Abstract
The aim of this study was to evaluate the activity of daily living (ADL) and surgical interventions in patients with mucopolysaccharidosis IVA (MPS IVA).
The factor(s) that affect ADL are age, clinical phenotypes, surgical interventions, therapeutic effect, and body mass index.
The ADL questionnaire comprises three domains: “Movement,” “Movement with cognition,” and “Cognition.” Each domain has four subcategories rated on a 5-point scale based on the level of assistance. The questionnaire was collected from 145 healthy controls and 82 patients with MPS IVA. The patient cohort consisted of 63 severe and 17 attenuated phenotypes (2 were undefined); 4 patients treated with hematopoietic stem cell transplantation (HSCT), 33 patients treated with enzyme replacement therapy (ERT) for more than a year, and 45 untreated patients.
MPS IVA patients show a decline in ADL scores after 10 years of age. Patients with a severe phenotype have a lower ADL score than healthy control subjects, and lower scores than patients with an attenuated phenotype in domains of “Movement” and “Movement with cognition.” Patients, who underwent HSCT and were followed up for over 10 years, had higher ADL scores and fewer surgical interventions than untreated patients. ADL scores for ERT patients (2.5 years follow-up on average) were similar with the-age-matched controls below 10 years of age, but declined in older patients. Surgical frequency was higher for severe phenotypic patients than attenuated ones. Surgical frequency for patients treated with ERT was not decreased compared to untreated patients.
In conclusion, we have shown the utility of the proposed ADL questionnaire and frequency of surgical interventions in patients with MPS IVA to evaluate the clinical severity and therapeutic efficacy compared with age-matched controls.
Keywords: ADL, MPS IVA, ERT, HSCT, Surgical intervention
1. Introduction
Mucopolysaccharidosis IVA (MPS IVA; Morquio A syndrome) is a lysosomal storage disorder with an autosomal recessive trait, caused by a deficiency of N-acetylgalactosamine-6-sulfate sulfatase (GALNS) [1–5]. The deficiency of this enzyme leads to systemic accumulation of the glycosaminoglycans (GAGs) keratan sulfate (KS) and chondroitin-6-sulfate (C6S), primarily in cells and extracellular matrix of cartilage [6]. Accumulation of these GAGs causes incomplete endochondral ossification, leading to characteristic skeletal features such as disproportionate dwarfism with a short trunk and neck, pectus carinatum, joint laxity, kyphoscoliosis, genu valgum, and pes planus [1,2,7–16]. Radiographic findings show dysostosis multiplex with universal platyspondyly, anterior beaking of the lumbar spine, flaring of the rib cage, tilted ulna, coxa valga, flattering femoral head, and epiphyseal dysplasia of joints [2,7,8,17–28]. Most patients become wheelchair-bound in their second decade of life and undergo multiple orthopedic surgeries to alleviate serious medical complications [14,23–25]. Common mortality and morbidity are due to spinal cord injury that leads to spinal cord compression and instability of C1–C2 joint and later, respiratory failure caused by obstructive trachea and restrictive lung [14,23–25,27–29]. Severe tracheal obstruction leads to a high mortality and morbidity [28,30,31]. Most patients with MPS IVA patients need multiple surgical procedures with a high-risk anesthetic care throughout their lives [32–36].
The surgical interventions for patients with MPS IVA include a wide range of tissues such as adenoid/tonsil, ear, mandibula, spine, trachea, hand, hip, leg, knee, and ankle, that are required at different ages of development [14,16,37].
The two major therapeutic options for MPS patients are enzyme replacement therapy (ERT) and hematopoietic stem cell transplantation (HSCT). When compared with ERT, HSCT showed a superior reduction in substrate burden [38,39], and was better able to ameliorate and/ or cure associated musculoskeletal and organ-specific complications [40–42]. Unlike conventional ERT, HSCT can access the central nervous system and bone tissue, allowing treatment of neurocognitive degeneration [25,38,43–45] and skeletal dysplasia [46,47]. The mortality rate of the HSCT procedure for patients with MPS is now approximately 5% in a facility with well-trained staff [48, personal communication with Dr. Yabe]. Using an improved HSCT protocol [48] and by careful selection of patients and donors, the risk of HSCT is minimized. ERT for diagnosed MPS patients is currently widely accepted. Early diagnosis and early treatment provide the most benefit to the patients with MPS [49,50].
There is no therapy to cure bone lesions in MPS IVA although ERT [51–54] and HSCT [52,54–56] are available in clinical practice. Studies have shown that weekly ERT reduces urinary GAG levels and improves endurance (6-min walk test; 6MWT) and lung function [57,58]. However, in an extension trial, patients who had received placebo and subsequently received ERT for 48 weeks, did not show any improvement in the 6MWT, compared with placebo group [59]. The long-term follow-up after early introduction of HSCT has shown a therapeutic effect in amelioration of progression of the disease, suggesting that HSCT is a therapeutic option for patients with Morquio A [56]. Regardless of treatment approach, early treatment yields the most significant impact in ameliorating disease symptoms [25,52,56,60,61].
To assess the ADL in patients with MPS, several questionnaires have been designed. The Pediatric Evaluation of Disability Inventory (PEDI) is used to measure general child health [62]. The Functional Independence Measure (FIM) is widely used to evaluate ADL in patients with Hunter syndrome [22,23,62]. The PEDI and FIM questionnaire covers both motor and cognitive function. Moreover, a modified version of the Brief Assessment Examination (BAE) is used to assess cognitive functions for MPS III, which includes both ADL and evaluation of MPS-specific symptoms. [63]. However, these questionnaires need a trained professional, making the assessment inconvenient and time-consuming for the patients and their families. We have developed a simple ADL questionnaire and validated it for normal healthy children and patients with Hunter syndrome, distinguishing patients from control subjects [44]. This ADL questionnaire comprises three domains: “Movement,” “Movement with Cognition,” and “Cognition”, distinguishing it from other questionnaires comprised of, at most, two domains. We demonstrated that the ADL score correlates with clinical phenotype and therapeutic mode in Hunter syndrome and that HSCT provides a higher ADL score than ERT [44, see Supplemental Fig 1]. Early HSCT provided a higher score than late HSCT. Another advantage of this ADL questionnaire is that it can be self-administered and completed within 15 min. These findings indicated that this questionnaire could probably be applied to other types of MPS.
To date, several studies have evaluated the clinical status of MPS IVA [62,64–67]; however, the effect of ERT and HSCT on ADL and surgical interventions has not been compared with age-matched control subjects and untreated patients.
In this study, we have compared the ADL score and surgical interventions in treated, untreated, and age-matched control groups and have assessed the correlation between ADL and MPS symptom scores.
2. Subjects and methods
2.1. Subjects
All healthy controls were enrolled with informed consent at Gifu University. The MPS IVA patients were enrolled, with informed consent at Gifu University and Alfred I. duPont Hospital for Children (AIDHC). Age, gender, height and weight, ADL and MPS questionnaires, and surgical, ERT, and HSCT history were collected. Normal control subjects (n = 145; 72 males, 73 females; age range 0.33–43.55 years old; average age, 9.19 ± 8.00 years, median 7.08) and patients with MPS IVA (n = 82; 39 males, 43 females; age range, 0.71–70.46 years old; average age 22.40 ± 13.13 years, median 19.85) were enrolled. Sixty-three patients were diagnosed with a severe phenotype, and 17 patients were with an attenuated phenotype according to the criteria by height [68,69].
The FDA report indicates that a group of patients, who received placebo for 24 weeks in phase 3 clinical trial and subsequently received ERT for other 48 weeks in an extension trial, did not show therapeutic improvement of 6MWT, compared with the baseline in placebo group [58]. Therefore, patients with more than one-year ERT were defined as ERT-group. Thirty-three patients were treated with ERT including two patients who underwent corrective tracheal surgery during treatment (see below) [31], 4 patients were treated with HSCT [56], and 45 patients were untreated (including 12 patients with less than one-year ERT). In our study, the average starting age of ERT was 16.5 ± 12.7 years, and the average duration of ERT was 2.5 ± 1.0 years. The average starting age of HSCT was 10.6 ± 5.6 years, and the average observation period after HSCT was 19.0 ± 7.6 years.
Patients treated with ERT for more than one year had participated in a clinical trial [64]. Patients, who could not walk >30 m in a 6 min walk test (6 MWT), were not eligible to participate in the trial. Patients, who underwent surgical interventions within 3 months before initiation of ERT and had planned them during the clinical trials, were also excluded.
Therefore, patients in the ERT group did not include the most severe phenotype.
Six patients (2 patients with less than one-year ERT; 4 patients with more than one-year ERT) discontinued ERT because of either 1) adverse effects (2 patients) or 2) inability to strictly comply with the protocol (4–5 h weekly infusions, 4 patients).
For adults aged 20 years or older, body mass index [BMI: weight (kg)/height (m)2] was used to determine standard weight status categories. Four categories of weight status associated with BMI for adults are classified as follows: below 18.5 as “Underweight,” 18.5–24.9 as “Normal or healthy Weight,” 25.0–29.9 as “Overweight,” and 30.0 and above as “Obese.” For children and teens, aged 2 through 19 years old, the corresponding BMI-for-age percentile was assigned based upon a CDC BMI-for-age growth chart. BMI-for-age weight status categories and the corresponding percentiles are shown as follows [68]; less than the 5th percentile as “Underweight,” 5th percentile to less than the 85th percentile as “Normal or healthy Weight,” 85th to less than the 95th percentile as “Overweight,” and Equal to or greater than the 95th percentile as “Obese.”
2.2. ADL and MPS questionnaires and surgical history
In the first section, the ADL questionnaire used in this study comprised three domains (“Movement,” “Movement with cognition,” and “Cognition”); each with four subcategories scored from 0 to 5 (0 being the inability to perform task without maximum assistance and 5 being the ability to perform a task without any assistance) (see Supplemental Fig. 1: [44]). The questionnaire has previously been used for patients with Hunter syndrome [44]. The first domain, “Movement” includes basic motor skills needed for normal daily function with 4 subcategories: 1) walking, 2) movement on stairs, 3) grasping/finger movement, and 4) endurance in a 6 MWT. The second domain, “Movement with cognition,” includes movement that requires the cognitive function to some extent, comprising 1) toileting, 2) changing clothes, 3) bathing, and 4) eating. The third domain, “Cognition,” includes 1) understanding of everyday conversation, 2) conversation and speaking with others, 3) social participation, and 4) problem solving. The maximum score was 20 points per domain for a total of 60 points.
In the second section, an MPS questionnaire was also conducted that includes 12 symptoms specific to MPS: work/study, behavioral problems, sleep, pain, joint flexion, respiratory status, infection, vision, hearing, skin, hair, and appetite. These were all scored on a 0–5 scale. This questionnaire was used and validated for patients with Hunter syndrome [44].
