Abstract
Myelin is a multilayer wrapping of insulation formed by glial cells around axons that is essential for rapid impulse transmission, but how glial cells accomplish this cellular choreography has long intrigued researchers. In this issue of Cell, Snaidero et al., provide new insights into how myelin forms and is remodeled.
Five hundred million years ago an extraordinary development in cellular evolution occurred: the formation of an insulating sheath (myelin) on nerve fibers (axons) in vertebrates. The myelin sheath transformed the way neural impulses are transmitted, by forcing action potentials to “jump” rapidly between periodic breaks in myelin (nodes of Ranvier), thus dramatically increasing transmission speed and elevating nervous function well beyond that of invertebrates. Not until the development of electron microscopy was the surprising submicroscopic structure of myelin revealed. Rather than being a secretion of the axon, myelin was found to be a thick wrapping of highly compacted layers of cell membrane spun around the axon by nonneuronal cells (glia). Myelin and the nodes of Ranvier are the most complex cell-cell junctions known, requiring precise cell-cell recognition, synthesis of vast quantities of specialized cell membrane, and intricate cell motility to wrap up to 100 layers of membrane around axons. Damage to myelin is the source of much disease and disability, and recently, myelin has attracted attention as a possible new cellular mechanism participating in learning (Fields, 2010). The studies by Snaidero et al. (2014), provide new information on the cellular dynamics and molecular signaling controlling myelin formation and remodeling. The work advances understanding of how myelin membrane is added to the existing sheath, which has significance for nervous system development, disease, and understanding of how myelin may be remodeled to optimize function.
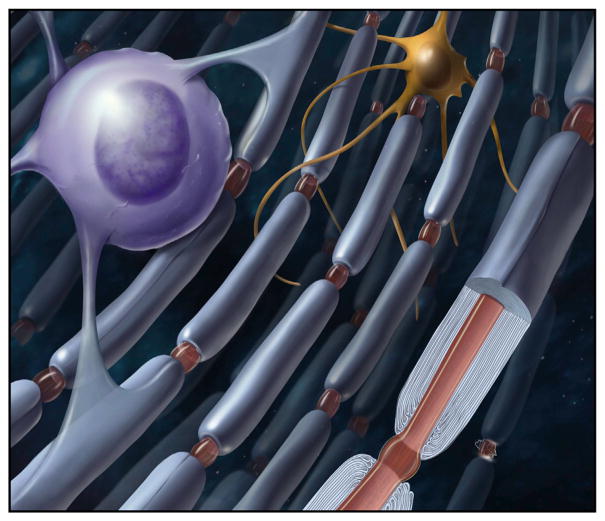
In the central nervous system, myelin is formed by multipolar glia, oligodendrocytes, that can extend dozens of slender cell processes to ensheath multiple axons simultaneously. Wrapping multiple layers of membrane around an axon as one would wind electrical tape on a wire is a topological impossibility for a multipolar cell. Myelin is formed in the PNS (peripheral nervous system) and CNS by the innermost sheet-like glial process in contact with the axon spiraling around it and spinning out multiple layers of overlapping membrane. Cytoplasm becomes expelled from all but the innermost and outermost layers of the myelin sheath. In the intervening layers, the cell membranes come together to form compact myelin by the action of myelin basic protein (MBP), found preferentially in the compacted layers of myelin. The process of myelination begins when an oligodendrocyte cell process contacts an axon and forms a specialized membrane junction “spot weld,” as described by Luse in 1959. This junction is now understood to be a specialized membrane domain for intercellular communication between the glial cell process and axon (Wake et al., 2011). The glial process then expands laterally along the axon and begins to encircle it in a nonuniform manner (Luse, 1959). Because the segment of myelin between each node of Ranvier is several times larger than an oligodendrocyte, as it wraps, the glial cell process expands laterally into a ribbon that broadens in width to wrap the entire internodal length. This can be seen in live imaging studies, where the process has been likened to making a croissant from a triangular piece of dough (Sobottka et al., 2011). Using similar methods and serial block face imaging of myelination in zebrafish, Snaidero et al., provide data consistent with this mechanism of myelin formation (Figure 1).
Figure 1. An Oligodendrocyte Extends Processes that Wrap around the Nerve Fiber in a Croissant-like Layer of Membranes.
Image credit: Alan Hoofring, NIH.
Snaidero and colleagues address the question of how membrane and proteins are delivered to the advancing inner tongue of myelin not only during development but throughout life because the length of the myelin sheath must expand and additional layers of myelin are added as axons grow in caliber and length with body growth.
Oligodendrocytes are highly polarized cells that synthesize vast quantities of specialized membrane to ensheath axons. Consequently, trafficking of vesicles, specific mRNAs, and proteins is highly polarized and precisely sorted in oligodendrocytes to generate and maintain the unique composition of the myelin sheath and cell body membrane domains. Vesicular stomatitis virus glycoprotein (VSC-G), a marker of trafficking to the basolateral region of cells, is trafficked away from the cell body and accumulates selectively in the myelin sheath sub-cellular domain of oligodendrocytes in cell culture (Baron et al., 1999). Delivery of VSC to the membrane depends on submembrane F-actin at the leading edge, as shown by disrupting the cytoskeleton or altering actin polymerization with protein kinases. Snaidero et al., replicate these cell culture results and show that this also occurs in vivo by injecting the virus into the brain during myelination of the corpus callosum and observing VSC accumulating at the inner tongue of myelin adjacent to the axon membrane.
