Abstract
Background
To revise the model for perioperative risk for esophagectomy for cancer utilizing the Society of Thoracic Surgeons General Thoracic Surgery Database in order to provide enhanced risk stratification and quality improvement measures for contributing centers.
Methods
The Society of Thoracic Surgeons General Thoracic Surgery Database was queried for all patients treated for esophageal cancer with esophagectomy between July 1, 2011 and June 30, 2014. Multivariable risk models for major morbidity, perioperative mortality and combined morbidity and mortality were created with the inclusion of surgical approach as a risk factor.
Results
4321 esophagectomies were performed by 164 participating centers. The most common procedures included: Ivor Lewis (32.5%), Transhiatal (21.7%), Minimally Invasive esophagectomy, Ivor Lewis type (21.4%) and McKeown (10.0%). Sixty-nine percent of patients received induction therapy. Perioperative mortality (inpatient and 30-day) was 135/4321 (3.4%). Major morbidity occurred in 1429 patients (33.1%). Major morbidities include unexpected return to OR (15.6%), anastomotic leak (12.9%), reintubation (12.2%), initial ventilation beyond 48 hours (3.5%), pneumonia (12.2%), renal failure (2.0%), recurrent laryngeal nerve paresis (2.0%). Statistically significant predictors of combined major morbidity or mortality included: age >65, BMI ≥35, preoperative congestive heart failure, Zubrod score >1, McKeown Esophagectomy, current or former smoker and squamous cell histology.
Conclusion
Thoracic surgeons participating in the Society of Thoracic Surgeons General Thoracic Surgery Database perform esophagectomy with low morbidity and mortality. McKeown esophagectomy is an independent predictor of combined postoperative morbidity or mortality. Revised predictors for perioperative outcome were identified to facilitate quality improvement processes and hospital comparisons.
Keywords: Esophageal cancer, esophageal surgery, quality care, outcomes
There will be an estimated 17,000 new cases of esophageal cancer in 2015 in the United States. Furthermore, esophageal cancer will account for almost 16,000 deaths in the same year, 2.6% of all cancer deaths.[1] The demographics of esophageal cancer have evolved dramatically over the past 30 years[2]. Not surprisingly, treatment modalities have also evolved into a complex array of diagnostic and therapeutic choices optimally managed by a multidisciplinary team. Esophagectomy is a vital component of curative therapy for esophageal carcinoma. Historically, esophagectomy has been associated with significant perioperative morbidity and mortality.[3, 4] As the disease, medical science, and technology evolve, so have the surgical techniques which now includes at least seven different surgical procedures that can be labeled “esophagectomy”. Due to the complexities of care for this disease, the Society of Thoracic Surgeons produced its first outcome model for esophagectomy for esophageal cancer in 2009.[5] This effort has provided valuable risk adjusted analysis to participants for quality improvement and establishment of performance benchmarks. Since the time of this original study, the number of contributing centers has risen from 73 to 164 and the volume of cases contributed annually has almost doubled.
The purpose of this study was to develop updated models of perioperative morbidity and mortality following esophagectomy for esophageal cancer that better represent current surgical practice. We examined three different outcome measures: operative mortality, major morbidity and combined mortality or major morbidity. We also evaluated these models to determine if they could measure variation in hospital performance.
Patients and Methods
Society of Thoracic Surgeons Database
The Society of Thoracic Surgeons (STS) established the General Thoracic Surgery Database (GTSD) in 2002 as a component of the STS National Database as a voluntary registry to support quality improvement efforts of Thoracic surgeons and hospitals.[6] Participating institutions receive biannual reports containing center-specific results as well as risk adjusted national benchmarks for lung and esophageal resection. The STS GTSD has been externally audited since 2010.[7] Audits have revealed a high degree of accuracy and completeness of data. The initial participation in the STS GTSD requires Institutional Review Board approval. Analyses of de-identified data in accordance with the Health Insurance Portability and Accountability Act, such as this study, are exempted from additional IRB approval.
Patient Population
The GTSD was queried for all patients undergoing elective esophagectomy for primary esophageal cancer between January 1, 2012 and December 31,2014. Patients were excluded for benign disease and discordance between declared diagnosis and staging information. Patients were also excluded if they were missing the following data elements: age, gender, perioperative mortality. For the purposes of multivariable analysis, patients with missing clinical stage and tumor histology were excluded and thus of the 4321 patients initially identified, 3942 were included in the multivariable analyses.
