Introduction
Cancer is the uncontrolled proliferation of abnormal cells. Different treatment options are available to treat the cancers and chemotherapy is one of them. Numerous chemotherapeutic agents have been developed. Depending on the type of cancer, the particular type of chemotherapeutic agent is used. Unfortunately almost all of these drugs suffer from severe side effects and drug resistances. Besides, some of the organic drugs such as alkylating agents, nitrogen mustards, nitrosoureas, alkylsulfonates, triazines, and ethylenimines can eventually lead to leukemia. Long term use of breast cancer drug tamoxifen leads to endometrial cancer. Drugs derived from inorganic coordination compounds such as cisplatin, carboplatin, etc. are highly effective to several cancers. Fortunately they don't cause leukemia later on. However, they too suffer from severe toxicity and drug resistances. Compared to classical coordination metal complexes, organometallics offer opportunities for the design of novel drugs with metal specific modes of action [1]. For example, numerous organometallic tamoxifen derivatives have been found to be active against both estrogen receptor-positive and estrogen receptor-negative breast cancer cells [2]. On the contrary, tamoxifen can be used for estrogen receptor-positive breast cancer only. It is not at all effective on estrogen receptor-negative breast cancers. Additionally, it leads to endometrial cancer for its prolonged uses. Majority of the organometallic tamoxifen derivatives have been found to have much lower IC-50 values than the IC-50 value of tamoxifen. In fact, organometallic diphenols have IC-50 values of 0.7 and 0.6 μM for estrogen receptor-positive MCF-7 and estrogen receptor-negative MDAMB-231 breast cancer cells, respectively [3]. Many tricarbonyl rhenium (I) compounds have been found to be active against several cancer cell lines [4] and surprisingly they are not active against normal cells. Based on these facts, we embarked on the study of the cytotoxic properties of the rhenium tosylato complex, (CO)3(5,6-dimethyl-1,10-phenanthroline)ReOTs (abbreviated as TOS5) on BxPC-3 pancreatic cancer and pentylcarbonato complex, (CO)3(2,2’-Bipyridyl) ReOC(O)OC5H11 (abbreviated as PC1) on LNCaP and MDA prostate cancer cell lines.
Materials and Methods
Synthesis of the rhenium complexes
Synthesis of PC1
We previously reported its synthesis [5]. In brief, a mixture of one equivalent of Re2(CO)10 and two equivalents of 2,2’-bipyridyl was allowed to reflux in 1-pentanol for 24 hours while carbon dioxide was bubbled through the reaction mixture. Cooling the mixture to room temperature and subsequent filtration afforded yellow to red crystals of PC1 in high yield.
Synthesis of TOS5
We previously synthesized PC5 according to the method of synthesis of PC1 as noted above. A mixture of one equivalent of PC5 and one equivalent of ptoluenesulfonic acid in 20 mL of dichloromethane was allowed to stir for 30 minutes. The solvent was then removed on a rotary evaporator. The solid residue was recrystallized in a mixture of dichloromethane and hexane. The yield was nearly quantitative.
Cell Culture
The pancreatic cancer cell lines BxPC-3 and prostate cancer cell lines LNCaP and MDA obtained from ATCC (VA, USA) were grown in RPMI1640 medium supplemented with 10% serum and antibiotics penicillin and streptomycin using a 37° C carbon dioxide incubator.
MTT Assay
BxPC-3 pancreatic cancer cell death after treatment with TOS dissolved in DMSO was assayed by following standard MTT protocol using a Biotek 96-well plate reader.
Flow Cytometric Assay for Apoptosis and cell death
MDA and LNCaP prostate cancer cell lines were treated with PC1 dissolved in DMSO and analyzed for apoptosis and cell death using a Millipore Flow Cytometer with VIACOUNT (Millipore, USA) reagents.
DNA-Binding Studies using UV-Vis Spectrophotometry
The DNA-binding studies of PC1 and TOS5 were performed through UV titrations of PC1 and TOS5 with calf thymus DNA. The UV titrations of PC1 were reported previously [6]. The UV titrations of TOS5 were carried out in a similar manner. In brief, a DMSO solution of TOS5 diluted in Tris-HCl buffer saline into a cuvette was mixed gradually with an increasing amount of DNA and the spectrum was recorded after each addition of DNA.