In the third section, patients and/or their parent/guardian were asked about growth (height and weight) and Morquiorelated surgical history [44].
Questionnaires were sent or given to families with MPS IVA patients, completed directly by the patient and/or the patient’s parent/guardian, and then returned to the study group. Fourteen of the 82 patients provided multiple questionnaires, with at least two being one year apart (8 untreated, 4 ERT, 1 ERT before and after corrective tracheal surgery, and 1 HSCT).
ADL questionnaires were obtained from healthy control individuals after informed consent and then de-identified before inclusion in the study.
2.3. Surgical intervention
We obtained a surgical history from 75 patients in the ADL study (gender; 36 males and 39 females, age range; 2.45–70.46 years old, average age 22.70 ± 13.22 years, clinical phenotype; 59 severe and 16 attenuated). The surgical type was grouped by the location as the spine, hip, leg, hand/humerus, trachea, jaw, C-section, adenoid/tonsil, ear (tube placement), eye, hernia, HSCT, and others including breast abscess and teeth surgery.
Moreover, the patients were divided into untreated, before-ERT, after-ERT, pre-HSCT, and post-HSCT groups. The patients were also based on age groups; 0–5, 5–10,10–15,15–20, and >20 years of age. “Surgical frequency per year” is represented as total surgical times/ total observation year period. The frequency of surgical interventions (surgical times/observation years) was compared in untreated, ERT, and HSCT groups. Surgical rate (number of patients with surgical intervention/total number of patients × 100) was compared in treated and untreated groups as well as in age-groups.
2.4. Statistical analysis
Means and standard deviations of the total score and each domain were calculated in age-groups 0–5, 5–10,10–15,15–20, and >20 years of age. Student’s t-test was used to compare 1) MPS IVA patients with age-matched normal controls over 5 years of age, 2) patients with the severe phenotypes to patients with attenuated phenotypes, 3) ERT patients with untreated patients, and 4) HSCT patients with untreated patients. HSCT and ERT groups were not directly compared since the average treatment time between two therapies was markedly different (average: 18.1 years vs. 2.5 years). The equal variance assumption of the t-test was tested using the F test. The significance level was defined at p < 0.05.
Sixty-three of 82 patients were classified as severe, and seventeen were defined as attenuated. Two were undefined. There were insufficient data to compare treatments for patients with an attenuated phenotype.
3. Results
3.1. Demographics
The clinical phenotype was defined as severe (63 patients) if the patient’s height was below the 75th percentile height of a growth chart of MPS IVA patients and as attenuated (17 patients) if above the 75th percentile [68,69]. In height, 32.5% of the patients were below the 25th percentile, 29% between the 25 and 50th percentile, 17.5% between the 50–75th percentile and 21.3%, more than the 75 percentile [69,70] (Table 1).
Table 1.
Demographics of patients with MPS IVA in this study.
| Category | All MPS IVA | Attenuated | Severe | |
|---|---|---|---|---|
| N | 82 | 17 | 63 | |
| Gender: male: female | 39:43 | 7: 10 | 31:32 | |
| Age (years) | 22.40 ± 13.13 | 25.28 ± 13.08 | 21.67 ± 13.32 | |
| * Height (%) | <10% | 11.25 | 0.00 | 14.29 |
| <25% | 21.25 | 0.00 | 26.98 | |
| <50% | 28.75 | 0.00 | 36.51 | |
| <75% | 17.50 | 0.00 | 22.22 | |
| <90% | 16.25 | 76.47 | 0.00 | |
| above 90% | 5.00 | 23.53 | 0.00 | |
| Weight (%)* | <10% | 17.72 | 0.00 | 22.58 |
| <25% | 21.52 | 0.00 | 27.42 | |
| <50% | 25.32 | 5.88 | 30.65 | |
| <75% | 18.99 | 35.29 | 14.52 | |
| <90% | 12.66 | 41.18 | 4.84 | |
| above 90% | 3.80 | 17.65 | 0.00 | |
| ** Operations (%) | Spine | 49.33 | 37.50 | 52.54 |
| Hip | 20.00 | 37.50 | 15.25 | |
| Leg (knee, femur, tibia, ankle) | 52.00 | 37.50 | 55.93 | |
| Hand | 4.00 | 6.25 | 3.39 | |
| Trachea | 5.33 | 0.00 | 6.78 | |
| Inguinal and umbilical Hernia | 6.67 | 0.00 | 8.47 | |
| Adeno and tonsillectomy | 18.67 | 12.50 | 20.34 | |
| C-section | 4.00 | 12.50 | 1.69 |
For body weight, 39% patients were below the 25th percentile, 25% were between 25 and 50 percentile, 19% between 50 and 75th percentile, and 16%, more than the 75th percentile, using weight charts for patients with MPS IVA [69,70].
3.2. ADL scores in control subjects
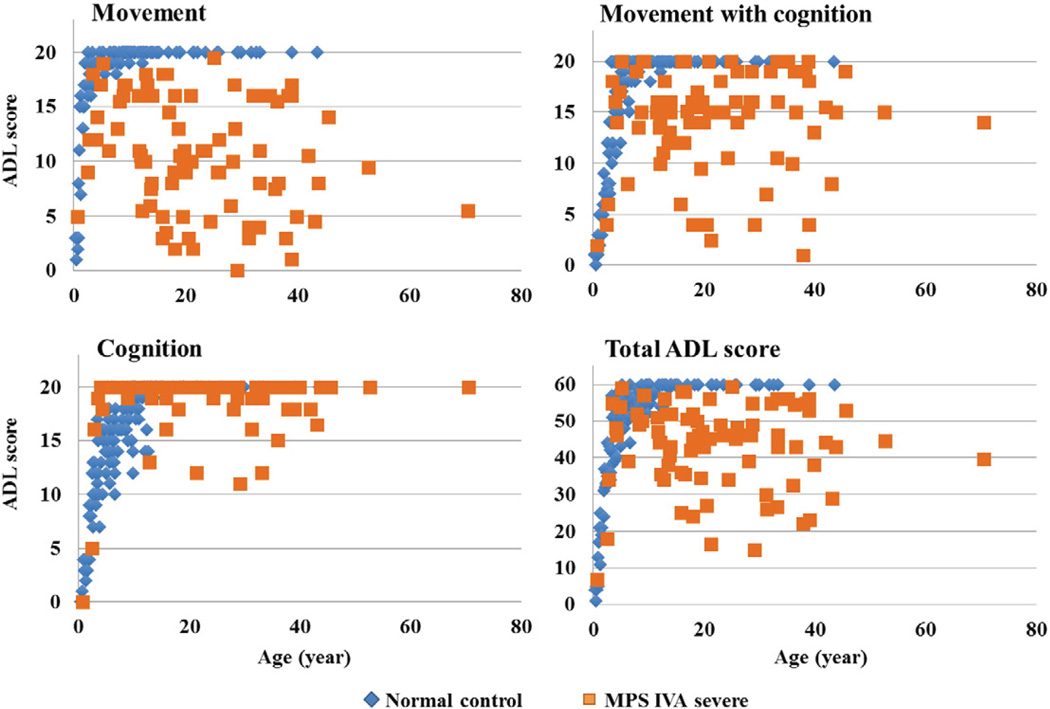
In control subjects, all scores increased sharply with age (Fig. 1) [44]. The total score reached a plateau at 10 years old, and all control subjects older than 15 years old obtained the maximum score, 60 (Fig. 1). “Movement” scores reached a plateau at 7 years old (Fig. 1), and “Movement with cognition” scores reached a plateau at 8 years old (Fig. 1). “Cognition” scores rose more slowly, reaching a plateau at 11 years old (Fig. 1).
Fig. 1.
ADL score in patients with MPS IVA and the age-matched control subjects.
3.3. Comparison of ADL scores between controls and patients with a severe or attenuated phenotype
In patients with MPS IVA, all scores increased sharply and reached a plateau by 10 years of age, as seen in control subjects (Fig. 1; Table 2). However, total scores became more variable in older patients. While some patients maintained scores just below the average of the control subjects, scores for others declined with age (Fig. 1). The decline of the score in patients was primarily observed in the domains, “Movement” and “Movement with cognition.”
Table 2.
ADL scores for age-matched controls, patients with attenuated phenotypes, and patients with severe phenotypes.
| Subject age range (years) | ||||||
|---|---|---|---|---|---|---|
| 0–5 | 5–10 | 10–15 | 15–20 | 20< | ||
| Control | n | 42 | 58 | 26 | 5 | 14 |
| Movement | 14.57 ± 6.26 | 19.40 ± 0.56 | 19.96 ± 0.20 | 20.00 ± 0.00 | 20.00 ± 0.00 | |
| Movement Cognition | 8.79 ± 6.54 | 19.19 ± 1.89 | 19.88 ± 0.43 | 20.00 ± 0.00 | 20.00 ± 0.00 | |
| Cognition | 8.38 ± 5.49 | 15.93 ± 2.55 | 18.81 ± 1.83 | 20.00 ± 0.00 | 20.00 ± 0.00 | |
| Severe | n | 7 | 4 | 11 | 13 | 28 |
| Movement | 12.43 ± 4.50 | 14.88 ± 2.66 | 10.68 ± 4.05 | 8.46 ± 4.67 | 8.23 ± 5.32 | |
| pa | 0.3908 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |
| Movement with Cognition | 11.00 ± 6.76 | 14.13 ± 4.94 | 13.77 ± 2.09 | 12.88 ± 4.10 | 12.46 ± 5.78 | |
| pa | 0.4129 | p < 0.0001 | p < 0.0001 | p < 0.005 | p < 0.0001 | |
| Cognition | 14.00 ± 8.10 | 19.75 ± 0.50 | 19.27 ± 2.10 | 19.38 ± 1.19 | 18.23 ± 2.69 | |
| pa | p < 0.05 | p < 0.005 | 0.5038 | 0.2743 | p < 0.05 | |
| Attenuated | n | - | 3 | 1 | 3 | 10 |
| Movement | - | 16.33 ± 3.06 | 18.00 | 14.50 ± 3.50 | 13.25 ± 3.58 | |
| pa | - | p < 0.0001 | NA | p < 0.01 | p < 0.0001 | |
| Movement with Cognition | - | 19.67 ± 0.58 | 18.00 | 17.33 ± 2.31 | 17.70 ± 2.06 | |
| pa | - | 0.6661 | NA | p < 0.05 | p < 0.001 | |
| Cognition | - | 20.00 ± 0.00 | 20.00 | 20.00 ± 0.00 | 19.70 ± 0.67 | |
| pa | - | p < 0.01 | NA | NA | 0.1074 | |
| t-Test between phenotypes | Movement | - | 0.5289 | NA | 0.0559 | p < 0.01 |
| Movement with Cognition | - | 0.1177 | NA | 0.0961 | p < 0.01 | |
| Cognition | - | 0.4366 | NA | 0.3990 | 0.0986 | |
Control vs. severe or attenuated phenotype.
