Abstract
Only a portion of the population exposed to trauma will develop persistent emotional alterations characteristic of posttraumatic stress disorder (PTSD), which illustrates the necessity for identifying vulnerability factors and novel pharmacotherapeutic alternatives. Interestingly, clinical evidence suggests that novelty seeking is a good predictor for vulnerability to the development of excessive and persistent fear. Here, we first tested this hypothesis by analyzing contextual and cued fear responses of rats selected for their high (high responders, HR) or low (low responders, LR) exploration of a novel environment, indicator of novelty seeking. While HR and LR rats exhibited similar sensitivity to the shock and cued fear memory retention, fewer extinction sessions were required in HR than LR animals to reach extinction, indicating faster contextual and cued memory extinction. In a second part, we found an effective disruption of contextual fear reconsolidation by the N-methyl-D-aspartate receptor antagonist ketamine, associated with a down-regulation of early growth response 1 (Egr1) in the hippocampal CA1 area, and up-regulation of brain-derived neurotrophic factor (Bdnf) mRNA levels in the prelimbic and infralimbic cortices. Altogether, these data demonstrate a link between novelty seeking and conditioned fear extinction, and highlight a promising novel role of ketamine in affecting established fear memory.
Keywords: fear conditioning, novelty seeking, individual differences, fear extinction, ketamine, fear memory reconsolidation
1. Introduction
Following exposure to a trauma, some individuals will develop excessive and persistent negative symptoms characteristic of posttraumatic stress disorder (PTSD) such as fear, hypervigilance and hyperarousal to reminders of the traumatic event. From both a diagnostic and therapeutic perspective, the presence of such inter-individual variations strengthens the importance of identifying the factors that influence an individual’s resilience or vulnerability to the development of PTSD symptoms following exposure to trauma. In accordance with the preponderance of individual variability in the PTSD pathology (Kessler et al., 1995), heterogeneity in animal response represents a key criterion for the development of clinically-relevant animal models (Borghans and Homberg, 2015; Matar et al., 2013; Siegmund and Wotjak, 2006; Whitaker et al., 2014; Yehuda and Antelman, 1993). However, while the mechanisms underlying variations in response to a trauma are somewhat understood, little is known about the factors able to predict an individual’s vulnerability prior to the traumatic event.
In humans, evidence supports a link between the novelty seeking personality trait and the development of PTSD. Indeed, among other personality traits, PTSD patients display higher scores of novelty seeking, which is predictive of PTSD symptoms severity in a population of combat-related PTSD patients (Evren et al., 2010; Jakšić et al., 2012; Richman and Frueh, 1997; Wang et al., 1997). However, one study analyzing personality traits prior to the exposure of a traumatic event revealed that novelty seeking was negatively associated with the risk for developing PTSD, which suggests a predictive value for novelty seeking in PTSD vulnerability and resiliency (Gil, 2005). Interestingly, novelty seeking can also be measured in rats, where some individuals display high rates of exploratory locomotion (termed high responders, HR) to a novel environment, while others exhibit low rates of exploratory locomotion (low responders, LR)(Piazza et al., 1989). The locomotor response to a novel environment not only predicts subsequent response to drugs of abuse such as amphetamine and cocaine (Hooks et al., 1992, 1991; Piazza et al., 2000; Pierre and Vezina, 1997), but also predicts differences in anxiety- and depression-related behaviors at baseline and in response to stress. Indeed, we demonstrated that HR animals have lower anxiety-levels at baseline (Kabbaj et al., 2000; Kabbaj and Akil, 2001), but present with a higher vulnerability to the development of depressive-like behaviors and impaired neuroendocrine regulations in response to repeated social defeat (Calvo et al., 2011; Duclot et al., 2011; Duclot and Kabbaj, 2013; Hollis et al., 2011), an effect mediated in part by a differential regulation of the brain-derived neurotrophic factor (Bdnf) gene (Duclot and Kabbaj, 2013). Of particular interest, HR and LR rats differ in the sensitivity and persistence of freezing behavior when re-exposed to the context where the social defeat occurred (Duclot et al., 2011). In addition to providing evidence for differences in fear response between HR and LR rats, these observations further suggest a connection between novelty seeking in rats and vulnerability to a traumatic stressor.
The necessity in improving the ability to predict an individual’s vulnerability to develop PTSD symptoms is further strengthened by the poor efficacy of current pharmacological treatments, mainly selective serotonin reuptake inhibitors, in preventing PTSD onset (Amos et al., 1996) and a non-response rate of more than 40% (Stein et al., 2006). Recently, however, the N-methyl-D-aspartate (NMDA) receptor antagonist ketamine has emerged as potential therapeutic for PTSD. Indeed, in a randomized clinical trial, acute ketamine treatment induced a rapid reduction of core PTSD symptoms lasting up to two weeks (Feder et al., 2014). In rodents, however, studies of ketamine’s effects on fear and anxiety are controversial and indicate either beneficial effects (Amann et al., 2009; Pietersen et al., 2007, 2006; Zhang et al., 2015), no effect (Groeber Travis et al., 2015), or detrimental effects (Juven-Wetzler et al., 2014). It is important to note that such discrepancy likely results from a wide range of experimental designs with variable translational relevance to PTSD and thus warrants further investigation of ketamine’s effect on fear memory in a clinically-relevant paradigm.
In view of the low efficacy of currently available pharmacotherapies, the combined use of pharmaco- and psychotherapies targeting fear memory extinction or blockade of its reconsolidation represent a very promising approach (Hendriksen et al., 2014; Pitman, 2011). Upon retrieval, the fear memory enters a labile state allowing for update and incorporation of new information, but importantly requires reconsolidation if it is to persist, a process involving brain structures critical to fear memory conditioning processes such as the hippocampus, the medial prefrontal cortex (mPFC), and the amygdala (Baldi and Bucherelli, 2015; Orsini and Maren, 2012; Tovote et al., 2015). Blocking memory reconsolidation thus offers an interesting opportunity to reduce the original fear memory, and several pharmacological agents, including NMDA receptor-targeting compounds, showed positive effects (Baldi and Bucherelli, 2015; Steckler and Risbrough, 2012). Nevertheless, it remains unknown whether ketamine, which showed promising results in clinical trial (Feder et al., 2014), could prove efficient in blocking fear memory reconsolidation.
Altogether, these observations suggest that novelty seeking may predict individual differences in fear response upon exposure to a stressful event. To test this hypothesis, we aimed at characterizing the response of adult HR and LR rats following a contextual and cued fear conditioning paradigm. Next, we assessed the potential of ketamine in disrupting reconsolidation of the contextual fear memory complemented by an exploratory analysis of the underlying neuronal activation.
2. Materials and Methods
2.1 Animals and drugs
Eight week-old male Sprague-Dawley rats weighing 225–260 g (Charles River Laboratories, Wilmington, MA, USA), randomly pair-housed in Plexiglas cages (45.2 × 26.5 × 20.3 cm), were used in this study. Rats were maintained on a 12-h light/dark cycle (lights off at 7:00 p.m.) with food and water available ad libitum except during testing. As the determination of the HR/LR phenotype was performed 5 days after reception at the vivarium, the distribution of HR/LR phenotypes among cages was random. Ketamine hydrochloride (Henry Schein Animal Health, Dublin, OH, USA) was dissolved in 0.9% sterile saline solution at 10 or 20 mg/kg and injected intraperitoneally (i.p.). All experiments were performed during the first 6 hours of the light phase of the light/dark cycle and were all conducted in accordance with the guidelines of the Animal Care and Use Committee of Florida State University and National Institute of Health guidelines.
2.2 Experimental design
Following five days of habituation to the animal facility and daily handling, all animals were first screened for their locomotor activity in order to be assigned to an HR or LR group, and after 3 days of rest, were subjected to the fear conditioning procedure. Two separate cohorts of animals were used. The first underwent fear conditioning training, contextual and cued fear memory tests, extinction of contextual and then cued memory, followed by the assessment of ketamine’s effects in disrupting reconsolidation memory. The second cohort of animals underwent the same training protocol as the first cohort, were tested for contextual fear 48 hrs later, followed 10 days later by contextual memory reactivation for the assessment of ketamine effects. It is important to note that shocked animals in both cohorts exhibited similar freezing behavior upon memory reactivation (first cohort: 46.4 ± 9.4%, second cohort: 56.0 ± 6.0%, t19 = 0.88, p = 0.389, d = 0.39, unpaired two-tailed t test), indicating that subsequent differences in reconsolidation would not result from cohort-dependent differences in contextual fear memory retrieval.
