Abstract
In adult rats, perinatal taurine depletion followed by high sugar intake alters neural and renal control of arterial pressure via the renin-angiotensin system. This study tests the hypothesis that perinatal taurine supplementation predisposes adult female rats to the adverse arterial pressure effect of high sugar intake via the renin-angiotensin system, rather than via estrogen. Female Sprague-Dawley (SD) rats were fed normal rat chow with 3% taurine (TS) or water alone (C) from conception to weaning. Their female offspring were fed normal rat chow with either 5% glucose in tap water (TSG, CG) or tap water alone (TSW, CW). At 7-8 weeks of age, the female offspring's renin-angiotensin system or estrogen receptors were inhibited by captopril or tamoxifen, respectively. Body weight, heart weight, kidney weight, mean arterial pressures (MAP) and heart rates were not significantly different among control groups (without captopril or tamoxifen). Captopril (but not tamoxifen) decreased MAP but not heart rates in all groups. In TSG compared to TSW, CW and CG groups, baroreflex sensitivity of heart rate (BS-HR) and renal nerve activity (BS-RN) were significantly decreased. Neither captopril nor tamoxifen altered BS-HR in TSG, but tamoxifen (but not captopril) restored TSG BS-RN to CW or CG control levels. Perinatal taurine imbalance (either increase or decrease) did not disturb sympathetic and parasympathetic nerve activity in the adult rats on high or basal sugar intake. Compared to its effect in CW and CG groups, tamoxifen increased sympathetic but decreased parasympathetic activity less in TSG and TSW groups. Inhibition of the renin-angiotensin system did not affect autonomic nerve activity in any group. These data suggest that in adult female rats that are perinatally supplemented of taurine, high sugar intake after weaning blunts arterial baroreflex via an estrogen (but not renin-angiotensin) mechanism.
Keywords: arterial pressure, autonomic nervous system, baroreflex, estrogen, high sugar, perinatal taurine, renal sympathetic nerve, renin-angiotensin system
1.1 INTRODUCTION
The perinatal environment influences not only growth and development of the fetus and newborn, but the phenotypic expression of the adult offspring. For examples, inhibition of the renin-angiotensin system from conception throughout life, or during the perinatal period alone, attenuates or prevents hypertension and other related damage in adult spontaneously hypertensive rats (Wyss el al. 1994). However, such inhibition produces salt-sensitive hypertension in adult normotensive rats. Several lines of evidence have reported that perinatal over or under dietary exposure programs adult function and dysfunction (Barnes and Ozanne 2011; Marino el al. 2011; Zhang el al. 2011). Previous experiments indicates that either taurine depletion or supplementation in early life alters renal function in adult male and female rats (Roysommuti el al. 2009a; Roysommuti el al. 2010a; Roysommuti el al. 2010b), while perinatal taurine depletion paired with a high sugar diet after weaning blunts baroreflex function and depresses the autonomic nervous system control of arterial pressure (Roysommuti el al. 2009b; Thaeomor el al. 2010). These abnormalities are improved by acute inhibition of renin-angiotensin system (Thaeomor el al. 2010).
The effect of perinatal taurine exposure on adult function is sex dependent (Roysommuti el al. 2009c), suggesting the possible role of sex hormones particularly estrogen in these effects. Without high sugar intake after weaning, perinatal taurine depletion only slightly but significantly blunts baroreflex sensitivity in male but not female rats. In contrast, a high sugar diet markedly depresses baroreflex function and increases sympathetic nerve activity in both sexes (Roysommuti el al. 2009b; Thaeomor el al. 2010). The significant role of estrogen on arterial pressure control is also supported by studies in spontaneously hypertensive rats (Bonacasa el al. 2008) and salt-induced hypertensive and ovarectomized rats (Fang el al. 2001). The low incidence of hypertension in pre- compared to postmenopausal women also supports a role for estrogen in cardiovascular disorders (Leuzzi and Modena 2011; Yanes and Reckelhoff 2011).
