bain O., Förster I., Kirschning C. J., Martin C., and Hoerauf A. (2011) CCL17 controls mast cells for the defense against filarial larval entry. J. Immunol. 186, 4845–4852 [DOI] [PubMed] [Google Scholar]
- 41. Jordà M. A., Verbakel S. E., Valk P. J., Vankan-Berkhoudt Y. V., Maccarrone M., Finazzi-Agrò A., Löwenberg B., and Delwel R. (2002) Hematopoietic cells expressing the peripheral cannabinoid receptor migrate in response to the endocannabinoid 2-arachidonoylglycerol. Blood 99, 2786–2793 [DOI] [PubMed] [Google Scholar]
- 42. Ghosh S., Preet A., Groopman J. E., and Ganju R. K. (2006) Cannabinoid receptor CB2 modulates the CXCL12/CXCR4-mediated chemotaxis of T lymphocytes. Mol. Immunol. 43, 2169–2179 [DOI] [PubMed] [Google Scholar]
- 43. Pereira J. P., An J., Xu Y., Huang Y., and Cyster J. G. (2009) Cannabinoid receptor 2 mediates the retention of immature B cells in bone marrow sinusoids. Nat. Immunol. 10, 403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miller A. M., and Stella N. (2008) CB2 receptor-mediated migration of immune cells: it can go either way. Br. J. Pharmacol. 153, 299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Besnard A. G., Guabiraba R., Niedbala W., Palomo J., Reverchon F., Shaw T. N., Couper K. N., Ryffel B., and Liew F. Y. (2015) IL-33-mediated protection against experimental cerebral malaria is linked to induction of type 2 innate lymphoid cells, M2 macrophages and regulatory T cells. PLoS Pathog. 11, e1004607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bose T. O., Pham Q. M., Jellison E. R., Mouries J., Ballantyne C. M., and Lefrançois L. (2013) CD11a regulates effector CD8 T cell differentiation and central memory development in response to infection with Listeria monocytogenes. Infect. Immun. 81, 1140–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Backer R., Schwandt T., Greuter M., Oosting M., Jüngerkes F., Tüting T., Boon L., O'Toole T., Kraal G., Limmer A., and den Haan J. M. (2010) Effective collaboration between marginal metallophilic macrophages and CD8+ dendritic cells in the generation of cytotoxic T cells. Proc. Natl. Acad. Sci. U.S.A. 107, 216–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mukhopadhyay S., Plüddemann A., and Gordon S. (2009) Macrophage pattern recognition receptors in immunity, homeostasis and self tolerance. Adv. Exp. Med. Biol. 653, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kossodo S., Monso C., Juillard P., Velu T., Goldman M., and Grau G. E. (1997) Interleukin-10 modulates susceptibility in experimental cerebral malaria. Immunology 91, 536–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Youn J. I., and Gabrilovich D. I. (2010) The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur. J. Immunol. 40, 2969–2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cripps J. G., and Gorham J. D. (2011) MDSC in autoimmunity. Int. Immunopharmacol. 11, 789–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Van Ginderachter J. A., Beschin A., De Baetselier P., and Raes G. (2010) Myeloid-derived suppressor cells in parasitic infections. Eur. J. Immunol. 40, 2976–2985 [DOI] [PubMed] [Google Scholar]
- 53. Hegde V. L., Nagarkatti M., and Nagarkatti P. S. (2010) Cannabinoid receptor activation leads to massive mobilization of myeloid-derived suppressor cells with potent immunosuppressive properties. Eur. J. Immunol. 40, 3358–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Louvet A., Teixeira-Clerc F., Chobert M. N., Deveaux V., Pavoine C., Zimmer A., Pecker F., Mallat A., and Lotersztajn S. (2011) Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology 54, 1217–1226 [DOI] [PubMed] [Google Scholar]
- 55. Zarruk J. G., Fernández-López D., García-Yébenes I., García-Gutiérrez M. S., Vivancos J., Nombela F., Torres M., Burguete M. C., Manzanares J., Lizasoain I., and Moro M. A. (2012) Cannabinoid type 2 receptor activation downregulates stroke-induced classic and alternative brain macrophage/microglial activation concomitant to neuroprotection. Stroke 43, 211–219 [DOI] [PubMed] [Google Scholar]