2.3 Determination of HR/LR phenotype
The HR/LR phenotype was determined as previously described using circular activity chambers (Med Associates Inc., St. Albans, VT, USA) (Hollis et al., 2011). Rats were free to explore for one hour the circular runway chamber divided in four equal quadrants by photobeam sensors, and the resulting photobeam breaks were recorded as an index of locomotion and used to assign rats to the HR or LR phenotype if their locomotion scored higher or lower, respectively, than the median (Dietz et al., 2005). Notably, the distribution of these locomotion scores were similar to the normal distribution recorded in an extensive population of comparable male rats (n = 919, Fig. 1A background). In an effort to minimize animal use, rats with the highest and lowest locomotion scores were assigned to the HR and LR groups receiving shock during the fear conditioning procedure, respectively, while those scoring the closest to the median were assigned to the control group (not receiving shock). Notably, because exposure to a conspecific receiving footshock can enhance fear by itself (Ito et al., 2015), the locomotor score of the cagemate was also taken into consideration when distributing rats among experimental groups so that both cagemates received the same treatment.
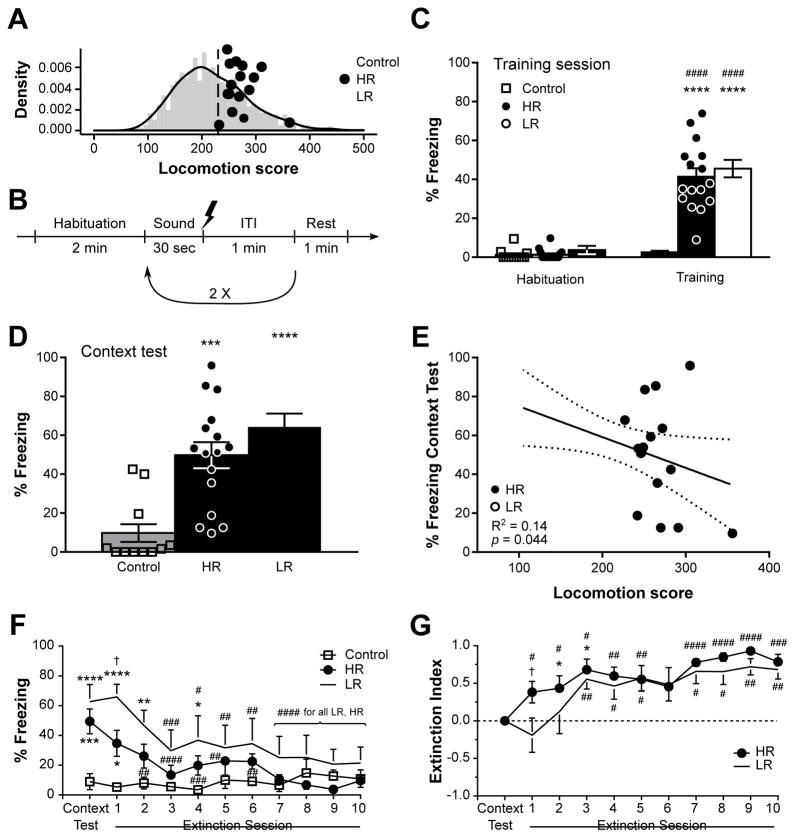
Figure 1.
Contextual fear memory in high-(HR) and low-responders (LR) rats. In (A) the locomotion scores of each rat are depicted against the normal distribution of an extensive population of rats (n = 919). In the training session detailed in (B) and performed in context A, rats were exposed to a total of 3 repeats of a sound co-terminating with a shock (except for “Control” group). While the percentage of time spent freezing during the training session (C) revealed no differences between HR and LR rats, LR animals spent slightly more time freezing during the context test (D) performed 48 hrs later. A linear regression analysis revealed a negative link between locomotion scores and freezing behavior during the context test (E). Beginning on the following day, rats were subjected to a daily contextual fear extinction for 10 days, which revealed faster extinction in HR animals as revealed by analysis of percentage of time spent freezing (F) and index of extinction (G). Each individual data point is depicted in panels (A,C–E), whereas n = 6–8 in (F,G). Throughout the figure, while white squares represent control animals, black circles and white circles represent HR and LR rats, respectively. In (C–F), *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs Control of same session; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 vs same phenotype in “Habituation” or “Context Test”; In (G), *p < 0.05 vs “Context Test”, #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 vs an index of “0”. †p < 0.05 HR vs LR. ITI: intertrial interval. Data are represented as mean ± SEM.
In order to further confirm the HR/LR phenotype, the locomotor response in a novel environment was also measured the following day in a three-chamber conditioned place preference apparatus equipped with photobeam breaks (Med Associates Inc.) for one hour. Using a similar median split method, the HR/LR phenotype of 80% of the rats of the HR and LR groups could thus be confirmed.
2.4 Fear conditioning
The fear conditioning apparatus (Bioseb, Pinellas Park, FL, USA), enclosed in a soundproof cabinet, is composed of a 25 × 25 × 25 cm enclosure with Plexiglas walls and a grid floor placed on a pressure plate linked to a computer running Freezing (v1.3.05, Panlab Harvard Apparatus, Barcelona, Spain) for the acquisition and measurement of the freezing behavior. The floor and walls are modulatory, allowing the creation of different contexts. Context A (training context) consisted of black smooth Plexiglas walls, steel grid floor, and was wiped clean before each session with 70% Ethanol, whereas context B consisted in white smooth metal covers securely mounted on each wall and the grid floor, and was cleaned with 70% Isopropanol before each session.
The first day (day 1), rats were placed in context A for the training session. After 2 min of habituation to the context, a tone (4 kHz, 75 dB) was presented for 30 sec co-terminating during the last second with a shock (0.5 mA, 1 sec). After an intertrial interval (ITI) of 1 min, rats were exposed to two additional conditioning cycles (3 total) and let an additional minute in the context before being placed back in their home cage. Control animals were exposed to the same protocol without shock delivery. Shock intensity, duration, and repetitions were determined based on the literature (Cordero et al., 1998; Luyten et al., 2011; Phillips and LeDoux, 1992) and a pilot experiment, and designed to ensure a consistent but not excessive contextual fear response, thereby preventing interference of ceiling effects with the observation of inter-individual variations in fear response. Contextual fear memory was measured two days later (day 3, Context test), and then subjected to extinction by daily sessions consisting in 5 min-long exposure to context A for 10 additional days (days 4–13). The cue memory was then tested the following day (day 14) in the context B using the same protocol as during the training session but without shock delivery, before being subject to extinction 6 days later (day 20). The extinction of cue memory was performed as for the cue test, but with 10 exposures to the tone without shock delivery. Freezing behavior was recorded throughout the procedure as an indicator of fear. Furthermore, to provide a quantitative measurement of each rat’s extinction, an extinction index was calculated as follows: (%Freezing during first session of extinction - %Freezing during current session)/%Freezing during first session of extinction. An index of 1 thus represents a complete extinction of the fear memory, while an index of 0 corresponds to an absence of extinction.