Perinatal taurine supplementation is common in pregnant women and as a dietary supplement in newborns (McPherson and Hardy 2011; Wu 2009; Yamori el al. 2010). Although adverse effects of taurine excess have not been definitively demonstrated in humans, taurine supplementation during late pregnancy in rats stimulates postnatal growth and induces obesity and insulin resistance in adult offspring (Hultman el al. 2007). However, perinatal taurine supplementation's effect and possible mechanisms of action on arterial pressure control has not been established. The present study tests the hypothesis that perinatal taurine supplementation predisposes female adult rats to adverse effects of high sugar intake on arterial pressure control by a renin-angiotensin (rather than estrogen) mechanism.
1.2 METHODS
1.2.1 Experimental protocol
Sprague-Dawley (SD) rats were bred at the animal unit of Faculty of Medicine, Khon Kaen University and maintained at constant humidity (60 ± 5%), temperature (24 ± 1° C), and light cycle (06.00–18.00 h). Female SD rats were fed normal rat chow with 3% beta-alanine (taurine depletion, TD), 3% taurine (taurine supplementation, TS) or water alone (control, C) from conception to weaning. Their female offspring were fed with the normal rat chow with either 5% glucose in tap water (TD with glucose, TDG; TS with glucose, TSG; C with glucose, CG) or tap water alone (TDW, TSW, CW) throughout the experiment. All experimental procedures were pre-approved by the Universities Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health guidelines.
To test the possible role of renin-angiotensin system, another six separated groups had been treated with captopril in drinking water (an angiotensin converting enzyme inhibitor, 400 mg/L) from 7 days before parameter measurements until the end of experiment (CW+Cap, CG+Cap, TDW+Cap, TDG+Cap, TSW+Cap, TSG+Cap). In addition to test the possible role of estrogen, more six separated groups had been treated with an estrogen receptor antagonist (tamoxifen, 10 mg/kg/day, oral) since 7 days before the study (CW+Tam, CG+Tam, TDW+Tam, TDG+Tam, TSW+Tam, TSG+Tam).
At 7-8 weeks of age, under thiopental anesthesia, all female rats were implanted with femoral arterial and venous catheters. Three days later, arterial pressure, heart rate, arterial baroreflex sensitivity control of heart rate (BSHR) were measured in conscious freely moving rats before and during infusion of phenylephrine (BSHR-phenylephrine)) or sodium nitroprusside (BSHR-nitroprusside). After 24 hours resting, rats were anesthetized with thiopental sodium, were tracheostomized, and their arterial pressures were recorded continuously, respectively. Renal sympathetic nerve activity was continuously recorded by using stainless steel electrodes (12 MΩ, 0.01 Taper, A-M System, Sequim, Washington, USA) connected to DAM-80 amplifier (World Precision Instruments, Sarasota, Florida, USA) and BioPac Systems (Goleta, California, USA), respectively. Multiunit recording of renal nerve activity was conducted only on nerve units that responded to changes in arterial pressure following nitroprusside or phenylephrine infusion. Body temperature was servo-control at 37±0.5°C by a rectal probe connected to a temperature regulator controlling an overhead heating lamp. At the end of experiment, all animals were terminated by a high dose of thiopental anesthesia and kidney and heart weights were then collected.
1.2.2 Data analyses
All data were analyzed by Acknowledge software version 3.9.1 (BioPac Systems, Goleta, California, USA). Frequency domains of arterial pressure pulse spectrum were analyzed using the Acknowledge software to indirectly estimate the sympathetic (low frequency 0.3–0.5 Hz, LF) and the parasympathetic nerve activities (high frequency 0.5–4.0 Hz, HF). The percent power spectral densities of LF or HF to the total power of LF plus HF indicate sympathetic and parasympathetic nerve activity, respectively (Roysommuti S 2009; Thaeomor A 2010). Baroreflex sensitivity values were calculated from changes in heart rates (BSHR) or renal nerve activity (BSRA) per changes in mean arterial pressures.
1.2.3 Statistical analyses
All data are expressed as mean ± SEM and were statistically analyzed using one-way ANOVA and a post hoc Duncan's Multi-Range test. Statistically significant difference is p-values < 0.05.