- 56. Sponaas A. M., Cadman E. T., Voisine C., Harrison V., Boonstra A., O'Garra A., and Langhorne J. (2006) Malaria infection changes the ability of splenic dendritic cell populations to stimulate antigen-specific T cells. J. Exp. Med. 203, 1427–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Voisine C., Mastelic B., Sponaas A. M., and Langhorne J. (2010) Classical CD11c+ dendritic cells, not plasmacytoid dendritic cells, induce T cell responses to Plasmodium chabaudi malaria. Int. J. Parasitol. 40, 711–719 [DOI] [PubMed] [Google Scholar]
- 58. deWalick S., Amante F. H., McSweeney K. A., Randall L. M., Stanley A. C., Haque A., Kuns R. D., MacDonald K. P., Hill G. R., and Engwerda C. R. (2007) Cutting edge: conventional dendritic cells are the critical APC required for the induction of experimental cerebral malaria. J. Immunol. 178, 6033–6037 [DOI] [PubMed] [Google Scholar]
- 59. Lundie R. J., de Koning-Ward T. F., Davey G. M., Nie C. Q., Hansen D. S., Lau L. S., Mintern J. D., Belz G. T., Schofield L., Carbone F. R., Villadangos J. A., Crabb B. S., and Heath W. R. (2008) Blood-stage Plasmodium infection induces CD8+ T lymphocytes to parasite-expressed antigens, largely regulated by CD8α+ dendritic cells. Proc. Natl. Acad. Sci. U.S.A. 105, 14509–14514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yañez D. M., Manning D. D., Cooley A. J., Weidanz W. P., and van der Heyde H. C. (1996) Participation of lymphocyte subpopulations in the pathogenesis of experimental murine cerebral malaria. J. Immunol. 157, 1620–1624 [PubMed] [Google Scholar]
- 61. Potter S., Chan-Ling T., Ball H. J., Mansour H., Mitchell A., Maluish L., and Hunt N. H. (2006) Perforin mediated apoptosis of cerebral microvascular endothelial cells during experimental cerebral malaria. Int. J. Parasitol. 36, 485–496 [DOI] [PubMed] [Google Scholar]
- 62. Nitcheu J., Bonduelle O., Combadiere C., Tefit M., Seilhean D., Mazier D., and Combadiere B. (2003) Perforin-dependent brain-infiltrating cytotoxic CD8+ T lymphocytes mediate experimental cerebral malaria pathogenesis. J. Immunol. 170, 2221–2228 [DOI] [PubMed] [Google Scholar]
- 63. Mebius R. E., and Kraal G. (2005) Structure and function of the spleen. Nat. Rev. Immunol. 5, 606–616 [DOI] [PubMed] [Google Scholar]
- 64. Poppensieker K., Otte D. M., Schürmann B., Limmer A., Dresing P., Drews E., Schumak B., Klotz L., Raasch J., Mildner A., Waisman A., Scheu S., Knolle P., Forster I., Prinz M., et al. (2012) CC chemokine receptor 4 is required for experimental autoimmune encephalomyelitis by regulating GM-CSF and IL-23 production in dendritic cells. Proc. Natl. Acad. Sci. U.S.A. 109, 3897–3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sigel E., Baur R., Rácz I., Marazzi J., Smart T. G., Zimmer A., and Gertsch J. (2011) The major central endocannabinoid directly acts at GABAA receptors. Proc. Natl. Acad. Sci. U.S.A. 108, 18150–18155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Montecucco F., Matias I., Lenglet S., Petrosino S., Burger F., Pelli G., Braunersreuther V., Mach F., Steffens S., and Di Marzo V. (2009) Regulation and possible role of endocannabinoids and related mediators in hypercholesterolemic mice with atherosclerosis. Atherosclerosis 205, 433–441 [DOI] [PubMed] [Google Scholar]
- 67. Capasso R., Izzo A. A., Fezza F., Pinto A., Capasso F., Mascolo N., and Di Marzo V. (2001) Inhibitory effect of palmitoylethanolamide on gastrointestinal motility in mice. Br. J. Pharmacol. 134, 945–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Russo R., Loverme J., La Rana G., Compton T. R., Parrott J., Duranti A., Tontini A., Mor M., Tarzia G., Calignano A., and Piomelli D. (2007) The fatty acid amide hydrolase inhibitor URB597 (cyclohexylcarbamic acid 3′-carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice. J. Pharmacol. Exp. Ther. 322, 236–242 [DOI] [PubMed] [Google Scholar]
- 69. Specht S., Arriens S., and Hoerauf A. (2006) Induction of chronic colitis in IL-10 deficient mice requires IL-4. Microbes Infect. 8, 694–703 [DOI] [PubMed] [Google Scholar]