Structured Abstract
Purpose
To develop a nomogram based on clinicopathologic factors to quantify the risk of local recurrence (LR) after limb-sparing surgery without adjuvant radiation (RT).
Methods
Review of our prospective sarcoma database identified 684 patients with primary, nonmetastatic, extremity STS treated with limb-sparing surgery alone between 6/1982–12/2006. No patient received adjuvant radiation or chemotherapy. Age, sex, grade, depth, size, site, margin status and histology were analyzed for prognostic significance with respect to local recurrence rates using Gray’s test. Variables which were significant in univariate analysis at the 0.05 level were entered into a multivariate competing risk regression model. Based upon the multivariate analysis, a nomogram for predicting the 3- and 5-year risk of LR was developed using R libraries cmprsk and QHScrnomo. Concordance index (C-index) was calculated to evaluate the discriminatory power of the prognostic model.
Results
With a median follow-up of 58 months for censored patients (73 months for all patients), the overall 3- and 5-year actuarial local recurrence rates were 11% and 13%, respectively. Factors included in the nomogram were age (≤50 vs >50), size (≤5 vs >5cm), margin status (negative vs positive), grade (low vs high), and histology (atypical lipomatous tumor/well differentiated liposarcoma vs other). The STS nomogram predicted the local recurrence rate with a C-index of 0.73.
Conclusions
A nomogram for extremity STS that includes age, size, margin status, grade of tumor, and histology predicts the 3- and 5-year risk of local recurrence after limb-sparing surgery in the absence of adjuvant RT.
Introduction
Two randomized trials comparing limb-sparing surgery (LSS) versus LSS and postoperative radiation in patients with extremity soft tissue sarcomas demonstrated a 20–25% reduction in the risk of local recurrence with radiation. Based on this, many recommend adjuvant radiation as the standard treatment for most patients with extremity soft tissue sarcomas (STS).1,2
However, the conclusion by Yang et al from the NCI randomized trial was that “although postoperative radiation therapy is highly effective in preventing LRs, selected patients with extremity soft tissue sarcoma may not require adjuvant XRT after limb-sparing surgery.”2
So, who are these patients who do not need adjuvant radiation? There has been a major effort in the sarcoma community for the past two decades to better characterize this subset of patients. Prognostic factors, which impact the risk of LR, have been identified. Such prognostic factors include margin status, age, status at presentation (primary vs. recurrent), tumor location, grade, size, extent of surgery, and histology.3,4,5–12,9,12–14 Coindre et al found a nearly two-fold increase in LR for patients with a simple local excision versus a more extensive resection.13 A large series from MSKCC showed that patients >50 years old were 1.6-times more likely to develop a LR compared to younger patients.9
Unfortunately, despite the identification of the aforementioned prognostic factors, it remains difficult to quantify the risk of LR for an individual patient, and it is often physician bias, experience and gestalt, which ultimately determine adjuvant therapy6,14 Because significant controversy still remains regarding which patients should receive adjuvant radiation, a tool incorporating all relevant prognostic factors that can reliably and accurately assess the risk of LR is needed. Nomograms are predictive tools which create a simple graphical representation of a statistical predictive model and are used to generate a numerical probability of a clinical event. They have been developed to address the inherent limitations of either individual prognostic factors or the TNM staging system by incorporating multiple risk factors in an attempt to better predict outcomes.
A postoperative nomogram to predict the risk of sarcoma-related death has been reported from our institution, however, to our knowledge, a nomogram to predict the risk of LR does not exist. The purpose of this study is to develop a nomogram that can predict the risk of local recurrence in a group of patients treated with limb-sparing surgery and without adjuvant radiation or chemotherapy using the clinicopathologic data from our prospective database for primary, extremity soft tissue sarcomas.
Methods
Patients
Review of the Memorial Sloan-Kettering Cancer Center (MSKCC) prospective sarcoma database identified 684 patients with primary, non-metastatic, extremity STS treated with LSS surgery alone between 6/1982–12/2006. Patients who required amputation as part of their upfront definitive management were excluded. Patients who received any type of radiotherapy or chemotherapy as part of their initial treatment regimen were excluded. All histologies were included except dermatofibrosarcoma and desmoid tumors due to their unique natural history and management.