RESULTS
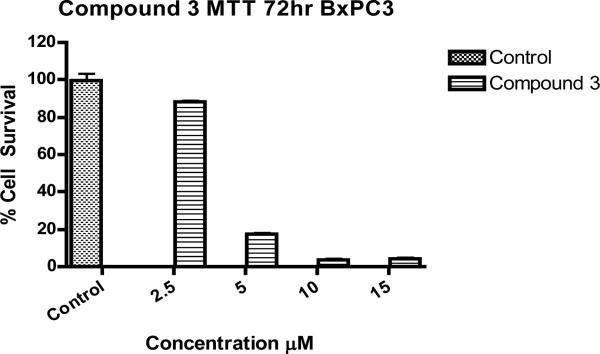
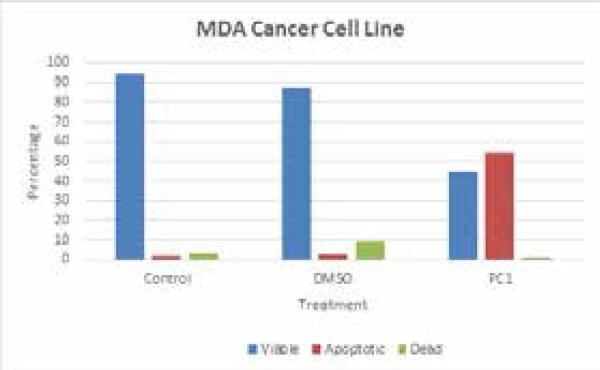
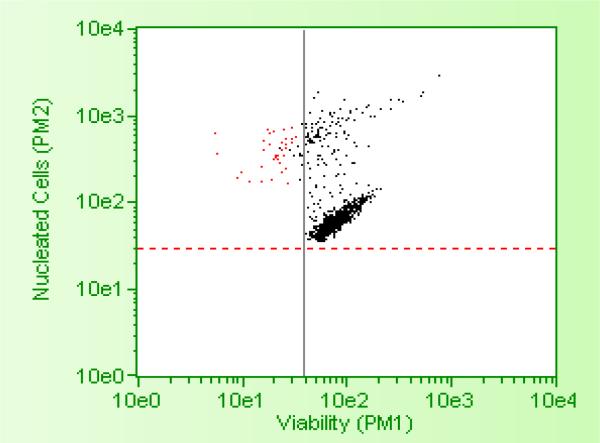
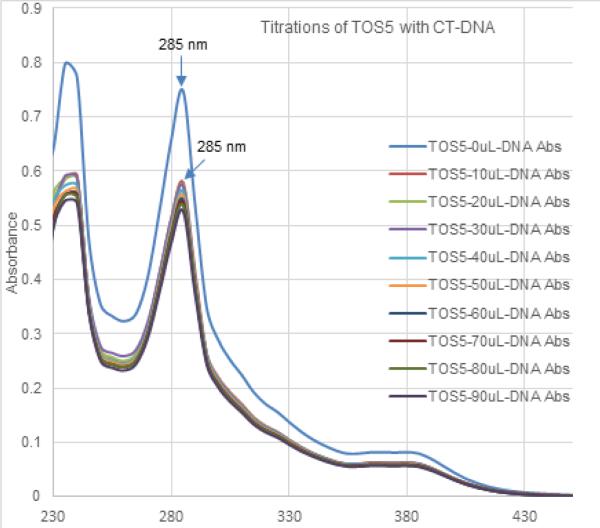
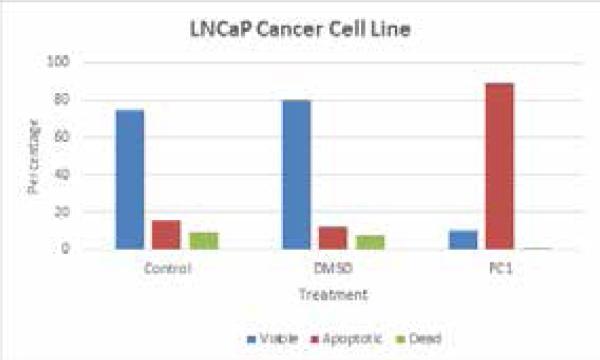
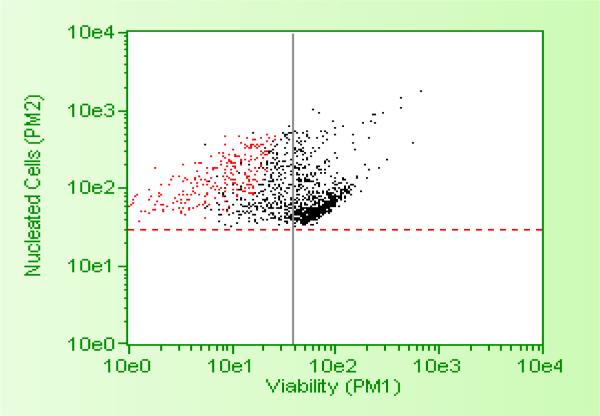
MTT assay showed TOS5 to be effective at a IC-50 dose of less than 3 μM (see, Figure 1), while flow cytometric analysis showed PC1 to be effective at around 5 μM IC-50 dose, successfully inducing cell death and apoptosis (Figures 2 - 5) in LNCaP and MDA prostate cancer cell lines. The UV titrations of TOS5 show that the absorbance decreases with each addition of CT-DNA (see, Figure 6).