1. 3 RESULTS
At 7-8 weeks of age, all groups displayed similar body, heart and kidney weights as previously reported (Roysommuti S 2009). Mean arterial pressures were not significantly different among untreated control groups, and acute inhibition of renin-angiotensin system by captopril significantly decreased mean arterial pressures to a similar extent (about 15 mm Hg decreases). Compared to control treatment, inhibition of the estrogen receptors by tamoxifen did not significantly alter mean arterial pressures in any group, but the values of CG and TSW were slightly and significantly higher than those of CW and TSG (Fig. 1).
Fig. 1.
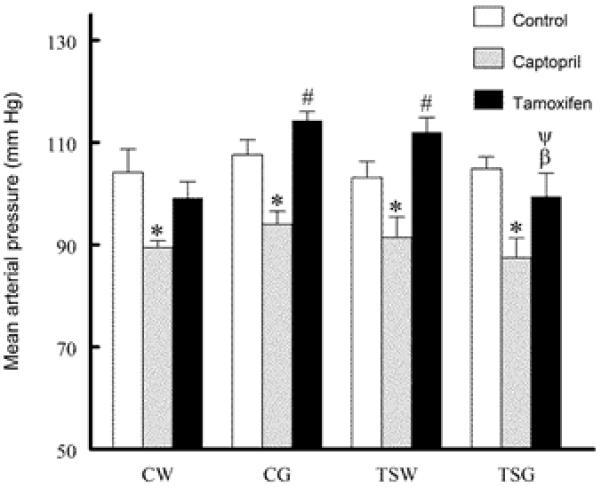
Comparison of mean arterial pressure among groups (CW, control with water intake alone; CW+Cap, CW plus captopril treatment; CW+Tam, CW plus tamoxifen treatment; CG, control with high sugar intake; CG+Cap, CG plus captopril treatment; CG+Tam, CG plus tamoxifen treatment; TSW, perinatal taurine supplementation with water intake alone; TSW+Cap, TSW plus captopril treatment; TSW+Tam, TSW plus tamoxifen treatment; TSG, perinatal taurine supplementation with high sugar intake; TSG+Cap, TSG plus captopril treatment; TSG+Tam, TSG plus tamoxifen treatment; * p < 0.05 vs. control; # p < 0.05 vs. CW of same treatment; β p < 0.05 vs. CG of same treatment, ψ p< 0.05 vs. TSW of same treatment).
Heart rates were not significantly different among groups (Fig. 2). Baroreflex sensitivity control of heart rate was not significantly different among control CW, CG and TSW, but it was impaired significantly in control TSG (Fig. 3). While tamoxifen treatment significantly decreased baroreflex sensitivity in CW, CG and TSW, captopril treatment significantly decreased only in TSW. Neither captopril nor tamoxifen altered the baroreflex sensitivity controls of heart rate in TSG. Compared to CW and TSW, baroreflex sensitivity of renal nerve activity was impaired significantly in CG and TSG rats (Fig. 4). While tamoxifen treatment (compared to control) significantly decreased baroreflex sensitivity in CW, CG and TSW, captopril treatment significantly decreased in CW and CG (only BSRA-nitroprusside). Tamoxifen but not captopril treatment restored baroreflex sensitivity of TSG compared to TSW and CG.
Fig. 2.