2.5 Effects of ketamine on contextual fear memory reconsolidation
Ten days following contextual memory test, the contextual memory was reactivated by placing the animals back in context A (training context) for 5 min. In order to recover the fear memory in animals of the first cohort, which underwent both contextual and cued extinction, these animals were re-exposed to the training protocol in a novel context 18 days after cue extinction, and then subjected to a context test 2 days later to verify the re-acquisition of fear to the training context (Freezing: 2.15 ± 0.8% for non-shocked controls, 59.10 ± 10.8% for shocked rats, t9.104 = 5.23, p = 0.0005, d = 2.14) necessary for the assessment of ketamine’s effect (Supplementary Fig. S1). At the end of the reactivation session, rats were injected with saline or ketamine (10 or 20 mg/kg, i.p.) and placed back in their home cage. Notably, ketamine reduced PTSD symptoms in a clinical trial (Feder et al., 2014) at the same dose it induces antidepressant effects (Murrough et al., 2013). We thus chose a minimal dose of 10 mg/kg (i.p.) based on its ability to modulate conditioned fear in rats (Honsberger et al., 2015), and its antidepressant effects consistent throughout the literature (Carrier and Kabbaj, 2013; Li et al., 2010). Twenty-four hours later, the contextual fear memory was tested again in context A for 5 min. Twenty-five minutes after the end of the test, rats were killed by rapid decapitation, the brain quickly dissected out, snap-frozen in 2-methylbutane, and stored at −80°C until processing for in situ hybridization.
2.6 In situ hybridization
Six brains from saline- and ketamine-treated shocked animals were processed for in situ hybridization as previously described (Stack et al., 2010). Each brain was sectioned on a cryostat at 20 μm, and a series of sections taken at 100 μm intervals (200 μm at the level of mPFC) were mounted on poly-L-lysine-coated slides. The sections were first fixed in 4% paraformaldehyde for 1 hr, followed by three washes in 2X saline sodium citrate (SSC), before being placed in 0.25% acetic anhydride in triethanolamine (0.1 M, pH 8) for 10 min at room temperature, rinsed in distilled water, and dehydrated through graded alcohols (50, 75, 85, 95, and 100%). After air-drying, the sections were hybridized with a 35S-labeled Egr1 (Stack et al., 2010) or Bdnf (donated by Drs Gall and Lauterborn, and described in Isackson et al., 1991) cRNA probes, which were labeled at 37°C for 2 hrs in a reaction mixture containing 1 μg of linearized plasmid, 5X transcription buffer (Promega, Madison, WI, USA), 125 μCi [35S]uridicine triphosphate, 125 μCi [35S]cytosine triphosphate, 600 μM each of adenosine triphosphate, and guanidine triphosphate, 6 mM dithiothreitol, 80U RNase inhibitor, and 40U of polymerase. The probes were then separated from unincorporated nucleotides over Micro Bio Spin Chromatography columns (Bio-Rad, Hercules, CA, USA), and then diluted in hybridization buffer (containing 50% formamide, 10% dextran sulfate, 3X SSC, 50 mM sodium phosphate buffer pH 7.4, 1X Denhardt’s solution, 0.1 mg/mL yeast tRNA, and 10 mM dithiothreitol) to yield 106 cpm/70 μL. Following overnight hybridization at 55°C in an humidified environment, the sections were rinsed and washed twice in 2X SSC for 5 min each and then incubated for 1 hr in RNase (200 μg/mL in Tris buffer containing 0.5 M NaCl, pH 8) at 37°C. The reactions were then washed in increasingly stringent solutions of SSC, 2X, 1X, and 0.5X for 5 min each, followed by incubation for 1 hr in 0.1X SSC at 65°C. Following a final rinse in distilled water, the sections were dehydrated through graded alcohols, air-dried, and exposed to Kodak XAR film (Eastman Kodak, Rochester, NY, USA), for 14 (Egr1) or 22 days (Bdnf). Notably, sections pretreated with RNAse or incubated with sense riboprobes were used as negative controls (data not shown).
Quantification of the radioactive signal was performed on six equivalent sections per region per rat as previously described (Stack et al., 2010) in each of the following brain regions: anterior cingulate (ACC), prelimbic (PLC), and infralimbic (ILC) cortices of the mPFC sampled from 3.7 mm to 1.7 mm from bregma (Paxinos and Watson, 1998), CA1, CA3, and dentate gyrus (DG) of the dorsal hippocampus sampled from −2.12 mm to −3.8 mm from bregma, and basolateral (BLA) and central (CeA) amygdala nuclei sampled from −1.40 mm to −2.8 from bregma.
2.7 Statistical analyses
The percentage of time spent freezing during the training session and the cued-fear memory test were analyzed with a two-way repeated measures ANOVA with “session” as within-subject factor, and “phenotype” as between-subject factor, followed by Bonferonni’s (within phenotype) and Tukey’s (within session) post-hoc tests. Data from the contextual fear memory test, however, were analyzed with a one-way ANOVA followed by Tukey’s post-hoc test. The percentage of time spent freezing during extinction protocols was analyzed first with a two-way repeated measures ANOVA with “session” as within-subject factor, and “phenotype” as between-subject factor, followed by Bonferonni’s (within phenotype) and Tukey’s (within session) post-hoc tests to identify main effects of extinction, phenotype, and their interaction. Because extinction index data did not follow a normal distribution, the evolution of the index across sessions was first analyzed within each phenotype with a Friedman’s test followed by a Dunn’s post-hoc test, and then between phenotypes at each session separately with a two-tailed Mann-Whitney. Notably, the latter analysis was complemented by a Wilcoxon signed-rank test test performed at each exposure within each phenotype against a hypothetical index value of “0” indicating the presence or absence of extinction at this specific exposure session. Similarly, the effect of ketamine on contextual fear memory reconsolidation was analyzed by two-way repeated measures ANOVA with “session” as within-subject factor, and “treatment” as between-subject factor, followed by Bonferonni’s (within treatment) and Tukey’s (within session) post-hoc tests, whereas the corresponding index of extinction was analyzed with a one-way ANOVA between treatment groups, and a one-sample t-test against a null value within each treatment group. Finally, in situ signals were analyzed within each structure by a two-tailed unpaired t-test (performed on optical densities before transformation to percentage of saline-treated rats). For all pairwise comparisons, the Cohen’s d effect size was estimated from each group’s mean, standard deviation, and sample size, while the effect size for ANOVA was estimated from the eta squared (η2). All tests were performed using Prism 6.07 (GraphPad Software, Inc., La Jolla, CA, USA) with alpha set at 0.05, and after verification that each test’s assumptions of homoscedasticity or normality were met before running any ANOVA.
3. Results
3.1 Novelty seeking predicts rate of contextual fear memory extinction
To investigate the relationship between novelty seeking and fear memory, HR and LR rats (Fig. 1A) were subjected to a classical contextual and cued fear conditioning in which a tone was presented in association or not with a mild foot-shock, repeated three times (Fig. 1B). Although a substantial individual variability in the percentage of time spent freezing was observed following presentation of the shock, both HR and LR rats exhibited a similar level of freezing behavior (F1,39 = 120.3, p < 0.0001, η2 = 0.38 for Session, F2,39 = 36.6, p < 0.0001, η2 = 0.18 for Phenotype, and F2,39 = 25.2, p < 0.0001, η2 = 0.16 for the interaction, Fig. 1C), indicating similar fear response between HR and LR animals upon presentation of the shock. Similarly, when rats were re-exposed to context A for the contextual fear memory test 24 hrs later, both HR and LR rats exhibited freezing behavior higher than non-shocked control rats (F2,39 = 18.0, p < 0.0001, η2 = 0.48, Fig. 1D). Nevertheless, although no significant differences were detected (p = 0.231), HR rats tended to spend less time freezing than LR rats, and the locomotion score was negatively linked to freezing behavior in the contextual test (Fig. 1E) but not during fear conditioning training (R2 = 0.006, p = 0.687, data not shown), indicating that response to a novel environment can, in part, predict the subsequent level of contextual freezing. Notably, HR and LR rats exhibited a similar evolution of freezing behavior throughout the contextual test session, denoting that differences between both phenotypes were consistent (Supplementary Fig. S2).
