Abstract
The CoASTAL cohort represents the first community cohort assembled to study a HAB related illness. It is comprised of three Native American tribes in the Pacific NW for the purpose of studying the health impacts of chronic, low level domoic acid (DA) exposure through razor clam consumption. This cohort is at risk of domoic acid (DA) toxicity by virtue of their geographic location (access to beaches with a history of elevated DA levels in razor clams) and the cultural and traditional significance of razor clams in their diet. In this prospective, longitudinal study, Wave 1 of the cohort is comprised of 678 members across the lifespan with both sexes represented within child, adult and geriatric age groups. All participants are followed annually with standard measures of medical and social history; neuropsychological functions, psychological status, and dietary exposure. DA concentration levels are measured at both public and reservation beaches where razor clams are sourced and multiple metrics have been piloted to further determine exposure. Baseline data indicates that all cognitive and psychological functions are within normal limits. In addition there is considerable variability in razor clam exposure. Therefore, the CoASTAL cohort offers a unique opportunity to investigate the potential health effects of chronic, low level exposure to DA over time.
1. Introduction
The role that ocean waters play in global health is increasingly recognized as an important public health issue (Knap et al., 2002). The growing demand for fish and shellfish, coupled with widespread contamination and development in coastal areas, has challenged the fragile ecological balance that exists between land-dwelling and marine populations. Health concerns stemming from the ocean generally revolve around exposure to agents in the marine ecosystem such as environmental pollutants, metals and biotoxins. Marine scientists have identified the phenomenon of harmful algal blooms (HABs) as an important source of marine biotoxins (Erdner et al., 2008). While microscopic algae are essential for ocean life, there are a few dozen species that produce powerful biotoxins. When these algae bloom in excess, there can be devastating health effects in seabirds, marine mammals and humans. Harmful algal blooms have been linked to human death and illness and mass killings of fish, marine mammals and seabirds (Stommel and Waters, 2004). Oceanographic monitoring data suggest that there are more toxic algal species, frequency of HABs appears to be increasing and toxic strains of algae are spreading across new geographical regions (Luckas et al., 2005).
There are five clinical poisoning syndromes associated with HABs but only one, Amnesic Shellfish Poisoning, has a well defined neurologic, gastrointestinal and neuropsychological syndrome characterized by profound memory disorder. Domoic acid (DA), the causative agent in Amnesic Shellfish Poisoning, is produced by the microscopic algae Pseudo-nitzschia (Trainer et al., 1998). The potential risk of DA to human health was discovered in 1987 in Montreal, Canada where 153 cases of acute intoxication were documented (including 4 deaths) (Perl et al., 1990a,b; Teitelbaum et al., 1990a,b). These clinical cases were linked to the consumption of DA-contaminated mussels harvested from the Cardigan River. Symptomology included nausea, vomiting, abdominal cramping, excessive respiratory secretions, coma and death. Neurological symptoms such as headaches, hallucinations, confusion and memory impairment were also reported in exposed individuals. Clinical evaluations of 14 adults after the outbreak revealed that 12/14 had severe antegrade memory deficits with relative preservation of higher cortical functions (Teitelbaum et al., 1990a,b). Affected individuals had difficulty remembering events that had occurred after DA exposure. Subsequent to this incident, environmental sampling revealed blooms of Pseudo-nitzschia in the river where the mussels had been harvested. This episode firmly established the serious and complex health effects associated with the oral ingestion of DA-contaminated shellfish.
In response to the Canadian episode of DA poisoning, scientific investigations of DA toxicity and neurotoxicity were initiated in the laboratory with animal models. The neurobehavioral signature of this compound has been detailed by Grant and colleagues (2010). Domoic acid acts as a strong emetic and is associated with adverse changes in both brain and behavior. The hippocampus is the primary site of injury in the central nervous system (CNS). The adverse consequences of DA exposure are not limited to a single behavioral effect and treatment-related changes have been documented in motor, cognitive and emotionality domains. Research has also demonstrated that functional losses can be persistent and injuries to the CNS can be progressive over time. Given that DA is an increasingly prevalent biotoxin in the world's oceans, there is an urgent need to identify study populations that have documented (and measurable) low-dose, chronic exposures to domoic acid; which can be assessed for evidence of clinical (and subclinical) exposure effects; and which, ideally, can be followed longitudinally to evaluate changes across time. We report here the identification and initial characterization of such an exposure cohort.
2. Materials, Methods, and Results
2.1 Study Background and Community Involvement
The Communities Advancing the Studies of Tribal Nations Across the Lifespan (CoASTAL) cohort evolved from a community based, environmental health initiative of the Quileute Indian Nation in response to concerns about rising levels of DA in the Pacific Northwest, USA. Shellfish managers, Tribe Council members, and the Quileute Health Clinic participated in the design, implementation and interpretation of a pilot study that raised the possibility of DA neurotoxicity among some people in their community. However, since exposure and cognition are both complex processes, further investigation with a larger sample over time was needed to clarify and extend the findings. Subsequently, neighboring Pacific coast Tribal Nations both north (Makah Tribe) and south (Quinault Indian Nation) joined the study team to further examine the potential health effects of low levels of domoic acid exposure by razor clam consumption. Using a community based participatory model, the contributions of these Tribal Nations to the cohort study included assistance with research design and implementation; recruitment, data collection, interpretation of results, and community outreach and education. This occurred through hiring local study coordinators (Tribal members) and assembling local data collection field teams, community advisory boards and medical advisory groups. Tribal fisheries experts, shellfish researchers and environmental health managers assisted with providing environmental; razor clam harvest and DA data; and critical linkages with Tribal Councils and other community leaders. Community events such as health fairs, elder dinners and treaty celebrations were used as venues to introduce the study to the Tribal Nations, distribute informational brochures about the study and educational materials about memory, DA and healthy diets. The CoASTAL cohort study was funded by the National Institute of Environmental Health Science and data collection began in the summer of 2005.
2.2 The CoASTAL Cohort
2.2.1 Recruitment
This study represents the methodology and baseline data collection for wave 1 (the first 5 years) of a prospective longitudinal cohort study of DA neurotoxicity in the Pacific Northwest. The cohort was comprised of 678 participants. Recruitment was initially limited to individuals in the following age groups: school age children (6-12 years), adults (18-64 years) and geriatric (65+ years). Sampling frames for each site were derived through tribal registries from the Quinault, Quileute, and Makah reservations. After sorting by age, potential participants were randomly selected based upon the total number of enrolled members for each study site. A letter of invitation was sent to each selected adult participant and to the parents or primary caregiver of child participants. The letter of invitation explained the nature, purpose and general procedures of the study.
Field coordinators scheduled baseline visits for all participants who agreed to take part in the study. If a participant declined participation, their name was removed from the list and an alternate was randomly selected within their age group. This sequence was continued until the goal recruitment numbers were met at each location or until the lists of potential participants were exhausted. An alternative methodology was used to recruit the infant sample as they were not included on the tribal enrollment registries. Flyers were posted in health clinics, on bulletin boards and in local newspapers on each reservation asking parents/primary caregivers to volunteer their infants (ages 9-16 months) for participation in the study. Infant participants received the same reimbursement as child participants for the completion of the developmental exam and exposure history (obtaining mother's shellfish consumption and nursing history).
2.2.2 Cohort characteristics
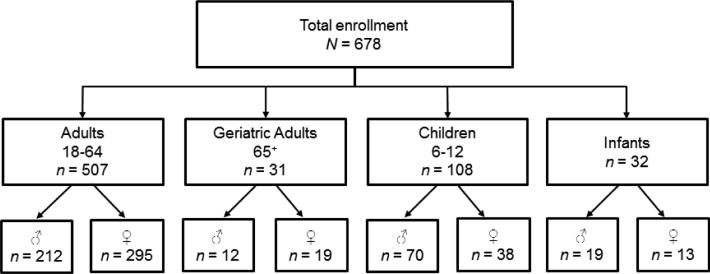
Figure 1 provides a summary of cohort enrollment (N=678). One hundred eight children between the ages of 6-12, 507 adults between 18-64 years, 31 geriatric participants, and 32 infants were enrolled in the CoASTAL study. The cohort consisted of 58% women and 42% men; this is similar to the population distribution of cohorts previously reported for tribal peoples (e.g., Slattery et al., 2007). Descriptive data for the characteristics of participants enrolled in the cohort are presented in Tables 2-4. The average age of adult participants was 40.4 years with a range of 18 to 83 years. The majority of adults had at least a high school education, and women had significantly more years of education than men (p < 0.01). At the time of enrollment, 48% of participants were unemployed. The mean age of children was 8.4 years and 35% of child participants were girls. A limited number of infants were also included in the cohort at this study phase and their mean age was 10.8 months.
Figure 1. Cohort enrollment by age and sex.
