Abstract
Ultrasound guidance for steroid injection in de Quervain disease is useful in identifying the presence of subcompartments and effectively injecting the drug into tendon sheath. We prospectively studied 50 patients with features of de Quervain disease to determine the effectiveness of ultrasound in positioning of needle for steroid injection and effectiveness of single versus multiple injections in the presence of subcompartments. Scalp vein set was inserted into the tendon sheath under ultrasound guidance and sterile conditions. Mixture containing 1 mL of methylprednisolone 40 mg with 1 mL of 2% lignocaine was injected and the patient followed for 6 months. In patients having subcompartments, improvement was better when two separate injections into each subcompartment were given compared with single. Ultrasound guidance is helpful in identifying the existence of subcompartment and injecting the subcompartments separately. Scalp vein set may be very effective in ultrasound-guided injection. This is a level III study.
Keywords: ultrasound guidance, de Quervain disease, steroid injection, scalp vein
Introduction
De Quervain tenosynovitis is a condition involving the first dorsal compartment of wrist, which is a stenosing tenosynovitis of abductor pollicis longus (APL) and extensor pollicis brevis (EPB) tendons.1 2 Clinical features of the condition include pain and swelling over the radial styloid process, painful thumb and wrist movements, and sometimes palpable thickening of the tendon sheath. Nonoperative treatment modalities include nonsteroidal anti-inflammatory drugs, splinting, and local steroid injection. Steroid injection is given into the tendon sheath and it is useful in the early stage of disease (tenosynovitis), which is a safe and cost-effective method. Failure of this method is commonly due to inaccurate technique of injection and anatomical variations in the first dorsal compartment in the form of subcompartments and multiple tendons.3 Response to injection is poor when the injection does not enter the compartment or all subcompartments. Operative treatment in the form of surgical decompression is indicated when conservative treatment fails and in late stage of the disease (tenovaginitis). To know the effectiveness of ultrasound in the management of de Quervain disease, we undertook the study to determine the following: (1) the effectiveness of ultrasound in needle positioning for steroid injection; (2) functional outcome of steroid injection; (3) prevalence of anatomical variation in the form of multiple tendons and subcompartments in the study population; and (4) effectiveness of single versus multiple injections in the presence of subcompartments in symptomatic de Quervain disease.
Materials and Methods
We studied 51 wrists in 50 patients prospectively who presented to our outpatient department with the clinical features of de Quervain disease over a period of 26 months between August 2012 and October 2014.
Inclusion criteria included patients presenting at an early stage with clinical features of de Quervain disease who did not respond favorably with rest, anti-inflammatory drugs, and splintage for a period of 3 weeks.
Exclusion criteria included patients presenting at an advanced stage (tenovaginitis), patients having received prior steroid injection, and patients with recurrence of the disease following any method of treatment.
After obtaining informed consent from the patient, scalp vein set was inserted into the tendon sheath under sterile conditions after painting with 10% povidone iodine and draping with sterile sheet (Fig. 1). Sterile, transparent Tegaderm film was applied to the ultrasound probe to maintain sterility. We used scalp vein set (butterfly needle/winged infusion set), as it was easy to position and helpful in creating working space for ultrasound probe and effective delivery of the drug. The position of the needle was confirmed under ultrasound guidance. In 33 wrists (65%) where there were no subcompartments, a mixture of 1 mL of methylprednisolone 40 mg (Depo-Medrol) and 1 mL of 2% lignocaine was injected into the tendon sheath, and drug filling the space was confirmed by ultrasound (Fig. 2). In the other 18 wrists (35%) in which two subcompartments were encountered, we injected only the most affected subcompartment in the first eight cases (APL/radial of the two subcompartments). However, in the next 10 cases, we split the dose 50/50 and injected into each of two subcompartments. Patients were followed up at regular intervals (2 weeks, 6 weeks, and 6 months) to assess the pain, functional outcome, and improvement. We assessed pain by using numeric pain rating scale (0–10)4 before injection and compared with scores at follow-up. Functional outcome was assessed by direct treatment response based on consensus between physician and participant (0 = no response, 1 = partial response but not satisfactory warranting further treatment, 2 = partial response satisfactory and not warranting further treatment, 3 = complete resolution of symptoms).5 Improvement was assessed by using 5-point ordinal scale as perceived by patient (−2 = much worse, −1 = worse, 0 = not better/not worse, +1 = better, +2 = much better).