Clinicopathologic covariates that could potentially predict for local recurrence were collected and analyzed including: age, sex, site, depth, size, histopathology, grade, and margin status. Grade was classified according to a two-tiered high and low grade system. Tumor depth was evaluated relative to the investing fascia of the extremity, with tumors being characterized as either deep or superficial. Tumor size was defined as the maximum diameter of the tumor at pathologic analysis. Age and size were analyzed as categorical and continuous variables. Margin status was defined as classified as either close/positive or negative. A positive margin was defined as tumor cells at the inked margin, and a close margin contained cells <1mm from the inked margin. Because of the similar natural history of well differentiated liposarcoma (WDL) and atypical lipomatous tumors (ATL) and their relatively low risk of local recurrence,15 these two histologic subtypes were grouped together and compared to all other histologies in the statistical analysis.
Statistics
To examine the association between clinicopathological factors and risk of local recurrence, a competing risk survival analysis was conducted treating deaths as a competing risk, as a significant amount of patients died during the follow-up without the development of LR. Followup time was calculated from the time of the primary resection. Variables with p-value<0.05 in univariate analyses were entered to a multivariate model. Categorizations of continuous variables were determined before the analysis. Based on the multivariate model, a competing risk nomogram was developed to estimate the probability of local recurrence.16,17
Nomogram validation included two components. First, a concordance index was obtained by ten-fold cross-validation. The entire data were randomly split into 10 groups; each time the multivariate model with same set of variables was derived on 9 groups and used to predict the probability of local recurrence in the remaining one group. This process was repeated for each group in order to provide predictions for all patients. To protect against the influence of random split, the ten-fold cross-validation was repeated 100 times and the average concordance index was calculated. The concordance index indicates that, among any pair of patients, the probability of one patient experiencing local recurrence earlier if the predicted probability for this patient is higher. Secondly, the predicted probability of LR generated using ten-fold cross validation was compared with the observed local recurrence probability for the entire study cohort. All analyses were performed using R software with library cmprsk for competing risk univariate and multivariate analysis, and library QHScrnomo for competing risk nomogram and validation.
Results
The clinicopathologic characteristics of the cohort are listed in Table 1. The median age at the time of diagnosis was 53 (range, 16 to 95) with 56% (n=381) of patients ≥ 50 years old. Men constituted 55% (n=379) of the patient population. Tumor site was in the upper extremity (at or beyond the shoulder) in 27% of patients (n=185) and lower extremity (at or beyond the groin) in 73% (n=499). Fifty six percent of patients (n=299) had high grade tumors and 44% (n=385) had low grade tumors. Forty seven percent of patients (n=318) had tumors ≤5cm and 53% of patients had tumors >5cm (n=366). The microscopic margin of resection was negative in 73% (495/684), close (<1mm from the inked margin) in 16% (n = 116), and positive in 11% (n = 78). Of the 78 patients with positive margin, 49 (63%) were low grade with atypical lipomatous tumors and well differentiated liposarcomas comprising 29 out of the 49. The remaining 29/78 patients with high grade lesions and positive margin didn’t receive adjuvant radiation because they were either included in MSKCC randomized trial1 or due to patients preference. Tumors were staged using the 2002 AJCC classification; AJCC stage grouping was as follows: 47% (n=318) stage I, 38% (n=258) stage II, and 16% (n=108) stage III. Histopathologic classification, in decreasing order of incidence, is as follows: liposarcoma (n=260, 38%) followed by malignant fibrous histiocytoma (n=153, 22%), leiomyosarcoma (n=95, 14%), synovial sarcoma (n=38, 6%), fibrosarcoma (n=35, 5%), and other (n = 103, 15%). Of the 260 liposarcomas, 71 were atypical lipomatous tumors (ALT), 88 well differentiated liposarcomas (WDL), and 101 other liposarcoma subtypes.
Table 1.