Please find cleaned-up version of drawing shown here- same image as Power Point document.)
Table 2.
Characteristics of Children Enrolled in the CoASTAL Cohort Study
| Full sample n = 108 | Boys n = 70 | Girls n = 38 | p value* | |
|---|---|---|---|---|
| Gender (Female) | 35 | 0 | 100 | - |
| Preterm birth | 16 | 15 | 16 | 1.00 |
| Birth weight (g)** | 3490 ± 600 | 3510 ± 630 | 3460 ± 600 | 0.69 |
| Attend tribal school | 19 | 17 | 21 | 0.80 |
| Received tutoring | 25 | 30 | 16 | 0.16 |
| Diagnosed with learning disability | 9 | 13 | 3 | 0.10 |
| Difficulty with speech | 12 | 17 | 3 | 0.03 |
| Difficulty with language | 7 | 10 | 0 | 0.05 |
| Birth trauma | 11 | 10 | 11 | 1.00 |
| Asthma | 18 | 24 | 8 | 0.06 |
| Exposed to hazardous chemicals | 2 | 3 | 0 | 0.54 |
Data presented as percentage, except birth weight presented as mean ± SD.
p values from chi-square test, Fisher's exact test or t-test, as appropriate
n=84 (53 males, 31 females)
Table 4.
Health and Medical History of Adult and Geriatric Participants
| Full sample n = 538 | Men n = 224 | Women n = 314 | p value* | |
|---|---|---|---|---|
| Body Mass Index (Mean) | 32.4 ± 7.4 | 31.2 ± 6.4 | 33.2 ± 7.9 | 0.003 |
| Normal | 13 | 15 | 12 | |
| Overweight | 29 | 30 | 28 | |
| Obese | 57 | 54 | 60 | |
| Current smoker | 47 | 47 | 47 | 0.951 |
| Alcohol use | <0.001 | |||
| Never | 51 | 39 | 59 | |
| Monthly | 21 | 25 | 19 | |
| 2-4 times a month | 20 | 26 | 16 | |
| 2-3 times a week | 6 | 8 | 5 | |
| 4 or more times a week | 2 | 3 | 1 | |
| Ever smoked marijuana | 0.019 | |||
| Never | 34 | 35 | 34 | |
| Fewer than 3 times | 22 | 16 | 26 | |
| More than 3 times | 44 | 50 | 41 | |
| Ever tried cocaine | 36 | 36 | 36 | 0.841 |
| Ever tried heroin | 4 | 3 | 5 | 0.208 |
| Ever tried other hallucinogen | 19 | 25 | 15 | 0.016 |
| Ever tried other non-prescription psychotropic drug | 19 | 18 | 20 | 0.732 |
| Ever diagnosed or received treatment for alcohol or substance abuse problems | 29 | 36 | 24 | 0.002 |
| Co-morbid medical conditions | ||||
| Continuous | 1.63 ± 1.84 | 1.33 ± 1.94 | 1.83 ± 1.74 | 0.006 |
| Number of co-morbidities | ||||
| 0 | 29 | 38 | 24 | 0.004 |
| 1 | 30 | 32 | 30 | |
| 2 | 17 | 13 | 19 | |
| 3 | 24 | 18 | 28 | |
| Diabetes | 14 | 13 | 15 | 0.464 |
| Cancer | 6 | 3 | 7 | 0.083 |
| Stroke | 5 | 6 | 5 | 0.668 |
| Seizures/Epilepsy | 4 | 7 | 3 | 0.028 |
| Multiple Sclerosis | 0.9 | 1.2 | 0.8 | 0.646 |
| Dementia (AD, PD, HD) | 0.5 | 1.2 | 0 | 0.153 |
| Lupus | 1.4 | 0.6 | 1.9 | 0.411 |
| Head injury | 12 | 14 | 11 | 0.331 |
| Loss of consciousness due to head injury (n = 64) | 3 | 0 | 5 | 0.510 |
| High blood pressure | 26 | 26 | 25 | 0.846 |
| Memory problems (n = 56) | 13 | 16 | 10 | 0.688 |
| Tremors | 1.2 | 1.0 | 1.3 | 1.000 |
| Depression, anxiety, and other psychiatric problems | 25 | 12 | 34 | <0.001 |
| Taking anticonvulsants† | 14 | 5 | 9 | - |
| Taking anti-depressants† | 75 | 12 | 63 | - |
| Taking anti-hypertensives† | 89 | 34 | 55 | - |
Data presented as percentage, except Body Mass Index presented as mean ± SD.
p values from chi-square test, Fisher's exact test or t-test, as appropriate
Value reported is total number of individuals that reported taking the drug in question
Dementia diagnoses: Alzheimer's disease, Parkinson's disease, Huntington's disease
2.2.3 Baseline Visit Procedures
Written informed consent was obtained from all participants at the beginning of their baseline visit in compliance with standard procedures required by the University of Maryland Institutional Review Board. All visits were conducted by trained examiners in private offices on the participant's respective field site. Exclusionary criteria included a history of severe dementia, severe head injury or other psychiatric or neurological disorder which precluded understanding informed consent or assessment procedures. Consent was obtained from the parents or primary caregivers for each child and the cognitive exam procedures were explained to all children to insure they were comfortable taking part in the study.
The baseline evaluation for the school age children, adults and geriatric samples included a background/medical history and confounding exposure questionnaire; shellfish consumption, dietary intake questionnaires, and records; and a cognitive screening exam. Parents or primary caregivers completed all questionnaires based on their child's history. All adult participants received $50 each year for the completion of a cognitive exam and providing medical history and dietary consumption data. In addition, adult participants received $10 for each one-day food intake record four times a year. Child participants received a $50 gift card (upon completion of the annual exam) and a $10 gift card (upon providing a one-day food record) for reimbursement.
Total time for the baseline visit was approximately 2.5 hours, 75 minutes for the obtaining informed consent and completing the surveys and questionnaires; and an additional 75 minutes for the cognitive screening exam with rest breaks as needed. Upon completion of the exam, participants were also asked to take home and complete a one-day food record to be returned within 5 days. The same consenting procedure used for the school age children was replicated for the infants. The infants’ parent or primary caregiver was required to be in the testing room and take part in the developmental exam. The exam took approximately 1.15 hours, which included a 15 minute play break for the infant. Infant participants also received a $50 and a $10 gift card, as well as a blanket and small toy.
2.3 Measures
All data were collected using standardized instruments, interviews or cognitive assessment procedures. Measurement selection for cognitive instruments were based upon the following criteria: 1) the ability to screen the major functional domains of human cognition in standard fashion without redundancy; 2) the ability to assess memory functions; 3) suitability of measures for each age group; 4) well documented reliability and validity for cognitively intact or neurologically impaired populations; 5) cross-cultural validity and suitability of measures for the assessment of Native Americans; and 6) brevity to minimize participant burden (maximum length of 75 minutes for the cognitive exam). Data for all measures described below were collected for all participants at the baseline visit and each year thereafter for a total of 4 visits. Univariate analyses were performed to describe demographic, health history, psychological functioning, and dietary characteristics of the cohort. Differences on categorical variables were examined using Pearson's chi-squared test; differences in continuous variables were evaluated by Student's two-sample t-test. All statistical analyses were performed using Stata 10 software (Stata Corp, College Station, TX, USA). Statistical significance for all analyses was defined as p < 0.05.
2.3.1 Health history
Demographic data, developmental, academic, social, occupational, medical, neurologic, and psychiatric history were assessed using a modified version of the Boston Occupational and Environmental Health Questionnaire (Feldman, 1999) for adults and a modified version of the Pediatric Neuropsychology and Environmental Questionnaire (Feldman, 1999). The Brief Michigan Alcohol Screening Test (BMAST; Pokorny et al., 1972) and Alcohol Use Disorders Identification Test (AUDIT; Babor et al., 1989) were used to identify individuals with alcohol use problems.
2.3.2 Anthropometry
Trained field coordinators measured weight and height. Participants wore lightweight clothing, removed their shoes, and emptied their pockets prior to measurements. Height was measured to the nearest inch using a portable stadiometer (Shorr Infant/Child/Adult Portable Height-Length Measuring Board, Olney, Maryland). Weight was measured on a calibrated electronic scale and recorded to the nearest pound (SECA Digital Floor scale, Hanover, Maryland). Body mass index (BMI) was calculated using the formula weight (kg)/height(m)2.
Health status and medical history of adult participants is summarized in Table 5. The mean BMI was 32.4; 29% of adults were categorized as overweight and 57.3% were categorized as obese. Women were significantly more likely than men to be categorized as obese (60% v. 54%, respectively). Among adult participants, 47% were current smokers, and 49% reported at least monthly alcohol use; men drank significantly more often than women (p < 0.001). With respect to substance use, 66% reported some marijuana use, 36% reported previous cocaine use, 19% reported previous use of hallucinogens, and 19% reported use of non-prescription psychotropic drugs. Twenty-nine percent of participants reported previous diagnosis or treatment for an alcohol or substance use problem. Men reported history of diagnosis or treatment for alcohol or substance use more frequently than women (p = 0.002). Twenty five percent of participants reported a history of hypertension and 14% reported a history of diabetes.