NA: not applicable.
Patients with MPS IVA had a similar or higher ADL score in the “Cognition” domain than normal control before the age of 10 years and were indistinguishable from normal controls between10 and 20 years of age. Only the group of patients older than 20 years had a significantly lower score for “Cognition” than control subjects (Table 2).
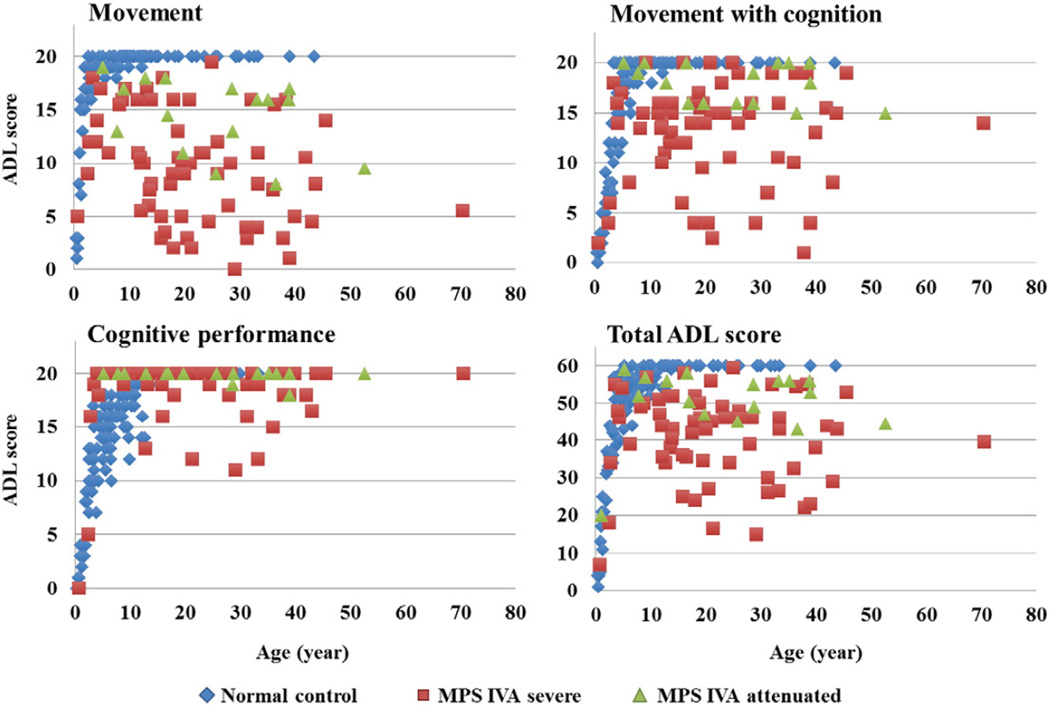
Total ADL scores for patients with a severe phenotype of MPS IVA ranged widely at all ages, from 7 to 59.5 (Fig. 2). The average ADL score for patients with a severe phenotype was 40.50 ±11.7 while the ADL score for patients with an attenuated phenotype was 52.1 ± 5.1. The total average score of MPS IVA patients with a severe form increased until around 9 years of age and declined after 10 years old. “Movement” score in patients with MPS IVA was lower than that in normal control subjects and declined after 10 years of age (Table 2). The “Movement with cognition” score followed a similar trend, but to a lower extent (Table 2). However, most patients maintained a near-normal cognitive function score through all ages. Several patients with lower cognitive function scores had physical severe handicaps including a bedridden condition, difficulty in breathing, and/or hearing loss, indicating that reduced cognition could in part be due to difficulties in learning social activity.
Fig. 2.
Correlation between ADL scores and clinical phenotypes.
Patients with an attenuated phenotype of MPS IVA also showed a decline in “Movement” scores with age, but to a lower extent than seen in patients with severe phenotypes (Fig. 2, Table 2). The difference in ADL scores for “Movement” and “Movement with cognition” between attenuated phenotype vs. severe phenotype was significant in patients over 20 years of age (Table 2).
A decline of “Movement with cognition” score was observed in several patients. Overall, patients with attenuated phenotypes had a higher mean score in all three domains, compared with those with severe phenotypes; however, total and subclasses of ADL scores substantially overlapped between severe phenotypic patients with a high score and attenuated patients (Table 2).
3.4. ADL scores in HSCT patients
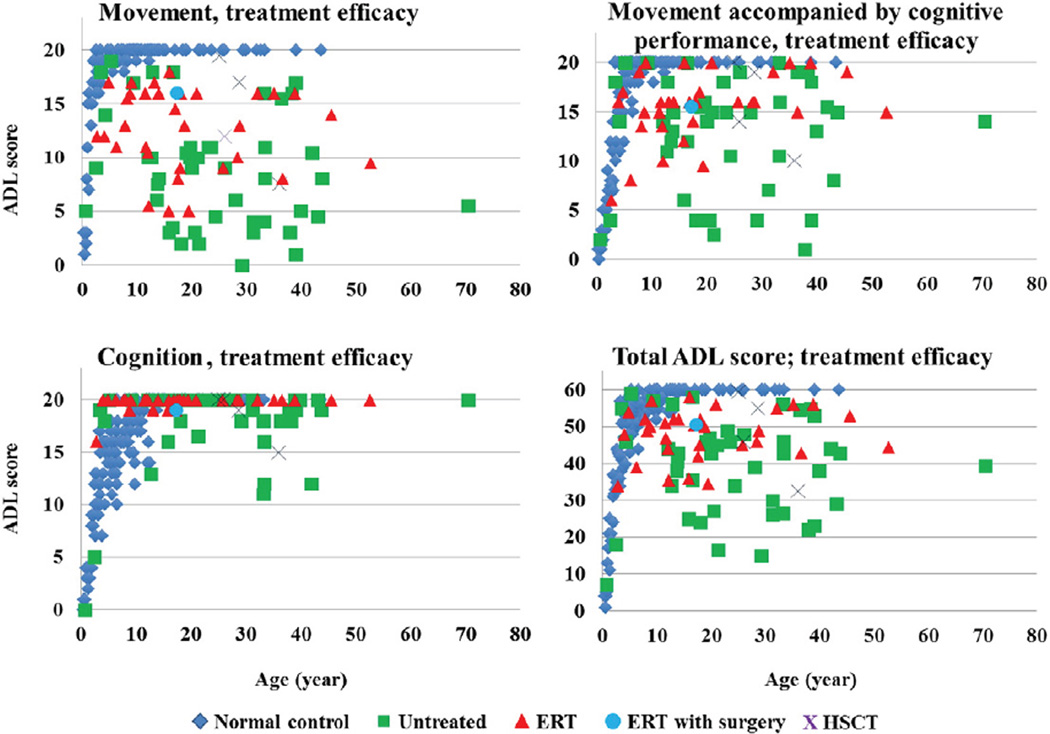
Four patients had been treated with HSCT, (three severe, one attenuated) when aged 4.0–15.6 years (mean 10.6 ± 5.6 years). This follow-up study was 11.2–28.0 years after treatment (mean 18.1 ± 7.6 years) when they are between 25.0 and 36.0 years of age (mean 28.9 ± 5.0 years). In the 4 patients treated with HSCT, their overall average ADL score was higher (48.3 ± 11.9), compared with age-matched untreated patients (38.0 ± 12.5). The movement score for HSCT treated patients was significantly higher than for untreated patients (p < 0.05). A 26-year-old female patient, who had been diagnosed with a severe phenotype and underwent HSCT at 4 years of age, had the highest score, 59.5 points, among all patients investigated in this study [56] (Supplemental Video Fig. 2). A 31-year-old patient with an attenuated phenotype scored 55 in total ADL.
3.5. ADL scores in ERT patients
Thirty-three patients in this study had been treated with ERT for more than one year. The average period of treatment was 2.5 ± 1.0 years. In patients treated with ERT (mean present age 19.2 ± 12.4 years), the average total ADL score was 48.1 ± 6.7. Scores were lower than age-matched controls but higher than untreated patients (Fig. 3).
Fig. 3.
ADL score in patients treated with ERT and HSCT. ERT with surgery: the patient with 2.5-year ERT received a corrective tracheal surgery.
Although the mean ADL score for ERT was similar to that for HSCT, a direct comparison cannot be made between these two groups. The ERT group had only been treated for an average of 2.5 years while the HSCT group had been treatedanaverageof18.1years. The ERT group does not include the most severe patients since patients, who could not walk ≥30 m in the 6 MWT, were excluded from the clinical trial of ERT.
3.6. Comparison of ADL scores by BMI
We investigated the comparison of ADL scores by BMI in 76 patients investigated here (Table 3). Regarding BMI, 2.6% patients were classified as “Underweight,” 53.8% as “Normal or Healthy Weight,” 32.1% as “Overweight,” and 11.5% as “Obese.” The “Obese” group had a lower total ADL score than the “Normal or Healthy Weight” and “Overweight” groups (p < 0.04 and p < 0.02, respectively) (Table 3). Only the domain of “Movement with cognition” was significantly lower on its own in the “Obese” group (p < 0.02 and p < 0.002, respectively).
Table 3.
Comparison of ADL scores by BMI groups.
| Category | All ages (n) | 2–19 years (n) | Over 20 years (n) | ADL score | Movement | Movement with Cognition | Cognition | |
|---|---|---|---|---|---|---|---|---|
| BMI | Underweight | 2 | 0 | 2 | 38.50 ± 23.3 | 10 | 10 | 18.5 |
| Normal or healthy weight | 42 | 25 | 17 | 44.54 ± 10.67 | 10.99 ± 5.30 | 14.26 ± 4.60 | 19.29 ± 1.86 | |
| Overweight | 25 | 9 | 16 | 45.42 ± 9.24 | 10.98 ± 4.79 | 15.50 ± 3.54 | 18.94 ± 2.15 | |
| Obese | 9 | 5 | 4 | 35.67 ± 12.40* | 8.78 ± 4.89 | 9.89 ± 5.46* | 17.00 ± 4.90 |
statistically significant.