Patient and treatment characteristics
| Characteristics | n | % |
|---|---|---|
|
| ||
| Age | ||
| <50 | 381 | (56) |
| ≥50 | 303 | (44) |
|
| ||
| Gender | ||
| Male | 379 | (55) |
| Female | 305 | (45) |
|
| ||
| Size | ||
| ≤ 5cm | 318 | (47) |
| >5cm | 366 | (53) |
|
| ||
| Depth | ||
| Superficial | 235 | (34) |
| Deep | 449 | (66) |
|
| ||
| Margin | ||
| Negative | 497 | (73) |
| Close or Positive | 187 | (27) |
|
| ||
| Site | ||
| Upper | 185 | (27) |
| Lower | 499 | (73) |
|
| ||
| Grade | ||
| Low | 385 | (44) |
| High | 299 | (56) |
|
| ||
| Histology | ||
| Liposarcoma | 260 | (38) |
| Well differentiated liposarcoma | 88 | |
| Atypical lipomatous tumor | 71 | |
| Other | 101 | |
| Malignant fibrous histiocytoma | 153 | (22) |
| Leiomyosarcoma | 95 | (14) |
| Synovial sarcoma | 38 | (6) |
| Fibrosarcoma | 35 | (5) |
| Other | 103 | (15) |
|
| ||
| AJCC stage | ||
| I | 318 | (47) |
| II | 258 | (38) |
| III | 108 | (16) |
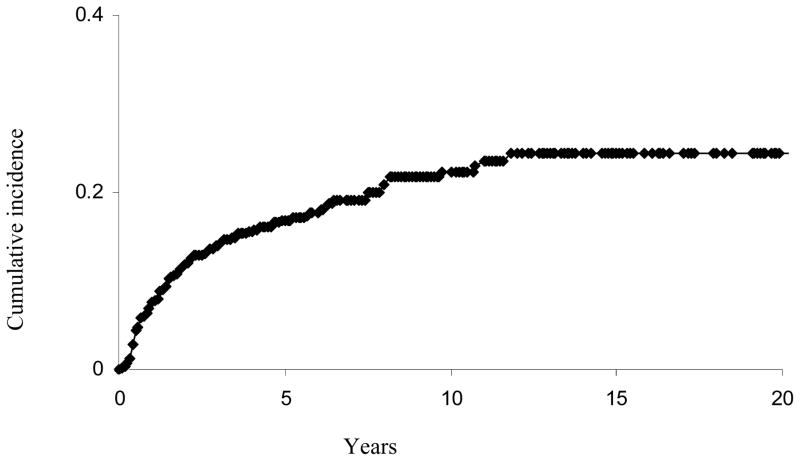
With a median followup of 58 months for censored patients (73 months for all patients), a total of 92 patients developed a LR (Figure 1). The 3-, 5-, and 10- year actuarial risk of LR was 11%, 13%, and 19%, respectively. As shown in Table 2, in univariate analysis, age >50, size >5cm, positive/close margin, high grade, and histology other than WDL or ATL were all associated with increased risk of LR. Sex, depth and site did not affect the rate of LR. Table 3 shows the results of the competing risk regression model for LR in this patient population. In multivariate analysis, age (p=0.02), size (p=0.05), margin status (p<0.01), histology (p=0.01), and grade (p<0.01) all remained independent prognostic factors for LR.
Figure 1.
Cumulative incidence curve for local recurrence for the entire cohort.
Table 2.
Univariate analysis for predictors of local recurrence.
| Covariate | 3 year cumulative incidence of LR | 5 year cumulative incidence of LR | p-value |
|---|---|---|---|
| Age: | |||
| <=50 | 7 | 9 | |
| >50 | 15 | 17 | <0.001 |
|
| |||
| Sex: | |||
| Male | 11 | 12 | |
| Female | 11 | 14 | 0.61 |
|
| |||
| Site: | |||
| Lower | 11 | 13 | |
| Upper | 8 | 13 | 0.65 |
|
| |||
| Size | |||
| <=5cm | 8 | 10 | |
| >5cm | 13 | 16 | 0.017 |
|
| |||
| Depth: | |||
| Superficial | 10 | 12 | |
| Deep | 11 | 13 | 0.34 |
|
| |||
| Histology: | |||
| Others | 13 | 15 | |
| WDL/ALT | 2 | 6 | 0.003 |
|
| |||
| Grade: | |||
| Low | 6 | 8 | |
| High | 16 | 19 | <0.001 |
|
| |||
| Margin: | |||
| Negative | 8 | 10 | |
| Positive/close | 17 | 22 | <0.001 |
Table 3.