Table 5.
Health and medical history of children in the CoASTAL cohort
| Full sample n = 108 | Boys n = 70 | Girls n = 38 | p value* | |
|---|---|---|---|---|
| Age | 8.4 ± 1.5 | 8.4 ± 1.5 | 8.5 ± 1.5 | 0.75 |
| Gender (Female) | 35 | 0 | 100 | - |
| Body Mass Index | 20.5 ± 4.7 | 20.1 ± 4.2 | 21.2 ±5.4 | 0.29 |
| Normal | 79 | 79 | 78 | |
| Overweight | 14 | 16 | 10 | |
| Obese | 7 | 4 | 12 | |
| Preterm birth | 16 | 15 | 16 | 1.00 |
| Birth weight (g)** | 3490 ± 600 | 3510 ± 630 | 3460 ± 600 | 0.69 |
| Attend tribal school | 19 | 17 | 21 | 0.80 |
| Received tutoring | 25 | 30 | 16 | 0.16 |
| Diagnosed with learning disability | 9 | 13 | 3 | 0.10 |
| Difficulty with speech | 12 | 17 | 3 | 0.03 |
| Difficulty with language | 7 | 10 | 0 | 0.05 |
| Birth trauma | 11 | 10 | 11 | 1.00 |
| Asthma | 18 | 24 | 8 | 0.06 |
| Exposed to hazardous chemicals | 2 | 3 | 0 | 0.54 |
Data presented as percentage, except age, Body Mass Index, and birth weight presented as mean ± SD
p values from chi-square test, Fisher's exact test or t-test, as appropriate
n=84 (53 males, 31 females)
Health status and medical history of children and infants enrolled in the cohort are summarized in Tables 6 and 7. For school age children, the mean BMI was 20.5, with 14% of children categorized as overweight and 7% categorized as obese. Sixteen percent of children were born prematurely. Boys were significantly more likely than girls to be diagnosed with a learning disability (13% v. 3%, respectively) and to have difficulties with speech or language.
Table 6.
Health and Medical History of Infants in the CoASTAL Cohort
| Characteristic | Full sample n = 32 | Boys n = 19 | Girls n = 13 | p value* |
|---|---|---|---|---|
| Age (months) | 10.8 ± 1.7 | 10.8 ± 1.6 | 10.8 ± 1.9 | 0.99 |
| Premature birth | 30 | 25 | 36 | 0.68 |
| Birth weight (g) | 3470 ± 670 | 3520 ± 690 | 3410 ± 650 | 0.67 |
| Breast fed | 77 | 88 | 62 | 0.19 |
| Exposed to hazardous chemicals | 3 | 0 | 8 | 0.43 |
| Mother smoked tobacco during pregnancy | 55 | 50 | 63 | 0.68 |
Data presented as percentage, except for age and birth weight presented as mean ± SD.
p values are from t-test or Fisher's exact test, as appropriate.
Table 7.
Findings of children in the CoASTAL Cohort
| Measure and | Full sample n=108 | Boys n=70 | Girls n=38 | p value* |
|---|---|---|---|---|
| Child Depression Inventory | ||||
| Total symptoms | 47 | 47 | 46 | 0.564 |
| Negative Mood | 48 | 49 | 48 | 0.756 |
| Interpersonal Problems | 50 | 49 | 52 | 0.442 |
| Ineffectiveness | 46 | 46 | 46 | 0.928 |
| Anhedonia | 51 | 52 | 49 | 0.288 |
| Negative Self Esteem | 44 | 45 | 42 | 0.135 |
| Child Behavior Checklist | ||||
| Internalizing problems | 48 | 49 | 48 | 0.678 |
| Externalizing problems | 50 | 51 | 49 | 0.521 |
| Total behavior problems | 48 | 49 | 48 | 0.798 |
p values from chi-square test, Fisher's exact test or t-test, as appropriate
2.3.3 Cognitive assessment
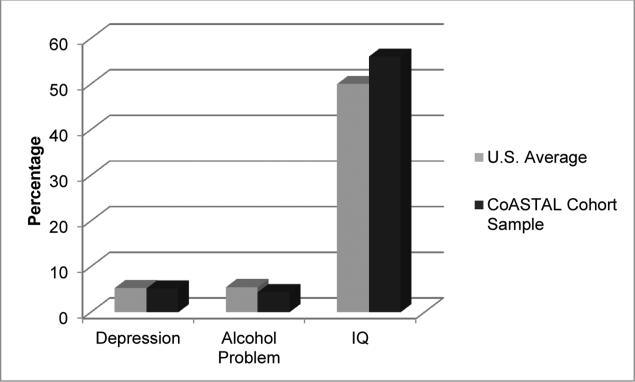
Cognitive functions were assessed with standardized neuropsychological test batteries conducted by trained examiners unaware of exposure data. The critical cognitive domains assessed included: Simple and Complex attention and Concentration [Wechsler Adult Intelligence Scale, Third Edition (WAIS-III) Digit Span, WAIS-III Coding (Wechsler, 1997); Trail Making Test, Parts A and B; Kiddie Trails (Reitan, 1992)], Constructional praxis [WAIS-III Block Design (Wechsler, 1997), Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV) Block Design (Wechsler, 2003)], Verbal memory [California Verbal Learning Test, Second Edition (CVLT-II) Standard, Short and Children's versions (Delis et al., 2000)] Visual memory [Children's Memory Scale (CMS) Dot Location Test (Cohen, 1997)] , Psychomotor speed [Trail Making Test, Part A] and Dexterity [Lafayette Grooved Pegboard (Lafayette Instrument Company, 2002)] and Cognitive flexibility [Trail Making Test, Part B (Reitan, 1992)]. Intelligence quotient (IQ) estimates were made from the WAIS-III subtests. Assessment of early learning, memory and intelligence in infants was evaluated with the Mullen Scales of Early Learning (Mullen, 1995). Overall, the baseline cognitive status was normal for all participants. For the cognitive status of adults, please refer to Figure 2.
Figure 2.
Behavioral findings of adults in the CoASTAL Cohort
2.3.4 Psychological adjustment
Psychological functioning was measured using standard measures of depression and anxiety in both adults and children. Depression was assessed using the Beck Depression Inventory-II (BDI-II; Beck et al., 1996) in adults and the Children's Depression Inventory (CDI; Kovacs, 1992) in children. The BDI-II is a 21 item self-report inventory that measures severity of depressive symptoms. Respondents are asked to rate each question on a scale from 0-3, which reflect varying degrees of agreement with depressive symptoms. The total score ranges from 0-63 with higher total scores suggesting more severe symptoms of depression. Scores in the range of 14-19 indicate mild depression, scores from 20-28 indicate moderate levels of depression, and scores from 29-63 indicate severe depression. CDI scores range from 0-54. Both the BDI-II and CDI evaluate the individual's mood in the 2 weeks prior to the assessment. Symptoms of anxiety were assessed using the Spielberger State-Trait Anxiety Inventory (STAI; Spielberger et al., 1983). The STAI is a 40 item self-report measure designed to measure an individual's current anxiety level as well as his/her typical anxiety levels. Items are scored on a 4-point Likert-type scale (1=not at all; 4=very much so).
Children's overall behavioral adjustment was assessed with the Achenbach Child Behavior Checklist (Achenbach et al., 2001). The CBCL is a 118 item questionnaire designed to assess children's social competence and behavior problems as reported by parents (Achenbach et al., 2001). Items are rated on a 3 point scale (0=not true; 2=very true or often true). The CBCL yields scores for internalizing behavior problems, externalizing problems, and total behavior problems as well as subareas of social withdrawal, somatic complaints, anxiety and depression, destructive behavior, social problems, thought problems, attention problems, aggressive behavior and delinquent behaviors. Test-retest reliabilities range from 0.95 to 1.00; internal consistency reliability coefficients for subscales range from 0.78 to 0.97; and criterion validity has been found to be acceptable (National Center for Community-Based Child Abuse Prevention, 2005). T scores were used for all analyses.
In summary, findings of psychological adjustment indicated that 25% of adults reported a history of depression, anxiety, or other psychological problems, with women reporting significantly more symptoms of depression than men (BDI: mean = 11.8 v. 8.3, p<0.01.) Men and women reported similar levels of state anxiety, and women reported significantly higher trait anxiety (STAI: mean = 54.4 v. 51.9, p=0.01). Both boys and girls scored in the average range for total symptoms of depression, negative mood, interpersonal problems, ineffectiveness, anhedonia, as measured on the CDI; scores on the negative self-esteem scale were slightly below average for both boys and girls. No significant differences were noted between boys and girls for any of the CDI scale scores. Similarly, scores on the Internalizing, Externalizing, and Total Behavior problem scales of the CBCL were also in the average range with no significant differences in behavior problems noted between boys and girls.