Outcome Measures
Postoperative events were those defined by the STS GTSD guidelines.[8] The primary outcomes were perioperative mortality or major morbidity, similar to the prior STS GTSD risk models.[5] Death during the index hospitalization for surgery or within 30 days of the procedure were both considered perioperative mortality. Major morbidity was defined as the presence of one or more of the following postoperative events: unexpected return to the operating room, anastomotic leak, reintubation, initial ventilatory support greater than 48 hours, pneumonia, renal failure, and recurrent nerve paresis. These measures were a consensus agreement based upon the prior risk model[5], clinical judgement, literature review, and preliminary data analysis. Notably, in comparison to the first esophagectomy model[5], renal failure and recurrent nerve paresis were added to the list of major complications. Additionally, bleeding requiring return to the OR was broadened to any unexpected return to the operating room. Three separate outcomes were examined: mortality, major morbidity (at least on major postoperative morbidity) and combined mortality or major morbidity defined as the presence of either mortality or major morbidity.
Covariate Selection
Covariates selected for the risk adjustment models were identified from the two most recent versions of the STS GTSD (v2.2). BMI was imputed based upon the median for gender in the 2% of patients with missing data. Age was considered as a continuous variable but with separate estimates for those older than 65 and less than 65. Odds ratios were determined based upon a 10 year increase in age. Zubrod score was divided into three groups: 0, 1, and 2-5 due to the small patient numbers in the groups three, four and five. BMI was divided into five groups based upon the commonly accepted classification: underweight (BMI < 18.5 kg/m2), Normal weight (BMI ≥18.5 and <25.0 kg/m2), overweight (BMI ≥25.0 and <30.0 kg/m2), obesity class I (BMI ≥30 and < 35 kg/m2), obesity class II or III (BMI ≥35 kg/m2).[9]
Procedures
There are numerous techniques for performing an esophagectomy. The STS GTSD has divided these techniques into seven basic groups. Four of these require “open surgery” and include:
-
1)
Ivor Lewis esophagectomy –Laparotomy and thoracotomy with anastomosis constructed in the chest.
-
2)
Transhiatal esophagectomy – Laparotomy and cervical incision with the anastomosis constructed in the neck. No chest incisions are made other than for drain placement.
-
3)
McKeown or “Three hole” esophagectomy – Laparotomy, thoracotomy and cervical incision with anastomosis constructed in the neck.
-
4)
Thoracoabdominal esophagectomy – A thoracoabdominal incision with division of the costal margin. The anastomosis can be placed in the chest or a separate cervical incision can be made for construction of the anastomosis in the neck.
Minimally invasive esophagectomy requires the use of video technology to perform the procedure without the aid of large incisions which require soft tissue retractors or rib spreading for visualization. These procedures can also be performed robotically.
-
5)
Minimally invasive esophagectomy, Ivor Lewis type (MIE-IL)- Thoracoscopy and laparoscopy are utilized. The anastomosis is created in the chest.
-
6)
Minimally invasive esophagectomy, transhiatal type (MIE-THE)- Laparoscopy and a cervical incision are utilized. The anastomosis is constructed in the neck.
-
7)
Minimally invasive esophagectomy, McKeown type (MIE-McK)- Thoracoscopy, laparoscopy and a cervical incision are utilized. The anastomosis is constructed in the neck.
The type of procedure utilized was determined by the primary site.
Statistical Analyses
Three multivariable logistic regression models were created to determine association of independent predictors of the primary outcome measures: morbidity, mortality, combined morbidity or mortality. All covariates were retained in the models. A hierarchical model with center specific random effects was utilized to account for statistical dependence between outcomes of patients at the same centers. Model discrimination was assessed by examining the area under the receiver operator curve (C statistic). Finally, model calibration was assessed with the Hosmer-Lemeshow goodness-of-fit test.
We further examined in hospital performance for the combined outcome of mortality or major morbidity. The same hierarchical model as above was utilized but the Bayesian approach facilitated computation of a standardized incidence ratio (SIR) for each hospital (participant), along with 95% Bayesian credible intervals. Hospital specific standardized incidence ratios with 95% Bayesian credible intervals summarize performance variation, as previously described [5, 10]. The SIR is the ratio between the participant’s risk-adjusted rate and the risk-adjusted rate of a hypothetical average participant. A SIR of > 1 is consistent with a higher risk-adjusted mortality or major morbidity in comparison to that hypothetical average participant. Analyses were performed using SAS 9.4 statistical package utilizing the GLIMMIX and MCMC modules.