3.7. MPS questionnaire
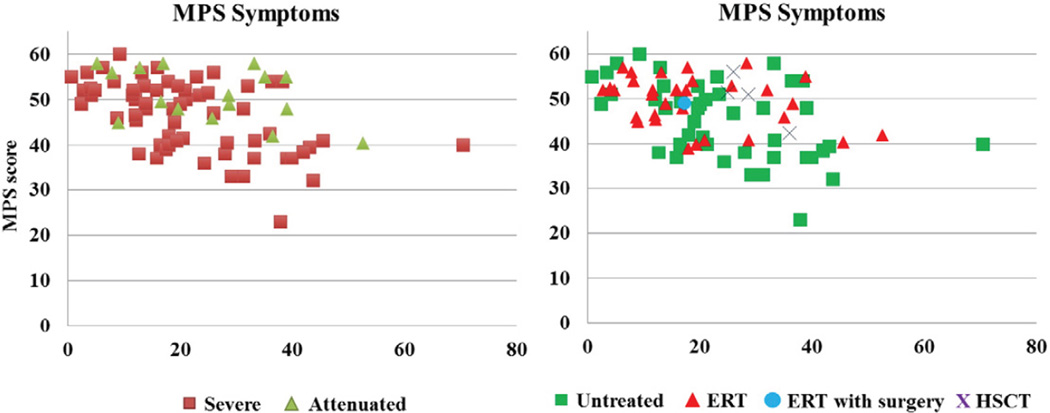
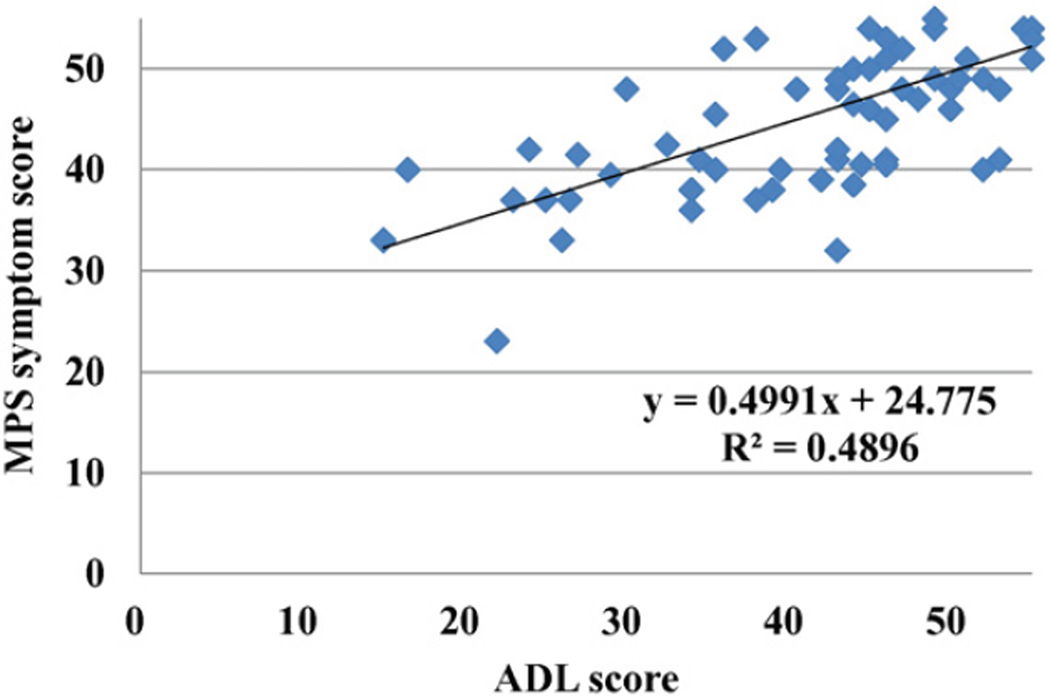
The MPS score declined in patients >10 years old (under 5 years, 52.5 ± 2.4; 5–10 years, 53.7 ± 5.9; 10–15 years, 49.5 ± 5.1; 15– < 20 years, 46.9 ± 6.5; > 20 years, 44.3 ± 8.2), suggesting disease progression with time. Patients with a severe phenotype had variable and lower average scores which declined with age. Among the symptoms related to MPS, patients had lowest scores in pain and joint flexion problems and also had low scores in work/study habits and respiratory status. Patients, who had been treated with HSCT (n = 4), had a higher score (50.3 ± 5.6), compared with those in the age-matched untreated patients (42.2 ± 8.8) (Fig. 4). As seen for ADL scores, patients treated with ERT who were older than 20 years (n = 9) had better MPS scores than untreated patients (47.1 ± 5.9 vs 43.5 ± 8.6), although this may be due to inclusion of more severely affected patients in the untreated group. There was a moderate correlation between ADL and MPS scores (Fig. 5).
Fig. 4.
MPS score in MPS IVA patients. ERT with surgery: the patient with 2.5-year ERT received a corrective tracheal surgery.
Fig. 5.
Correlation between ADL and MPS symptom score.
3.8. Longitudinal ADL and MPS scores
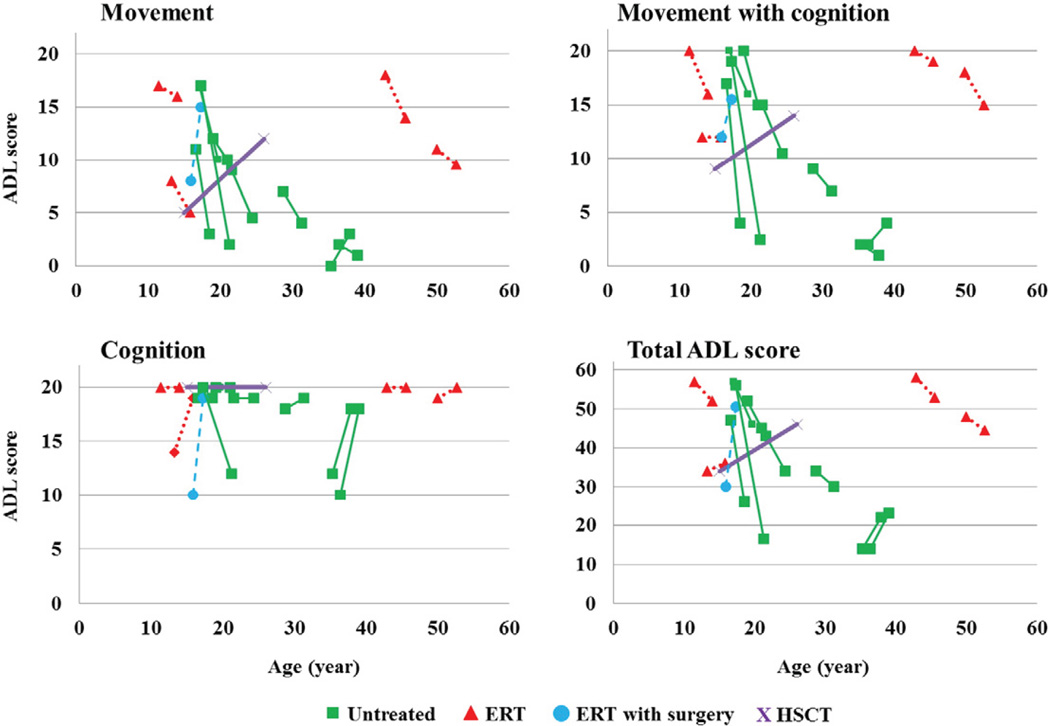
Fourteen patients (one, attenuated; thirteen, severe) completed more than one questionnaire over a period on more than one year. Four patients completed the ADL study before and after ERT; one before and after HSCT and leg surgery; one before and after ERT and corrective tracheal surgery; and eight untreated patients at different time points. A 16-year-old male patient had been undergoing ERT for over 2.5 years but was experiencing worsening tracheal obstruction and severe respiratory distress (ADL score 30 at 15.9 years [28,70]). Near fatal tracheal obstruction was relieved by urgent trachea vascular reconstruction with marked improvement of his respiratory symptoms (ADL score 50.5 at 17.3 years). This patient provided the largest improvement in ADL score among all patients investigated in longitudinal study group. The improvement in ADL score is most probably due to increased mobility after surgery and unrelated to ERT. Three of the 4 patients treated with ERT alone, showed a decline in ADL scores after treatment, similar to that seen in most untreated patients (Fig. 6).
Fig. 6.
Longitudinal ADL score. ERT with surgery: the patient with 2.5-year ERT received a corrective tracheal surgery. Twelve patients have a severe phenotype while a 52.6-year-old patient has an attenuated phenotype.
The only patient treated with HSCT who had taken the ADL survey more than once showed an improved score from 34 (at 15.0 years, pre-HSCT) to 46 (26.0 years old: 11 years later post-HSCT).
3.9. Surgical intervention
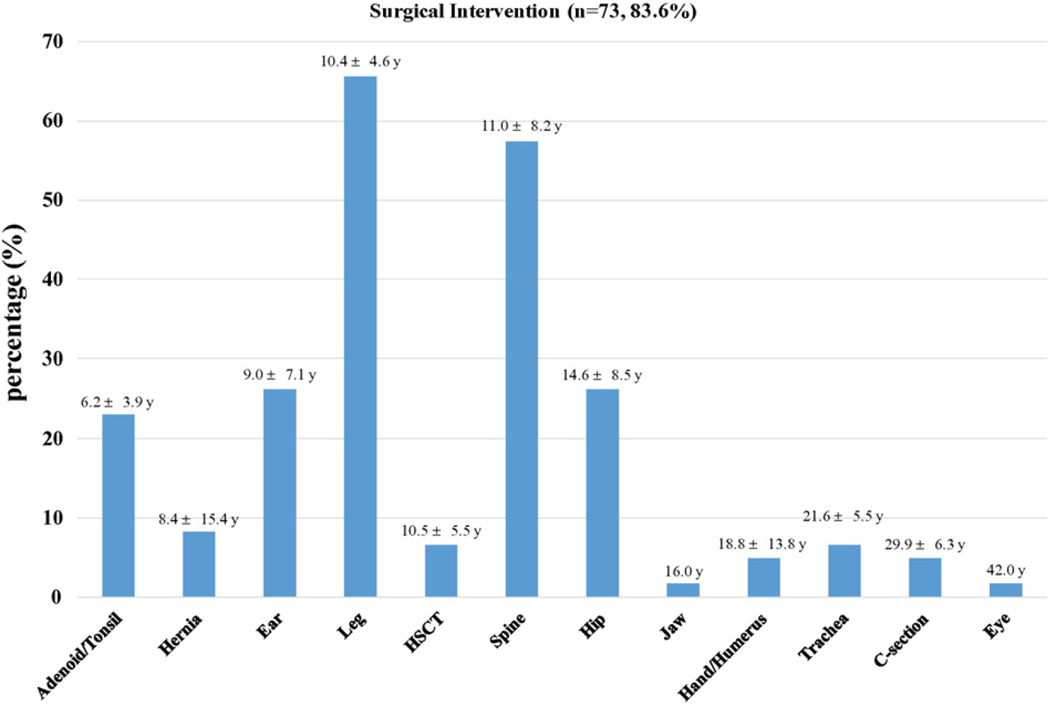
In the present study, 91.5% of patients (n = 75) had received at least one surgical intervention (average: 3 surgeries per patient for a total of 223, Fig. 7).
Fig. 7.