2.3.5 Domoic Acid Exposure Assessment
Since the primary mechanism for DA exposure is through razor clam consumption, shellfish consumption and general dietary intakes, these exposures were assessed using multiple methods. The utility of the dietary assessment methods used in the CoASTAL cohort have been previously described (Fialkowski et al., 2010a) and will be briefly summarized here. Environmental data with respect to DA levels was also obtained.
2.3.5.1 Food frequency questionnaire
Participants completed the Block Food Frequency Questionnaire, (FFQ; Block et al., 1986), to characterize usual frequency of consumption of specific foods or food groups. The Block FFQ is a self-administered, quantitative food frequency questionnaire that asks respondents to recall their usual frequency of consuming specific foods or food groups; it also asks respondents to provide estimates of portion sizes using standardized portion photos. The period of dietary recall was the year prior to the baseline study visit. The Block FFQ has been extensively validated with diverse populations of adults (Cummings et al., 1987; Sobell et al., 1989; Block et al., 1990; Coates et al., 1991; Block et al., 1992; Subar et al., 2001). The Block FFQ compares favorably with other FFQ instruments (Subar et al., 2001). For participants with self-identified limitations in reading skills, the Block FFQ was completed by a standarized interview developed specifically for this study.
2.3.5.2 Dietary Records
Dietary records were completed every 4 months as two 1 day dietary records and one set of 2 days of dietary records for a total of 4 dietary records over 1 year. Respondents were assigned a grouping of days of the week based on the day of their first visit. At least one of the assigned days included a weekend day. This approach was designed to capture seasonal and day of the week variation and decrease respondent fatigue. Field coordinators trained the participants in record keeping techniques, provided a tool kit of calibrated utensils (e.g. measuring cups and spoons), provided recording materials, followed up with phone calls, and reviewed the dietary record upon completion. Data coding and entry were performed by staff trained in the use of the Nutrition Data System for Research (NDS-R) Database Version 4.07 (© Regents of the University of Minnesota). The intakes from the dietary records were calculated as the mean of the number of days reported. At least 2-4 days were reported by the respondents. The results for the dietary intakes as assessed with the dietary records among the adults of the CoASTAL cohort have been reported previously (see Fialkowski et al., 2010a,b; Fialkowski et al., 2012). In general, the men and women did not record intakes that met Dietary Reference Intake (DRI) recommendations for total fat, saturated fat, cholesterol, protein, vitamin A, vitamin E, vitamin C, magnesium, and zinc (Fialkowski et al., 2010b). When seeking to find a dietary pattern reflecting the presence of indigenous foods, such a pattern was not detected (Fialkowski et al., 2012).
Estimates from the FFQ of the dietary intakes of the men and women are shown in Table 9. These intakes from the CoASTAL cohort were compared to the intakes of men and women 20 years and older reported in the What We Eat in America (WWEIA), National Health and Nutrition Examination Survey (NHANES) from 2011-2012. The intakes of dietary cholesterol for both men and women were higher than the intakes reported in NHANES, i.e., 338 mg for men, 229 mg for women. For dietary fiber, the men's intakes were slightly lower than men in NHANES at 20.3 g; however, the intakes for women were similar to women in NHANES at 16.1 g. For the fatty acids, the intakes of omega-3 fatty acids were similar to the men and women in NHANES. For example, the men from the NHANES sample had a mean intake of 2.4 g and the women 1.8 g. Given the access to seafood rich in omega-3 fatty acids among the Tribal Nations of the Pacific Northwest, this is surprising. However, the FFQ may have underestimated the seafood consumption by limiting choices of fish relevant to the Pacific coastal waters. On the other hand, the intakes of the monounsaturated fatty acids (MUFA) were high among the men compared to NHANES, i.e., 34.8 g MUFA and the polyunsaturated fatty acids (PUFA) were lower, i.e., 28.7 g. The women were consistently higher than their NHANES counterparts, i.e., 24.3 g MUFA and 16.8 g PUFA. The Acceptable Macronutrient Distribution Range (AMDR) set as the Dietary Reference Intakes (DRI) for fat is 20-35% of energy (kcal). Both the men and women of the CoASTAL cohort at 37.2% and 36.5%, respectively, had slightly higher fat intake than the acceptable range.
Table 9.
Mean dietary intakes for children 5-17 years old from a food frequency questionnaire
| Total N = 99 | Boys n = 65 | Girls n = 34 | p-value* | |
|---|---|---|---|---|
| mean ± SD | ||||
| Cholesterol, mg | 269 ± 160 | 279 ±178 | 249 ± 117 | 0.390 |
| Dietary fiber, g | 17.2 ± 10.1 | 17.6 ± 11.2 | 16.4 ± 7.9 | 0.566 |
| Omega-3 fatty acids, g | 1.73 ± 1.20 | 1.83 ± 1.42 | 1.59 ± 0.70 | 0.422 |
| MUFA, g | 36.9 ± 22.3 | 38.6 ± 25.4 | 33.8 ± 14.5 | 0.311 |
| PUFA, g | 21.2 ± 13.7 | 22.4 ± 15.8 | 18.9 ± 8.1 | 0.234 |
| % energy fat | 36.5 ± 4.9 | 36.6 ± 4.9 | 36.4 ± 4.8 | 0.810 |
| % energy SFA | 12.0 ± 2.0 | 11.9 ± 1.9 | 12.1 ± 2.0 | 0.688 |
| % energy protein | 14.3 ± 2.5 | 14.3 ± 2.6 | 14.4 ± 2.4 | 0.736 |
| % energy carbohydrate | 50.8 ± 6.3 | 50.8 ± 6.3 | 50.8 ± 6.3 | 0.980 |
MUFA = Monounsaturated fatty acids
PUFA = Polyunsaturated fatty acids
SFA = Saturated fatty acids
The dietary intakes of the boys and girls from the CoASTAL cohort displayed in Table 10 were also compared to intakes reported in the WWEIA, NHANES from 2011-2012 for boys and girls 2-19 years old. The intakes of dietary cholesterol for both boys and girls were higher than the intakes reported in NHANES, i.e., 250 mg for boys, 197 mg for girls. For dietary fiber, the boy's intakes were slightly higher than boys in NHANES, 15.9 g and the intakes for girls were slightly higher than girls in NHANES, 12.9 g. For the fatty acids, the intakes of omega-3 fatty acids were slightly higher compared to the boys and girls in NHANES. For example, the boys had a mean intake of 1.73 g and the girls 1.45 g. In contrast to the adults, the children's intakes of omega-3 fatty acids were more consistent with the access to seafood rich in omega-3 fatty acids among the Tribal Nations of the Pacific Northwest. The intakes of the MUFA were higher among the boys compared to NHANES, i.e., 28.1 g MUFA and the PUFA were higher, i.e., 18.0 g. The girls were also consistently higher than their NHANES counterparts, i.e., 22.5 g MUFA and 15.0 PUFA. The Acceptable Macronutrient Distribution Range (AMDR) set as the Dietary Reference Intakes (DRI) for fat is 20-35% of energy (kcal). Both the boys and girls, at 36.6% and 36.4%, respectively, were slightly higher than the acceptable range. Thus, the greater intakes in fatty acids observed among the Native American children can partially be explained by the higher proportion of fat consumed in the diets.
Table 10.
