Abstract
Case description
A 71-year-old female accidentally received thiothixene (Navane), an antipsychotic, instead of her anti-hypertensive medication amlodipine (Norvasc) for 3 months. She sustained physical and psychological harm including ambulatory dysfunction, tremors, mood swings, and personality changes. Despite the many opportunities for intervention, multiple health care providers overlooked her symptoms.
Discussion
Errors occurred at multiple care levels, including prescribing, initial pharmacy dispensation, hospitalization, and subsequent outpatient follow-up. This exemplifies the Swiss Cheese Model of how errors can occur within a system. Adverse drug events (ADEs) account for more than 3.5 million physician office visits and 1 million emergency department visits each year. It is believed that preventable medication errors impact more than 7 million patients and cost almost $21 billion annually across all care settings. About 30% of hospitalized patients have at least one discrepancy on discharge medication reconciliation. Medication errors and ADEs are an underreported burden that adversely affects patients, providers, and the economy.
Conclusion
Medication reconciliation including an ‘indication review’ for each prescription is an important aspect of patient safety. The decreasing frequency of pill bottle reviews, suboptimal patient education, and poor communication between healthcare providers are factors that threaten patient safety. Medication error and ADEs cost billions of health care dollars and are detrimental to the provider–patient relationship.
Keywords: medication error, patient safety, quality improvement, adverse drug event, healthcare dollars, drug-induced parkinsonism, Swiss Cheese Model
A recently widowed 71-year-old female was hospitalized for uncontrolled hypertension and acute kidney injury. Past medical history was significant for coronary artery disease with bypass grafting, heart failure with preserved ejection fraction, hypertension, and type 2 diabetes mellitus. The patient was a reformed cigarette smoker and under significant stress at home due to the death of her husband. During the hospitalization, she received temporary hemodialysis, her anti-hypertensive medications were adjusted, and she clinically improved. At the time of discharge, her prescription medications included amlodipine (Norvasc) 10 mg twice daily (with two refills), metoprolol 50 mg twice daily, doxazosin 2 mg daily, and torsemide 30 mg daily.
Over the next 3 months, she experienced worsening fatigue, slow movements, lethargy, personality changes, and a ‘stoic’ facial expression, as noted in her medical records. Her blood pressure was not optimally controlled. During this time period, she was re-hospitalized for chest pain and underwent angioplasty. During her admission, she encountered multiple specialists and ancillary staff. As an outpatient, she was seen by her family physician twice. After several weeks, she was eventually diagnosed with anxiety and depression for which she was prescribed citalopram and alprazolam.
Thereafter, the patient presented for the third time to our emergency room after a fall with light-headedness and poor ambulation. She demonstrated a shuffling gait, blank facies, and bradykinesia. Laboratory work was notable for an elevated creatinine. CT of the head and brain without contrast revealed no acute abnormalities. Admission medication reconciliation (MED REC) revealed that she was taking metoprolol, doxazosin, alprazolam, citalopram, and thiothixene (Navane) 10 mg twice daily.
Upon review of her pill bottles, it was found that her outpatient pharmacy accidentally dispensed Navane (an antipsychotic) instead of Norvasc, and she dutifully took this medication for 3 months. The written prescription was deemed legible. A diagnosis of thiothixene-related drug-induced Parkinsonism was made. Thiothixene was discontinued and her clinical status improved.
Discussion of errors
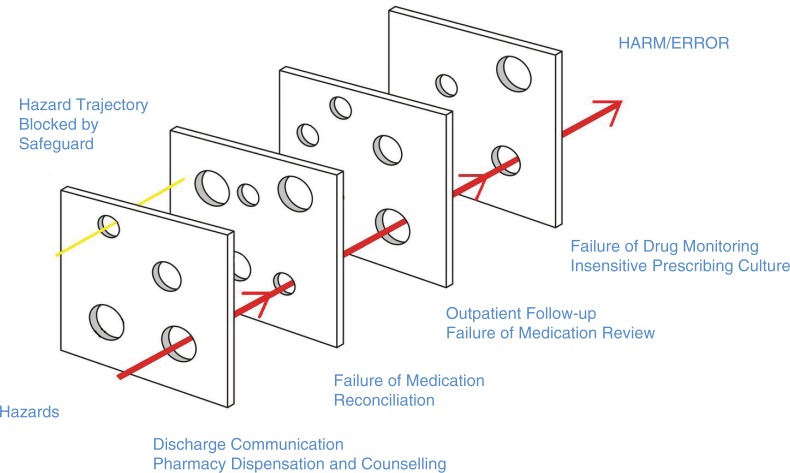
Our patient experienced harm because of a multisystem fall out. This case is a perfect demonstration of the Swiss Cheese Model of system accidents (1). In order for errors to occur, there must be failures at multiple levels (Fig. 1).