Results
The STS GTSD identified 4321 patients having an esophagectomy for esophageal cancer from 164 participating centers. Patient demographics for the study population are presented in Table 1. The average age of a patient was 64 years and only one third had a normal Body Mass Index (BMI). Interestingly only 2.8% of the population had a below normal BMI. Three quarters of the population were active or former smokers. The majority of the population (95%) had a good performance status (Zubrod Score 0 or 1). Over 70% of the study population received induction therapy prior to surgery. The clinical stage at presentation and the location of disease within the esophagus are listed in Table 2.
Table 1.
Presenting Characteristics
| Characteristic | N(%) or Mean (SD) |
|---|---|
| Age (yrs) | 63.6 (+-9.5) |
| Male gender | 3588 (83%) |
| Black race | 155 (3.6%) |
| Body Mass Index (kg/m2) | |
| <18.5 | 118 (2.7%) |
| >18.5 and <25.0 | 1340 (31.0%) |
| >25.0 and <30.0 | 1587 (36.7%) |
| >30.0 and <35.0 | 829 (19.2%) |
| >35.0 | 447 (10.3%) |
| Smoking History | |
| Current | 631 (14.6%) |
| Former | 2594 (60.0%) |
| Never | 1096 (25.4%) |
| Hypertension | 2500 (57.9%) |
| Diabetes Mellitus (DM) | 952 (22.0%) |
| Congestive heart failure | 103 (2.4%) |
| Coronary artery disease | 848 (19.6%) |
| Peripheral vascular disease | 202 (4.7%) |
| Renal dysfunction* | 55 (1.3%) |
| Corticosteroid use | 67 (1.6%) |
| History of Induction therapy** | 2930 (67.8%) |
| Zubrod Score | |
| 0=Normal activity, no symptoms | 936 (21.7%) |
| 1=Symptoms but fully ambulatory | 3172 (73.4%) |
| 2=Symptoms, in bed<50% of time | 185 (4.3%) |
| 3=Symptoms, in bed >50% of time | 20 (0.5%) |
| 4=Bedridden | 7 (0.2%) |
| 5=Moribund | 1 (0.02%) |
| ASA Risk Classification | |
| I | 15 (0.3%) |
| II | 667 (15.4%) |
| III | 3287 (76.1%) |
| IV | 350 (8.1%) |
| V | 2 (0.05%) |
History of Cr>=2 or Dialysis dependency,
Treatment with chemotherapy or radiotherapy within 6 mos of surgery for esophageal cancer
Table 2.
Clinical Stage at Presentation
| Stage | N (%) |
|---|---|
| I | 651 (15.7%) |
| II | 1873 (45.2%) |
| III | 1547 (37.4%) |
| IV | 71 (1.7%) |
| Site of Cancer | |
| Upper third | 62 (1.4%) |
| Middle third | 305 (7.1%) |
| Lower third | 2619 (60.6%) |
| Gastroesophageal junction | 1335 (30.9%) |
The distribution of procedure types are depicted in Figure 1. Not surprisingly, there is significant heterogeneity in the type of procedure performed. The most common procedures performed were the Ivor Lewis Esophagectomy (32.5%) followed by the Transhiatal Esophagectomy (21.7%) and the Minimally Invasive, Ivor Lewis-type Esophagectomy (21.4%). Overall, minimally invasive techniques were utilized to perform 1489 (33.8%) of the total cases.
Figure 1.
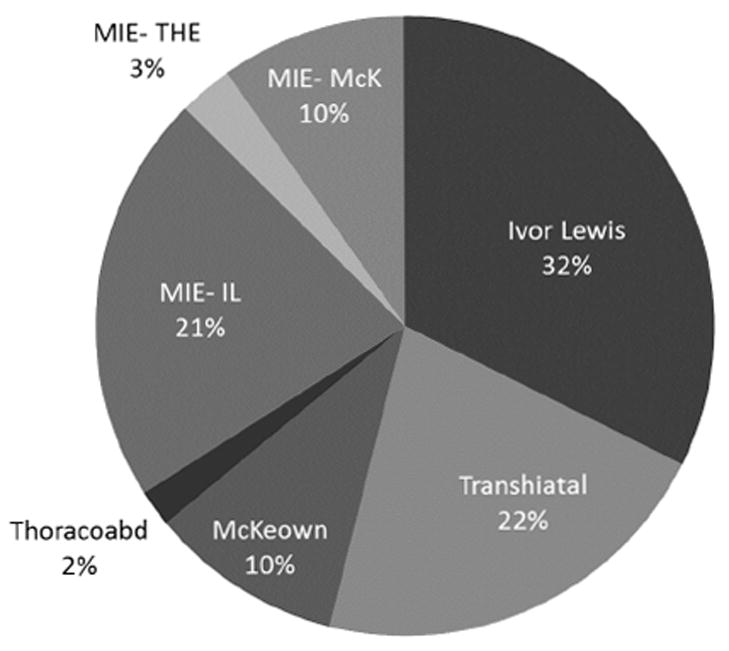
The distribution of surgical techniques
The perioperative mortality rate following esophagectomy was 3.1% (N=135). At least one major morbidity occurred in 33.1% (N=1429) of patients. The breakdown of mortality and morbidity based on procedure type is listed in Table 3. Table 4 lists morbidity and mortality based upon preoperative BMI and, separately, preoperative smoking status. Interestingly, the mortality rate was highest amongst patients with below normal BMI (5.9%) although this did not reach statistical significance on univariable analysis (p=0.54). On the other hand, univariable analysis did reveal a statistically higher morbidity rate in the highest BMI category as compared to normal BMI (OR=1.41; 95% CI [1.13 - 1.77]) but below normal BMI did not reach statistical significance (OR=1.36; 95% CI [0.91 - 2.00]).