The following day, the contextual fear memory was submitted to extinction by repeating the exposure to context A without the shock, for 10 additional days. While both HR and LR rats exhibited a reduction in the percentage of time spent freezing over consecutive days, the novelty seeking phenotype influenced the profile of extinction (F10,190 = 9.33, p < 0.0001, η2 = 0.11 for Session, F20,190 = 4.50, p = 0.025, η2 = 0.18 for Phenotype, and F20,190 = 3.61, p < 0.0001, η2 = 0.08 for the interaction, Fig. 1F). Indeed, only three exposures to context A without shock (Extinction Session #2) are required for HR rats to statistically present with a reduction in freezing behavior and become indistinguishable from non-shocked controls, whereas an additional repeat (Extinction Session #3) is necessary for LR animals (Fig. 1F). Accordingly, the level of freezing between HR and LR rats differed during the second exposure to the context (Extinction Session #1, p = 0.022). It is important to note however, that the slight difference in freezing level between HR and LR rats during the initial exposure to context A without shock (Context Test) could account for the difference observed in extinction. We therefore developed an extinction index providing a quantitative description of each group’s extinction profile while accounting for baseline differences in freezing behavior, and confirmed the faster rate of contextual fear memory extinction in HR rats when compared to LR animals. Indeed, while the extinction indexes increased over sessions in both HR and LR groups (χ210 = 27.8, p = 0.002 for HR; χ210 = 27.7, p = 0.002 for LR, Fig. 1G), HR and LR animals differed during the second exposure to the context A (Extinction Session #1, U = 8, p = 0.043). Furthermore, a significant extinction is detected since Extinction Session #1 in HR animals, but two additional sessions are required to see such effect in LR rats (Extinction Session #3).
Altogether, these data suggest that despite a similar immediate freezing response to the shock, the novelty seeking phenotype can predict part of the resulting contextual fear memory retention, with higher locomotor scores being linked to lower freezing behavior in response to the context, and a faster extinction of the contextual fear memory.
3.2 Faster extinction of cued-fear memory in HR than LR rats
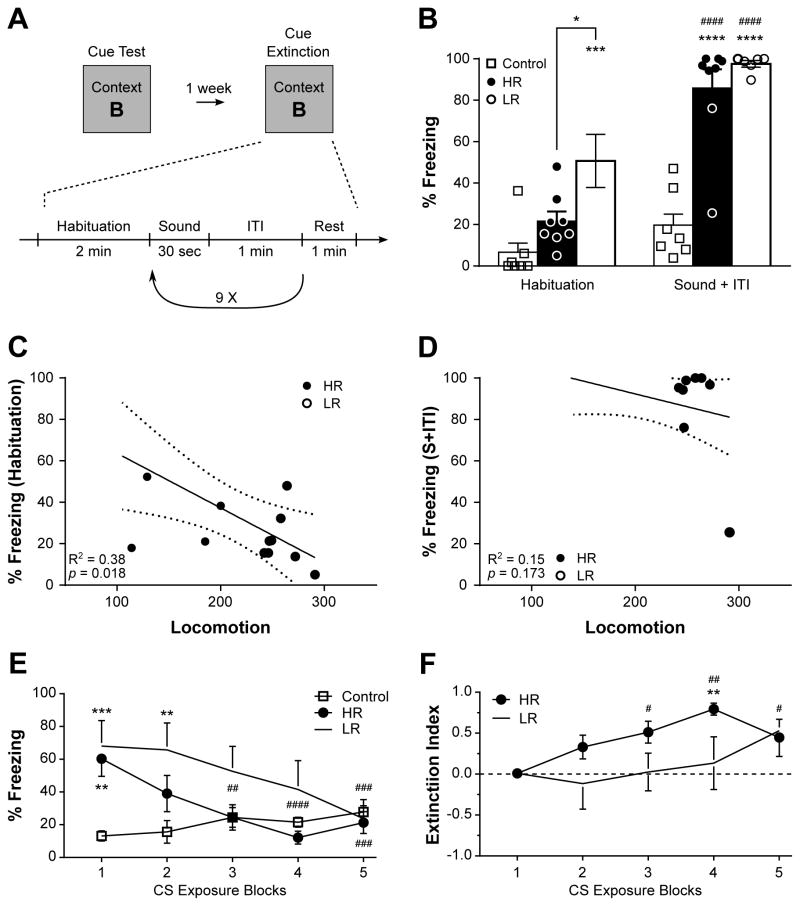
Following extinction of the contextual fear memory, the cued component of the fear memory was tested the following day by placing the animals in context B and presenting 3 repeats of the conditioning tone under the same protocol as during the training session, but without shock delivery. In response to the tone, both HR and LR animals, but not non-shocked controls, displayed an enhanced and similar freezing behavior (F1,19 = 89.7, p < 0.0001, η2 = 0.28 for Session, F2,19 = 27.3, p < 0.0001, η2 = 0.42 for Phenotype, and F2,19 = 13.1, p = 0.0003, η2 = 0.08 for the interaction, Fig. 2B). Interestingly, LR animals exhibited substantially higher freezing behavior than HR or non-shocked controls during the habituation period to the novel context B (p = 0.019 vs. HR, p = 0.0003 vs. Control, Fig. 2B). This observation was further supported by a negative association between locomotion and percentage of time spent freezing during the habituation period (Fig. 2C), but not in response to the tone (Fig. 2D).
Figure 2.
Cued fear memory in high-(HR) and low-responders (LR) rats. (A) Timeline of Cue Test and Cue Extinction conducted in context B, during which rats were exposed to a total of 10 presentations of the tone. During the Cue Test (B), while both HR and LR rats exhibited a strong freezing behavior, LR animals spent more time freezing than HR or control rats. Accordingly, a significant negative regression was observed between the locomotion score and the percentage of time spent freezing during the Habituation period of the Cue Test (C) but not during the presentation of the sound (S + ITI, D). Furthermore, HR rats showed a faster extinction of the cued fear memory than LR animals, as revealed by the percentage of time spent freezing (E) and index of extinction (F). Each individual data point is depicted in panel (B), whereas n = 6–8 in (E,F). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs Control of same session; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 vs same phenotype in “Habituation” (B) or first exposure block (E). In (F), **p < 0.05 vs first exposure block, #p < 0.05, ##p < 0.01, vs an index of “0”. Data are represented as mean ± SEM.
The cued fear memory was then subjected to extinction by repeated presentations (10 total) of the tone without shock delivery (Fig. 2A). Both HR and LR rats showed a reduction in freezing behavior over repeated presentations of the tone (displayed as five blocks of two for clarity), indicating extinction of cued fear memory in both phenotypes (F4,76 = 5.63, p = 0.0005, η2 = 0.08 for Session, F2,19 = 4.17, p = 0.032, η2 = 0.15 for Phenotype, and F8,76 = 4.46, p = 0.0002, η2 = 0.13 for the interaction, Fig. 2E). However, a significant reduction in freezing behavior, when compared to the first tone presentation, is detected from the third exposure block in HR animals, whereas two additional blocks of tone presentation are required to see the same effect in LR animals (Fig. 2E). Similarly, only 2 exposure blocks are required for HR rats to be statistically indistinguishable from non-shocked controls, while 5 are required in LR animals. Supporting this observation, while both HR and LR rats showed an increase of extinction index over the exposure blocks (χ24 = 13.4, p = 0.009 for HR, χ24 = 11.6, p = 0.021 for LR, Fig. 2F), HR animals showed a significant increase in the extinction index at the third exposure block, whereas LR animals do not. Moreover, extinction was statistically detected as early as the third exposure block in HR rats, while two more exposure blocks were needed in LR animals (Fig. 2F).
Altogether, these data indicate that the novelty seeking phenotype can predict freezing behavior in an environment not previously associated with the shock, with low novelty seeking being associated with higher freezing response. Despite a similar strong freezing in both groups in response to the tone, however, the cued fear memory could be extinguished faster in HR than LR rats.
3.3 Disruption of contextual fear memory reconsolidation by ketamine
In the second part of this study, we investigated the therapeutic potential of ketamine on fear memory by focusing on the analysis of its effects on the reconsolidation of fear memory. To this aim, the contextual fear memory was reactivated ten days following the last context test by re-exposing the animals to context A without shock delivery. At the end of this session, rats were injected with saline or ketamine (10 or 20 mg/kg, i.p.), placed back in their home cage, and then re-exposed to context A twenty-four hours later to measure the level of contextual fear memory (Fig. 3A).