Figure 1.
Effect of TOS5 (compound 3) on BxPC3 pancreatic cancer cell lines with DMSO control.
Figure 2.
Effect of PC1 on MDA prostate cancer cell lines
Figure 5.
Flow cytometric data showing the effect of 5μ PC1 on MDA prostate cancer cell lines.
Figure 6.
UV titrations of TOS5 with calf thymus DNA
Discussion
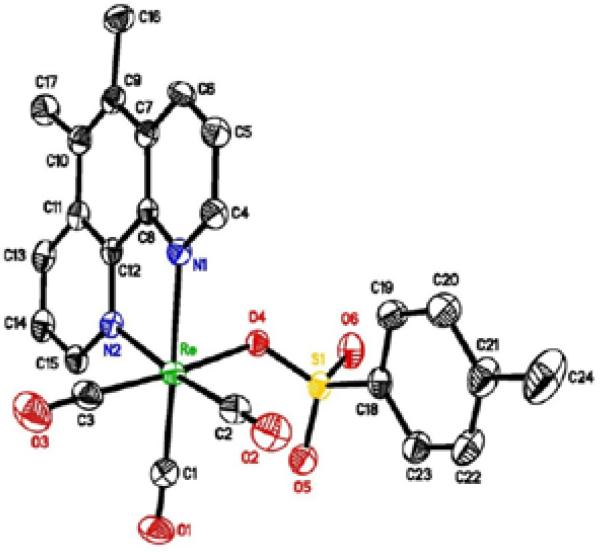
Our results show that both the rhenium compounds, TOS5 and PC1 were very effective in killing the cancer cell lines tested. As reported previously [6], many of the PC series compounds follow DNA intercalation mechanism due to the presence of planar aromatic (phenanthrolines) rings. Recently we solved the crystal structure of TOS5 which is shown in Figure 7 [7]. The structure indicates that the phenanthroline ring is planar. It is, therefore, expected that the TOS5 compound will follow intercalation mechanism. Figure 6 shows that each addition of DNA to TOS5 decreases the absorbance (hypochromic shift) without any red shift (bathochromic shift or shift to longer wavelength). It is, therefore, likely that TOS5 does not follow intercalation mechanism. Instead, it binds with the grooves of the DNA double helix which was observed in related rhenium complex [8]. In the absence of other techniques, it is highly premature to make a statement that TOS5 follows a groove binding mode. However, UV titration of complexes with DNA is a common and powerful technique to elucidate the mechanism of action.
Figure 7.
X-ray structure of TOS5
Pancreatic cancer is the second most prevalent cancer of the gastrointestinal area, and about 46,000 cases are diagnosed each year in the United States. About 90 percent of pancreatic cancers develop in duct cells. The disease can be difficult to detect or diagnose early because patients often have no symptoms or have symptoms that are similar to other illnesses and very difficult to treat. Other than skin cancer, prostate cancer is the most common cancer in American men. The American Cancer Society's estimates for prostate cancer in the United States for 2015 are about 220,800 new cases of prostate cancer with 27,540 deaths. Thus both the cancer types needs novel drugs for both cure and prevention of recurrence. In this study, we have presented the effectiveness of two novel rhenium-based compounds in treating pancreatic prostate cancer. Both the compounds showed cytotoxicity and bioactivity, and on farther investigation showed external DNA binding as their mode of action. We will be looking forward to future in vivo research studies like ADME and cancer model mice in order to take these drugs from bench to bed side.
Figure 3.
Effect of PC1 on LNCaP prostate cancer cell lines.
Figure 4.
Flow cytometric data showing the effect of 5 μM PC1 on LNCaP prostate cancer cell lines.