Table 3.
Morbidity and Mortality based on Procedure Type
| Procedure | Mortality [N (%)] | Morbidity [N (%)] |
|---|---|---|
| Ivor Lewis Esophagectomy | 54 (3.8%) | 446 (31.8%) |
| Transhiatal Esophagectomy | 22 (2.4%) | 334 (35.7%) |
| McKeown or “Three hole” Esophagectomy | 22 (5.1%) | 166 (38.2%) |
| Thoracoabdominal Esophagectomy | 2 (2.4%) | 31 (36.5%) |
| Minimally invasive, Ivor Lewis Type | 21 (2.3%) | 270 (29.3%) |
| Minimally invasive, Transhiatal Type | 4 (3.3%) | 41 (34.2%) |
| Minimally invasive, McKeown Type | 10 (2.4%) | 141 (33.7%) |
Table 4.
Morbidity and Mortality based upon Body Mass Index and Smoking Status
| BMI | Mortality [N (%)] | Morbidity [N (%)] |
|---|---|---|
| <18.5 | 7 (5.9%) | 47 (39.8%) |
| >18.5 and <25.0 | 41 (3.1%) | 438 (32.7%) |
| >25.0 and <30.0 | 51 (3.2%) | 492 (31.0%) |
| >30.0 and <35.0 | 23 (2.8%) | 267 (32.2%) |
| >35.0 | 13 (2.9%) | 185 (41.4%) |
| Smoking History | ||
| Current | 19 (3.0%) | 243 (38.5%) |
| Former | 83 (3.2%) | 876 (33.8%) |
| Never | 33 (3.0%) | 310 (28.3%) |
Three separate multivariable logistic regression models were created to identify independent predictors of perioperative mortality, major morbidity and combined mortality or major morbidity. The results of this analysis are listed in Table 5. Interestingly, Ivor Lewis-Type Minimally Invasive Esophagectomy offers a statistically significant perioperative survival advantage when compared to Ivor Lewis esophagectomy (OR for mortality alone (OR=0.50, 95% CI [0.28 - 0.89]; P=0.04) in the mortality analysis however in the combined model, there is no statistical difference (OR=0.86, 95% CI [0.69-1.10]). Figure 2 demonstrates the independent predictors of combined perioperative morbidity or mortality. These include age >65, congestive heart failure, Zubrod Score>1, past or current smoking status, BMI >35, squamous histology and McKeown or “three hole” esophagectomy. Figure 3 demonstrates the odds ratios for combined perioperative morbidity and mortality based upon procedure with Ivor Lewis esophagectomy, the most common procedure, as the base line comparator.
Table 5.