Fig.1.
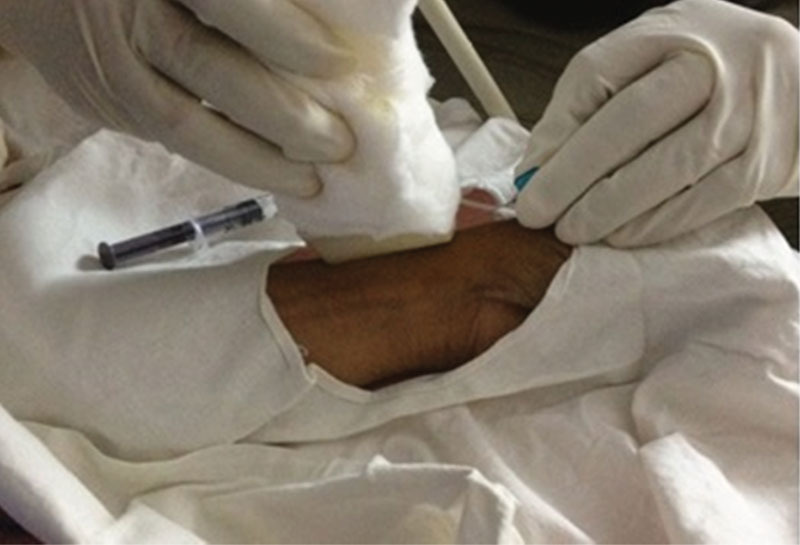
Injection technique with scalp vein set under ultrasound guidance.
Fig. 2.
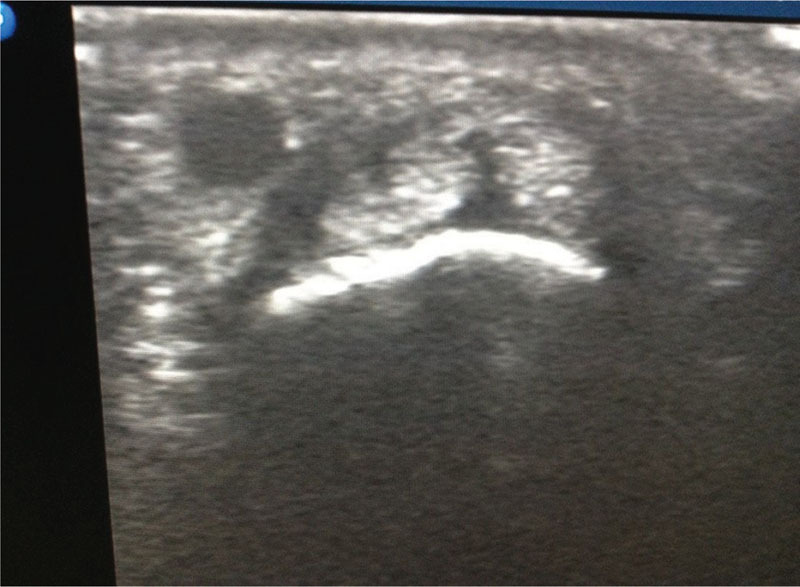
Ultrasound picture showing drug distribution after injection.
Results
In this study, 51 wrists in 50 patients presented with acute symptoms of de Quervain disease were analyzed. The compartment size was found to be increased in all the affected wrists (100% of the cases) as compared with the unaffected wrists. Out of the 51 wrists, 18 (35%) were found to have separate subcompartment for APL and EPB. Among these 18 wrists, we injected into only one subcompartment in the first 8 cases and into both subcompartments separately in rest of 10 cases. Out of eight wrists where single subcompartment was injected, five wrists had complete resolution of symptoms, while the other three cases had incomplete resolution of symptoms warranting further treatment. All the 10 cases in which we injected both subcompartments separately had complete resolution of symptoms without warranting any further treatment. The other 33 wrists (65%) where there was no subcompartment were treated by single injection, and all had complete resolution of symptoms. Pain assessment mean scores were 7.80 before the injection and 1.30, 1.30, and 1.30 at 2 weeks, 4 weeks, and 6 months follow-up, respectively (Table 1). Direct treatment response showed values of 2.42, 2.45, and 2.67 at 2 weeks, 6 weeks, and 6 months follow-up, respectively. Assessment of improvement showed mean scores of 1.78 at 2 weeks, 1.75 at 6 weeks, and 1.80 at 6 months (Table 2).