Multivariate analysis for predictors of local recurrence.
| Covariate | HR | 95% CI | P-value |
|---|---|---|---|
| Margin (positive/close) | 2.37 | (1.49, 3.77) | <0.001 |
| Grade (high) | 2.02 | (1.27, 3.22) | <0.001 |
| Age (≥50) | 1.72 | (1.09, 2.72) | 0.02 |
| Size (>5cm) | 1.59 | (1.00, 2.52) | 0.05 |
| Histology (Other than WDL or ALT) | 2.86 | ((1.28, 6.42) | 0.001 |
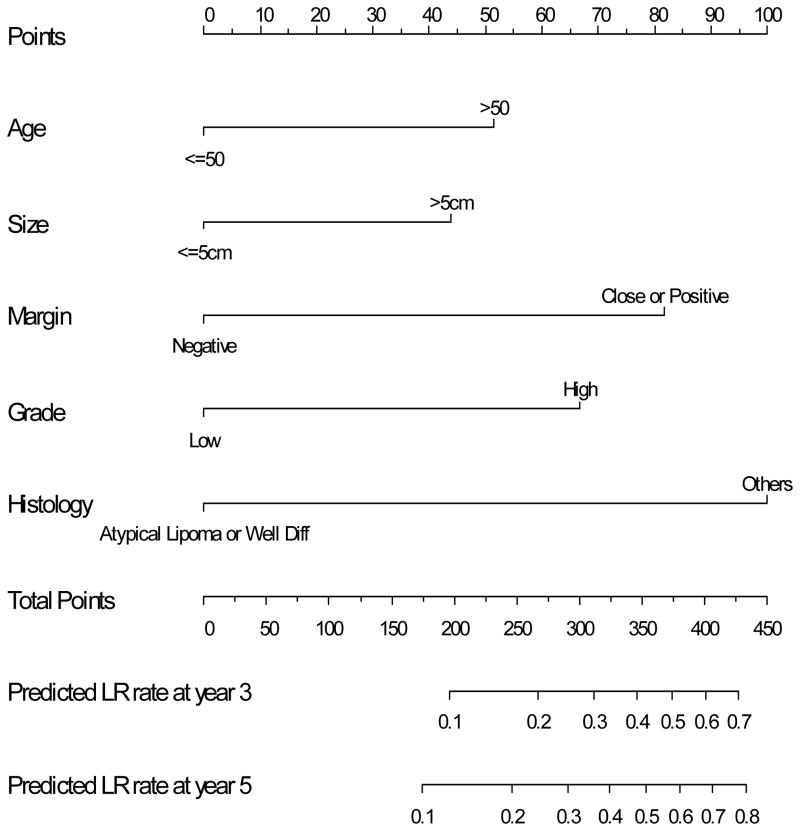
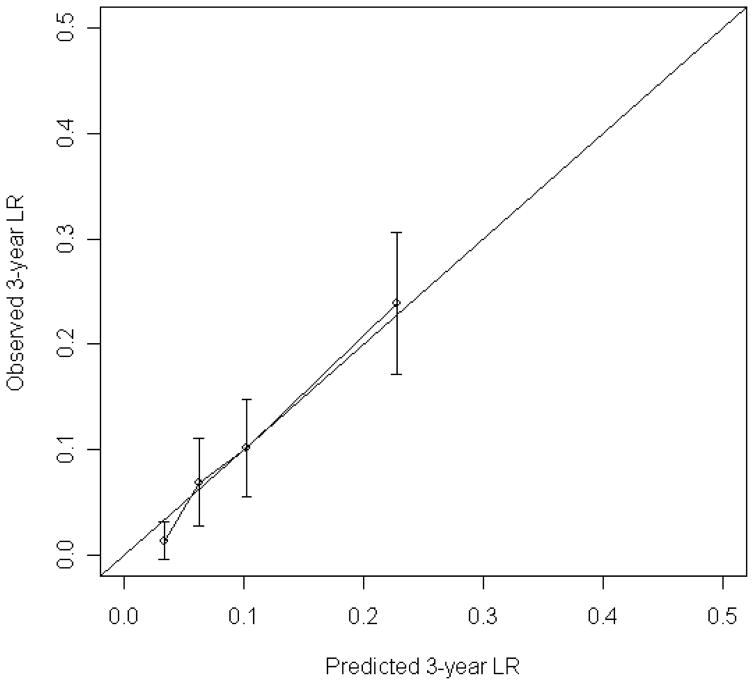
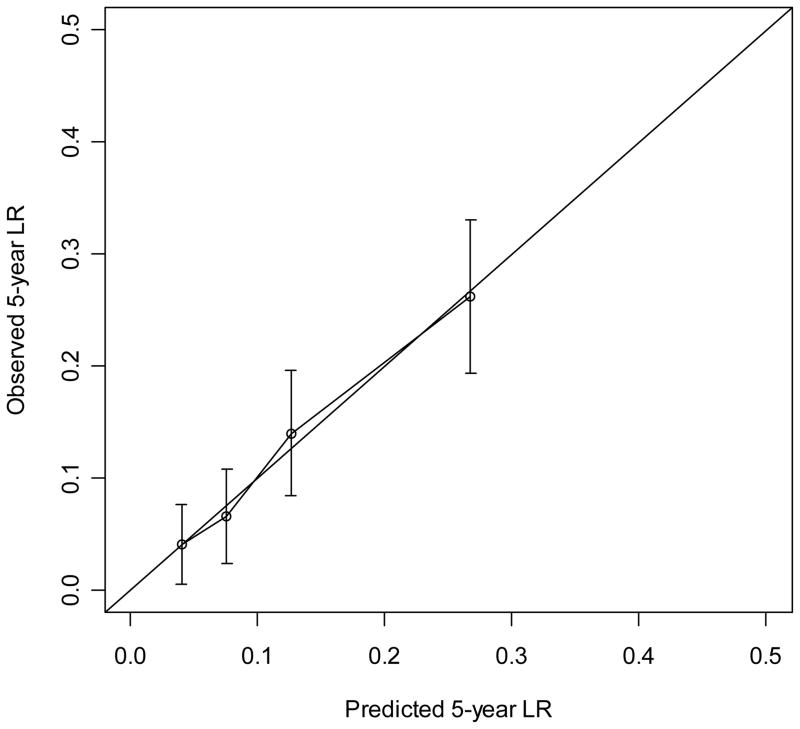
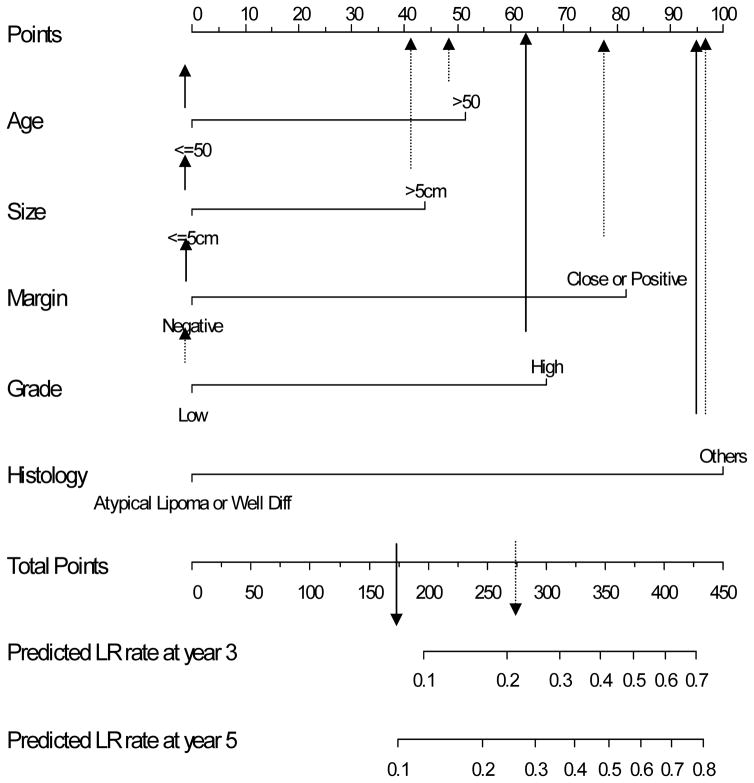
The nomogram to predict the 3- and 5-year risk of LR, incorporating the five independent prognostic factors for LR (age, grade, size, margin status, and histology) is shown in Figure 2. The C-index of the nomogram is 0.74 and 0.73, respectively. When age and size were incorporated as continuous variables using natural splines, the C-index decreased to 0.72, thus the final nomogram contains both as categorical variables. Figure 3 demonstrates the validation of the nomogram, plotting the mean nomogram predictions against the actual outcomes for the patients in this data set. As shown in the figure, the nomogram appears well calibrated and there was good correlation between predicted and observed outcome across the spectrum of predictions. The 5-year rate of LR for AJCC stage 1, 2, and 3 was 8%, 15%, and 21%, respectively (p<0.001). The predictive power of the nomogram, however, is better than the AJCC staging system in determining the rate of LR. The C-index for the nomogram is 0.74 compared to 0.61 for the AJCC stage.