Predictors of Major Morbidity or Mortality
| Morbidity Model | Morbidity Model | Combined Morbidity and Mortality Model | ||||
|---|---|---|---|---|---|---|
| Variable | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p |
| Age (per 10 yr increase below 65 yrs) | 1.03 (0.90-1.17) | 0.70 | 1.40 (0.92-2.13) | 0.11 | 1.04 (0.91-1.18) | 0.60 |
| Age (per 10 yr increase above 65 yrs) | 1.32 (1.11-1.56) | 0.001 | 1.68 (1.12-2.50) | 0.01 | 1.32 (1.12-1.57) | <0.001 |
| Female gender | 0.85 (0.71-1.04) | 0.12 | 0.64 (0.37-1.11) | 0.11 | 0.86 (0.71-1.05) | 0.13 |
| Black race | 1.14 (0.78-1.66) | 0.51 | 0.64 (0.21-1.89) | 0.41 | 1.16 (0.79-1.69) | 0.45 |
| Congestive Heart Failure | 2.18(1.40-3.40) | <0.001 | 1.98 (0.89-4.54) | 0.11 | 2.25 (1.44-3.51) | <0.001 |
| Coronary Artery Disease | 1.06(0.88-1.28) | 0.54 | 1.19 (0.76-1.86) | 0.45 | 1.05 (0.87-1.27) | 0.59 |
| Peripheral Vascular Disease | 1.37 (0.99-1.88) | 0.06 | 0.68 (0.28-1.66) | 0.39 | 1.34 (0.97-1.85) | 0.08 |
| Zubrod Score (vs. 0) | <0.001 | <0.001 | <0.001 | |||
| 1 | 0.91 (0.76-1.10) | 0.99 (0.61-1.60) | 0.92 (0.77-1.10) | |||
| >1 | 1.89 (1.34-2.67) | 3.31 (1.70-6.44) | 1.91 (1.35-2.69) | |||
| ASA Risk Class (vs. I or II) | 0.11 | 0.63 | 0.07 | |||
| III | 1.18(0.95-1.45) | 1.29 (0.68-2.41) | 1.20 (0.98-1.48) | |||
| IV or V | 1.41 (1.02-1.94) | 1.49 (0.66-3.37) | 1.44 (1.05-1.99) | |||
| Diabetes Mellitus | 1.03 (0.87-1.23) | 0.72 | 1.29 (0.85-1.98) | 0.24 | 1.06 (0.89-1.26) | 0.50 |
| Hypertension | 1.12 (0.96-1.31) | 0.15 | 1.05 (0.69-1.60) | 0.82 | 1.12 (0.95-1.30) | 0.17 |
| Corticosteroid Use | 1.50 (0.88-2.58) | 0.14 | 3.47 (1.39-8.68) | 0.008 | 1.60 (0.93-2.74) | 0.09 |
| Renal Dysfunction | 1.61 (0.89-2.92) | 0.11 | 1.45 (0.42–5.00) | 0.55 | 1.58 (0.87-2.86) | 0.13 |
| Induction therapy | 0.85 (0.71-1.02) | 0.08 | 1.13 (0.70-1.80) | 0.63 | 0.86 (0.71-1.03) | 0.09 |
| Cigarette smoking (vs. never) | <0.001 | 0.79 | <0.001 | |||
| Past | 1.25 (1.05-1.48) | 0.85 (0.55-1.31) | 1.25 (1.05-1.48) | |||
| Current | 1.68 (1.33-2.12) | 0.96 (0.52-1.79) | 1.65 (1.31-2.09) | |||
| BMI (vs. normal) | <0.001 | 0.83 | <0.001 | |||
| <18.5 | 1.21 (0.79-1.88) | 1.71 (0.67-4.36) | 1.25 (0.81-1.93) | |||
| ≥25 and <30 | 0.96 (0.80-1.14) | 1.20 (0.71-1.76) | 0.96 (0.80-1.14) | |||
| ≥30 and <35 | 1.06 (0.86-1.31) | 1.01 (0.57-1.78) | 1.08 (0.88-1.33) | |||
| ≥35 | 1.69 (1.31-2.18) | 1.14 (0.57-2.34) | 1.69 (1.30-2.17) | |||
| Clinical Stage (vs. I) | 0.89 | 0.81 | 0.76 | |||
| II | 0.96 (0.77-1.20) | 0.90 (0.49-1.65) | 0.94 (0.75-1.17) | |||
| III | 0.92 (0.72-1.18) | 1.08 (0.57-2.08) | 0.89 (0.70-1.13) | |||
| IV | 0.86 (0.47-1.56) | 1.31 (0.33-5.13) | 0.81 (0.45-1.45) | |||
| Squamous Cancer (vs. Adenocarcinoma) | 1.45 (1.18-1.78) | <0.001 | 1.68 (1.04-2.72) | 0.03 | 1.46 (1.19-1.79) | <0.001 |
| Procedure (vs. Ivor Lewis) | <0.001 | 0.04 | 0.004 | |||
| Transhiatal | 1.08 (0.86-1.36) | 0.62 (0.36-1.07) | 1.08 (0.86-1.36) | |||
| McKeown | 1.58 (1.19-2.09) | 1.50 (0.85-2.65) | 1.59 (1.20-2.10) | |||
| Thoracoabdominal | 1.46 (0.87-2.46) | 0.66 (0.14-3.06) | 1.43 (0.85-2.40) | |||
| MIE-IL | 0.87 (0.69-1.08) | 0.50 (0.28-0.89) | 0.86 (0.69-1.08) | |||
| MIE-THE | 1.06 (0.67-1.66) | 0.65 (0.19-2.21) | 1.04 (0.66-1.63) | |||
| MIE-McK | 1.26 (0.94-1.70) | 0.63 (0.30-1.34) | 1.25 (0.93-1.68) | |||
| C statistic | 0.63 | 0.71 | 0.63 | |||
Figure 2.