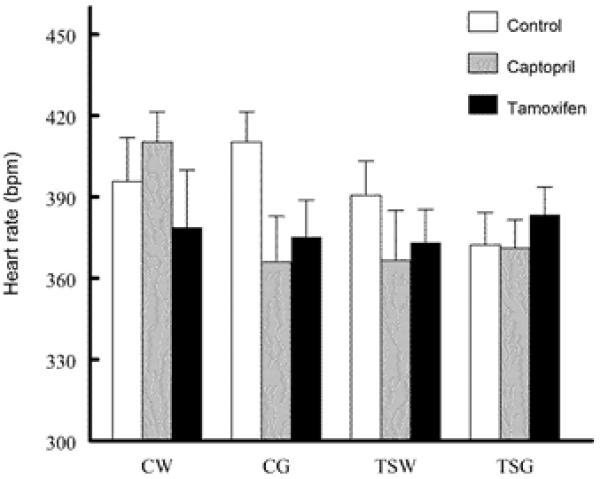
Comparison of heart rate among groups (CW, control with water intake alone; CW+Cap, CW plus captopril treatment; CW+Tam, CW plus tamoxifen treatment; CG, control with high sugar intake; CG+Cap, CG plus captopril treatment; CG+Tam, CG plus tamoxifen treatment; TSW, perinatal taurine supplementation with water intake alone; TSW+Cap, TSW plus captopril treatment; TSW+Tam, TSW plus tamoxifen treatment; TSG, perinatal taurine supplementation with high sugar intake; TSG+Cap, TSG plus captopril treatment; TSG+Tam, TSG plus tamoxifen treatment; no significant difference was observed among groups).
Fig. 3.
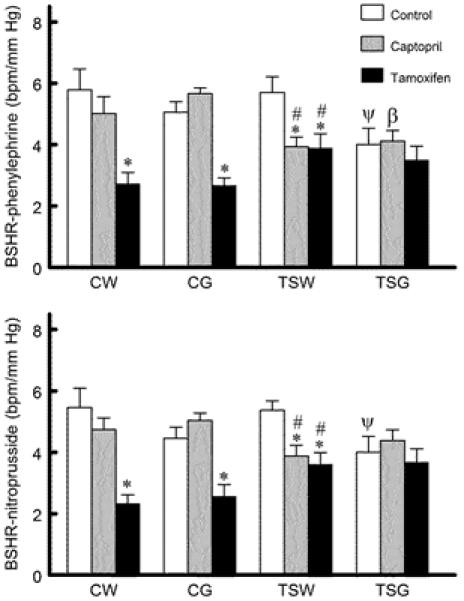
Comparison of baroreflex sensitivity control of heart rate (BSHR) among groups (BSHR-phenylephrine, BSHR tested by phenylephrine infusion; BSHR-nitroprusside, BSHR tested by sodium nitroprusside infusion; CW, control with water intake alone; CW+Cap, CW plus captopril treatment; CW+Tam, CW plus tamoxifen treatment; CG, control with high sugar intake; CG+Cap, CG plus captopril treatment; CG+Tam, CG plus tamoxifen treatment; TSW, perinatal taurine supplementation with water intake alone; TSW+Cap, TSW plus captopril treatment; TSW+Tam, TSW plus tamoxifen treatment; TSG, perinatal taurine supplementation with high sugar intake; TSG+Cap, TSG plus captopril treatment; TSG+Tam, TSG plus tamoxifen treatment; * p < 0.05 vs. control; # p < 0.05 vs. CW of same treatment; β p < 0.05 vs. CG of same treatment, ψ p< 0.05 vs. TSW of same treatment).
Fig. 4.
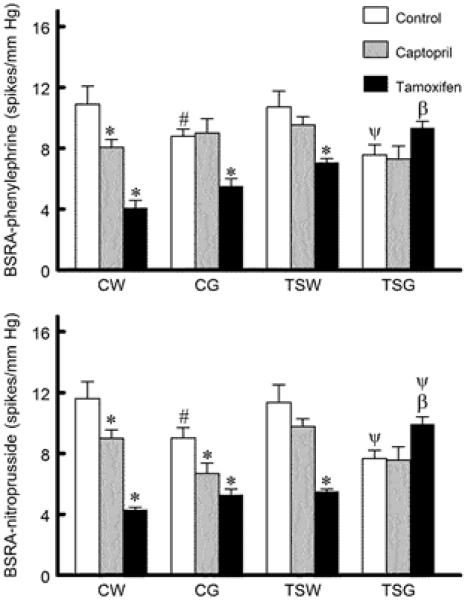
Comparison of baroreflex sensitivity control of renal nerve activity (BSRA) among groups (BSRA-phenylephrine, BSRA tested by phenylephrine infusion; BSRA-nitroprusside, BSRA tested by sodium nitroprusside infusion; CW, control with water intake alone; CW+Cap, CW plus captopril treatment; CW+Tam, CW plus tamoxifen treatment; CG, control with high sugar intake; CG+Cap, CG plus captopril treatment; CG+Tam, CG plus tamoxifen treatment; TSW, perinatal taurine supplementation with water intake alone; TSW+Cap, TSW plus captopril treatment; TSW+Tam, TSW plus tamoxifen treatment; TSG, perinatal taurine supplementation with high sugar intake; TSG+Cap, TSG plus captopril treatment; TSG+Tam, TSG plus tamoxifen treatment; * p < 0.05 vs. control; # p < 0.05 vs. CW of same treatment; β p < 0.05 vs. CG of same treatment, ψ p< 0.05 vs. TSW of same treatment).