Adult seafood consumption as reported on Shellfish Assessment Survey
| Total N = 526 | Men n = 217 | Women n = 309 | p value* | |
|---|---|---|---|---|
| Eat fish and/or shellfish (%; n = 524) | 96 | 97 | 95 | 0.229 |
| Eat razor clams (%; n = 519) | 83 | 87 | 80 | 0.025 |
| Razor clams consumed/month Fall/Winter (mean ± SD; n = 492) | 13 ± 16 | 15 ± 17 | 13 ± 16 | 0.139 |
| Razor clams consumed/month annually (mean ± SD; n = 455) | 13 ± 15 | 14 ± 16 | 12 ± 14 | 0.087 |
| Razor clam meals/month during Fall/Winter (%; n = 505) | 0.018 | |||
| 0 | 18 | 14 | 21 | |
| 1 to 2 | 36 | 40 | 33 | |
| 3 to 4 | 24 | 29 | 21 | |
| 5 to 6 | 11 | 8 | 13 | |
| 7 to 8 | 5 | 4 | 5 | |
| 9 to 10 | 6 | 5 | 7 | |
| Number of razor clams per meal in Fall/Winter (%; n = 498) | <0.001 | |||
| 0 | 18 | 14 | 21 | |
| 1 to 2 | 18 | 15 | 21 | |
| 3 to 5 | 41 | 40 | 42 | |
| 6 to 9 | 15 | 18 | 12 | |
| 10 or more | 8 | 13 | 4 | |
| Eat razor clam chowder (n = 473) | 78 | 80 | 76 | 0.369 |
| Razor clam chowder meals/month in Fall/Winter (%; n = 470) | 0.488 | |||
| 0 | 22 | 20 | 24 | |
| 1 to 2 | 40 | 43 | 37 | |
| 3 to 4 | 22 | 24 | 21 | |
| 5 to 6 | 9 | 7 | 10 | |
| 7 to 8 | 5 | 4 | 5 | |
| 9 to 10 | 2 | 3 | 2 | |
| Cups of razor clam chowder per meal in Fall/Winter (%; n = 479) | 0.011 | |||
| 0 | 23 | 20 | 25 | |
| 1 | 20 | 18 | 22 | |
| 2 | 35 | 32 | 37 | |
| 3 | 15 | 19 | 12 | |
| 4 | 7 | 11 | 4 | |
| Razor clam meals/month in Spring/Summer (%; n = 479) | 0.062 | |||
| 0 | 19 | 15 | 22 | |
| 1 to 2 | 42 | 46 | 39 | |
| 3 to 4 | 26 | 28 | 24 | |
| 5 to 6 | 6 | 7 | 6 | |
| 7 to 8 | 3 | 1 | 5 | |
| 9 to 10 | 4 | 4 | 4 | |
| Number of razor clams per meal in Spring/Summer (%; n = 471) | 0.001 | |||
| 0 | 19 | 15 | 22 | |
| 1 to 2 | 19 | 18 | 20 | |
| 3 to 5 | 41 | 38 | 43 | |
| 6 to 9 | 15 | 19 | 12 | |
| 10 or more | 6 | 11 | 3 | |
| Eat crab (%; n=496) | 22 | 25 | 20 | 0.199 |
| Number of crab meals in Fall/Winter (mean ± SD; n = 487) | 0.6 ± 1.7 | 0.8 ± 2.1 | 0.5 ± 1.3 | 0.049 |
| Number of crab meals in Spring/Summer (mean ± SD; n = 483) | 0.5 ± 1.6 | 0.5 ± 1.4 | 0.5 ± 1.8 | 0.868 |
| Number of little neck clams consumed yearly (mean ± SD; n = 229)† | 19 ± 40 | 19 ± 36 | 19 ± 43 | 0.956 |
| Number of butter clams consumed yearly (mean ± SD; n = 225)† | 20 ± 42 | 19 ± 34 | 20 ± 48 | 0.917 |
| Number of horse clams consumed yearly (mean ± SD; n = 221)† | 5.7 ± 29 | 7.0 ± 36 | 4.9 ± 23 | 0.600 |
p values from chi-square test, Fisher's exact test or t-test, as appropriate
Clam data is very unstable due to a few large values
2.3.5.3 Shellfish consumption
Shellfish consumption was assessed using the ShellfishAssessment Survey (SAS), specifically developed and validated in collaboration with participating tribes for this population (Fialkowski et al., 2010a). The SAS was piloted on each reservation and adjustments were made for clarity, readability and relevance. The final version of the questionnaire asks for the frequency, types, and preparation of shellfish as well as locations from which shellfish were harvested. Each participant took 10 to 15 minutes to complete the SAS upon entry into the cohort, reporting their consumption of shellfish over the previous month.
Shellfish consumption for adults and children is summarized in Tables 11 and 12. The majority of adults and children reported consumption of fish and/or shellfish (96% and 92%, respectively). Razor clam consumption was also relatively high with adults consuming more razor clams (83%) than children (58%). However, there was considerable variability in the number of razor clams consumed annually or seasonally.
Table 11.
Children's seafood consumption as reported on Shellfish Assessment Survey
| Total N = 108 | Boys n = 70 | Girls n = 38 | p value* | |
|---|---|---|---|---|
| Eat fish and/or shellfish (%; n = 108) | 92 | 90 | 95 | 0.489 |
| Eat razor clams (%; n = 105) | 58 | 56 | 62 | 0.533 |
| Razor clams consumed/month Fall/winter (mean ± SD; n = 99) | 4.6 ± 6.6 | 4.0 ± 5.5 | 5.6 ± 8.0 | 0.256 |
| Razor clams consumed/month annually (mean ± SD; n = 97) | 4.3 ± 6.9 | 4.0 ± 6.5 | 4.9 ± 7.5 | 0.535 |
| Razor clam meals/month during Fall/Winter (%; n = 103) | 0.487 | |||
| 0 | 43 | 45 | 38 | |
| 1 to 2 | 29 | 30 | 27 | |
| 3 to 4 | 20 | 20 | 22 | |
| 5 to 6 | 6 | 5 | 8 | |
| 7 to 8 | 1 | 0 | 3 | |
| 9 to 10 | 1 | 0 | 3 | |
| Number of razor clams per meal in Fall/Winter (%; n = 99) | 0.205 | |||
| 0 | 44 | 48 | 38 | |
| 1 to 2 | 30 | 26 | 38 | |
| 3 to 5 | 22 | 24 | 19 | |
| 6 to 9 | 2 | 0 | 5 | |
| 10 or more | 1 | 2 | 0 | |
| Eat razor clam chowder (%; n = 94) | 63 | 63 | 62 | 0.922 |
| Razor clam chowder meals/month in Fall/Winter (%; n = 91) | 0.927 | |||
| 0 | 38 | 38 | 39 | |
| 1 to 2 | 37 | 38 | 36 | |
| 3 to 4 | 19 | 16 | 22 | |
| 5 to 6 | 4 | 5 | 3 | |
| 7 to 8 | 1 | 2 | 0 | |
| 9 to 10 | 0 | 0 | 0 | |
| Cups of razor clam chowder per meal in Fall/Winter (%; n = 92) | 0.711 | |||
| 0 | 38 | 38 | 38 | |
| 1 | 34 | 31 | 38 | |
| 2 | 24 | 27 | 19 | |
| 3 | 3 | 4 | 3 | |
| 4 | 1 | 0 | 3 | |
| Razor clam meals/month in Spring/Summer (%; n = 102) | 0.573 | |||
| 0 | 43 | 45 | 39 | |
| 1 to 2 | 38 | 35 | 44 | |
| 3 to 4 | 14 | 15 | 11 | |
| 5 to 6 | 2 | 2 | 3 | |
| 7 to 8 | 2 | 3 | 0 | |
| 9 to 10 | 1 | 0 | 3 | |
| Number of razor clams per meal in Spring/Summer (%; n = 99) | 0.274 | |||
| 0 | 44 | 48 | 39 | |
| 1 to 2 | 33 | 30 | 39 | |
| 3 to 5 | 18 | 21 | 14 | |
| 6 to 9 | 4 | 2 | 8 | |
| 10 or more | 0 | 0 | 0 | |
| Eat crab (%; n = 106) | 16 | 19 | 11 | 0.285 |
| Number of crab meals in Fall/Winter (mean ± SD; n = 106) | 0.6 ± 1.5 | 0.7 ± 1.7 | 0.4 ± 1.1 | 0.346 |
| Number of crab meals in Spring/Summer (mean ± SD; n = 106) | 0.5 ± 1.3 | 0.6 ± 1.5 | 0.2 ± 0.8 | 0.187 |
| Number of little neck clams consumed yearly (mean ± SD; n = 73)† | 5.2 ± 17 | 4.3 ± 13 | 7.1 ± 21.9 | 0.510 |
| Number of butter clams consumed yearly (mean ± SD; n=74)† | 5.0 ± 12.9 | 3.1 ± 8.6 | 8.9 ± 18.7 | 0.072 |
| Number of horse clams consumed yearly (mean ± SD; n=69)† | 0.6 ± 2.4 | 0.4 ± 1.6 | 1.1 ± 3.8 | 0.262 |
p values from chi-square test, Fisher's exact test or t-test, as appropriate
Clam data is very unstable due to a few large values
2.3.5.4 Shellfish sampling and analyses
Shellfish samples were collected twice monthly to coincide with the summer-fall HAB season (June through October) and on a monthly basis the remainder of the year. The shellfish of greatest interest were razor clams because razor clams can hold DA in tissue for up to one year and thus produce the greatest human health risk. Domoic acid sampling occurred on a weekly basis during the summer-fall HAB season.