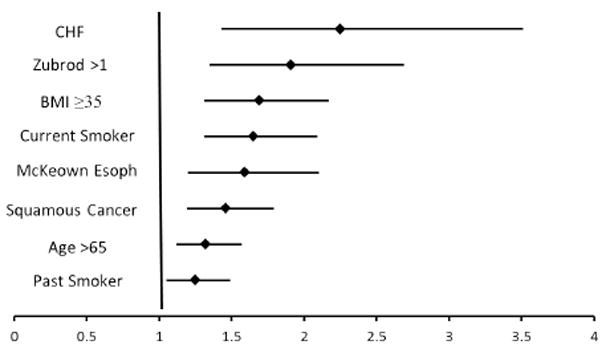
Independent predictors of combined perioperative morbidity and mortality. (Odds Ratio with 95% confidence intervals)
Figure 3.
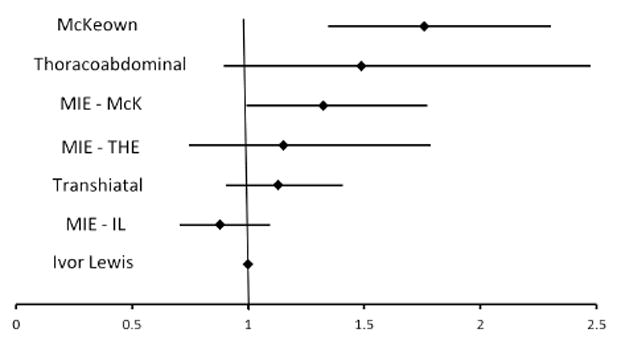
Multivariate analysis of Procedure Type compared to Ivor Lewis Esophagectomy (Odds Ratio with 95% confidence intervals)
The individual program performance is presented in Figure 4. A gradient of performance is readily evident with few statistically significant outliers on the favorable (SIR <1) and unfavorable (SIR >1) ends of the spectrum suggesting the model provides a useful comparison.
Figure 4.
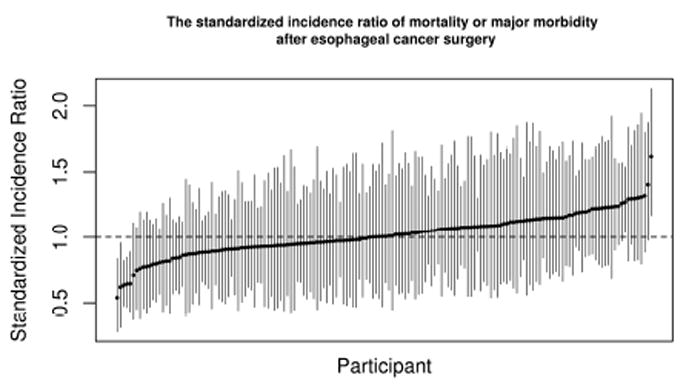
Participant performance variability demonstrated as individual program SIR with 95% Bayesian probability intervals.
Comment
Sites participating in the STS GTSD continue to demonstrate a low mortality and morbidity following esophagectomy for esophageal cancer. In the current study, the perioperative mortality rate was 3.1%. This is similar to several recent large series including a 3.4% perioperative mortality reported from the analysis of the Japanese National Clinical Database including 5345 esophagectomies[11] and 3.0% mortality rate in a recent American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) study[12] including 1032 esophagectomies. This rate lands favorably within the range of 30 day mortality of 0 -11% reported by Blencowe and colleagues[13] in a meta-analysis of outcomes after esophagectomy including 122 studies reporting on over 50,000 esophagectomies. At first glance, it may seem that this rate is higher than the reported 2.7% mortality in the first risk adjustment model for esophagectomy from the STS GTSD.[5] It is important to note, however, that the definition of mortality has broadened from in hospital mortality to perioperative mortality which includes any death during the index hospitalization or within 30 days of surgery. This is due to efforts by the Society of Thoracic Surgeons to improve data completeness. It is important because clinically significant differences exist between these different operative mortality definitions with perioperative mortality being higher than inhospital or 30 day.[14]
Thirty three per cent of patients experienced at least one major morbidity following esophagectomy in the current study. Again, the definition has broadened in comparison to the prior STS risk model[5] to include renal failure, recurrent laryngeal nerve injury, and unexpected return to the Operating Room thus potentially explaining the difference from the prior rate of 24%. It is quite difficult to compare morbidity rates between studies from different databases due to the varying definitions of morbidity[4]. For example, in the aforementioned NSQIP study[12] of 1032 esophagectomy patients, the reported morbidity rate was 50% but the list of morbidities is broader due to differences in database structure and study design. Clearly harmonizing definitions would be of considerable benefit when comparing data from different data sources.