Table 1. Pain assessment by numeric pain rating scale.
| Time of pain assessmen | N | Mean | Standard deviation | Median | 95% confidence interval for mean | Friedman test value | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Lower bound | Lower bound | |||||||
| Pain before injection | 51 | 7.80 | 1.217 | 8.00 | 7.46 | 8.15 | 0.000 | |
| At 2-wk follow-up | 51 | 1.28 | 1.283 | 1.00 | 0.93 | 1.63 | 94.120 | |
| At 6-wk follow-up | 51 | 1.29 | 1.285 | 1.00 | 0.92 | 1.66 | ||
| At 6-mo follow-up | 51 | 1.31 | 1.197 | 1.00 | 0.98 | 1.64 | ||
Table 2. Improvement using 5-point ordinal scale.
| Improvement | Score | Chi-square test p-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| −2 | −1 | 0 | +1 | +2 | |||||||
| No. of patients | % | No. of patients | % | No. of patients | % | No. of patients | % | No. of patients | % | ||
| At 2 wk | 0 | 0 | 0 | 0 | 2 | 3.9 | 5 | 9.8 | 44 | 86.3 | <0.001 |
| At 6 wk | 0 | 0 | 0 | 0 | 3 | 5.9 | 5 | 9.8 | 43 | 84.3 | |
| At 6 mo | 0 | 0 | 0 | 0 | 3 | 5.9 | 4 | 7.8 | 44 | 86.3 | |
Discussion
Functional outcome following steroid injection mainly depends on the presence of anatomical variations such as subcompartments and multiple tendon slips. Recent studies determined that the role of compartment septation (subcompartment) is more important than multiple tendon slips.6 7 8 Detecting this anatomical variation assists injection accuracy and improves treatment results, since the EPB compartment can be missed because of its separate, small, and deep location.9 10 11 Ultrasonography is a valuable asset in the diagnosis and treatment of de Quervain tenosynovitis. Not only does it show the thickening of the tendons, but it also allows direct visualization of the needle entering the tendon sheath. It may help decrease the incidence of intratendinous injection and some complications of subcutaneous corticosteroid injection such as fat atrophy and skin hypopigmentation. It is especially beneficial in confirming injection into the correct subcompartment when a dividing septum is present in the tendon sheath.12 13
Richie and Briner,14 who reviewed seven current reputable papers and surveyed the treatment outcomes, concluded that the efficacy rate of injecting the steroid was 83%. In our study, 94.1% (48) of the patients had complete resolution of symptoms after ultrasound-guided steroid injection at the end of 6 months. We attribute this high percentage of resolution of symptoms to the following: (1) usage of scalp vein set which gave enough working space for the surgeon and the sonologist; and (2) addressing both the involved subcompartments separately by independent injections. In three patients (5.9%), there was incomplete resolution of symptoms, and all of them had subcompartment and were treated by single injection. Out of the three patients with incomplete resolution of symptoms, one was given second injection and the other two opted for surgical release at a later date. McDermott et al,13 in their study, used hypodermic needle for injection, and if subcompartment is present, both subcompartments were entered by piercing the septum. In our study, the first eight patients having subcompartments were treated with single injection. In subsequent 10 patients with subcompartments, we changed our protocol to inject both subcompartments separately. Zingas et al11 reported that a higher rate of symptom relief was attained in patients with successful steroid injections into the APL and EPB compartments than that with a steroid injection only into the APL or none of the compartments. Exact delivery of steroids into both compartments under the guidance of sonography may lead to improved outcomes. We found that using scalp vein set and injecting both subcompartments separately had better results compared with single injection.