Distribution of surgical operations performed on MPS IVA patients (n = 73, 83.6% of a total number of patients). The numbers shown above each bar depict the mean age and standard deviation of the patients who underwent surgery. A total number of Morquio-related surgical interventions is 223.
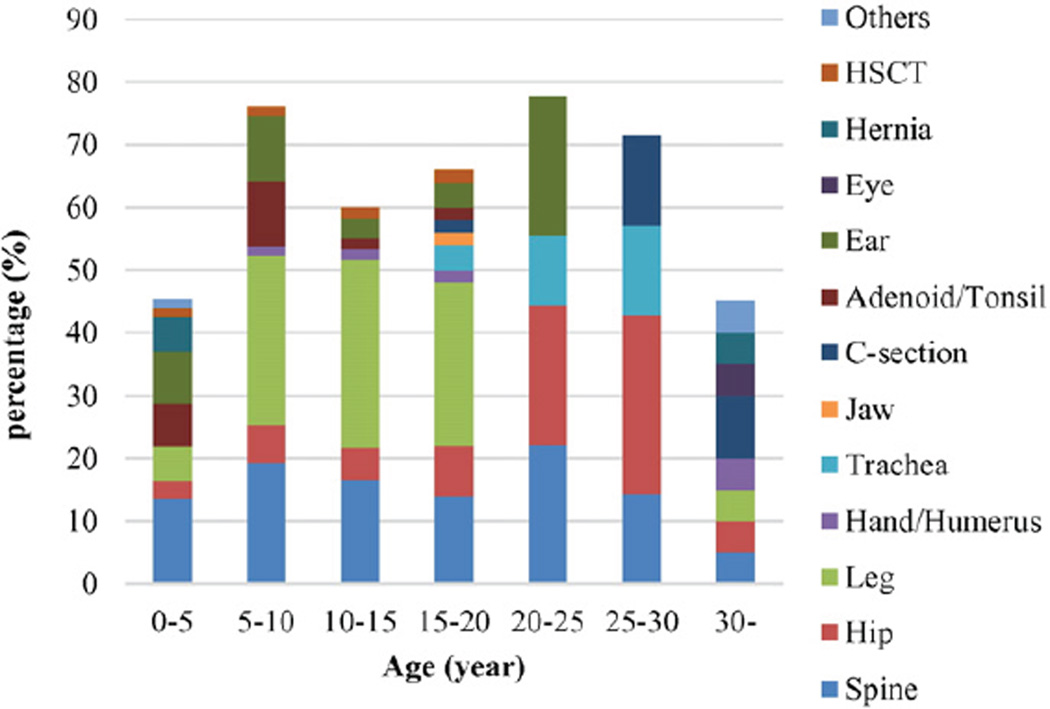
Surgeries are required throughout life, with certain surgeries more common at different stages of development (Fig. 8). Adenoid/tonsil surgery is most prevalent in children younger than 10 years of age. Leg surgery ismost often required during the growth period (10–20 years of age). Surgery in the trachea is most commonly conducted after 15 years of age. Hip surgery is most prevalent in young adults between 20 and 30 years of age. Spine surgery can be needed at any age.
Fig. 8.
Surgical rate by age. The surgical rate is expressed as the number of patients with surgical intervention(s) divided by a total number of patients × 100 in every 5-year observation term except over 30 years of age. Total number of patient in 0–5, 5–10, 10–15, 15–20, 20–25, 25–30, and over 30-years of age are 73, 67, 60, 50, 9,7, and 20, respectively. Others include one breast abscess surgery and one teeth surgery.
Patients with a severe phenotype require more surgeries (0.15/year) and at a younger average age 20.6 ± 11.2 years) compared to patients with an attenuated phenotype (0.10/year and 26.3 ± 13.5 years) (Supplemental Fig. 3).
The frequency of surgical intervention in all MPS IVA patients studied here was 0.14 time per year. The effect of treatment on surgical intervention was compared. Surgical frequency in the after-ERT group was not decreased compared with that in the before-ERT group. ERT group had a higher tendency to surgical frequency compared with the untreated group in age groups of 0–5 years, 5–10 years, and 15– 20 years of age (Supplemental Fig. 4). No orthopedic surgical intervention except the bilateral osteotomies in one of 4 patients had been performed after HSCT. In a post-HSCT group, surgical intervention was not observed in groups with 0–15 years and after 20 years of age. HSCT group had a lower surgical frequency compared with the untreated group (Supplemental Fig. 4).
Three female patients had C-sections. One female patient with over 1-year ERT treatment had four while the other two untreated patients had one each. Their babies were delivered without complication and not affected by MPS IVA. Four untreated patients died of respiratory failure with tracheal obstruction at ages of 20, 23, 28, and 37 years. A 20-year-old male patient had a cervical fusion. He could not make extubation post-surgery with a severe tracheal obstruction and died of respiratory failure one week after surgery [9]. One patient had a tracheostomy at 18 years old and died of respiratory failure at 37 years. Three patients (16, 24, and 26 years old) underwent a newly-designed corrective tracheal surgery for patients with MPS IVA. We have reported a 16-year-old case, who underwent the 2.5 year ERT, with a follow-up after 10-months of tracheal surgery [31]. The other two (one is untreated, and the other one is under one-year ERT) cases have had the surgery in the past month and have not yet been evaluated for ADL post-operatively.
4. Discussion
Our study has demonstrated that 1) the ADL questionnaire shows age-dependency and distinguishes MPS IVA patients from age-matched control subjects, 2) ADL scores in MPS IVA patients decline with time, especially in the domains of “Movement” and “Movement with cognition,” 3) ADL scores in patients with a severe form are more variable and lower compared to those with an attenuated form, 4) patients treated with HSCT, especially if treated at an early age, have a better ADL [56], 5) surgical correction of tracheal obstruction with ERT provides a dramatic improvement of ADL, 6) the MPS questionnaire shows age-dependency of MPS symptom, and there is a moderate correlation between ADL and the MPS questionnaire, and 7) the frequency and location of the surgical intervention are age- and phenotype-dependent.
MPS IVA is a progressive skeletal dysplasia with an imbalance of growth, leading to multiple bone deformities, tracheal obstruction, and restrictive lung. Unique skeletal features in MPS IVA provide a serious impact on the ADL and consequent frequent surgical interventions. Therefore, characterization of their ADL and surgical intervention should provide a better understanding of the obstacles for patients with MPS IVA, enabling physicians and patients to observe the progression of the disease and to evaluate and monitor therapeutic efficacy.
A sample size of 82 for patients with MPS IVA in this study is sufficient to evaluate whether ADL score can distinguish attenuated vs. severe and controls vs. MPS IVA patients with its simplicity and suitability of the questionnaire. It is also noteworthy that patients with Hunter syndrome showed a marked decline of ADL score in “Cognition” and “Movement with cognition” with age but maintained “Movement [44].” This finding in ADL is different from that in patients with MPS IVA, and therefore, the proposed ADL questionnaire can distinguish MPS IVA from other types of MPS that have CNS involvement. In contrast, the BAE questionnaire is not suitable for MPS IVA since it does not assess a movement function.
Most patients with MPS IVA keep a high score in cognitive function, and, therefore, reduced ADL scores are primarily due to reduced movement and movement with cognition. ADL scores measured in 14 patients over a one-year period indicate that most patients decline in score, particularly in the two areas that involve movement.
Some patients show substantially reduced ADL scores even in the cognitive domain. This may be caused in part by their physically handicapped condition and respiratory restriction that keep them away from social participation in environments where they can learn problem-solving skills and social activity.
A 26-year-old HSCT patient improved the ADL score from 34 to 46 over a 10-year period [55,56]. Another 25-year-old patient, who had undergone HSCT at 4 years of age, had the highest score of 59.5 among the patients investigated in this study. These results indicate the positive effect of HSCT and that it may be even more beneficial if performed at an early stage [56]. These results indicated that ADL questionnaire can evaluate the efficacy of treatments for MPS IVA.
A 16-year-old male patient, who received lumbar epidural-general anesthesia for bilateral distal femoral osteotomies, sustained a severe thoracic spinal cord infarction. Pre-operative imaging showed a moderate thoracic spinal stenosis and a mild degree of spinal cord compression [71]. The epidural anesthesia led to considerable delay in the recognition of the diagnosis of paraplegia. ADL score markedly decreased from 47 at preoperation to 26 at post-operation when aged 18.5 years. This experience indicates that it is critical to avoid the use of epidural anesthesia without firm indication in the presence of spinal stenosis in patients with MPS IVA. We had 4 patients with a severe tracheal obstruction who had lower ADL and MPS scores (31.25 ± 11.4; 37.5 ± 10.8, respectively) who later died due to respiratory failure. Two-thirds of patients with MPS IVA die of respiratory failure [30]. We have shown that the severity of tracheal obstruction increases with age and that all patients over 15 years of age have moderate to severe tracheal obstruction (>50% obstruction) [28]. Therefore, it is critical to understand how tracheal obstruction provides an impact to ADL with age and how current therapies such as ERT and HSCT provide an impact to tracheal obstruction. A 16-year-old patient with near fatal tracheal obstruction was rescued by surgical procedure without the need for tracheostomy, leading to marked improvement of ADL. Two additional patients over 20 years old (one has been on ERT for one-year, and the other is untreated) have also received tracheal reconstruction surgery recently and are under the follow-up (unpublished data). It is anticipated that improvements in their respiratory function will lead to improved mobility and consequently improved ADL scores. This surgical intervention may provide a new paradigm for the treatment of a tracheal obstruction in MPS IVA, resulting in rescuing the lives of patients with MPS IVA with a significant improvement of ADL [28,31].
Surgical interventions in patients with MPS IVA are very common throughout life, and each surgical procedure requiring anesthesia provides a high risk because of difficult airway [9,71,72]. Minimization of surgical frequency by management and use of non-surgical treatments should lower risk factors for these patients.
Our identifcation of leg, spine, and hip surgeries as the most frequent surgical procedures confirms and extends our previous findings [14].
Leg surgeries including osteotomies and 8-plate are most common in the 5–20 year age groups, suggesting that the abnormal growth of long bone during this period is a major factor causing rapid genu valgum. Hip surgery was more common after 20 years of age since deterioration of femur cap and the hip shelf is slowly progressive with age, compared with a leg deformity. There is no age-dependency of spine surgical rate since the progression of spinal involvement such as cervical instability and spinal cord compression at cervical, thoracic, and lumbar levels is different in individual patients and clinical phenotype [73]. The slowly progressive form may not become evident until late childhood or adolescence often first manifesting as hip problems [74].