Due to improvements in participation and design of the STS database, we are now able to evaluate individual surgical techniques. Given the heterogeneity of techniques, we can provide a meaningful comparison of technique using prospectively collected clinical data. A provocative finding of our analysis is the demonstration of McKeown esophagectomy as an independent predictor of increased perioperative morbidity or mortality, independent of tumor histology. This is not entirely surprising as the McKeown esophagectomy essentially combines the perioperative risks of a cervical dissection inherent to the Transhiatal esophagectomy with the risks of a thoracotomy inherent to the Ivor Lewis approach. Furthermore, in the mortality alone model, McKeown esophagectomy was not an outlier (OR=1.50 95% CI [0.85-2.65] in contrast to the morbidity alone model where there was a significant difference (OR=1.56 95% CI [1.19-2.09]. What remains to be seen, however, is whether or not the oncologic value of the McKeown approach could translate into an improved five year survival from esophageal cancer. Of further note was the perioperative survival advantage seen for Minimally Invasive Ivor Lewis esophagectomy. This finding warrants further investigation as there are inherent biases when selecting an open or minimally invasive procedure that may favor the minimally invasive approach. An additional finding of interest is that one third of all esophagectomy procedures were performed using minimally invasive techniques. Although it was not statistically significant, the potential reduction in combined morbidity or mortality for MIE-IL will be worth following as experience with this technique continues to grow. There is mounting evidence demonstrating the potential benefits of the minimally invasive approach with respect to reduction of perioperative complications[15-17] but selection bias remains a concern which would be best addressed with a randomized trial.
An additional notable finding was the demonstration of squamous cell cancer histology as a significant predictor morbidity, mortality and combined morbidity and mortality. This is consistent with the latest edition of esophageal cancer staging which revealed a rather striking worsening of outcomes for early stage squamous cell carcinoma of the esophagus when compared to adenocarcinoma after extensive risk adjustment.[18] The relatively early effect in this study would suggest an association between squamous cell carcinoma and additional, unmeasured preoperative variables such as malnutrition or socioeconomic status that may be affecting the outcome of the surgery.
Other predictors of perioperative morbidity or mortality include CHF, functional status (Zubrod score >1), smoking history, age and morbid obesity. Clearly these factors emphasize the importance of careful patient selection for this relatively high risk operation. A notable difference from the first analysis of the STS data[6] was the differentiation of smoking status into current and past smoking history. Both current and past smoking history were independent predictors of perioperative morbidity and mortality although current smoking demonstrates a more significant effect suggesting a potential benefit of smoking cessation prior to surgery. Another notable difference with the prior analysis is the elimination of several patient comorbidities as predictors of perioperative adverse events including CAD, diabetes mellitus, steroid use and hypertension. One would hope this represents an overall improvement in medical management of these conditions leading to a decline in adverse events as the relative frequency of comorbidities is remarkably similar between the two studies.
Limitations of this study include the potential for bias related to the voluntary nature of participation in the STS GTSD. This likely selects out surgeons with particular interest in quality improvement who can utilize this tool to continuously refine their programs. A such, these surgeons are likely a skewed group of the total physicians in the United States performing esophagectomy as supported by the more than 50% reduction in perioperative mortality in the STS GTSD (3.1%) when compared to recent NIS evaluation (8.7%)[19]. In comparison to the prior risk model [5], the GTSD now has a regularly scheduled auditing program to ensure the accuracy of data collection. Furthermore, data completeness has been significantly improved. An example of this is the reduction in missing pathologic stage from 33% in the initial study to 2.3% currently. Disappointingly, we continue to be unable to utilize pulmonary function testing (PFT) as a variable due to incompleteness of data. This is likely not a random effect as patients with good performance status or no smoking history are less likely to have undergone pulmonary function tests preoperatively. A further limitation is the inability to account for hybrid procedures which combine open and thoracoscopic or laparoscopic techniques. Future versions of the STS database will need to address this issue due to the increasing heterogeneity of esophageal surgical techniques. Finally, these analyses measure perioperative outcomes and do not track 90-day, 1-year or 5-year end points. These are critical outcomes measures and to address this the GTSD added long-term survival to the database in January 2015.