Fig. 1.
Case-specific demonstration of the Swiss Cheese Model of System Error. Modeled after James Reason's Swiss Cheese Model, published in 2000 (Reason (1)).
An adverse event is defined as an injury resulting from either medical intervention or omission, while an adverse drug event (ADE) is any injury due to a medication. A medication error is any error occurring in the medication use process, including during prescribing, transcribing, dispensing, administration, adherence, and/or monitoring (2, 3). Medication error may not always result in injury and therefore will not always be an ADE. ADEs may be preventable or non-preventable. Non-preventable ADEs are also known as adverse drug reactions and may include allergies and the correct use of medications that were not tolerated (2).
Dispensing/transcribing: The case described above was initially a pharmacy dispensation error, possibly related to under-staffing and on-going pressure to fill timely prescriptions. Commonly, a pharmacy technician to pharmacist ratio is between 2:1 and 4:1, depending on local regulation and laws. A single pharmacist may be handling double (or more) the amount of medications as their technicians, and it is known that pharmacist errors are associated with increasing length of shift and number of verified prescriptions (4). Pharmacy technicians have substantial access to medications and a great potential to catch impending error, especially in the face of pharmacist fatigue. The State of Pennsylvania has few requirements for pharmacy technicians. According to Pennsylvania State code, a technician may be unlicensed and neither certification nor continuing education is required. Furthermore, the State allows local pharmacy managers to assign responsibilities and duty limitations within each institution. This means that pharmacy technician responsibilities vary across the State of Pennsylvania (5). Many other States have more requirements such as minimum age, minimum education level, continuing education, and licensure. It is conceivable that this patient's pharmacy may have been understaffed, under trained, and fatigued.
Prescribing/monitoring: Multiple physicians overlooked the patient's use of Navane for several months, even during her second hospitalization. She had unmonitored outpatient access due to the refills prescribed during hospital discharge. While all of her outpatient and previous hospitalization records likely stated Norvasc/amlodipine, the only way to truly confirm her medication regimen would have been a thorough pill bottle review. Navane/Norvasc is one of many sound-alike, look-alike drug names, and a valuable list can be found at the Institute for Safe Medication Practices (Table 1).
- Prescribing/monitoring: The dose of amlodipine this patient received exceeded the recommended daily maximum of 10 mg (6). Thiothixene, which carries a black box warning for increased risk of death in elderly patients, is typically started at low doses around 2 mg and titrated up. The dose of thiothixene she received (20 mg) was for severe schizophrenia. Given the nature of thiothixene, the pharmacy staff would ideally notice a brand new medication at an unusually high starting dose and communicate their concerns to the provider. If this had occurred, the patient may never have received the wrong medication. If a conversation had taken place between the pharmacist and the patient about her new medication, it may have been noted that it was not for her blood pressure. This raises a concern about the relationship between prescribers and pharmacy staff, as well as pharmacy drug education.
- High prescription volume pharmacies have devised ways to increase speed, including allowing patients to bypass counseling (insertion of written material and signing that additional counseling is not desired) which has removed the direct pharmacist–patient interaction. The pharmacist is one of the last lines of defense in medication profile safety. Do high volume pharmacies and cessation of counseling threaten patient safety?
- Have prescribers created a culture and atmosphere of being unavailable and resistant to pharmacy input regarding prescriptions? Do physicians avoid consulting with pharmacists because of a culture where weaknesses are hidden? Do physicians indirectly act as if they are superior to pharmacists? Importantly, is this dynamic effecting patient care?
- One study established very different opinions between community physicians/prescribers and pharmacists: pharmacists were in favor of increasing their own prescribing power and vaccine administration, while physicians were not. Seventy-three percent of community prescribers and 43% of pharmacists agreed that communication between the two professions was very good (7).
Table 1.