2.3.5.5 Domoic acid sampling data
Serial samples were collected from 11 beach areas on the North, North Central and Central Region Pacific coast of Washington where people reported sourcing razor clams. Concentrations of domoic acid in razor clams were determined by the Shellfish Program in the Office of Environmental Health and Safety (Washington State Department of Health) and the Northwest Fisheries Science Center (National Oceanic and Atmospheric Administration) using the method described by Hatfield et al., (1994), which uses a methanol/water extraction and analysis by high-performance liquid chromatography. Mean levels of domoic acid across all sites were 4.04 (SD=4.97) during the first year of the study, with the highest median level reported from a single sample collected at Second Beach (13.59 ppm) and the most consistent elevation level noted in samples obtained from Kalaloch Beach (mean=15.69). These beaches historically have the highest elevations of DA, are closely monitored by regulatory agencies and closed to shellfish harvesting when levels begin to rise toward 20 ppm.
2.3.5.6 Exposure Assessment
In addition to examining levels of razor clam consumption (the primary vector of DA exposure), DA toxicity level data were collected from 11 beaches of 8 regions of the Northwest Coast of Washington where razor clams were sourced. Data collection ranged from the Columbia River to Neah Bay (Figure 1) and included tribal as well as non-tribal beaches. Since there was considerable variability in the sources of razor clams among participants, three exposure methods were studied in Wave 1. The first involved defining exposure as the product of (the average number of razor clams consumed per month that year) times (the mean measure domoic concentration, in parts per million, in razor clams at all beach(es) from which the participant ate clams that year). The second involved establishing a “typical” exposure level based upon DA concentrations in regions of most frequently sourced clams. Finally, the third exposure method was based on consumption data, dividing the group into high and low consumers based upon the overall distribution of scores. Individuals consuming 15 or more razor clams/month were considered high consumers and people who ate fewer were low consumers.
2.3.5.7 Mercury Exposure
Fish and seafood comprise a large part of the CoASTAL cohort's diet. Therefore this population as a whole may be at risk of methylmercury exposure. Since elevated levels of methylmercury exposure may also be associated with cognitive and neurological symptoms, methylmercury is a potential confounding factor in this study. For this reason, the concentration of total mercury in hair samples was analyzed for 20% of adults and children at all study sites. Of the 228 samples, more than half were below the level of quantification and more than one-third of these were below the level of detection. Where levels were detected for women and children, they remained below the means reported by the 1999-2000 NHANES in each age group (McDowell et al., 2004; Centers for Disease Control and Prevention, 2005). Moreover, the adult women from the CoASTAL cohort had an approximately 4 fold lower mean concentration than the means reported from NHANES. There was no difference in the means between men and women. The reasons for the relatively low mercury concentrations in the CoASTAL cohort is most likely because the Tribal Nations are located in a remote part of Northwest Washington state, where there is a relatively low risk for exposure to toxicants due to the absence of mining and industrial sources.
3. Discussion
The CoASTAL cohort represents the first community cohort of Native Americans assembled to study a HAB-related illness, specifically Amnesic Shellfish Poisoning. The cohort is unique to the extent that it is geographically located in a region with low level DA exposures through razor clam consumption and has a wide range of consumers as well as non-consumers in the population. Moreover, by virtue of tribe membership and reservation living, the cohort is relatively stable for longitudinal studies. Finally, close monitoring of DA levels in razor clams from both tribe and state natural resources and fisheries programs provide frequent measures of DA levels and widespread monitoring. Thus, there is access to the best available DA exposure data in the United States and possibly the world. From a public health perspective, this cohort is particularly important to study as they are at greatest risk for chronic, low level DA related toxicity if it exists.
DA levels during the study period remained relatively low, thus direct comparison to the documented cases of Amnesic Shellfish Poisoning associated with the Prince Edward Island outbreak (Perl et al., 1990a,b; Teitlebaum et al., 1990a,b) cannot be made. However, since the convergence of data from human autopsy and non-human primate studies suggests temporal lobe abnormalities at the root of the serious and persistent memory difficulties (Sutherland et al., 1990; Cendes et al., 1995; Slikker et al., 1998a,b;) lower level exposures might be expected to contribute to milder forms of memory problems. There is currently no available biomarker of human exposure to DA, therefore measures of exposure are necessarily linked to razor clam consumption and estimates of DA levels established from sourced beach sampling.
Overall, baseline cognitive data with the CoASTAL cohort suggests scores well within the average range for age and educational level for children and adults on all cognitive measures. Psychological assessment data found that one-fourth of adults reported a history of depression, anxiety, or other psychological problems, with women reporting more symptoms of depression than men. Men and women reported similar levels of state anxiety (current), and women reported significantly higher trait anxiety (long term, stable). Despite these statistical differences, scores on the psychological measures remained within normal limits. Similarly, the children demonstrated no evidence for problems with depression or interpersonal problems. Their relative low scores on the negative self-esteem scale suggest a healthy sense of liking themselves and feeling loved. Overall, the psychological adjustment of this cohort further supports their suitability for prospective studies.
Dietary data for the CoASTAL cohort sample shown in the tables 9 and 10 were derived from FFQs, whereas, the national data from NHANES used for comparison were derived from 24-hour dietary recalls. Thus, some of the observed differences may be an artifact of the different methodologies. Nonetheless, despite this study's data collection methods differing from NHANES - that large body of national data is the only reference available for comparison - and many of the results were similar. Further, we have previously published the results of the dietary records and compared the CoASTAL cohort intakes to NHANES with the same result of similar intakes (Fialkowski et al., 2010b). The dietary record as a methodology would be considered a closer method to the 24-hour dietary recall. Collectively, these results would support the merits of the CoASTAL cohort methods with regard to nationally vetted methods and high comparability to representative national samples of the United States.
The primary limitations of this study are exposure assessment and generalizability to non-Native American people. With respect to exposure assessment, there is currently no available method for detection of DA in human body fluids. Although environmental exposures are historically very difficult to assess (Nitta et al., 2010), every effort is made in this investigation to use the best available methods and models. However, as new methods become available, this well studied cohort would be in an optimal position to participate in validation studies and benefit from the evolving science. Meanwhile, the best available method for assessing exposure is through dietary consumption and razor clam source data. For the next wave of this study we will be piloting a technology assisted dietary assessment (TADA) method to potentially increase accuracy of dietary consumption data and make real time links to memory functions (Boushey et al., in press). Finally, DA exposure history also remains an unknown in this study. To date, careful medical history taking has allowed investigators to rule out any prior episodes of Amnesic Shellfish Poisoning in the CoASTAL cohort. Questions still remain regarding generalizability of this cohort to other, non-American Indian people. This is particularly relevant as 26% of the cohort believes that Pacific Northwest coastal Native American Indians have developed immunity to DA throughout many years and generations of exposure (see Roberts et al., in press). Accordingly, it might be true. This question could only be answered with future comparative studies (behavioral and genetic) within the context of more advanced detection methods and exposure models.
Meanwhile, the CoASTAL cohort offers a unique opportunity to investigate the potential health effects of chronic low level exposure to domoic acid over time. Findings may be relevant to protecting the health of this vulnerable population and high risk groups within it (infants, children, elderly), as well as other populations. Moreover, the results of this study may also be of public health significance by establishing guidelines for preventing DA illnesses associated with long term chronic exposure if indicated. This is particularly important as DA and its HAB precursors are being increasingly reported in other areas of the United States and the world.
The CoASTAL cohort represents the first community cohort of Native Americans assembled to study a HAB-related illness, specifically Amnesic Shellfish Poisoning. The cohort is unique to the extent that it is geographically located in a region with low level DA exposures through razor clam consumption and has a wide range of consumers as well as non-consumers in the population. Moreover, by virtue of tribe membership and reservation living, the cohort is relatively stable for longitudinal studies. Finally, close monitoring of DA levels in razor clams from both tribe and state natural resources and fisheries programs provide frequent measures of DA levels and widespread monitoring. Thus, there is access to the best available exposure data in the United States and possibly the world. From a public health perspective, this cohort is particularly important to study as they are at greatest risk for chronic, low level DA related toxicity if it exists.
DA levels during the study period remained relatively low, thus direct comparison to the documented cases of Amnesic Shellfish Poisoning associated with the Prince Edward Island outbreak (Perl et al., 1990a,b; Teitlebaum et al., 1990a,b) cannot be made. However, since the convergence of data from human autopsy and non-human primate studies suggests temporal lobe abnormalities at the root of the serious and persistent memory difficulties (Sutherland et al., 1990; Cendes et al., 1995; Slikker et al., 1998a,b;) lower level exposures might be expected to contribute to milder forms of memory problems. There is currently no available biomarker of human exposure to DA, therefore measures of exposure are necessarily linked to razor clam consumption and DA levels established from sampling from sourced beaches.