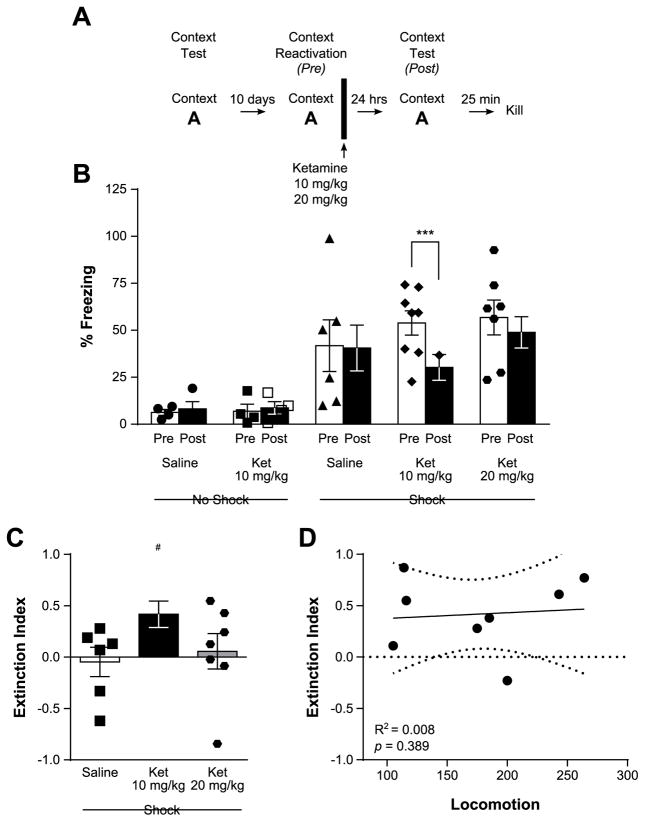
Figure 3.
Disruption of contextual fear memory reconsolidation by ketamine. In panel (A), the experimental timeline is depicted. The analysis of the percentage of time spent freezing (B) before (Pre) and 24 hrs after ketamine treatment (Post), reveals a reduction in freezing in rats treated with ketamine at the 10 mg/kg dose (i.p.), but not 20 mg/kg or saline. This translates into a positive extinction index (C) in 10 but not 20 mg/kg ketamine- or saline-treated animals. A linear regression analysis between Extinction Index in rats treated with 10 mg/kg and Locomotor score (D) revealed the absence of relationship between novelty seeking phenotype and response to ketamine. Each individual data point is depicted within each column. ***p < 0.001; #p < 0.05 vs an index of “0”. Data are represented as mean ± SEM.
During the contextual memory recall session (Pre), shocked animals displayed substantially more freezing behavior than non-shocked rats (No shock: 6.60 ± 1.89%, Shock: 51.44 ± 5.44%, t24.1 = 7.78, p < 0.0001, d = 2.07, unpaired two-tailed t-test), confirming the successful reactivation of the contextual fear memory. Notably, rats treated with 10 mg/kg but not 20 mg/kg ketamine immediately after memory recall displayed reduced contextual freezing the following day (p = 0.001 for 10 mg/kg, p = 0.90 for 20 mg/kg), whereas saline-treated rats did not (F1,24 = 3.92, p = 0.059, η2 = 0.01 for Session, F4,24 = 5.51, p = 0.003, η2 = 0.42 for Treatment, and F4,24 = 3.23, p = 0.03, η2 = 0.03 for the interaction, Fig. 3B). This suggests that ketamine treatment at the 10 mg/kg dose impaired memory reconsolidation, and was further confirmed by the positive extinction between the two contextual test sessions detected in rats treated with 10 mg/kg ketamine (t7 = 3.26, p = 0.014, d = 1.15) but not 20 mg/kg ketamine (t6 = 0.33, p = 0.454, d = 0.12) or saline (t5 = 0.32, p = 0.758, d = −0.13, two-tailed one-sample t test, Fig. 3C).
Notably, in light of the differences in contextual fear extinction we uncovered between HR and LR animals, we tested whether the locomotor response to a novel environment would be a predictor of response to 10 mg/kg ketamine by conducting a linear regression analysis. No link could thus be found between locomotion scores and index of extinction in saline- or ketamine-treated animals (saline: R2 = 0.009, p = 0.858; 10 mg/kg ketamine: R2 = 0.008, p = 0.829, Fig. 3D; 20 mg/kg ketamine: R2 = 0.02, p = 0.780), indicating that the novelty seeking phenotype does not predict subsequent contextual fear memory reconsolidation or its disruption by ketamine.
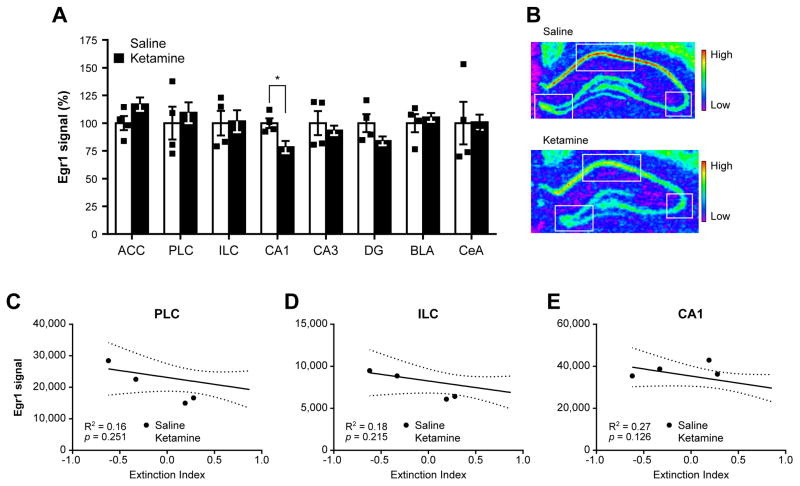
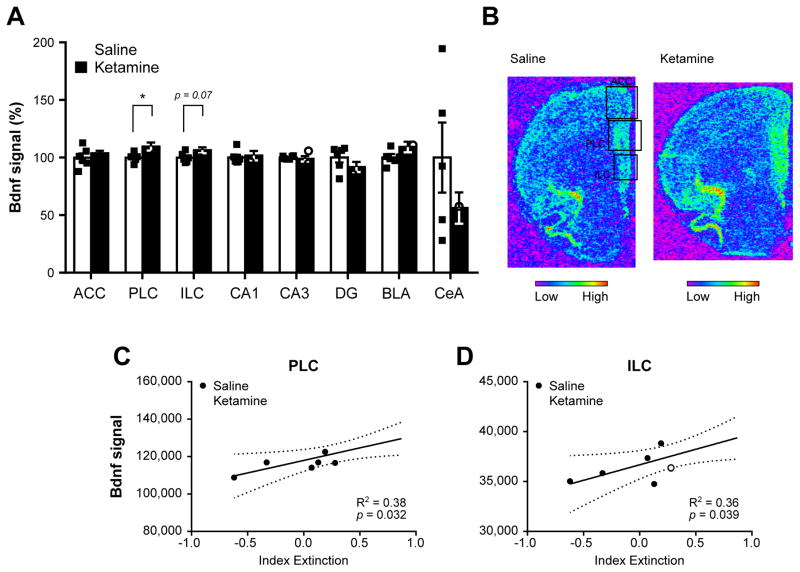
Contextual fear memory reconsolidation involves complex interactions within a neural circuit including the hippocampus, mPFC, and amygdala, mediated by dynamic changes in the expression of genes such as early growth response 1 (Egr1) and brain-derived neurotrophic factor (Bdnf) (Baldi and Bucherelli, 2015; Barnes et al., 2012). In an effort to investigate the underlying correlates of ketamine’s effects, we analyzed Egr1 and Bdnf mRNA levels in the hippocampal, mPFC, and amygdalar subregions of saline- and 10 mg/kg ketamine-treated rats by in situ hybridization 25 min following the last contextual fear test (Fig. 3A). In ketamine-treated rats, Egr1 mRNA levels were down-regulated in the CA1 area (t8 = 2.78, p = 0.04, d = −1.80, Fig. 4A,B), while Bdnf mRNA levels were up-regulated in the PLC (t10 = 2.52, p = 0.031, d = 1.45, Fig. 5A,B). Interestingly, the reduction in contextual fear memory tended to be negatively linked to Egr1 mRNA levels in the CA1, PLC, and ILC regions (Fig 4C–E), but was found positively associated with Bdnf mRNA levels in the PLC and ILC (Fig. 5C,D). No other differences between saline- and ketamine-treated rats, or regression with extinction index, were found in other areas investigated (Fig. 4, Fig. 5, Supplementary Fig. S3). In addition to its role in the regulation of learning and memory processes such as fear memory reconsolidation (Besnard et al., 2013; Lee et al., 2004; Veyrac et al., 2014) Egr1 expression levels can also be used to measure neuronal activation underlying learning and memory (Veyrac et al., 2014). By conducting a comparative correlative analysis of Egr1 mRNA levels signal between structures, we are therefore able to investigate alterations of neuronal connections in 10 mg/kg ketamine-treated rats. Notably, while Egr1 signals between all structures are highly correlated in saline-treated rats (Supplementary Fig. S4A), ketamine-treated rats exhibit a different profile of correlations affecting in particular those centered on the CA1 area. Indeed, when compared to saline-treated rats, these connections are reversed with the mPFC, lost within the hippocampus, and markedly weakened with the central amygdala (CeA) of ketamine-treated rats (Supplementary Fig. S4B, Supplementary Fig. S5).