Examples of sound-alike, look-alike drug names
| Actonel (risedronic acid) | Actos (pioglitazone) |
|---|---|
| Adderall (amphetamine and dextroamphetamine) | Inderal (propranolol) |
| Amaryl (glimepiride) | Reminyl (galantamine) |
| Amiodarone | Amantadine |
| Brilinta (ticagrelor) | Brintellix (vortioxetine) |
| Bupropion | Buspirone |
| Catapres (clonidine) | Klonopin (clonazepam) |
| Coumadin (warfarin) | Cardura ( doxazosin) |
| Doribax (doripenem) | Zovirax (acyclovir) |
| Hydralazine | Hydroxyzine |
| Lamictal (lamotrigine) | Lamisil (terbinafine hydrochloride) |
| Levothyroxine | Lanoxin (digoxin) |
| Lovenox (enoxaparin) | Levemir (insulin detemir) |
| Metformin | Metronidazole |
| Navane (thiothixene) | Norvasc (amlodipine) |
| Paxil (paroxetine) | Plavix (clopidogrel) |
| Plaquenil (hydroxychloroquine sulfate) | Provigil (modafinil) |
| Risperidone | Ropinirole |
| Sertraline | Cetirizine |
Modeled after the Institute for Safe Medication Practices (ISMP), list of confused drug names, updated February 2015.
A thorough MED REC and review of indications is an important aspect of patient safety. Pharmacy department, outpatient providers, hospitalists, and specialists should be reviewing medications and their respective indications, and providing education to patients. Office and hospital medication reconciliation should be ultimately done by the prescriber, without being solely delegated to ancillary staff. The development of unusual symptoms or poor treatment response should trigger an evaluation by the physician and/or pharmacist along with a pill bottle review.
Corrective actions
Electronic health records and computerized software allow institutions to import external pharmacy records and provides information such as pharmacy location, prescriber identification, date filled, and directions for administration. These are features that should be available in all electronic charting systems and should be utilized by all health care providers. We have also implemented a two-step medication review on admission and discharge. Licensed providers (MD, PA, APRN) document home medications and perform admission medication reconciliation, otherwise known as continuing necessary home medications and adding other medications deemed necessary. As part of the admission process, nursing staff independently reviews and documents home medications, which offer an opportunity for intervention. We release weekly reports naming physicians who have not properly completed a MED REC and direct them for further electronic health record training in an effort to promote accountability. A patient cannot be discharged until both a licensed prescriber and a nurse have reviewed and documented the discharge medications independently.
Our Hospitalists have been instructed not to provide medication refills except under special circumstances. A concept developed within our residency program was to write the indication on all prescriptions. For example, ‘Take 1 tablet by mouth once daily for blood pressure’. As the indication is part of the written instructions, it will appear on the pill bottle and serve as a label for the patient. Attending physicians were asked to prescribe medications in the same format.
In adjunction with our Antibiotic Stewardship Program, integrated work rounds have been instituted. Clinical pharmacists round with medical residents on the hospital teaching services. We have also begun integrated teaching rounds with a clinical pharmacist and the intensive care unit teams.
We encourage our resident physicians to question the indications, utility, dose, and safety of all medications. We hope to inspire a teaching atmosphere where all providers, regardless of hierarchy, can discuss medication utility and potential ADEs.
Statistics and data
The Medical Care Availability and Reduction of Error (MCARE) Act requires healthcare facilities to report several types of events. A serious event occurs, when a patient is harmed. An incident or ‘near miss’ is an event or error with the potential of harm that did not injure the patient (8).
In the State of Pennsylvania, the Patient Safety Authority is an independent agency that conducts data collection and analysis. Between the years 2004 and 2014, they received 2.2 million safety reports. The most common serious events reported were procedure-related complications and falls. Serious medication errors ranked 6th. Out of all the safety reports, medication error was the second most common incident (9).
The national data available regarding patient safety and ADEs were alarming. The Centers for Disease Control and Prevention (CDC) estimates that nearly half of the US population have used a prescription medication during the past 30 days (10). It is projected that by the year 2020, 157 million Americans will have more than one chronic condition (11). Patients with chronic conditions may see as many as 16 physicians annually; this creates a huge potential for ADEs, poor communication, and fall out (12). A recent article published in the British Medical Journal described medical error as the third leading cause of death. Their data analysis included greater than 400,000 deaths a year from medical error, none of which captured deaths outside inpatient care due to lack of ICD 10 coding. One of their foundations for improvement called for increased error awareness and the ability to discuss errors (13).
Statistics and reports often focus on certain areas and special populations including transitions of care, at-risk groups, hospitalization, and economic impacts. Below the (P) represents State of Pennsylvania, while (N) represents national data.
Transitions of care
The emergency department is the third most common source of medication errors (14) (P).
Surveillance data indicate ADEs account for more than 3.5 million physician office visits, one million emergency department visits, and 125,000 hospital admissions yearly (3) (N).