Overall, baseline cognitive data with the CoASTAL cohort suggests scores well within the average range for age and educational level for both children and adults on all cognitive measures. Psychological assessment data found that one-fourth of adults reported a history of depression, anxiety, or other psychological problems, with women reporting more symptoms of depression than men. Men and women reported similar levels of state anxiety (current), and women reported significantly higher trait anxiety (long term and more embedded in personality). Despite these differences, as a group, scores on the psychological measures remained within normal limits. Similarly, the children demonstrated no evidence for problems with depression or interpersonal problems. Their relative low scores on the negative self-esteem scale suggest a fairly healthy sense of liking themselves and feeling loved. Overall, the psychological adjustment of this cohort further supports their suitability for prospective studies.
Dietary data for the CoASTAL cohort sample shown in the tables 9 and 10 were derived from FFQs, whereas, the national data from NHANES used for comparison were derived from 24-hour dietary recalls. Thus, some of the observed differences may be an artifact of the different methodologies. Nonetheless, despite this study's data collection methods differing from NHANES, the large body of national data is the only reference available for comparison, and many of the results were similar. Further, we have previously published the results of the dietary records and compared the CoASTAL cohort intakes to NHANES with the same result of similar intakes (Fialkowski et al., 2010b). The dietary record as a methodology would be considered a closer method to the 24-hour dietary recall. Collectively, these results would support the merits of the CoASTAL cohort methods with regard to nationally vetted methods and high comparability to representative national samples of the United States.
The primary limitations of this study are exposure assessment and generalizability to non-Native American people. With respect to exposure assessment, there is currently no available method for detection of DA in human body fluids. Although environmental exposures are historically very difficult to assess (Nitta et al., 2010), every effort is made in this investigation to use the best available methods and models. As more advanced methods become available, this well studied cohort would be in an optimal position to participate in validation studies. Meanwhile, the best available method for assessing exposure is through dietary consumption and razor clam source data. For the next wave of this study we will be piloting a technology assisted dietary assessment (TADA) method to potentially increase accuracy of dietary consumption data and make real time links to memory functions (Boushey et al., in press). Finally, DA exposure history also remains an unknown in this study. To date, careful medical history taking has allowed investigators to rule out any prior episodes of Amnesic Shellfish Poisoning in the CoASTAL cohort. A final caveat regards the generalizability of this cohort to other, non-Native American people. This question could only be answered with future comparative studies (behavioral and genetic) with other at risk populations.
Meanwhile, the CoASTAL cohort offers a unique opportunity to investigate the potential health effects of chronic low level exposure to domoic acid over time. Findings may be relevant to protecting the health of this vulnerable population and high risk groups within it (infants, children, elderly), as well as others. Moreover, the results of this study may also be of public health significance by providing reassurance of no risk or establishing guidelines for preventing DA illnesses if indicated. This is particularly relevant as DA and its HAB precursors are being increasingly reported in other areas of the United States and the world.
Table 1.
Characteristics of Adult and Geriatric Participants Enrolled in the CoASTAL Cohort
| Full sample n = 538 | Men n = 224 | Women n = 314 | p value* | |
|---|---|---|---|---|
| Age | 40.4 ± 14.7 | 39.6 ± 14.8 | 41.0 ± 14.5 | 0.30 |
| Gender (Female) | 58 | 0 | 100 | - |
| Employed | 52 | 48 | 55 | 0.11 |
| Educational level (%) | <0.01 | |||
| < High school | 25 | 26 | 24 | |
| High school | 37 | 46 | 31 | |
| Vocational/technical/associates degree/some college | 33 | 25 | 38 | |
| Bachelor's/master's/doctoral degree | 6 | 4 | 8 |
Data presented as percentage, except age presented as mean ± SD.
p values from chi-square test, Fisher's exact test or t-test, as appropriate
Table 3.
Characteristics of Infants Enrolled in the CoASTAL cohort
| Characteristic | Full sample n = 32 | Boys n = 19 | Girls n = 13 | p value* |
|---|---|---|---|---|
| Age (months) | 10.8 ± 1.7 | 10.8 ± 1.6 | 10.8 ± 1.9 | 0.99 |
| Premature birth | 30 | 25 | 36 | 0.68 |
| Birth weight (g) | 3470 ± 670 | 3520 ± 690 | 3410 ± 650 | 0.67 |
| Breast fed | 77 | 88 | 62 | 0.19 |
| Exposed to hazardous chemicals | 3 | 0 | 8 | 0.43 |
| Mother smoked tobacco during pregnancy | 55 | 50 | 63 | 0.68 |
Data presented as percentage, except age and birth weight presented as mean ± SD.
p values are from t-test or Fisher's exact test, as appropriate.
Table 8.
Mean dietary intakes for adults from a food frequency questionnaire
| Total N = 529 | Men n = 221 | Women n = 308 | p-value* | |
|---|---|---|---|---|
| mean ± SD | ||||
| Cholesterol, mg | 311 ± 287 | 367 ± 317 | 271 ± 256 | <0.001 |
| Dietary fiber, g | 17.0 ± 13.4 | 17.7 ± 14.4 | 16.6 ± 12.7 | 0.348 |
| Omega-3 fatty acids, g | 2.1 ± 1.9 | 2.2 ± 1.9 | 1.9 ± 1.8 | 0.145 |
| MUFA, g | 37.8 ± 32.9 | 41.3 ± 35.1 | 35.2 ± 31.1 | 0.034 |
| PUFA, g | 22.3 ± 19.0 | 23.6 ± 20.0 | 21.3 ± 18.3 | 0.180 |
| % energy fat | 36.8 ± 6.5 | 37.2 ± 6.2 | 36.5 ± 6.8 | 0.225 |
| % energy SFA | 11.4 ± 2.4 | 11.7 ± 2.2 | 11.2 ± 2.5 | 0.043 |
| % energy protein | 14.2 ± 3.2 | 14.9 ± 3.2 | 13.7 ± 3.1 | <0.001 |
| % energy carbohydrate | 49.6 ± 8.4 | 47.9 ± 7.9 | 50.9 ± 8.5 | <0.001 |
MUFA = Monounsaturated fatty acids
PUFA = Polyunsaturated fatty acids
SFA = Saturated fatty acids
Acknowledgements
Funding Support: Support for this work came from a National Institute of Environmental Health Sciences grant (NIEHS: 5R01ES012459) awarded to Dr. Grattan. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIEHS.
The authors thank the Makah, Quinault, and Quileute Indian Nation Tribal Councils; Vincent Cooke, Rachel Johnson, and Steve Pendleton from the Makah Environmental Health Division; Bill Parkin from the Makah Marina; Cathy Salazar from the Quileute Department of Natural Resources; Joe Schumacker and Dawn Radonski from the Quinault Department of Fisheries; our Field Examiners, Mary Carter, Christiana Ausherman, Sue Zalokar, Pat Caver, Jean Gookins, and Marlie Gill; our tribal medical advisory board, Thomas Van Eaton of Makah Health Services, Robert Young of the Quinault Health Center, and Brenda Jaime-Nielson and Brad Krall of the Quileute Health Center; and our tribal advisory committee, Theresa Parker, Deanna Buzzell-Gray, June Williams, Melissa Peterson-Renault, Mary Jo Butterfield, and Edith Hottowe from the Makah Indian Nation; and Alena Lopez, Ervin Obi, and Carolyn Gennari from the Quinault Indian Nation for their contributions and participation. The authors also thank Marie Fialkowsi, Nicholas Schulterman, Alison Lydecker, and Lynda Ireland for assistance with data analysis and Sailor Holobaugh, Ryan Jollie, and Anna Cohen for assistance with manuscript preparation.
References
- Achenbach TM, Rescorla LA. Manual for ASEBA school-age forms & profiles. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT.: 2001. [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. The Alcohol Use Disorders Identification Test: guidelines for use in primary care. 2nd ed. Department of Mental Health and Substance Dependence, World Health Organization; Geneva, Switzerland: 2001. http://whqlibdoc.who.int/hq/2001/who_msd_msb_01.6a.pdf. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory (BDI II) 2nd ed. Psychological Corporation; San Antonio, TX.: 1996. [Google Scholar]
- Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A databased approach to diet questionnaire design and testing. Am. J. Epidemiol. 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire design and testing. Am. Clin. Epidemiol. 1990;43:1327–1335. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J. Am. Diet. Assoc. 1992;92:686–693. [PubMed] [Google Scholar]
- Cendes F, Andermann F, Carpenter S, Zatorre RJ, Cashman NR. Temporal lobe epilepsy caused by domoic acid intoxication: evidence for glutamate receptor-mediated excitotoxicity in humans. Ann. Neurol. 1995;37:123–6. doi: 10.1002/ana.410370125. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention National Health and Nutrition Examination Survey 1999-2000 data documentation, codebook, and frequencies: hair mercury. 2005 http://www.cdc.gov/nchs/nhanes/nhanes1999-2000/LAB22.htm.