All groups with or without captopril treatment displayed similar sympathetic and parasympathetic nerve activity while those with tamoxofen treatment significantly increased sympathetic and decreased parasympathetic nerve activity (Fig. 5). However, the autonomic nerve activity was less in TSW+Tam and TSG+Tam, compared to CW+Tam and CG+Tam, respectively.
Fig. 5.
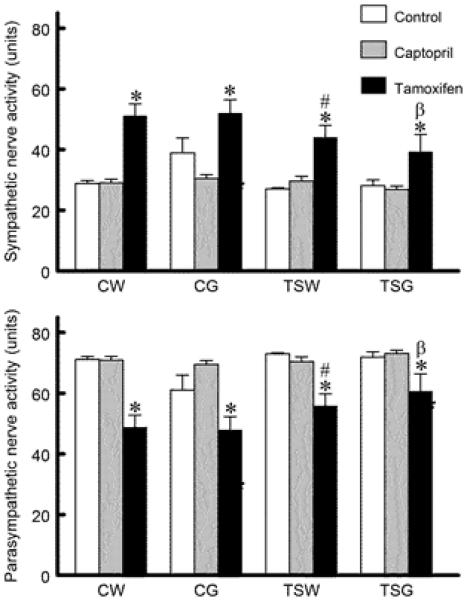
Comparison of sympathetic and parasympathetic nerve activity among groups (CW, control with water intake alone; CW+Cap, CW plus captopril treatment; CW+Tam, CW plus tamoxifen treatment; CG, control with high sugar intake; CG+Cap, CG plus captopril treatment; CG+Tam, CG plus tamoxifen treatment; TSW, perinatal taurine supplementation with water intake alone; TSW+Cap, TSW plus captopril treatment; TSW+Tam, TSW plus tamoxifen treatment; TSG, perinatal taurine supplementation with high sugar intake; TSG+Cap, TSG plus captopril treatment; TSG+Tam, TSG plus tamoxifen treatment; * p < 0.05 vs. control; # p < 0.05 vs. CW of same treatment; β p < 0.05 vs. CG of same treatment).
1.4 Discussion
Perinatal taurine depletion followed by high sugar intake after weaning blunts baroreceptor reflex function and increases sympathetic nerve activity in adult female rats, and this effect is abolished by short-term inhibition of the renin-angiotensin system (Thaeomor el al. 2010), but not by inhibition of estrogen receptors (Roysommuti el al. 2011). The present data indicate that in adult female rats that were prenatally taurine supplemented, estrogen (but not the renin-angiotensin system) contributes to the ability of high sugar intake to depress baroreflex renal nerve control but not baroreflex control of heart rate. In contrast, the renin-angiotensin system (but not estrogen) plays a more important on baroreflex impairments in heart rate control in TSW when compared to control rats. This phenomenon disappears after a high sugar diet. In addition, these changes may not relate to autonomic nervous system dysregulation.