Acknowledgement
This research was supported by a NCI disability supplement and NIH grant#T34 GM100831 to Dr. Hirendra Nath Banerjee.
Contributor Information
Hirendra Nath Banerjee, Natural, Pharmacy and Health Sciences Department, Elizabeth City State University, University of North Carolina, Elizabeth City, NC 27909, USA..
Ava Boston, Natural, Pharmacy and Health Sciences Department, Elizabeth City State University, University of North Carolina, Elizabeth City, NC 27909, USA..
Alexis Barfield, Natural, Pharmacy and Health Sciences Department, Elizabeth City State University, University of North Carolina, Elizabeth City, NC 27909, USA..
Monet Stevenson, Natural, Pharmacy and Health Sciences Department, Elizabeth City State University, University of North Carolina, Elizabeth City, NC 27909, USA..
Fazlul H. Sarkar, Department of Pathology, Wayne State University School of Medicine, Kormanos Cancer Center, Detroit, MI 48202, USA.
Dipak Giri, Department of Chemistry, Morgan State University, Baltimore, MD 21251, USA..
Angela Winstead, Department of Chemistry, Morgan State University, Baltimore, MD 21251, USA..
Jeanette A. Krause, Department of Chemistry, University of Cincinnati, OH 45221, USA.
Santosh K. Mandal, Department of Chemistry, Morgan State University, Baltimore, MD 21251, USA.
References
- 1.Gasser G, Ott I, Metzler-Nolte N. Organometallic Anticancer Compounds. J. Med. Chem. 2011;54:3–25. doi: 10.1021/jm100020w. and other references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hillard EA, Vessières A, Jaouen G. In: Medicinal Organometallic Chemistry. Jaouen G, Metzler-Nolte N, editors. Springer; Berlin: 2010. pp. 81–117. [Google Scholar]
- 3.a Hillard EA, Vessières A, Top S, Pigeon P, Kowalski K, Huché M, Jaouen G. Organometallic diphenols: The importance of the organometallic moiety on the expression of a cytotoxic effect on breast cancer cells. J. Organomet. Chem. 2007;692:1315–26. [Google Scholar]; b Cázares-Marinero JJ, Buriez O, Labbé E, Top S, Amatore C, Jaouen G. Synthesis, Characterization, and Antiproliferative Activities of Novel Ferrocenophanic Suberamides against Human Triple-Negative MDAMB-231 and Hormone-Dependent MCF-7 Breast Cancer Cells. Organometallics. 2013;32:5026–34. [Google Scholar]
- 4.Yan YK, Cho SE, Shaffer KA, Rowell JE, Barnes BJ, Hall IH. Cytotoxicity of rhenium(I) alkoxo and hydroxo carbonyl complexes in murine and human tumor cells. Pharmazie. 2000;55(4):307–13. [PubMed] [Google Scholar]
- 5.Mbagu MK, Kebulu DN, Winstead A, Pramanik SK, Banerjee HN, Iwunze MO, Wachira JM, Greco GE, Haynes GK, Sehmer A, Sarkar FH, Ho DM, Pike RD, Mandal SK. Fac-tricarbonyl(pentylcarbonato) (α-diimine)rhenium(I) complexes: One-pot synthesis, characterization, fluorescence studies, and cytotoxic activity against human MDA-MB-231 breast, CCl-227 colon and BxPC-3 pancreatic carcinoma cell lines. Inorg. Chem. Commun. 2012;21:35–38. [Google Scholar]
- 6.Medley J, Payne G, Banerjee HN, Giri D, Winstead A, Wachira JM, Krause JA, Shaw R, Pramanik SK, Mandal SK. DNA-Binding and Cytotoxic Efficacy Studies of Organorhenium Pentylcarbonate Compounds. Mol. Cell. Biochem. 2015;398(1-2):21–30. doi: 10.1007/s11010-014-2201-5. [DOI] [PubMed] [Google Scholar]
- 7.Crystal data and other structural information will be published elsewhere [Google Scholar]
- 8.Michael Kaplanis M, Stamatakis G, Papakonstantinou VD, Paravatou-Petsotas M, Demopoulos CA, Mitsopoulou CA. Re(I) tricarbonyl complex of 1,10-phenanthroline-5,6-dione: DNA binding, cytotoxicity, anti-inflammatory and anti-coagulant effects towards platelet activating factor. J. Inorg. Biochem. 2014;135:1–9. doi: 10.1016/j.jinorgbio.2014.02.003. [DOI] [PubMed] [Google Scholar]