The formation of dense layers of highly compacted cell membrane creates an impediment in delivering proteins and lipids to replace those lost from the compacted myelin sheath and to supply the inner tongue of uncompacted membrane where new layers of myelin are formed. The lateral cytoplasmic domains at the edge of each myelin layer remain uncompacted and in contact with the axonal membrane. These tubes of cytoplasm at the edge of each sheet move in a continuous helix around the axon toward the future node of Ranvier, where they stack up and form the paranodal loops as seen in cross section flanking the node. This long spiraling cytoplasmic channel provides a long distance pathway for transporting material from the cell body. Transport is also facilitated by fenestrated pockets of cytoplasm intruding between the layers of otherwise compacted myelin.
In addition to providing a conduit for transmitting cellular constituents across the compacted myelin, these cytoplasmic channels are thought to allow dynamic regulation of the myelin sheath to participate “in a dynamic process whereby the myelin lamellae are continually parting and coming together during life in response to physiological stresses and strains” (Robertson, 1958, as quoted in Velumian et al., 2011). Filling the cytoplasmic channels with the fluorescent dye Lucifer yellow shows that they can be in open or closed states, presumably associated with myelin stability and dynamics (Velumian et al., 2011). Snaidero et al., provide an important advance by showing that these channels can be regulated by stimulating myelin synthesis.
Inhibiting PI3K signaling is known to stimulate the formation of new layers of myelin by acting on AKT, mammalian target of rapamycin (mTOR), and other substrates to promote cell polarization, glial process outgrowth, and myelination. PIP3 is antagonized by the phosphatase and tenesin homolog (PTEN), which dephosphorylates PIP3 to PIP2. Previously members of this research team found that myelinating cells lacking PTEN have elevated PIP3 levels and hypermyelination, even when induced in mature oligodendrocytes (Goebbels et al., 2010).
Here Snaidero and colleagues report that when myelin synthesis is stimulated in this way (by conditional inactivation of Pten, which elevates PI(3,4,5)P3 levels) the number of cytoplasmic channels increased with the increase in myelination. Moreover, a large number of cytoplasmic rich inclusions were seen advancing along the length of the myelin sheath when viewed in long-section, explaining how new layers of myelin can be laid down underneath the existing layers of compact myelin.
There is current interest in the possibility that myelin remodeling could participate in learning, cognitive function, and psychiatric illness by adjusting conduction velocity for optimal function in an activity-dependent manner (Fields, 2010). Changes in anisotropy of water diffusion seen by diffusion tensor imaging in white matter regions of individuals after learning (Zatorre et al., 2012) could reflect changes in myelination or occur more rapidly from altered water diffusion through these cytoplasmic channels opened after learning.
Based on orientation of oligodendrocytes toward the cathode in cell cultures with an extracellular electrical field imposed (1V/cm), the authors speculate that elevated extracellular K+ concentration in the node of Ranvier produced by repetitive action potential firing could promote trafficking of membrane components and stimulate wrapping myelin at the node. Future research will be needed to determine if an electrical field of the proper polarity and intensity is generated at the developing node, but this mechanism may be more relevant to pathological effects on myelin during hyperexcitation than to normal development of the node.
The authors interpret the result as a direct action of PI(3,4,5)P3-dependent signaling on opening cytoplasmic channels, but in theory the cytoplasmic channels would need to reopen in response to any factor that increased myelinogenesis or prolongs myelination into adulthood, such as Akt signaling (Flores et al., 2008) or growth factor regulation. Other questions for the future include: How does the axon guide the myelination process? How is the nodal location and its structure determined and maintained? Is there a mechanism for thinning myelin, and if so, is it a reversal of the croissant-like process of myelinogenesis or a different process? Is action potential propagation influenced by changes in the cytoplasmic inclusions between layers of compacted myelin? How might disruption of the cytoplasmic channel dynamics participate in disease? Does action potential activity affect the opening or closing of the cytoplasmic channels in an activity-dependent manner to regulate conduction velocity? Clearly, these new findings open new avenues for investigation.
References
- Baron W, de Vries EJ, de Vries H, Hoekstra D. J Neurobiol. 1999;41:385–398. doi: 10.1002/(sici)1097-4695(19991115)41:3<385::aid-neu7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Fields RD. Science. 2010;330:768–769. doi: 10.1126/science.1199139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, Macklin WB. J Neurosci. 2008;28:7174–7183. doi: 10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebbels S, Oltrogge JH, Kemper R, Heilmann I, Bormuth I, Wolfer S, Wichert SP, Möbius W, Liu X, Lappe-Siefke C, et al. J Neurosci. 2010;30:8953–8964. doi: 10.1523/JNEUROSCI.0219-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luse SA. The fine structure of the morphogenesis of myelin. In: Korey SR, editor. The Biology of Myelin. New York: Paul B. Hoeber; 1959. p. 59. [PubMed] [Google Scholar]
- Snaidero N, Möbius W, Czopka T, Hekking LHP, Mathisen C, Verkleij D, Goebbels S, Edgar J, Merkler D, Lyons DS, et al. Cell. 2014;156:277–290. doi: 10.1016/j.cell.2013.11.044. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobottka B, Ziegler U, Kaech A, Becher B, Goebels N. Glia. 2011;59:1841–1849. doi: 10.1002/glia.21228. [DOI] [PubMed] [Google Scholar]
- Velumian AA, Samoilova M, Fehlings MG. Neuroimage. 2011;56:27–34. doi: 10.1016/j.neuroimage.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Wake H, Lee PR, Fields RD. Science. 2011;333:1647–1651. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen-Berg H. Nat Neurosci. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]