The effect of estrogen on baroreceptor function is complex. Direct infusion of phytoestrogen into isolated carotid sinus depresses the sinus nerve activity via inhibition of protein tyrosine kinase and decreased Ca2+ influx to stretch activated channels (Ma el al. 2005). In contrast, in rats brain estrogen enhances baroreflex sensitivity induced by phenylephrine via aortic nerve, and this baroreflex change can occur without any effect on arterial pressure (Mohamed el al. 1999). In addition, central estrogen enhances baroreceptor mediated increases in adrenal hormone secretion induced by hypotension in sheep (Purinton and Wood 2002). However, many brain areas respond differently to estrogen injection. Estrogen injection into the insular cortex increases renal sympathetic (but not vagal) nerve activity, while injection into the central nucleus of amygdala decreases sympathetic nerve activity (Saleh TM 2003). Although estrogen can modify baroreflex sensitivity and autonomic tone, its effect on arterial pressure is minimal (Saleh el al. 2005; Saleh and Connell 2003). This may explain why in the present study inhibition of estrogen receptors alters autonomic nerve activity and baroreflex sensitivity without any effect on arterial pressure and heart rate.
Perinatal taurine exposure influences brain growth and development and programs adult function and abnormalities (Aerts and Van Assche 2002; Huxtable 1992; Sturman 1993). It is likely that some brain areas in perinatal taurine supplemented rats respond to estrogen differently from control taurine treated animals, in such a manner that their estrogen-dependent baroreflex functions are depressed. This notation is confirmed by baroreflex sensitivity data in TSW+Tam (about 50% increases) compared to CW+Tam (about 30% increases) rats. This effect disappears after high sugar intake after weaning in TSG but not CG groups, suggesting that perinatal taurine supplementation changes the estrogen-mediated baroreflex interaction, making it more sensitive to some mechanisms related to glucose-insulin regulation.
The renin-angiotensin system underlies sugar-induced hypertension in many animal models (Erlich and Rosenthal 1995; Freitas el al. 2007; Rosenthal el al. 1995). High sugar intake (similar to that in the present study) induces renal dysregulation prior to its effects on hypertension and diabetes mellitus, and these changes are related to renin-angiotensin system overactivity (Roysommuti el al. 2002). The role of the renin-angiotensin system on autonomic nerve function, arterial pressure and heart rate is completely preserved in perinatal taurine supplemented rats, even with high sugar intake after weaning. Its inhibition decreased baroreflex control of heart rate in TSW but not in CW and CG, but it decreased baroreflex control of renal nerve in CW and CG but not in TSW. In addition, this effect disappears when these animals are treated with a high sugar diet after weaning. The present data suggest that perinatal taurine supplementation alters the interplay of renin-angiotensin system on baroreflex function but not autonomic tone. Angiotensin II generally affects baroreflex sensitivity by acting via the brain, especially the medulla. This study thus confirms that perinatal taurine supplementation programs baroreflex function by altering central nervous system.
1.5 CONCLUSION
In summary, perinatal taurine exposure programs adult health and disease. Many lines of evidence report that perinatal taurine depletion induces disorders in adult offspring, especially hypertension, renal dysfunction and metabolic disorders. In contrast, taurine supplementation, either during perinatal life or in the elderly has been accepted as beneficial for health. The present study suggests that although perinatal taurine excess alone may have minimal effect on cardiovascular control, high sugar intake after weaning can modify the baroreflex function mediated by estrogen and renin-angiotensin system. In the female, the estrogen rather than the renin-angiotensin system plays a protective role on the adverse effects of a high sugar diet.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand and by the USA National Institutes of Health (NIH) grants AT 00477 and NS057098 (JMW).