Conclusion
We conclude that ultrasound is very much helpful in identifying the existence of subcompartment and its guidance in injecting the subcompartments separately. Failure to identify the subcompartments and inject separately may lead to incomplete resolution of symptoms. Scalp vein set may be very effective in ultrasound-guided injection.
References
- 1.de Quervain F. On a form of chronic tendovaginitis by Dr. Fritz de Quervain in la Chaux-de-Fonds. 1895. Am J Orthop. 1997;26(9):641–644. [PubMed] [Google Scholar]
- 2.Finkelstein H. Stenosing tendovaginitis at the radial styloid process. J Bone Joint Surg Am. 1930;12(3):509–540. [Google Scholar]
- 3.Mirzanli C, Ozturk K, Esenyel C Z, Ayanoglu S, Imren Y, Aliustaoglu S. Accuracy of intrasheath injection techniques for de Quervain's disease: a cadaveric study. J Hand Surg Eur Vol. 2012;37(2):155–160. doi: 10.1177/1753193411409126. [DOI] [PubMed] [Google Scholar]
- 4.McCaffery M Pasero C 0–10 numeric pain rating scale. Pain: Clinical Manual St. Louis 199916 [Google Scholar]
- 5.Chung K C, Pillsbury M S, Walters M R, Hayward R A. Reliability and validity testing of the Michigan Hand Outcomes Questionnaire. J Hand Surg Am. 1998;23(4):575–587. doi: 10.1016/S0363-5023(98)80042-7. [DOI] [PubMed] [Google Scholar]
- 6.Visuthikosol V, Chanyasawat S. Surgical treatment of de Quervain's diseases: a clinical review of 42 cases. J Med Assoc Thai. 1988;71(11):637–639. [PubMed] [Google Scholar]
- 7.Harvey F J, Harvey P M, Horsley M W. De Quervain's disease: surgical or nonsurgical treatment. J Hand Surg Am. 1990;15(1):83–87. doi: 10.1016/s0363-5023(09)91110-8. [DOI] [PubMed] [Google Scholar]
- 8.Volpe A, Pavoni M, Marchetta A. et al. Ultrasound differentiation of two types of de Quervain's disease: the role of retinaculum. Ann Rheum Dis. 2010;69(5):938–939. doi: 10.1136/ard.2009.123026. [DOI] [PubMed] [Google Scholar]
- 9.Kwon B C, Choi S J, Koh S H, Shin D J, Baek G H. Sonographic identification of the intracompartmental septum in de Quervain's disease. Clin Orthop Relat Res. 2010;468(8):2129–2134. doi: 10.1007/s11999-009-1199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diop A N, Ba-Diop S, Sane J C. et al. Role of US in the management of de Quervain's tenosynovitis: review of 22 cases [in French] J Radiol. 2008;89(9, Pt 1):1081–1084. doi: 10.1016/s0221-0363(08)73912-x. [DOI] [PubMed] [Google Scholar]
- 11.Zingas C, Failla J M, Van Holsbeeck M. Injection accuracy and clinical relief of de Quervain's tendinitis. J Hand Surg Am. 1998;23(1):89–96. doi: 10.1016/S0363-5023(98)80095-6. [DOI] [PubMed] [Google Scholar]
- 12.Jeyapalan K, Choudhary S. Ultrasound-guided injection of triamcinolone and bupivacaine in the management of De Quervain's disease. Skeletal Radiol. 2009;38(11):1099–1103. doi: 10.1007/s00256-009-0721-y. [DOI] [PubMed] [Google Scholar]
- 13.McDermott J D, Ilyas A M, Nazarian L N, Leinberry C F. Ultrasound-guided injections for de Quervain's tenosynovitis. Clin Orthop Relat Res. 2012;470(7):1925–1931. doi: 10.1007/s11999-012-2369-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richie C A III, Briner W W Jr. Corticosteroid injection for treatment of de Quervain's tenosynovitis: a pooled quantitative literature evaluation. J Am Board Fam Pract. 2003;16(2):102–106. doi: 10.3122/jabfm.16.2.102. [DOI] [PubMed] [Google Scholar]