Figure 4.
Early growth response 1 (Egr1) expression profile in ketamine- and saline-treated animals following contextual fear memory reconsolidation test. The analysis of Egr1 mRNA levels by in situ hybridization in several subregions of the medial prefrontal cortex, hippocampus and amygdala (A) revealed a down-regulation of Egr1 in the hippocampal CA1 subregion in ketamine-treated animals when compared to rats injected with saline. Representative pictures of hippocampal Egr1 signal in saline-(top) and ketamine-treated (bottom) rats. Each square denotes the three hippocampal areas quantified. Regression analyses between the extinction index depicted in Fig. 3C and Egr1 signal in the prelimbic cortex (C), infralimbic cortex (D), and CA1 area (E) revealed a small but not significant negative link between Egr1 signal in the CA1 and contextual fear memory extinction. In (A), each individual data point is depicted within column. *p < 0.05. ACC: anterior cingulate cortex, PLC: prelimbic cortex, ILC: infralimbic cortex, CA1: cornu ammonis 1, CA3: cornu ammonis 3, DG: dentate gyrus, BLA: basolateral amygdala, CeA: central amygdala. Data are represented as mean ± SEM.
Figure 5.
Brain-derived neurotrophic factor (Bdnf) expression profile in ketamine- and saline-treated animals following contextual fear memory reconsolidation test. The analysis of Bdnf mRNA levels by in situ hybridization in several subregions of the medial prefrontal cortex, hippocampus and amygdala (A) revealed an up-regulation of Bdnf in the prelimbic and infralimbic areas of the medial prefrontal cortex (mPFC). Representative pictures of Bdnf signal in saline-(top) and ketamine-treated (bottom) rats in the mPFC. Each square denotes the three mPFC areas quantified. Regression analyses between the extinction index depicted in Fig. 3C and Bdnf signal in the prelimbic (C) and infralimbic cortices (D) revealed a significant positive link between Bdnf signal in these areas and contextual fear memory extinction. In (A), each individual data point is depicted within column. *p < 0.05. ACC: anterior cingulate cortex, PLC: prelimbic cortex, ILC: infralimbic cortex, CA1: cornu ammonis 1, CA3: cornu ammonis 3, DG: dentate gyrus, BLA: basolateral amygdala, CeA: central amygdala. Data are represented as mean ± SEM.
Altogether, these data suggest that ketamine, when injected immediately after contextual fear memory recall, can impair the reconsolidation of the memory engram, an effect associated with down-regulation of Egr1 expression in the hippocampal CA1 area, up-regulation of Bdnf expression in the prelimbic and infralimbic cortical regions, and alteration of neuronal connectivity between hippocampus, mPFC, and CeA.
4. Discussion
Identifying key susceptibility factors and developing novel pharmacotherapeutic strategies are critical in fear memory-related disorders such as PTSD. In this study, we first identified a link between novelty seeking in rats and individual differences in several aspects of fear memory. Then, we found that treatment with 10 but not 20 mg/kg ketamine immediately after contextual fear memory reactivation reduces freezing upon contextual retrieval the following day, an effect associated with down-regulation of Egr1 mRNA levels in the hippocampal CA1 region, and up-regulation of Bdnf mRNA levels in the prelimbic and infralimbic cortices. Although the exact mechanisms of ketamine actions remain to be identified, these observations provide the first evidence of ketamine’s therapeutic potential in a context related to fear memory reconsolidation blockade.
4.1 Novelty seeking predicts individual differences in fear memory
In the first part of this study, we tested whether novelty seeking can predict individual differences in fear response upon exposure to a stressful event. In this context, the intensity and duration of the aversive stimulus (foot shock) as well as its pairing with the conditioned stimuli (context and tone) were of critical importance to ensure the consistent induction of a conditioned fear, but within a range allowing for the observation of individual variability in the behavioral outcome, the freezing behavior. In this study, exposing rats to 3 repeats of the unconditioned stimulus (0.5 mA, 1 sec) during the training session resulted in immediate freezing of the same extent in both HR and LR animals, indicating similar sensitivity to the shock between phenotypes, and thereby ruling out interference with subsequent individual differences in freezing behavior. We nonetheless observed a wide variability in the contextual fear memory test, but not cued fear memory test in which both HR and LR freezing behavior were near or at maximum. Although we cannot rule out specific individual variability in contextual but not cued fear memory, it is important to note that responses to cues are commonly reported to condition faster and stronger than to a context (Curzon et al., 2011; Phillips and LeDoux, 1992), which would therefore suggest that HR/LR differences in cued fear memory retrieval could have been masked by the intensity of the conditioning protocol. This critical dependence on stimulus intensity is supported by the prediction of contextual fear response level by the locomotion score, despite the absence of significant differences between HR and LR rats in freezing response. Although this link was relatively weak (R2 = 0.14) and highly dependent on stimulus intensity, this represents a clear demonstration that the novelty seeking phenotype can, at least in part, predict individual differences in fear response. Notably, combined with the observation that the differences in time spent freezing between HR and LR rats were constant throughout the contextual test session (Supplementary Fig. S2), HR/LR differences in exploration are only observed in a novel environment and disappear upon re-exposure to the same context (Dellu et al., 1996), which indicates that the lower freezing observed in HR rats is unlikely to result from their higher exploratory activity in novel environments, but rather from a true reduction in fear-related behaviors.
Using an approach accounting for such baseline differences, we found that although HR and LR animals reach statistically indistinguishable levels of freezing at the end of the extinction protocols (both contextual and cued), HR rats exhibit faster fear extinction when compared to their LR counterparts. Because fear extinction involves acquisition of a new associative memory (Myers and Davis, 2007; Tovote et al., 2015), the faster extinction of contextual and cued fear memory in HR rats, or slower extinction in LR animals, could result from differences in extinction learning performances between HR and LR individuals. Notably, HR and LR Wistar rats exhibit similar spatial long-term memory in unstressed conditions, as measured in the water maze (Sandi and Touyarot, 2006; Touyarot et al., 2004), which suggests that the differences in contextual fear memory observed in our study do not reflect impaired spatial recognition in LR rats when compared to HR animals. Furthermore, it is interesting to note that exposure to chronic psychosocial or environmental stress impairs the subsequent acquisition of spatial memory in HR, but not LR, individuals (Sandi and Touyarot, 2006; Touyarot et al., 2004), which in our context would correspond to an impaired acquisition of extinction learning in HR animals. On the contrary, we found a faster acquisition of extinction learning in HR rats, combined with the presence of a similar profile in cued fear memory extinction—less dependent on spatial components—which thus opposes the interference from contextual and spatial recognition impairments with the observed fear extinction profiles. Furthermore, although our current study represents the first report of HR/LR differences in conditioned fear, it is worth noting that we observed similar differences in extinction of fear response following psychosocial stress. Indeed, while the freezing response of HR and LR rats does not differ when re-exposed to the social defeat context after four weeks—including both spatial and olfactory cues of the stressful episode—HR rats no longer display freezing behavior when re-exposed a second time two weeks later, suggesting better fear memory extinction in HR than LR animals (Duclot et al., 2011). Altogether, these data support the existence of differences in extinction learning between HR and LR rats.