Ear infections occur most commonly in young children because they are susceptible to infection, respiratory infections in particular, they have structurally and functionally immature Eustachian tubes, and their non-upright position during feeding [75]. Children often have obstructive problems from enlarged tonsils and adenoids without ever having had sore throats or “strep throat.” Adenoids usually begin to shrink after about 5 years of age and often practically disappear by the teen years. Therefore in our study, there is a high tendency of ear and adenoid/tonsil surgery being performed before 10 years of age. Patients with a severe form had a higher frequency of surgical intervention, compared with those with an attenuated form, suggesting a correlation between clinical phenotype and frequency of surgical intervention.
It is noteworthy that the present study population had a higher rate of surgical interventions compared to patients in our previous report [14], suggesting that the two studies may have included participants with differing surgical requirements.
The HSCT group had a lower surgical frequency compared with the untreated group, indicating that improvement of ADL in HSCT is not dependent on surgical intervention and that HSCT itself provides a positive impact to the bone as observed in bone pathology in MPS I [46]. Additional patients with HSCT are needed to determine the significance of these observations.
Obesity often leads to/results from physical inactivity, and we show that obese MPS IVA patients have lower ADL scores than non-obese patients. Obesity can provide a harmful effect on the body in various ways; 1) high blood pressure and high cholesterol, which are risk factors for cardiovascular disease [76], 2) breathing problems, such as sleep apnea and asthma [77,78], 3) joint problems and musculoskeletal discomfort [77,79], 4) psychological stress such as depression, behavioral problems, and issues in school [80–82], and 5) impaired social, physical, and emotional functioning [80]. All factors are closely associated with the clinical features and ADL in patients with MPS IVA One patient, who underwent HSCT at 15 years, had a BMI of 38.4 pre-transplantation (ADL score: 34) and this decreased to 32.0 at 26 years of age (ADL score: 46). Although the contribution of obesity to a reduced ADL remains unanswered, it is highly recommended that MPS IVA patients retain a near normal proportional stature.
Since patients with MPS IVA suffer from difficulties in ADL involving “Movement,” it is critical to assess not only impairment but also functionality. This ADL questionnaire indicates the level of assistance that the patient needs, leading to a more precise evaluation of ADL.
ERT does not seem to provide a short-term positive impact on the ADL score, and longer-term assessment is needed, especially as skeletal effects develop over many years during the progression of the disease. It will be of great interest to compare a long-term outcome of skeletal involvement in comparison with HSCT and untreated patients.
Evaluation of therapeutic effect by ADL showed a clear improvement after HSCT and trachea-corrective surgery. However, there are several limitations in this study that made some comparisons difficult; 1) the ERT group had a higher ADL score at the baseline than the untreated group, 2) only a limited number of patients were evaluated for change of longitudinal score, and 3) the therapeutic duration of ERT is much shorter than that of HSCT, 4) patients with the most severe phenotype were excluded from the ERT group, and 5) only a limited number of HSCT patients were studied.
In conclusion, we have determined the feasibility and reliability of an ADL questionnaire to show that it can distinguish control subjects from patients with MPS IVA and patients with severe phenotypes from attenuated phenotypes. We have also assessed the therapeutic efficacy of ADL questionnaire, confirming a long-term benefit of HSCT with fewer surgical interventions and an immediate effect of tracheal surgery. To compare HSCT and ERT, more patients of HSCT and longer duration of ERT are required.
Supplementary Material
Acknowledgments
This work was supported by grants from the Austrian MPS Society, The Bennett Foundation, and the International Morquio Organization (Carol Ann Foundation). This work was also supported by the Japanese MPS Family Society. Y.S. and K.E.O. were supported by the Ministry of Health, Labor, and Welfare of Japan. R.W.M. and S.T. were supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of NIH under grant numbers P20GM103464 and P30GM114736. S.T. and A.M.M. were supported by the National Institutes of Health grant R01HD065767. The content of the article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other sponsors. Editorial assistance to the manuscript was provided by Michelle Stofa at Nemours/ Alfred I. duPont Hospital for Children.
Abbreviations
- ADL
activity of daily living
- AIDHC
Alfred I. duPont Hospital for Children
- BMI
body mass index
- C6S
chondroitin-6-sulfate
- ERT
enzyme replacement therapy
- GAG
glycosaminoglycan
- GALNS
N-acetylgalactosamine-6-sulfate sulfatase
- HSCT
hematopoietic stem cell transplantation
- KS
keratan sulfate
- LSD
lysosomal storage disease
- MPS
mucopolysaccharidoses
- MPS IVA
mucopolysaccharidosis IVA
Appendix
Contributions to the project
Eriko Yasuda MSc is an expert of clinical pharmacy and contributed to the planning, data analysis, and reporting of the work described.
Yasuyuki Suzuki MD and Ph.D. is a clinical expert on HSCT and ERT on MPS over 30 years experience. He has contributed to the concept, development of ADL and MPS questionnaires, planning of the project, informed consent, analysis of data, and reporting of the work described. He and his team conducted the project and followed up with the patients.
Tsutomu Shimada Ph.D. is an expert of clinical pharmacy and has contributed to the concept of the project, planning, analysis of data, and reporting of the work described in the article.
Kazuki Sawamoto Ph.D. is an expert of clinical pharmacy and has contributed to the planning, data analysis, and reporting of the work described.
William G. Mackenzie MD is an orthopedic surgeon and has over 30 years of experience in Morquio surgery. He has contributed to the concept, treatment of patients, planning of the project, informed consent, analysis of data, and reporting of the work described.
Mary C. Theroux MD is an anesthesiologist and has over 20 years of experience in anesthesia for Morquio A. She has contributed to the concept, treatment of patients, planning of the project, informed consent, analysis of data, and reporting of the work described.
Christian Pizarro MD is a cardiologist and has established newlydevised corrective tracheal surgery for Morquio A. He has contributed to the concept, treatment of patients, planning of the project, informed consent, analysis of data, and reporting of the work described.
Freeman Miller MD is an orthopedic surgeon and expert on gait analysis on skeletal dysplasia. He has contributed to the concept, treatment of patients, planning of the project, informed consent, analysis of data, and reporting of the work described. Tariq Rahman PhD is an expert on the development of engineering device in the skeletal system. He has contributed to the concept, treatment of patients, planning of the project, informed consent, analysis of data, and reporting of the work described.
Heidi H. Kecskemethy is an expert on bone mineral density for skeletal dysplasia. She has contributed to the concept, treatment of patients, planning of the project, informed consent, analysis of data, and reporting of the work described.
Kyoko Nagao Ph.D. is an expert in hearing and speech sciences. She has contributed to the concept, planning of the project, informed consent, analysis of data, and reporting of the work described.
Thierry Morlet Ph.D. is an expert on hearing and neuroscience. He has contributed to the concept, planning of the project, analysis of data, and reporting of the work described.
Li Xie MSc is an expert on statistics and contributed to the planning of the article, statistical analysis of data, and reporting of the work described.
Thomas H. Shaffer Ph.D. is a Physiologist with over 40 years experience on the developing lung. He has contributed to the concept, treatment of patients, planning of the project, informed consent, analysis of data, and reporting of the work described.
Yasutsugu Chinen MD and Ph.D. is a clinical expert on HSCT and ERT and has contributed to HSCT on one patient with Morquio A for over 15 years follow-up, the planning, data analysis, and reporting of the work described.
Hiromasa Yabe MD and Ph.D. is a clinical expert on HSCT and has over 30 years of experience on HSCT He has contributed to HSCT in two patients with Morquio A for over 20 years follow-up. He has made the planning, data analysis, and reporting of the work described.
Akemi Tanaka MD and Ph.D. is a clinical expert on HSCT and ERT and has contributed to HSCT on one patient with Morquio A for over 25 years follow-up, data analysis, and reporting of the work described. She has deceased during the process of the manuscript.
Haruo Shintaku MD and Ph.D. is a clinical expert on HSCT and ERT and has contributed to the treatment of patients, data analysis, and reporting of the work described.
Kenji E. Orii MD and Ph.D. is a clinical expert on HSCT and ERT and has contributed to the treatment of patients, the planning, data analysis, and reporting of the work described.
Koji O. Orii MD and Ph.D. is a clinical expert on HSCT and ERT and has contributed to the data analysis of control subjects and reporting of the work described. Robert W. Mason Ph.D. is a biochemist and has contributed to data analysis, and reporting of the work described.
Adriana M. Montaño PhD has over 20 years of research experience in Morquio A disease. She has contributed to the planning, data analysis, and reporting of the work described.
Toshiyuki Fukao MD and Ph.D. is a clinical expert on HSCT and ERT and has contributed to the planning, data analysis, and reporting of the work described.
Tadao Orii MD is a principal investigator and has 50 years of clinical and research experiences in Morquio A. He has contributed to the planning, data analysis, and reporting of the work described.
Shunji Tomatsu MD and Ph.D. is a principal investigator and has 30 years of clinical and research experiences in Morquio A. He has contributed to the concept of the project, planning, analysis of data, and reporting of the work described in the article. He organized and communicated the entire team for this project.
International Morquio A investigational team
We have established a clinical research team to develop non-invasive assessment methods in Morquio A, leading to accurate and feasible evaluation of prognosis, therapeutic efficacy, and clinical severity. This multi-disciplinary team comprises the experts in the fields of orthopedics, tracheal-cardiology, anesthesiology, genetics, pharmacokinetics, molecular biology, gait pattern, bone mineral density, and pulmonary and hearing function, which are the keenest in Morquio A syndrome. The team has over 50 publications related to MPS IVA over the last five years in both basic and clinical research, including identification of biomarkers, diagnosis and treatments that encompass orthopedic and tracheal surgeries, ERT, HSCT, and gene therapy. This investigational team includes Japanese basic and clinical experts on Morquio A, who have abundant experience in gene cloning, clinical diagnosis, ERT, and HSCT for not only Morquio A but other types of MPS over a period of >50 years. The experts at Gifu University alone have over 100 publications related to MPS IVA since 1971. Thus, our investigational team members are internationally recognized in this field for their expertise ranging from basic science to clinical care.
Footnotes
Compliance with ethics
The study was approved by the Institutional Review Boards (IRB) at Gifu University, Saint Louis University, and Nemours/Alfred I. duPont Hospital for Children.
Conflict of interest
All the authors contributed to the Original Article and had no conflict of interest with any other party. Eriko Yasuda, Suzuki Yasuyuki, Kazuki Sawamoto, Tsutomu Shimada, William G. Mackenzie, Mary Theroux, Christian Pizarro, Freeman Miller, Heidi H. Kecskemethy, Kyoko Nagao, Thierry Morlet, Tariq Rahman, Thomas Shaffer, Yasutsugu Chinen, Hiromasa Yabe, Akemi Tanaka, Kenji E. Orii, Koji O. Orii, Robert W. Mason, AdrianaM. Montaño, Tadao Orii, and Shunji Tomatsu declare that they have no conflict of interests.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ymgme.2016.04.005.