Abbreviations
- CW
control with water intake alone
- CW+Cap
CW plus captopril treatment
- CW+Tam
CW plus tamoxifen treatment
- CG
control with high sugar intake
- CG+Cap
CG plus captopril treatment
- CG+Tam
CG plus tamoxifen treatment
- TSW
perinatal taurine supplementation with water intake alone
- TSW+Cap
TSW plus captopril treatment
- TSW+Tam
TSW plus tamoxifen treatment
- TSG
perinatal taurine supplementation with high sugar intake
- TSG+Cap
TSG plus captopril treatment
- TSG+Tam
TSG plus tamoxifen treatment
- SD
Sprague-Dawley
- HF
high frequency
- LF
low frequency
1.7 REFERENCES
- Aerts L, Van Assche FA. Taurine and taurine-deficiency in the perinatal period. J Perinat Med. 2002;30:281–286. doi: 10.1515/JPM.2002.040. [DOI] [PubMed] [Google Scholar]
- Barnes SK, Ozanne SE. Pathways linking the early environment to long-term health and lifespan. Prog Biophys Mol Biol. 2011;106:323–336. doi: 10.1016/j.pbiomolbio.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Bonacasa B, Sanchez ML, Rodriguez F, Lopez B, Quesada T, Fenoy FJ, Hernandez I. 2-Methoxyestradiol attenuates hypertension and coronary vascular remodeling in spontaneously hypertensive rats. Maturitas. 2008;61:310–316. doi: 10.1016/j.maturitas.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Erlich Y, Rosenthal T. Effect of angiotensin-converting enzyme inhibitors on fructose induced hypertension and hyperinsulinaemia in rats. Clin Exp Pharmacol Physiol. 1995;(Suppl 22):S347–S349. doi: 10.1111/j.1440-1681.1995.tb02949.x. [DOI] [PubMed] [Google Scholar]
- Fang Z, Carlson SH, Chen YF, Oparil S, Wyss JM. Estrogen depletion induces NaCl-sensitive hypertension in female spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1934–R1939. doi: 10.1152/ajpregu.2001.281.6.R1934. [DOI] [PubMed] [Google Scholar]
- Freitas RR, et al. Sympathetic and renin-angiotensin systems contribute to increased blood pressure in sucrose-fed rats. Am J Hypertens. 2007;20:692–698. doi: 10.1016/j.amjhyper.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Hultman K, Alexanderson C, Manneras L, Sandberg M, Holmang A, Jansson T. Maternal taurine supplementation in the late pregnant rat stimulates postnatal growth and induces obesity and insulin resistance in adult offspring. J Physiol. 2007;579:823–833. doi: 10.1113/jphysiol.2006.124610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- Leuzzi C, Modena MG. Hypertension in postmenopausal women: pathophysiology and treatment. High Blood Press Cardiovasc Prev. 2011;18:13–18. doi: 10.2165/11588030-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Ma HJ, Liu YX, Wang FW, Wang LX, He RR, Wu YM. Genistein inhibits carotid sinus baroreceptor activity in anesthetized male rats. Acta Pharmacol Sin. 2005;26:840–844. doi: 10.1111/j.1745-7254.2005.00127.x. [DOI] [PubMed] [Google Scholar]
- Marino M, Masella R, Bulzomi P, Campesi I, Malorni W, Franconi F. Nutrition and human health from a sex-gender perspective. Mol Aspects Med. 2011;32:1–70. doi: 10.1016/j.mam.2011.02.001. [DOI] [PubMed] [Google Scholar]
- McPherson RA, Hardy G. Clinical and nutritional benefits of cysteine-enriched protein supplements. Curr Opin Clin Nutr Metab Care. 2011;14:562–568. doi: 10.1097/MCO.0b013e32834c1780. [DOI] [PubMed] [Google Scholar]
- Mohamed MK, El-Mas MM, Abdel-Rahman AA. Estrogen enhancement of baroreflex sensitivity is centrally mediated. Am J Physiol. 1999;276:R1030–R1037. doi: 10.1152/ajpregu.1999.276.4.R1030. [DOI] [PubMed] [Google Scholar]
- Purinton SC, Wood CE. Oestrogen augments the fetal ovine hypothalamus- pituitary-adrenal axis in response to hypotension. J Physiol. 2002;544:919–929. doi: 10.1113/jphysiol.2002.025635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal T, Erlich Y, Rosenmann E, Grossman E, Cohen A. Enalapril improves glucose tolerance in two rat models: a new hypertensive diabetic strain and a fructose-induced hyperinsulinaemic rat. Clin Exp Pharmacol Physiol. 1995;(Suppl 22):S353–S354. doi: 10.1111/j.1440-1681.1995.tb02951.x. [DOI] [PubMed] [Google Scholar]