The differences in fear extinction between HR and LR rats despite similar initial fear acquisition are particularly interesting in relation with PTSD, often defined as an extinction learning disorder in which symptoms can be viewed as the result of impaired extinction (Koenigs and Grafman, 2009; VanElzakker et al., 2014). Interestingly, the segregation of rats based on their extinction rates revealed that weak extinction was associated with high anxiety-like behaviors, although anxiety levels prior to fear conditioning were not predictive of a weak extinction phenotype (Reznikov et al., 2015). Considering that heightened anxiety levels following fear conditioning is closely related to fear generalization in PTSD (Dunsmoor and Paz, 2015), it is particularly interesting to note that we observed a substantial freezing behavior in the habituation to the novel context B in shocked LR, but not HR animals, supported by a significant negative predictive link with locomotion score. This higher freezing behavior could simply result from the low locomotor response in a novel environment characteristic of LR rats, however, no freezing behavior was detected in LR animals during their first exposure to context A, which then was a novel environment. Although the interference of the low locomotor response of LR rats in a novel environment cannot be ruled out, this observation suggests that LR individuals present with heightened anxiety levels following fear conditioning that would be in line with lower extinction rates (Reznikov et al., 2015), and denote generalization of fear.
The observation of HR/LR differences in pathological hallmarks of PTSD such as fear extinction highlights an interesting link between the novelty seeking phenotype, core components of fear memory regulation, and PTSD. Notably, this association is further supported in animal models of individual differences in emotional response in which, for instance, predisposition to learned helplessness is predicted by novelty seeking and associated with differences in anxiety-like behaviors, acquisition of cued fear conditioning, and impaired fear extinction (Padilla et al., 2010; Shumake et al., 2005). Moreover, in combination with other personality traits, a few reports denote a predictive value for novelty seeking in PTSD vulnerability and symptoms vulnerability (Evren et al., 2010; Gil, 2005; Jakšić et al., 2012; Richman and Frueh, 1997; Wang et al., 1997), conferring both a preclinical and clinical relevance to the HR/LR model and individual differences in novelty seeking in a PTSD context. In addition, based on baseline differences in a behavioral paradigm with minimal impact on further experimental manipulations, the HR/LR model provides an interesting framework for the study of individual differences in vulnerability to the development of PTSD symptoms following exposure to traumatic stressor.
4.2 Disruption of fear memory reconsolidation by ketamine
In the second part of this study, we investigated whether ketamine could dampen fear memory, by focusing on the blockade of its reconsolidation for several reasons. First, together with extinction, reconsolidation presents with direct translational opportunities for intervention on established fear memories (Milad et al., 2006; Pitman, 2011). Second, while glutamatergic neurotransmission via NMDA receptors is involved in both extinction and reconsolidation processes, NMDA receptors antagonism would be predicted to impair extinction learning and thus promote persistence of fear, whereas it would be expected to reduce fear in a memory reconsolidation paradigm (Miller and Sweatt, 2006; Quirk and Mueller, 2008). Third, unlike facilitation of extinction learning, blockade of reconsolidation affects the pre-existing fear memory (Lattal and Wood, 2013), which provides unique therapeutic opportunities limiting the risk for fear return (Pitman, 2011).
As extinction learning and reconsolidation processes both consist in a re-exposure to the CS (Context A in our study), both can occur during contextual fear memory retrieval (Quirk and Mueller, 2008) and it can therefore appear unclear whether ketamine modulates extinction or reconsolidation processes. Interestingly, the duration of exposure to the CS is a deciding factor, with short exposures involving mainly reconsolidation processes, whereas prolonged or repeated exposures engage extinction events (Eisenberg et al., 2003; Pedreira and Maldonado, 2003; Quirk and Mueller, 2008; Sangha et al., 2003; Suzuki et al., 2004). In particular, pharmacological intervention in the 3 to 10 minutes following exposure to CS target reconsolidation of contextual fear (Suzuki et al., 2004), indicating that the 5-min exposure used in our study lies within the range of reconsolidation processes. In addition, the response to 10 mg/kg ketamine was independent of the novelty seeking phenotype, which we showed affects fear extinction performances and thereby suggests that extinction events are not predominant in our experimental paradigm. Moreover, based on the known requirement of NMDA receptors in both processes (Quirk and Mueller, 2008), the observation of a reduced fear in post-retrieval contextual test in ketamine-treated rats is in line with the outcome predicted by disruption of fear reconsolidation by NMDA receptors antagonism, but contradictory to the outcome predicted by NMDA receptors antagonism on fear extinction learning. Finally, the specific alteration of Egr1 but not Bdnf mRNA levels in the hippocampal CA1 area of 10 mg/kg ketamine-treated rats is coherent with the molecular signature of fear reconsolidation (Barnes et al., 2012; Kirtley and Thomas, 2010; Lee et al., 2004). Altogether, although the possibility of facilitation of extinction cannot be completely ruled out, these behavioral and molecular data support the engagement of reconsolidation events over fear extinction, and thus suggest that ketamine disrupts the reconsolidation of contextual fear memory.
Interestingly, this effect was observed at the 10 mg/kg, but not 20 mg/kg dose, which illustrates the dose-dependency of ketamine’s effects. This is in line with the conflicting reports on ketamine’s effects on fear and anxiety that likely originate from a wide variety of experimental designs difficult to translate into a preclinical context due to the use repeated injections at high doses or pre-conditioning treatment (Amann et al., 2009; Groeber Travis et al., 2015; Juven-Wetzler et al., 2014; Pietersen et al., 2007, 2006; Zhang et al., 2015). Notably, such dose-dependency is a common feature of ketamine’s effects on cognition or mood-related behaviors, with higher doses being ineffective (Chowdhury et al., 2016; Li et al., 2010), together with a high dependency on the behavioral paradigm and experimental timing (Browne and Lucki, 2013). Nevertheless, although the effect of ketamine on contextual fear memory reconsolidation processes remain unclear, NMDA receptors antagonism blocks reconsolidation of a variety of memories (Miller and Sweatt, 2006), including an appetitive Pavlovian memory (Lee and Everitt, 2008), or auditory fear conditioning (Merlo et al., 2014). On the contrary, pre-reactivation treatment with ketamine enhances Pavlovian fear memory in humans (Corlett et al., 2013), and auditory fear conditioning in rats without affecting contextual fear memory (Honsberger et al., 2015). Altogether, these data support a disruption of contextual fear memory reconsolidation by ketamine, resulting in reduced contextual fear memory upon subsequent contextual retrieval the following day.
Notably, this reduction was associated with a down-regulation of Egr1 but not Bdnf mRNA levels in the hippocampal CA1 area. This regulation is of particular interest due to increasing evidence supporting a specific hippocampal involvement of EGR1 in contextual fear memory reconsolidation. In particular, through the use of RNA interference, consolidation of a contextual fear memory was demonstrated to require hippocampal BDNF but not EGR1, whereas contextual fear memory reconsolidation is dependent on EGR1 but not BDNF (Barnes et al., 2012; Kirtley and Thomas, 2010; Lee et al., 2004). Moreover, Egr1 heterozygous mice display a selective deficit in contextual fear memory reconsolidation (Besnard et al., 2013). In addition to further support, at the molecular level, the predominance of reconsolidation processes in our experimental paradigm, these data highlight the down-regulation of Egr1 mRNA levels in the CA1 area of ketamine-treated rats as a good indicator of contextual fear memory blockade by ketamine, as underlined by the trend for a negative link between Egr1 mRNA levels in CA1 and contextual fear memory extinction index. Whether EGR1 is a mediator of ketamine’s effects, however, remains to be investigated.