References
- 1.Morquio L. The classics: on a form of familial osseous dystrophy. Clin. Orthop. Relat. Res. 1976;114:10–11. [PubMed] [Google Scholar]
- 2.Brailsford JF. The classics: chondro-osteo-dystrophy. Roentgenographic and clinical features of a child with dislocation of vertebrae. Clin. Orthop. Relat. Res. 1976;114:4–9. [PubMed] [Google Scholar]
- 3.Matalon R, Arbogast B, Justice P, Brandt IK, Dorfman A. Morquio’s syndrome: deficiency of a chondroitin sulfate N-acetylhexosamine sulfate. Biochem. Biophys. Res. Commun. 1974;61:759–765. doi: 10.1016/0006-291x(74)91022-5. [DOI] [PubMed] [Google Scholar]
- 4.Dorfman A, Arbogast B, Matalon R. The enzymic defects in Morquio and Maroteaux-Lamy syndrome. Adv. Exp. Med. Biol. 1976;68:261–276. doi: 10.1007/978-1-4684-7735-1_18. [DOI] [PubMed] [Google Scholar]
- 5.Di Ferrante N, Ginsberg LC, Donnelly PV, Di Ferrante DT, Caskey CT. Deficiencies of glucosamine-6-sulfate or galactosamine-6-sulfate sulfatases are responsible for different mucopolysaccharidoses. Science. 1978;199:79–81. doi: 10.1126/science.199.4324.79. [DOI] [PubMed] [Google Scholar]
- 6.Pedrini V, Lennzi L, Zambotti V. Isolation and identification of keratosulphate in urine of patients affected by Morquio-Ullrich disease. Proc. Soc. Exp. Biol. Med. 1962;110:847–849. doi: 10.3181/00379727-110-27668. [DOI] [PubMed] [Google Scholar]
- 7.Orii T, Minami R, Chiba G, et al. Study on Morquio syndrome. Bone Metab. (Japan.) 1971;5:72–78. [Google Scholar]
- 8.Sukegawa K, Orii T. Residual activity in fibroblasts from two brothers with the late-onset form of N-acetylgalactosamine-6-sulphate sulphatase deficiency. J. Inherit. Metab. Dis. 1982;5(4):231–232. doi: 10.1007/BF02179150. [DOI] [PubMed] [Google Scholar]
- 9.Yasuda E, Fushimi K, Suzuki Y, et al. Pathogenesis of Morquio A syndrome: an autopsied case reveals systemic storage disorder. Mol. Genet. Metab. 2013;109:301–311. doi: 10.1016/j.ymgme.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Neufeld EF, Muenzer J. The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. eighth. New York: McGraw-Hill; 2001. pp. 3421–3452. [Google Scholar]
- 11.Tomatsu S, Orii KO, Vogler C, et al. Mouse model for Galns−/− produced by targeted disruption of the gene defective in Morquio A disease. Hum. Mol. Genet. 2003;12:3349–3358. doi: 10.1093/hmg/ddg366. [DOI] [PubMed] [Google Scholar]
- 12.Tomatsu S, Gutiérrez MA, Nishioka T, et al. Development of MPS IVA mouse (Galnstm(hC79mC76)slu) tolerant hGALNS. Hum. Mol. Genet. 2005;14:3321–3336. doi: 10.1093/hmg/ddi364. [DOI] [PubMed] [Google Scholar]
- 13.Tomatsu S, Vogler C, Montaño AM, et al. Murine model (Galns(tm(C76S)slu)) of MPS IVA with missense mutation at the active site cysteine conserved among sulfatase proteins. Mol. Genet. Metab. 2007;91:251–258. doi: 10.1016/j.ymgme.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Montaño AM, Tomatsu S, Gottesman GS, Smith M, Orii T. International Morquio A registry: clinical manifestation and natural course of Morquio A disease. J. Inherit. Metab. Dis. 2007;301:165–174. doi: 10.1007/s10545-007-0529-7. [DOI] [PubMed] [Google Scholar]
- 15.Tomatsu S, Montaño AM, Oikawa H, et al. Mucopolysaccharidosis type IVA (Morquio A disease): clinical review and current treatment. Curr. Pharm. Biotechnol. 2011;12:931–945. doi: 10.2174/138920111795542615. [DOI] [PubMed] [Google Scholar]
- 16.Tomatsu S, Mackenzie WG, Theroux MC, et al. Current and emerging treatments and surgical interventions for Morquio A syndrome: a review. Res. Rep. Endocr. Disord. 2012;2:65–77. doi: 10.2147/RRED.S37278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hecht JT, Scott CI, Jr, Smith TK, Williams JC. Mild manifestations of the Morquio syndrome. Am. J. Med. Genet. 1984;18:369–371. doi: 10.1002/ajmg.1320180222. [DOI] [PubMed] [Google Scholar]
- 18.Hendriksz CJ, Harmatz P, Beck M, et al. Review of clinical presentation and diagnosis of mucopolysaccharidosis IVA. Mol. Genet. Metab. 2013;110:54–64. doi: 10.1016/j.ymgme.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin R, Beck M, Eng C, et al. Recognition and diagnosis of mucopolysaccharidosis II (Hunter syndrome) Pediatrics. 2008;121:e377–e386. doi: 10.1542/peds.2007-1350. [DOI] [PubMed] [Google Scholar]
- 20.Ochiai T, Ito K, Okada T, Chin M, Shichino H, Mugishima H. Significance of extensive Mongolian spots in Hunter’s syndrome. Br. J. Dermatol. 2003;148:1173–1178. doi: 10.1046/j.1365-2133.2003.05317.x. [DOI] [PubMed] [Google Scholar]
- 21.Young ID, Harper PS. The natural history of the severe form of Hunter’s syndrome: a study based on 52 cases. Dev. Med. Child Neurol. 1983;25:481–489. doi: 10.1111/j.1469-8749.1983.tb13794.x. [DOI] [PubMed] [Google Scholar]
- 22.Kuratsubo I, Suzuki Y, Orii KO, Kato T, Orii T, Kondo N. Psychological status of patients with mucopolysaccharidosis type II and their parents. Pediatr. Int. 2009;51:41–47. doi: 10.1111/j.1442-200X.2008.02652.x. [DOI] [PubMed] [Google Scholar]
- 23.Kato T, Kato Z, Kuratsubo I, et al. Evaluation of ADL in patients with Hunter disease using FIM score. Brain Dev. 2007;29:298–305. doi: 10.1016/j.braindev.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Patel P, Suzuki Y, Tanaka A, et al. Impact of enzyme replacement therapy and hematopoietic stem cell therapy on growth in patients with Hunter syndrome. Mol. Genet. Metab. Rep. 2014;1:184–196. doi: 10.1016/j.ymgmr.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka A, Okuyama T, Suzuki Y, et al. Long-term efficacy of hematopoietic stem cell transplantation on brain involvement in patients with mucopolysaccharidosis type II: a nationwide survey in Japan. Mol. Genet. Metab. 2012;107:513–520. doi: 10.1016/j.ymgme.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Tomatsu S, Fujii T, Fukushi M, et al. Newborn screening and diagnosis of mucopolysaccharidoses. Mol. Genet. Metab. 2013;110:42–53. doi: 10.1016/j.ymgme.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendelsohn NJ, Harmatz P, Bodamer O, et al. Importance of surgical history in diagnosing mucopolysaccharidosis type II (Hunter syndrome): data from the Hunter Outcome Survey. Genitourin. Med. 2010;12:816–822. doi: 10.1097/GIM.0b013e3181f6e74d. [DOI] [PubMed] [Google Scholar]
- 28.Tomatsu S, Averill LW, Sawamoto K, et al. Obstructive airway in Morquio A syndrome, the past, the present, and the future. Mol. Genet. Metab. 2016;117:150–156. doi: 10.1016/j.ymgme.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solanki GA, Martin KW, Theroux MC, et al. Spinal involvement in mucopolysaccharidosis IVA (Morquio-Brailsford or Morquio A syndrome): presentation, diagnosis and management. J. Inherit. Metab. Dis. 2013;36:339–355. doi: 10.1007/s10545-013-9586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavery C, Hendriksz C. Mortality in patients with morquio syndrome a. JIMD Rep. 2015;15:59–66. doi: 10.1007/8904_2014_298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pizarro C, Davies RR, Spurrier EA, et al. Surgical reconstruction for severe tracheal obstruction in Morquio A syndrome 2016. Ann. Thorac. Surg. doi: 10.1016/j.athoracsur.2016.02.113. (in press) [DOI] [PubMed] [Google Scholar]
- 32.Cooper GA, Southorn T, Eastwood DM, Bache CE. Lower extremity deformity management in MPS IVA, Morquio–Brailsford syndrome: preliminary report of Hemiepiphysiodesis correction of genu valgum. J. Pediatr. Orthop. 2015 Apr 3; doi: 10.1097/BPO.0000000000000464. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 33.Dhawale AA, Thacker MM, Belthur MV, Rogers K, Bober MB, Mackenzie WG. The lower extremity in Morquio syndrome. J. Pediatr. Orthop. 2012;32:534–540. doi: 10.1097/BPO.0b013e318259fe57. [DOI] [PubMed] [Google Scholar]
- 34.Ain MC, Chaichana KL, Schkrohowsky JG. Retrospective study of cervical arthrodesis in patients with various types of skeletal dysplasia. Spine (Phila Pa 1976) 2006;31:E169–E174. doi: 10.1097/01.brs.0000202758.61848.61. [DOI] [PubMed] [Google Scholar]
- 35.Defraia E, Marinelli A, Antonini A, Giuntini V. Abnormal mandibular growth after craniovertebral surgery in Morquio syndrome type a. Angle Orthod. 2005;75:461–464. doi: 10.1043/0003-3219(2005)75[461:AMGACS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 36.White KK, Jester A, Bache CE, et al. Orthopedic management of the extremities in patients with Morquio A syndrome. J. Child. Orthop. 2014;8:295–304. doi: 10.1007/s11832-014-0601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin HY, Chuang CK, Chen MR, et al. Natural history and clinical assessment of Taiwanese patients with mucopolysaccharidosis IVA. Orphanet J. Rare Dis. 2014;10(9):21. doi: 10.1186/1750-1172-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wynn RF, Wraith JE, Mercer J, et al. Improved metabolic correction in patients with lysosomal storage disease treated with hematopoietic stem cell transplant compared with enzyme replacement therapy. J. Pediatr. 2009;154:609–611. doi: 10.1016/j.jpeds.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Shimada T, Kelly J, LaMarr WA, et al. Novel heparan sulfate assay by using automated high-throughput mass spectrometry: application to monitoring and screening for mucopolysaccharidoses. Mol. Genet. Metab. 2014;113:92–99. doi: 10.1016/j.ymgme.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muenzer J, Wraith JE, Clarke LA, et al. Mucopolysaccharidosis I: management and treatment guidelines. Pediatrics. 2009;123:19–29. doi: 10.1542/peds.2008-0416. [DOI] [PubMed] [Google Scholar]
- 41.Whitley CB, Belani KG, Chang PN, et al. Long-term outcome of Hurler syndrome following bone marrow transplantation. Am. J. Med. Genet. 1993;46:209–218. doi: 10.1002/ajmg.1320460222. [DOI] [PubMed] [Google Scholar]
- 42.Souillet G, Guffon N, Maire I, et al. Outcome of 27 patients with Hurler’s syndrome transplanted from either related or unrelated haematopoietic stem cell sources. Bone Marrow Transplant. 2003;31:1105–1117. doi: 10.1038/sj.bmt.1704105. [DOI] [PubMed] [Google Scholar]