The emergency department is the third most common source of medication errors including wrong doses and overdoses (14) (P).
Special populations may be more likely affected and have adverse outcomes
The elderly and those with limited access to health care services, low health literacy, low socioeconomic status, and language barriers may be more often affected (15) (N)
Elderly patients are two to three times more likely to visit a physician office or emergency department, and seven times more likely to require hospitalization, due to ADEs (3) (N).
Recent reports found that language barriers were associated with increased falls and medication errors/ADEs (14) (P).
Hospitalization
In the elderly: 1 in 30 hospital admissions are due to an ADE (16) (N).
ADEs total one-third of total hospital adverse events (3) (N).
The average hospitalized patient experiences at least one medication error each day (17) (N).
In 2008, one in seven Medicare beneficiaries experienced an adverse event during their hospital stay. Forty-two percent of temporary harm events were related to medications, and 50% of all medication events were deemed preventable (18) (N).
Common high-risk medications include anticoagulants, opioids, insulin, and anti-diabetic agents (19) (P).
Discharge
Economic impact (United States)
Economic impacts have been inadequately studied.
Medication errors harm an estimated 1.5 million people every year, costing at least $3.5 billion annually (20) (N).
It is estimated that ADEs affect approximately 2 million hospital stays annually and prolong the length of stay by 1.7–4.6 days (3) (N).
In 2006, at least 1.5 million preventable ADEs occurred totaling more than $7 billion.
Preventable medication errors impact more than 7 million patients and cost almost $21 billion annually across all care settings (20) (N).
Spending in the United States for prescription drugs in 2010 was $259.1 billion and is expected to double over the next decade (3) (N).
Total expenditures on the Medicare Part D program alone in 2012 were $66.9 billion and are projected to reach $165.1 billion by 2022 (3) (N).
Conclusion
With a growing population and longer life expectancy, the frequent occurrences of ADEs, medication errors, as well as polypharmacy will likely increase. Efforts must be made to improve overall physician communication and transition of care. Important steps include clear patient instructions with indications for use on every prescription, utilization of EHR medication import (when available) to review outpatient prescription history, and creating a culture within the medical field of error discussion. Possibilities include medication teams who review admission and discharge reconciliations, team rounding with a pharmacist, encouraging postgraduate trainees and faculty to question indications and utility of medications, and distribution of national and institution data regarding errors, and adverse events. Mandatory training should occur for those providers who fail to document and reconcile medications properly. Unfortunately, there is no true way to monitor or enforce the critical thinking that is required for medication reconciliation.
When poor treatment response occurs or unusual symptoms develop, it is imperative that a review of medications and pill bottle review be part of the initial evaluation. We must implement and use multilevel safeguards, starting with error recognition. Medical error was recently described as the third leading cause of death; the emotional, professional, and economic impacts of errors and ADEs must be recognized. Only by creating a culture of humility, communication, and teamwork can we learn from our mistakes and hope to decrease preventable errors.
Acknowledgements
Special thank you to Richard W. Synder DO and Roman A. Tuma MD for your support.
Conflict of interest and funding
The authors have no conflicts of interest to declare.
Disclaimer
This case presentation is strictly for educational purposes with regards to adverse drug events and medical error. The views expressed represent the authors’ and are not an official position of the institution.
References
- 1.Reason J. Human error: Models and management. BMJ. 2000;320(7237):768–70. doi: 10.1136/bmj.320.7237.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aspden P, Wolcott J, Bootman JL, Cronenwett LR. Introduction. Preventing medication errors: Quality chasm series. Washington, DC: National Academies Press; 2007. pp. 36–37. [Google Scholar]
- 3.US. Department of Health and Human Services. Office of Disease Prevention and Health Promotion. Washington, DC: Plan for Adverse Drug Event Prevention; 2014. Available from: http://health.gov/hcq/pdfs/ADE-Action-Plan-508c.pdf [cited 18 February 2016]. [Google Scholar]
- 4.Gorbach C, Blanton L, Lukawski BA, Varkey AC, Pitman EP, Garey KW. Frequency of and risk factors for medication errors by pharmacists during order verification in a tertiary care medical center. Am J Health Syst Pharm. 2015;72(17):1471–4. doi: 10.2146/ajhp140673. doi: http://dx.doi.org/10.2146/ajhp140673. [DOI] [PubMed] [Google Scholar]
- 5.The Pennsylvania Code, Commonwealth of Pennsylvania. Chapter 27. State Board of Pharmacy. Fry Communications, Inc. [adopted June 1, 1973]. Available from: http://www.pacode.com/secure/data/049/chapter27/chap27toc.html [cited 15 February 2016].