Figure 2.
Nomogram to predict the rate of local recurrence at 3 and 5 years.
Figure 3.
Calibration of the nomogram. Nomogram predicted probabilities for LR within 3 year and 5 years are plotted on the x-axis. Observed probability for LR plotted on the y-axis.
Discussion
A recent report from MDACC demonstrated the importance of using tumor size and margin status in selecting patients for a surgery alone protocol.18 In that paper, Pisters et al reported the impressive outcomes of a prospective, nonrandomized trial from MDACC in which 74 patients with T1 tumors (≤5cm) and negative margins (R0 resections) were managed expectantly after LSS. After a median followup of more than six years, only five patients (7%) developed an isolated LR. It should be noted that prior to enrollment all patients were discussed at a multidisciplinary tumor board and a consensus was required regarding the appropriateness of omitting radiation for each patient. This trial showed that at an extremely experienced multidisciplinary sarcoma team can successfully identify patients who would have a low rate of local recurrence after surgery alone. What remains challenging, however, is how best to devise a tool that can objectively and accurately determine the risk of local recurrence for an individual patient in an everyday practice setting. This will help physicians who do not regularly treat sarcomas, better quantify the risk of local recurrence for an individual patient and make more informed decisions on the value of adjuvant radiation.
Nomograms have been developed for a variety of malignancies over the past decade to provide reliable prognostic information tailored to an individual and to assist in the treatment decision making process.19,20 In 2002, the MSKCC sarcoma nomogram was designed to predict the risk of death from sarcoma at 12 years.21 Since its publication, it has been used to counsel patients regarding prognosis, to design clinical trials and to guide treatment recommendations regarding adjuvant systemic therapy. In the current study a similar tool was devised to predict the risk of LR for a group of patients with primary extremity STS treated with LSS alone, without adjuvant radiation or chemotherapy. The nomogram was constructed to predict the 3- and 5- year risk of LR based on a combination of five independent prognostic factors identified in this study (older age, large size, high grade, histology other than well diff/atypical lipomatous tumor, and close/positive margin status). The concordance index of the nomogram was 0.73. The concordance index was the most robust when margin status was assessed as negative vs. close/positive. Patients who received chemotherapy were excluded because of the impact of chemotherapy on local control2,22,23 and many would argue that patients who need chemotherapy have sufficiently high risk factors to justify radiation as well.
Based on this nomogram, a hypothetical 40 year old patient with a 4 cm, high grade pleomorphic MFH of the extremity excised with negative margins has a total point score of 167 as shown in Figure 4. According to the local recurrence nomogram, this corresponds to a predicted LR rate at 3-years of <10%. Thus this young patient particularly if his/her tumor is in a location that a local recurrence would not preclude limb-sparing salvage therapy has a risk sufficiently low to consider treatment with surgery alone. Conversely, a second hypothetical 60 year old patient with a > 5 cm, low grade myxoid liposarcoma of the lower extremity excised with close margins since it is adjacent to the superficial femoral vessels of the thigh has a total point score of 278. This corresponds to an estimated LR rate at 3-years of 25%. Given that a local recurrence in such a location might preclude subsequent limb salvage, adjuvant radiation is a very reasonable choice for this patient.
Figure 4.
Two hypothetical cases. Case #1 (solid arrows) where total points were 167 based on age < 50, size < 5 cm, negative margin, but high grade histology yielding a predicted LR at 3 years < 10%. Compared to case # 2 (dashed arrows) where total points were 278 yielding a predicted 3 year LR that is 25%.