- Roysommuti S, Khongnakha T, Jirakulsomchok D, Wyss JM. Excess dietary glucose alters renal function before increasing arterial pressure and inducing insulin resistance. Am J Hypertens. 2002;15:773–779. doi: 10.1016/s0895-7061(02)02974-6. [DOI] [PubMed] [Google Scholar]
- Roysommuti S, Lerdweeraphon W, Malila P, Jirakulsomchok D, Wyss JM. Perinatal taurine alters arterial pressure control and renal function in adult offspring. Adv Exp Med Biol. 2009a;643:145–156. doi: 10.1007/978-0-387-75681-3_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roysommuti S, Malila P, Jirakulsomchok D, Wyss JM. Adult renal function is modified by perinatal taurine status in conscious male rats. J Biomed Sci. 2010a;17(Suppl 1):S31. doi: 10.1186/1423-0127-17-S1-S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roysommuti S, Malila P, Lerdweeraphon W, Jirakulsomchok D, Wyss JM. Perinatal taurine exposure alters renal potassium excretion mechanisms in adult conscious rats. J Biomed Sci. 2010b;17(Suppl 1):S29. doi: 10.1186/1423-0127-17-S1-S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roysommuti S, Suwanich A, Jirakulsomchok D, Wyss JM. Perinatal taurine depletion increases susceptibility to adult sugar-induced hypertension in rats. Adv Exp Med Biol. 2009b;643:123–133. doi: 10.1007/978-0-387-75681-3_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roysommuti S, Suwanich A, Lerdweeraphon W, Thaeomor A, Jirakulsomchok D, Wyss JM. Sex dependent effects of perinatal taurine exposure on the arterial pressure control in adult offspring. Adv Exp Med Biol. 2009c;643:135–144. doi: 10.1007/978-0-387-75681-3_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roysommuti S, Thaewmor A, Lerdweeraphon W, Khimsuksri S, Jirakulsomchok D, Schaffer SW. Perinatal taurine exposure alters neural control of arterial pressure via the renin-angiotensin system but not estrogen in rats. Amino Acids. 2011;41:S84. [Google Scholar]
- Saleh TM, Connell BJ. Central nuclei mediating estrogen-induced changes in autonomic tone and baroreceptor reflex in male rats. Brain Res. 2003;961:190–200. doi: 10.1016/s0006-8993(02)03928-8. [DOI] [PubMed] [Google Scholar]
- Saleh TM, Connell BJ, Cribb AE. Sympathoexcitatory effects of estrogen in the insular cortex are mediated by GABA. Brain Res. 2005;1037:114–122. doi: 10.1016/j.brainres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Sturman JA. Taurine in development. Physiol Rev. 1993;73:119–147. doi: 10.1152/physrev.1993.73.1.119. [DOI] [PubMed] [Google Scholar]
- Thaeomor A, Wyss JM, Jirakulsomchok D, Roysommuti S. High sugar intake via the renin-angiotensin system blunts the baroreceptor reflex in adult rats that were perinatally depleted of taurine. J Biomed Sci. 2010;17(Suppl 1):S30. doi: 10.1186/1423-0127-17-S1-S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Roysommuti S, King K, Kadisha I, Regan CP, Berecek KH. Salt-induced hypertension in normotensive spontaneously hypertensive rats. Hypertension. 1994;23:791–796. doi: 10.1161/01.hyp.23.6.791. [DOI] [PubMed] [Google Scholar]
- Yamori Y, Taguchi T, Hamada A, Kunimasa K, Mori H, Mori M. Taurine in health and diseases: consistent evidence from experimental and epidemiological studies. J Biomed Sci. 2010;17(Suppl 1):S6. doi: 10.1186/1423-0127-17-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanes LL, Reckelhoff JF. Postmenopausal hypertension. Am J Hypertens. 2011;24:740–749. doi: 10.1038/ajh.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Rattanatray L, McMillen IC, Suter CM, Morrison JL. Periconceptional nutrition and the early programming of a life of obesity or adversity. Prog Biophys Mol Biol. 2011;106:307–314. doi: 10.1016/j.pbiomolbio.2010.12.004. [DOI] [PubMed] [Google Scholar]