- 6.Uptodate. Topic 8627 Version 181.0. Available from: http://www.uptodate.com/contents/amlodipine-drug-information?source=search_result&search=amlodipine&selectedTitle=1%7E113 [cited 15 February 2016].
- 7.Moore T, Kennedy J, McCarthy S. Exploring the general practitioner–pharmacist relationship in the community setting in Ireland. Int J Pharm Pract. 2014;22(5):327–34. doi: 10.1111/ijpp.12084. doi: http://dx.doi.org/10.1111/ijpp.12084. [DOI] [PubMed] [Google Scholar]
- 8.Aspden P, Corrigan JM, Wolcott J, et al., editors. Institute of Medicine (US) Committee on Data Standards for Patient Safety. Introduction. In: Patient safety: Achieving a new standard for care. Washington, DC: National Academies Press (US); 2004. Available from: http://www.ncbi.nlm.nih.gov/books/NBK216083/ [cited 18 February 2016]. [PubMed] [Google Scholar]
- 9.Levine R. Pennsylvania Patient Safety Authority. 2014. Annual Report. 2015. Available from: http://patientsafetyauthority.org/PatientSafetyAuthority/Documents/Annual_Report_2014.pdf [cited 25 February 2016]
- 10.National Center for Health Statistics. Health, United States 2014: With special feature on adults aged 56–54. Hyattsville, MD: National Center for Health Statistics; 2015. [PubMed] [Google Scholar]
- 11.Tackling the burden of chronic diseases in the USA. Lancet. 2009;373(9659):185. doi: 10.1016/S0140-6736(09)60048-9. doi: http://dx.doi.org/10.1016/S0140-6736(09)60048-9. [DOI] [PubMed] [Google Scholar]
- 12.Bodenheimer T. Coordinating care – a perilous journey through the health system. N Engl J Med. 2008;358(10):1064–71. doi: 10.1056/NEJMhpr0706165. [DOI] [PubMed] [Google Scholar]
- 13.Makary MA, Daniel M. Medical error – the third leading cause of death in the US. BMJ. 2016;353:i2139. doi: 10.1136/bmj.i2139. [DOI] [PubMed] [Google Scholar]
- 14.Patient Safety Authority. News and Information Press Release. Pennsylvania Patient Safety Authority. 2011. Annual Report. Available from: http://patientsafetyauthority.org/PatientSafetyAuthority/Documents/FINAL 2011 Annual Report.pdf. March_1.pdf [cited 15 February 2016]
- 15.National Transition of Care Coalition. Improving transitions of care. 2010. Available from: http://www.ntocc.org/ [cited 24 February 2016]
- 16.Clin-Alert. Adverse drug reactions, hospitalizations due to adverse drug reactions in elderly patients. Available from: http://cla.sagepub.com/content/54/3.toc.pdf [cited 2 March 2016]
- 17.Philip Aspden, Julie Wolcott, Lyle Bootman J, Linda R, Cronenwett, Committee on Identifying and Preventing Medication Errors , editors. Board on Health Care Services; Institute of Medicine. Washington, DC: National Academies Press; 2007. [Google Scholar]
- 18.US. Department of Health and Human Services. Office of Inspector General. Adverse events in hospitals: National incidence among Medicare beneficiaries. Washington, DC: [updated November 2010]. Available from: http://oig.hhs.gov/oei/reports/oei-06-09-00090.pdf [cited 24 February 2016] [Google Scholar]
- 19.Yang A, Grissinger M. Wrong-patient medication errors. Pennsylvania patient safety advisory. Advisory Library website. 2013. Available from: http://patientsafetyauthority.org/ADVISORIES/AdvisoryLibrary/2013/Jun;10(2)/Pages/41.aspx [cited 15 February 2016]
- 20.Lahue BJ, Pyenson B, Iwasaki K, Blumen HE, Forray S, Rothschild JM. National burden of preventable adverse drug events associated with inpatient injectable medications: Healthcare and medical professional liability costs. Am Health Drug Benefits. 2012;5(7):1–10. [PMC free article] [PubMed] [Google Scholar]
- 21.Institute for Safe Medication Practices (ISMP) List of confused drug names. [updated February 2015]. Available from: http://www.ismp.org/Tools/confuseddrugnames.pdf [cited 20 June 2016]