The five prognostic factors incorporated in the nomogram have all been previously shown to be significant predictors of local recurrence.5,9,12 In previous reports from MSKCC, age >50 was identified as an independent risk factor for local recurrence on multivariate analysis.1,9 Zagars et al from MDACC showed that patients > 64 years old had a 1.8 times greater risk of developing a LR.12 Older age at diagnosis was also identified at Princess Margaret Hospital as independent risk factor for LR.24 It is well documented that the risk of LR is significantly higher for high grade tumors.12,13 The rate of LR for all low grade tumors, however, is not uniformly low. The NCI randomized trial found a significant reduction in LR after external beam radiation for both high grade and low grade tumors. Radiation reduced the risk of local recurrence from approximately 30% to 5% for low grade tumors.15 This indicates that there are some patients with low grade tumors that can be at increased risk for LR based on other adverse factors, highlighting the need to incorporate several prognostic factors, rather than relying on an individual factor. The impact of histology on local recurrence remains controversial. Some studies have shown that certain histopathologic subtypes such as MPNST, fibrosarcoma and epithelioid have higher rates of LR but other studies have not confirmed that histology affects LR.8,9,12 In the current study we did find a significantly lower risk of LR for patients with ALT/WDL subtypes compared to all other histologies. Kooby et al showed that ALT and WDL of the extremity and superficial trunk behave similarly.15 Among 91 patients in that study, there were no local recurrences at 5 years. In the current study, the influence of subtype was assessed only in liposarcoma due to lack of systematic classifications of other histologic subtypes especially in the MFH family.
Since the AJCC staging system for STS incorporates two (grade and size) of the five prognostic factors in the current nomogram model, the power of the AJCC in predicting local recurrence was assessed. Its concordance index was only 0.61 compared to 0.74 for the nomogram at three years. A concordence index of 0.74 may not be as high as that for predicting death from sarcoma. But it’s important to remember that what drives the concordance index is the number of events which will always be smaller for local recurrence than death in STS. Validation of this nomogram with other data sets is important before its widespread adoption, especially since selection bias may have influenced the decision on omitting adjuvant radiation in the current study.
In conclusion, we have constructed a nomogram for extremity STS that incorporates age, size, margin status, histology and grade of tumor to predict the 3- and 5- year risk of local recurrence after LSS in the absence of adjuvant RT. The nomogram should serve as a valuable clinical tool for assessing an individual patient’s risk of local recurrence and guiding adjuvant treatment decisions.
Acknowledgments
Supported by the NIH Soft Tissue Sarcoma Program Project (grant P01CA47179) Nicole Moraco and Alisa Pinkhasik for Data management support.
Footnotes
The authors report no potential conflicts of interest.
No outside funding was used to support this study.
Contributor Information
Oren Cahlon, Department of Radiation Oncology, Memorial Sloan-Kettering Cancer Center, New York, NY
Murray F. Brennan, Department of Surgery, Memorial Sloan-Kettering Cancer Center, New York, NY
Xiaoyu Jia, Department of Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY
Li-Xuan Qin, Department of Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY
Samuel Singer, Department of Surgery, Memorial Sloan-Kettering Cancer Center, New York, NY
Kaled M. Alektiar, Department of Radiation Oncology, Memorial Sloan-Kettering Cancer Center, New York, NY
References
- 1.Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14:859–868. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 2.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 3.Midis GPPR, Chen NP, Feig BW, Murphy A, Pollack A, PIsters PW. Locally recurrent soft tissue sarcoma of the extremity. Surgery. 1998;123:666–71. [PubMed] [Google Scholar]