We conclude that thoracic surgeons contributing to the STS GTSD perform esophagectomy with low perioperative morbidity and mortality. These analyses are the basis for improved risk-adjusted bi-annual feedback for GTSD participants. Ongoing monitoring of surgical outcomes with this resource may support practice improvements that translate into incremental reductions in perioperative mortality and morbidity. Clearly, improved patient selection, refinements in surgical technique, and enhancement of post-operative care remain vital to the advancement of care of patients with resectable esophageal cancer.
Acknowledgments
The authors would like to acknowledge the efforts of Donna McDonald MPH, RN, Senior Manager STS National Database & Patient Safety, in the development of this manuscript.
Footnotes
Presented at the Sixty-second Annual Meeting of the Southern Thoracic Surgical Association, Orlando, FL, November 4-7, 2015.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.SEER Stat Fact Sheets: Esophageal Cancer. 2015 [Google Scholar]
- 2.Pennathur A, et al. Oesophageal carcinoma. Lancet. 2013;381(9864):400–12. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 3.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364(22):2128–37. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Low DE, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG) Ann Surg. 2015;262(2):286–94. doi: 10.1097/SLA.0000000000001098. [DOI] [PubMed] [Google Scholar]
- 5.Wright CD, et al. Predictors of major morbidity and mortality after esophagectomy for esophageal cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk adjustment model. J Thorac Cardiovasc Surg. 2009;137(3):587–95. doi: 10.1016/j.jtcvs.2008.11.042. discussion 596. [DOI] [PubMed] [Google Scholar]
- 6.Wright CD, Edwards FH. The Society of Thoracic Surgeons General Thoracic Surgery Database. Ann Thorac Surg. 2007;83(3):893–4. doi: 10.1016/j.athoracsur.2006.09.078. [DOI] [PubMed] [Google Scholar]
- 7.Magee MJ, et al. External validation of the Society of Thoracic Surgeons General Thoracic Surgery Database. Ann Thorac Surg. 2013;96(5):1734–9. doi: 10.1016/j.athoracsur.2013.04.124. discussion 1738-9. [DOI] [PubMed] [Google Scholar]
- 8. [August 1, 2005];Society of Thoracic Surgery General Thoracic Surgery Database Guidelines. http://www.sts.org/national-databaseAccessed.
- 9.Obes Res. Suppl 2. Vol. 6. National Institutes of Health; 1998. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report; pp. 51S–209S. [PubMed] [Google Scholar]
- 10.Kozower BD, et al. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg. 2010;90(3):875–81. doi: 10.1016/j.athoracsur.2010.03.115. discussion 881-3. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi H, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg. 2014;260(2):259–66. doi: 10.1097/SLA.0000000000000644. [DOI] [PubMed] [Google Scholar]
- 12.Dhungel B, et al. Patient and peri-operative predictors of morbidity and mortality after esophagectomy: American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) 2005-2008. J Gastrointest Surg. 2010;14(10):1492–501. doi: 10.1007/s11605-010-1328-2. [DOI] [PubMed] [Google Scholar]
- 13.Blencowe NS, et al. Reporting of short-term clinical outcomes after esophagectomy: a systematic review. Ann Surg. 2012;255(4):658–66. doi: 10.1097/SLA.0b013e3182480a6a. [DOI] [PubMed] [Google Scholar]
- 14.Walters DM, et al. Understanding mortality as a quality indicator after esophagectomy. Ann Thorac Surg. 2014;98(2):506–11. doi: 10.1016/j.athoracsur.2014.03.041. discussion 511-2. [DOI] [PubMed] [Google Scholar]
- 15.Biere SS, Cuesta MA, van der Peet DL. Minimally invasive versus open esophagectomy for cancer: a systematic review and meta-analysis. Minerva Chir. 2009;64(2):121–33. [PubMed] [Google Scholar]
- 16.Sgourakis G, et al. Minimally invasive versus open esophagectomy: meta-analysis of outcomes. Dig Dis Sci. 2010;55(11):3031–40. doi: 10.1007/s10620-010-1153-1. [DOI] [PubMed] [Google Scholar]
- 17.Smithers BM, et al. Comparison of the outcomes between open and minimally invasive esophagectomy. Ann Surg. 2007;245(2):232–40. doi: 10.1097/01.sla.0000225093.58071.c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17(7):1721–4. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 19.Lapar DJ, et al. Differences in reported esophageal cancer resection outcomes between national clinical and administrative databases. J Thorac Cardiovasc Surg. 2012;144(5):1152–7. doi: 10.1016/j.jtcvs.2012.08.010. [DOI] [PubMed] [Google Scholar]