- 43.Algahim MF, Almassi GH. Current and emerging management options for patients with Morquio A syndrome. Ther. Clin. Risk Manag. 2013;9:45–53. doi: 10.2147/TCRM.S24771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanjuakio J, Suzuki Y, Patel P, et al. Activities of daily living in patients with Hunter syndrome: impact of enzyme replacement therapy and hematopoietic stem cell transplantation. Mol. Genet. Metab. 2015;114:161–169. doi: 10.1016/j.ymgme.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prasad VK, Mendizabal A, Parikh SH, et al. Unrelated donor umbilical cord blood transplantation for inherited metabolic disorders in 159 pediatric patients from a single center: influence of cellular composition of the graft on transplantation outcomes. Blood. 2008;112:2979–2989. doi: 10.1182/blood-2008-03-140830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yasuda E, Mackenzie W, Ruhnke K, et al. Molecular genetics and metabolism report long-term follow-up of post hematopoietic stem cell transplantation for Hurler syndrome: clinical, biochemical, and pathological improvements. Mol. Genet. Metab. Rep. 2015;2:65–76. doi: 10.1016/j.ymgmr.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee V, Li CK, Shing MM, et al. Umbilical cord blood transplantation for Maroteaux-Lamy syndrome (mucopolysaccharidosis type VI) Bone Marrow Transplant. 2000;26:455–458. doi: 10.1038/sj.bmt.1702528. [DOI] [PubMed] [Google Scholar]
- 48.Aldenhoven M, Jones SA, Bonney D, et al. Hematopoietic cell transplantation for mucopolysaccharidosis patients is safe and effective: results after implementation of international guidelines. Biol. Blood Marrow Transplant. 2015;21:1106–1109. doi: 10.1016/j.bbmt.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 49.Tomatsu S, Azario I, Sawamoto K, Pievani AS, Biondi A, Serafini M. Neonatal cellular and gene therapies for mucopolysaccharidoses: the earlier the better? J. Inherit. Metab. Dis. 2016;39(2):189–202. doi: 10.1007/s10545-015-9900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muenzer J. Early initiation of enzyme replacement therapy for the mucopolysaccharidoses. Mol. Genet. Metab. 2014;111:63–72. doi: 10.1016/j.ymgme.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 51.Tomatsu S, Yasuda E, Patel P, et al. Morquio A syndrome: diagnosis and current and future therapies. Pediatr. Endocrinol. Rev. 2014;12(Suppl. 1):141–151. [PMC free article] [PubMed] [Google Scholar]
- 52.Tomatsu S, Alméciga-Díaz CJ, Montaño AM, et al. Therapies for the bone in mucopolysaccharidoses. Mol. Genet. Metab. 2015;114:94–109. doi: 10.1016/j.ymgme.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tomatsu S, Sawamoto K, Shimada T, et al. Enzyme replacement therapy for treating mucopolysaccharidosis type IVA (Morquio A syndrome): effect and limitations. Expert Opin. Orphan. Drugs. 2015;3:1–12. doi: 10.1517/21678707.2015.1086640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tomatsu S, Sawamoto K, Alméciga-Díaz CJ, et al. Impact of enzyme replacement therapy and hematopoietic stem cell transplantation in patients with Morquio A syndrome. Drug Des. Devel. Ther. 2015;9:1937–1953. doi: 10.2147/DDDT.S68562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chinen Y, Higa T, Tomatsu S, Suzuki Y, Orii T, Hyakuna N. Long-term therapeutic efficacy of allogenic bone marrow transplantation in a patient with mucopolysaccharidosis IVA. Mol. Genet. Metab. Rep. 2014;1:31–41. doi: 10.1016/j.ymgmr.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yabe H, Tanaka A, Chinen Y, et al. Hematopoietic stem cell transplantation for Morquio A syndrome. Mol. Genet. Metab. 2016;117:84–94. doi: 10.1016/j.ymgme.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hendriksz ACJ, KIBerger KI, Giugliani R. et al. International guidelines for the management and treatment of Morquio A syndrome. Am. J. Med. Genet. A. 2015;167A:11–25. doi: 10.1002/ajmg.a.36833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.FDA Advisory Committee Briefing Document: Elosulfase alfa for Mucopolysaccharidosis Type IVA. http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm375126.pdf.
- 59.NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE: Elosulfase alfa for treating mucopolysaccharidosis type Iva. https://www.nice.org.uk/guidance/HST2/documents/mucopolysaccharidosis-type-iva-elosulfase-alfa-evaluation-consultation3.
- 60.Richard M, Arfi A, Seguin J, Gandolphe C, Scherman D. Widespread biochemical correction of murine mucopolysaccharidosis type VII pathology by liver hydrodynamic plasmid delivery. Gene Ther. 2009;16:746–756. doi: 10.1038/gt.2009.36. [DOI] [PubMed] [Google Scholar]
- 61.Jones SA, Bialer M, Parini R, et al. Safety and clinical activity of elosulfase alfa in pediatric patients with Morquio A syndrome (mucopolysaccharidosis IVA) < 5 years. Pediatr. Res. 2015;78(6):717–722. doi: 10.1038/pr.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guarany NR, Schwartz IV, Guarany FC, Giugliani R. Functional capacity evaluation of patients with mucopolysaccharidosis. J. Pediatr. Rehabil. Med. 2012;5:37–46. doi: 10.3233/PRM-2012-0194. [DOI] [PubMed] [Google Scholar]
- 63.Piotrowska E, Jakobkiewicz-Banecka J, Maryniak A, et al. Two-year follow-up of Sanfilippo disease patients treated with a genistein-rich isoflavone extract: assessment of effects on cognitive functions and general status of patients. Med. Sci. Monit. 2011;17:CR196–CR202. doi: 10.12659/MSM.881715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hendriksz C, Vellodi A, Jones S, et al. Long term outcomes of a phase1/2, multicenter, open-label, dose-escalation study to evaluate the safety, tolerability, and efficacy of BMN 110 in patients with mucopolysaccharidosis IVA (Morquio A syndrome) Mol. Genet. Metab. 2012;105:S35. [Google Scholar]
- 65.Hendriksz CJ, Giugliani R, Harmatz P, et al. Multi-domain impact of elosufase alfa in Morquio A syndrome in the pivotal phase III trial. Mol. Genet. Metab. 2015;114:178–185. doi: 10.1016/j.ymgme.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 66.Gelb MH, Turecek F, Scott CR, Chamoles NA. Direct multiplex assay of enzymes in dried blood spots by tandem mass spectrometry for the newborn screening of lysosomal storage disorders. J. Inherit. Metab. Dis. 2006;29:397–404. doi: 10.1007/s10545-006-0265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cotter EM, Burgio LD, Stevens AB, Roth DL, Gitlin LN. Correspondence of the functional independence measure (FIM) self-care subscale with real-time observations of dementia patients’ ADL performance in the home. Clin. Rehabil. 2002;16:36–45. doi: 10.1191/0269215502cr465oa. [DOI] [PubMed] [Google Scholar]
- 68.Centers for Disease Control and Prevention. http://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.htm.
- 69.Montaño AM, Tomatsu S, Brusius A, Smith M, Orii T. Growth charts for patients affected with Morquio A disease. Am. J. Med. Genet. A. 2008;146A:1286–1295. doi: 10.1002/ajmg.a.32281. [DOI] [PubMed] [Google Scholar]
- 70.Tomatsu S, Montaño AM, Oikawa H, et al. Impairment of body growth in mucopolysaccharidoses. In: Preedy VR, editor. Handbook of Growth and Growth Monitoring in Health and Disease. 2012. pp. 2091–2117. [Google Scholar]
- 71.Drummond JC, Krane EJ, Tomatsu S, Theroux MC, Lee RR. Paraplegia after epidural-general anesthesia in a Morquio patient with moderate thoracic spinal stenosis. Can. J. Anaesth. 2015;62:45–49. doi: 10.1007/s12630-014-0247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Theroux MC, Nerker T, Ditro C, Mackenzie WG. Anesthetic care and perioperative complications of children with Morquio syndrome. Paediatr. Anaesth. 2012;22:901–907. doi: 10.1111/j.1460-9592.2012.03904.x. [DOI] [PubMed] [Google Scholar]
- 73.Northover H, Cowie RA, Wraith JE. Mucopolysaccharidosis type IVA (Morquio syndrome): a clinical review. J. Inherit. Metab. Dis. 1996;19:357–365. doi: 10.1007/BF01799267. [DOI] [PubMed] [Google Scholar]
- 74.Debra S, Regier MDPhD, Matthew Oetgen MD, Pranoot Tanpaiboon MD. Mucopolysaccharidosis Type IVA. GeneReviews. Initial Posting. 2013 Jul 11; Last Revision: March 13, 2014. [Google Scholar]
- 75.Paradise JL. Otitis media in infants and children. Pediatrics. 1980;65(5):917–943. [PubMed] [Google Scholar]
- 76.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J. Pediatr. 2007;150:12–17. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 77.Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet. 2010;375:1737–1748. doi: 10.1016/S0140-6736(10)60171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sutherland ER. Obesity and asthma. Immunol. Allergy Clin. N. Am. 2008;28:589–602. doi: 10.1016/j.iac.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taylor ED, Theim KR, Mirch MC, et al. Orthopedic complications of overweight in children and adolescents. Pediatrics. 2006;117:2167–2174. doi: 10.1542/peds.2005-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morrison KM, Shin S, Tarnopolsky M, Taylor VH. Association of depression & health related quality of life with body composition in children and youth with obesity. J. Affect. Disord. 2015;172:18–23. doi: 10.1016/j.jad.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 81.Mustillo S, Worthman C, Erkanli A, Keeler G, Angold A, Costello EJ. Obesity and psychiatric disorder: developmental trajectories. Pediatrics. 2003;111:851–859. doi: 10.1542/peds.111.4.851. [DOI] [PubMed] [Google Scholar]
- 82.Halfon N, Larson K, Slusser W. Associations between obesity and comorbid mental health, developmental, and physical health conditions in a nationally representative sample of US children aged 10 to 17. Acad. Pediatr. 2013;13:6–13. doi: 10.1016/j.acap.2012.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.