- Coates RJ, Eley JW, Block G, Gunter EW, Sowell AL, Grossman C, Greenberg RS. An evaluation of a food frequency questionnaire for assessing dietary intake of specific carotenoids and vitamin E among low-income black women. Am. J. Epidemiol. 1991;134(6):658–671. doi: 10.1093/oxfordjournals.aje.a116138. [DOI] [PubMed] [Google Scholar]
- Cohen MJ. Manual for the Children's Memory Scale. Psychological Corporation; San Antonio, TX.: 1997. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2nd ed. Psychological Corporation; San Antonio, TX.: 2000. adult version manual. [Google Scholar]
- Erdner DL, Dyble J, Parsons ML, Stevens RL, Hubbard KA, Wrabel ML, Moore SK, Lefebvre KA, Anderson DM, Bienfang P, Bidigare RR, Parker MS, Moeller P, Brand LE, Trainer VL. Centers for Oceans and Human Health: a unified approach to the challenge of harmful algal blooms. Environ. Health. 2008;7(Suppl 2):S2. doi: 10.1186/1476-069X-7-S2-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman RG. Occupational and environmental neurotoxicology. Lippincott-Raven; Philadelphia, PA.: 1999. [Google Scholar]
- Fialkowski M, McCrory M, Roberts S, Tracy JK, Grattan L, Boushey C. Evaluation of dietary assessment tools used to assess the diet of adults participating in the Communities Advancing the Studies of Tribal Nations Across the Lifespan cohort. J. Am. Diet. Assoc. 2010a;110(1):65–73. doi: 10.1016/j.jada.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialkowski MK, McCrory MA, Roberts SM, Tracy JK, Grattan LM, Boushey CJ. Estimated nutrient intakes from food generally do not meet dietary reference intakes among adult members of Pacific Northwest Tribal Nations. J. Nutr. 2010b;140:992–998. doi: 10.3945/jn.109.114629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialkowski MK, McCrory MA, Roberts SM, Tracy JK, Grattan LM, Boushey CJ. Dietary patterns are associated with dietary recommendations but have limited relationship to BMI in the Communities Advancing the Studies of Tribal Nations Across the Lifespan (CoASTAL) cohort. Public Health Nutr. 2012;15:1948–1958. doi: 10.1017/S1368980012000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KS, Burbacher TM, Faustman EM, Gratttan L. Domoic acid: neurobehavioral consequences of exposure to a prevalent marine biotoxin. Neurotoxicol. Teratol. 2010;32(2):132–141. doi: 10.1016/j.ntt.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield CL, Wekell JC, Gauglitz EJ, Jr., Barnett HJ. Salt clean-up procedure for the determination of domoic acid by HPLC. Nat. Toxins. 1994;2:206–211. doi: 10.1002/nt.2620020409. [DOI] [PubMed] [Google Scholar]
- Knap A, Dewailly E, Furgal C, Galvin J, Baden D, Bowen RE, Depledge M, Dugay L, Fleming LE, Ford T, Moser F, Owen R, Suk WA, Unluata U. Indicators of ocean health and human health: developing a research and monitoring framework. Environ. Health Perspect. 2002;110(9):839–845. doi: 10.1289/ehp.02110839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. Model 32025 Grooved Pegboard test user instructions. Lafayette Instrument Company; Lafayette, IN: 1992. Children Depression Inventory (CDI) manual. Multi-Health Systems, Toronto, Ontario. Lafayette Instrument Company, 2002. [Google Scholar]
- Luckas B, Dahlmann J, Erler K, Gerdts G, Wasmund N, Hummert C, Hansen PD. Overview of key phytoplankton toxins and their recent occurrence in the North and Baltic Seas. Environ. Toxicol. 2005;20(1):1–17. doi: 10.1002/tox.20072. [DOI] [PubMed] [Google Scholar]
- McDowell MA, Dillon CF, Osterloh J, Bolger PM, Pellizzari E, Fernando R, Montes de Oca R, Schober SE, Sinks T, Jones RL, Mahaffey KR. Hair mercury levels in US children and women of childbearing age: reference range data from NHANES 1999-2000. Environ. Health Perspect. 2004;112(11):1165–1171. doi: 10.1289/ehp.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. AGS ed. American Guidance Service; Circle Pines, MN.: 1995. [Google Scholar]
- National Center for Community-Based Child Abuse Prevention Child Behavior Check List [fact sheet] 2005 http://friendsnrc.org/joomdocs/cbcl.pdf.
- Nitta H, Yamazaki S, Omon T, Sato T. An introduction to Epidemiological and statistical methods useful in environmental epidemiology. J Epidemol. 2010;20:177–184. doi: 10.2188/jea.JE20100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl TM, Bedard L, Kosatsky T, Hockin JC, Todd ECD, McNutt LA, Remis RS. Amnesic shellfish poisoning: a new clinical syndrome due to domoic acid. In: Hynie I, Todd ECD, editors. Proceedings of a Symposium, Domoic Acid Toxicity. Canada Disease Weekly Report. Ottawa, Ontario: 1990a. pp. 7–8. [PubMed] [Google Scholar]
- Perl TM, Bedard L, Kosatsky T, Hockin JC, Todd ECD, Remis RS. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. New Engl. J. Med. 1990b;322:1775–1780. doi: 10.1056/NEJM199006213222504. [DOI] [PubMed] [Google Scholar]
- Pokorny AD, Miller BA, Kaplan H. The Brief MAST: a shortened version of the Michigan Alcoholism Screening Test. Am. J. Psychiat. 1972;129:342–345. doi: 10.1176/ajp.129.3.342. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Trail Making Test: manual for administration and scoring. Reitan Neuropsychology Laboratory; Tucson, AZ.: 1992. [Google Scholar]
- Slattery ML, Schumacher MC, Lanier AP, Edwards S, Edwards R, Murtaugh MA, Sandidge J, Day GE, Kaufman D, Kanekar S, Tom-Orme L, Henderson JA. A prospective cohort of American Indian and Alaska Native people: study design, methods, and implementation. Am. J. Epidemiol. 2007;166(5):606–615. doi: 10.1093/aje/kwm109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slikker W, Scallet AC, Gaylor DW. Biologically-based dose-response model for neurotoxicity risk assessment. Toxicol. Lett. 1998a;102-103:429–433. doi: 10.1016/s0378-4274(98)00335-x. [DOI] [PubMed] [Google Scholar]
- Slikker W, Scallet AC, Gaylor DW. Risk assessment of domoic acid with the use of quantitative histological techniques. Toxicol. Lett. 1998b;95(Suppl 1):21–22. [Google Scholar]
- Sobell J, Block G, Koslowe P, Tobin J, Andres R. Validation of a retrospective questionnaire assessing diet 10–15 years ago. Am. J Epidemiol. 1989;130(1):173–187. doi: 10.1093/oxfordjournals.aje.a115310. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. State-Trait Anxiety Inventory for Adults: manual, instrument, and scoring guide. Mind Garden; Menlo Park, CA.: 1983. [Google Scholar]
- StataCorp . Stata Statistical Software: Release 10. StataCorp LP.; College Station, TX: 2007. [Google Scholar]
- Stommel EW, Watters MR. Marine neurotoxins: ingestible toxins. Curr. Treat. Option. N. 2004;6(2):115–123. doi: 10.1007/s11940-004-0020-9. [DOI] [PubMed] [Google Scholar]
- Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at American's Table Study. Am. J. Epidemiol. 2001;154:1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Hoesing JM, Whishaw IQ. Domoic acid, an environmental toxin, produces hippocampal damage and severe memory impairment. Neurosci. Lett. 1990;120(2):221–223. doi: 10.1016/0304-3940(90)90043-9. [DOI] [PubMed] [Google Scholar]
- Teitelbaum J. Clinical presentation of acute intoxication by domoic acid: case observations. In: Hynie I, Todd ECD, editors. Proceedings of a Symposium, Domoic Acid Toxicity. Canada Disease Weekly Report. Ottawa, Ontario: 1990a. pp. 5–6. [Google Scholar]
- Teitelbaum JS, Zatorre RJ, Carpenter S, Genderon D, Evans AC, Gjedde A, Cashman NR. Neurological sequelae of domoic acid intoxication due to the ingestion of contaminated mussels. New Engl. J. Med. 1990b;322:1781–1787. doi: 10.1056/NEJM199006213222505. [DOI] [PubMed] [Google Scholar]
- Trainer VL, Adams NG, Bill BD, Anulacio BF, Wekell JC. Concentration and dispersal of a Pseudo nitzschia bloom in Penn Cove, Washington, USA. Nat. Toxins. 1998;6:113–126. doi: 10.1002/(sici)1522-7189(199805/08)6:3/4<113::aid-nt14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children: technical and interpretative manual. 4th ed. Psychological Corporation; San Antonio, TX.: 2003. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale: administration and scoring manual. 3rd ed. Psychological Corporation; San Antonio, TX.: 1997. [Google Scholar]