- 4.Torres MA, Ballo MT, Butler CE, et al. Management of locally recurrent soft-tissue sarcoma after prior surgery and radiation therapy. Int J Radiat Oncol Biol Phys. 2007;67:1124–1129. doi: 10.1016/j.ijrobp.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 5.Alektiar KM, Leung D, Zelefsky MJ, et al. Adjuvant radiation for stage II-B soft tissue sarcoma of the extremity. J Clin Oncol. 2002;20:1643–1650. doi: 10.1200/JCO.2002.20.6.1643. [DOI] [PubMed] [Google Scholar]
- 6.Baldini EH, Goldberg J, Jenner C, et al. Long-term outcomes after function-sparing surgery without radiotherapy for soft tissue sarcoma of the extremities and trunk. J Clin Oncol. 1999;17:3252–3259. doi: 10.1200/JCO.1999.17.10.3252. [DOI] [PubMed] [Google Scholar]
- 7.Fleming JB, Cantor SB, Varma DG, et al. Utility of chest computed tomography for staging in patients with T1 extremity soft tissue sarcomas. Cancer. 2001;92:863–8. doi: 10.1002/1097-0142(20010815)92:4<863::aid-cncr1394>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 8.Gronchi A, Casali PG, Mariani L, et al. Status of surgical margins and prognosis in adult soft tissue sarcomas of the extremities: A series of patients treated at a single institution. J Clin Oncol. 2005;23:96–104. doi: 10.1200/JCO.2005.04.160. [DOI] [PubMed] [Google Scholar]
- 9.Pisters PW, Leung DH, Woodruff J, et al. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–89. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 10.Stojadinovic A, Leung D, Hoos A, et al. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg. 2002;235:424–434. doi: 10.1097/00000658-200203000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trovik CS, Bauer HCF, Alvegard TA, et al. Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically-treated patients from the Scandinavian Sarcoma Group Register. Eur J Cancer. 2000;36:710–716. doi: 10.1016/s0959-8049(99)00287-7. [DOI] [PubMed] [Google Scholar]
- 12.Zagars GK, Ballo MT, Pisters PW, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer. 2003;97:2530–43. doi: 10.1002/cncr.11365. [DOI] [PubMed] [Google Scholar]
- 13.Coindre JM, Terrier P, Bui NB, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol. 1996;14:869–877. doi: 10.1200/JCO.1996.14.3.869. [DOI] [PubMed] [Google Scholar]
- 14.Fabrizio PL, Stafford SL, Pritchard DJ. Extremity soft-tissue sarcomas selectively treated with surgery alone. Int J Radiat Oncol Biol Phys. 2000;48:227–232. doi: 10.1016/s0360-3016(00)00601-5. [DOI] [PubMed] [Google Scholar]
- 15.Kooby DA, Antonescu CR, Brennan MF, et al. Atypical Lipomatous Tumor/Well-Differentiated Liposarcoma of the Extremity and Trunk Wall: Importance of Histological Subtype With Treatment Recommendations. Ann Surg Oncol. 2004;11:78–84. doi: 10.1007/BF02524350. [DOI] [PubMed] [Google Scholar]
- 16.Harrel FE, Jr, Lee KL, Mark DB. Multivariate prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Kattan MW, Heller G, Brennan MF. A competing-risks nomogram for sarcoma-specific death following local recurrence. Stat Med. 2003;22:3515–3525. doi: 10.1002/sim.1574. [DOI] [PubMed] [Google Scholar]
- 18.Pisters PW, Pollock RE, Lewis VO, et al. Long-term results of prospective trial of surgery alone with selective use of radiation for patients with T1 extremity and trunk soft tissue sarcomas. Ann Surg. 2007;246:675–682. doi: 10.1097/SLA.0b013e318155a9ae. [DOI] [PubMed] [Google Scholar]
- 19.Iasonos A, Schrag D, Raj GV, et al. How To Build and Interpret a Nomogram for Cancer Prognosis. J Clin Oncol. 2008;26:1364–1370. doi: 10.1200/JCO.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 20.Van Zee KJ, Manasseh D-ME, Bevilacqua JLB, et al. A Nomogram for Predicting the Likelihood of Additional Nodal Metastases in Breast Cancer Patients With a Positive Sentinel Node Biopsy. Ann Surg Oncol. 2003;10:1140–1151. doi: 10.1245/aso.2003.03.015. [DOI] [PubMed] [Google Scholar]
- 21.Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20:791–6. doi: 10.1200/JCO.2002.20.3.791. [DOI] [PubMed] [Google Scholar]
- 22.Pisters PW, O’Sullivan B, Maki RG. Evidence-based recommendations for local therapy for soft tissue sarcomas. J Clin Oncol. 2007;25:1003–8. doi: 10.1200/JCO.2006.09.8525. [DOI] [PubMed] [Google Scholar]
- 23.Rhomberg W, Hassenstein EOM, Gefeller D. Radiotherapy vs. radiotherapy and razoxane in the treatment of soft tissue sarcomas: Final results of a randomized study. Int J Radiat Oncol Biol Phys. 1996;36:1077–1084. doi: 10.1016/s0360-3016(96)00433-6. [DOI] [PubMed] [Google Scholar]
- 24.LeVay J, O’Sullivan B, Catton C, et al. Outcome and prognostic factors in soft tissue sarcoma in the adult. Int J Radiat Oncol Biol Phys. 1993;27:1091–9. doi: 10.1016/0360-3016(93)90529-5. [DOI] [PubMed] [Google Scholar]