Although the neuronal activation in response to the final contextual test cannot be isolated from baseline neuronal connectivity due to the absence of naive animals, we could observe a marked alteration of connection between CA1 area and the prelimbic and infralimbic cortices where the correlations were reversed in ketamine-treated rats when compared to saline-injected animals (Supplementary Fig. S4). Interestingly, such switch in connectivity between hippocampus and mPFC has been reported in an auditory conditioning context, with higher hippocampus-mPFC connectivity being associated with low fear in extinguished animals (Sotres-Bayon et al., 2012). Furthermore, despite similar Egr1 mRNA levels in the CeA between saline- and ketamine-treated rats, the connection between CeA and CA1 was highly positively correlated in saline-treated rats, but slightly negatively correlated in ketamine-treated animals. As the CeA is one of the major mediators of fear output signals (Tovote et al., 2015), this further supports the hypothesis that the disruption of fear memory reconsolidation by ketamine involves alterations of the neuronal circuitry promoting fear response.
Despite evidence for the involvement of extra-hippocampal structures in the blockade of fear memory reconsolidation by NMDA receptors antagonism (Lee et al., 2006), D-AP5 infusions into the basolateral amygdala prevent memory destabilization upon retrieval, but not its reconsolidation (Ben Mamou et al., 2006), suggesting that the amygdala does not mediate blockade of contextual fear memory reconsolidation by ketamine. Accordingly, amygdalar Bdnf and Egr1 mRNA levels were similar in ketamine- and saline-treated rats. We observed, however, an upregulation of Bdnf mRNA levels in the prelimbic and infralimbic cortices in ketamine-treated animals, which was positively associated with fear reduction. Although the regulation of BDNF in the mPFC during contextual fear memory reconsolidation remains unclear, it is interesting to note that Bdnf mRNA levels are up-regulated during contextual fear extinction in the mPFC (Bredy et al., 2007). In view of the specific involvement of BDNF in memory consolidation but not reconsolidation, albeit demonstrated in the hippocampus (Barnes et al., 2012; Lee et al., 2004; Miller and Sweatt, 2006), and its positive association with fear reduction in our study, it is possible to hypothesize that the up-regulation of Bdnf mRNA levels observed in the mPFC reflects an enhanced consolidation of contextual fear memory extinction. While this hypothesis is further supported by the facilitation of fear extinction by BDNF in the infralimbic cortex (Peters et al., 2010; Rosas-Vidal et al., 2014), the increase in Bdnf mRNA levels we observed in the prelimbic cortex of ketamine-treated animals appears inconsistent at first, due to the known involvement of this structure in promoting fear (Tovote et al., 2015). Nevertheless, BDNF in the prelimbic cortex does not affect and is not required for fear extinction (Choi et al., 2010; Rosas-Vidal et al., 2014), which would thus suggest that its increase in the mPFC is not contradictory to an enhanced consolidation of fear extinction by ketamine. It would, however, be in contradiction with a recent report that ketamine, albeit at higher dose, does not alter fear extinction in rats (Groeber Travis et al., 2015).
Alternatively, such up-regulation of Bdnf mRNA levels could simply be related to the known up-regulation of BDNF protein levels by ketamine (Autry et al., 2011; Björkholm and Monteggia, 2016; Yang et al., 2013). Consistent with the similar Bdnf mRNA levels detected in the hippocampus of saline- and ketamine-treated rats (Fig. 5), however, this effect in the hippocampus is observed at 30 min following ketamine injection but is not maintained 24 hrs later, and affects proteins but not mRNA levels (Autry et al., 2011), thereby reflecting the transient effect of ketamine on Bdnf mRNA translation. Although the effects of ketamine on regulators of protein translation in the mPFC are well established (Li et al., 2010), its effects on Bdnf mRNA levels in the mPFC still remain unclear and would thus warrant further investigation.
4.3. Conclusions
In this study, we first found that differences in novelty seeking in rats can predict subsequent response to fear conditioning and fear memory extinction in particular. These observations not only add new evidence to the link between novelty seeking and the development of PTSD-like symptoms (Evren et al., 2010; Jakšić et al., 2012; Richman and Frueh, 1997; Wang et al., 1997), they suggest that novelty seeking can predict some aspects of an individual’s response to fear following exposure to a traumatic stressor. The HR/LR model thereby appears as a valuable tool with predictive value for the investigation of individual differences in vulnerability to the development of PTSD-like symptoms. Novelty seeking was not linked, however, to the disruption of contextual fear memory reconsolidation by ketamine. Indeed, 10 mg/kg ketamine treatment following contextual fear memory reactivation impairs fear memory reconsolidation in both high and low novelty seeking rats, resulting in lower freezing behavior upon subsequent memory retrieval the following day. Although the exact molecular correlates of ketamine’s effects remain to be uncovered, these findings depict a first and promising novel therapeutic role for ketamine in affecting established fear memory.
Supplementary Material
Highlights.
Individual differences in novelty seeking in rats predict rate of fear extinction.
High novelty seeking rats show faster fear extinction than low novelty seeking rats.
Ketamine impairs reconsolidation of contextual fear memory.
Ketamine’s effects are associated with early growth response 1 down-regulation in CA1.
Acknowledgments
This work was supported by grants from the National Institute of Mental Health (NIMH) MHR01 MH87583 and MH099085 to M.K. The funding source was not involved in the study’s design, data collection, analysis, and interpretation, or in the manuscript writing and submission processes.
Abbreviations
- ACC
Anterior cingulate cortex
- Bdnf
Brain-derived neurotrophic factor
- BLA
Basolateral amygdala
- CA1
Cornu Ammonis 1
- CA3
Cornu Ammonis 3
- CeA
Central amygdala
- CS
Conditioned stimulus
- DG
Dentate gyrus
- Egr1
Early growth response 1
- HR
High responder
- ILC
Infralimbic cortex
- ITI
Inter-trial interval
- LR
Low responder
- mPFC
medial prefrontal cortex
- NMDA
N-methyl-D-aspartate
- PLC
Prelimbic cortex
- PTSD
Posttraumatic stress disorder
- SSC
Saline Sodium Citrate
Footnotes
Chemical compounds studied in this article: Ketamine hydrochloride (PubChem CID: 3821).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amann LC, Halene TB, Ehrlichman RS, Luminais SN, Ma N, Abel T, Siegel SJ. Chronic ketamine impairs fear conditioning and produces long-lasting reductions in auditory evoked potentials. Neurobiol Dis. 2009;35:311–317. doi: 10.1016/j.nbd.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos T, Stein DJ, Ipser JC. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd; 1996. Pharmacological interventions for preventing post-traumatic stress disorder (PTSD) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi E, Bucherelli C. Brain sites involved in fear memory reconsolidation and extinction of rodents. Neurosci Biobehav Rev. 2015;53:160–190. doi: 10.1016/j.neubiorev.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Barnes P, Kirtley A, Thomas KL. Quantitatively and qualitatively different cellular processes are engaged in CA1 during the consolidation and reconsolidation of contextual fear memory. Hippocampus. 2012;22:149–171. doi: 10.1002/hipo.20879. [DOI] [PubMed] [Google Scholar]
- Ben Mamou C, Gamache K, Nader K. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat Neurosci. 2006;9:1237–1239. doi: 10.1038/nn1778. [DOI] [PubMed] [Google Scholar]
- Besnard A, Caboche J, Laroche S. Recall and reconsolidation of contextual fear memory: differential control by ERK and Zif268 expression dosage. PLoS One. 2013;8:e72006. doi: 10.1371/journal.pone.0072006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkholm C, Monteggia LM. BDNF--a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghans B, Homberg JR. Animal models for posttraumatic stress disorder: An overview of what is used in research. World J Psychiatry. 2015;5:387–396. doi: 10.5498/wjp.v5.i4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem. 2007;14:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne CA, Lucki I. Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants. Front Pharmacol. 2013;4:161. doi: 10.3389/fphar.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo N, Cecchi M, Kabbaj M, Watson SJ, Akil H. Differential effects of social defeat in rats with high and low locomotor response to novelty. Neuroscience. 2011;183:81–89. doi: 10.1016/j.neuroscience.2011.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology. 2013;70:27–34. doi: 10.1016/j.neuropharm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Choi DC, Maguschak KA, Ye K, Jang SW, Myers KM, Ressler KJ. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc Natl Acad Sci U S A. 2010;107:2675–2680. doi: 10.1073/pnas.0909359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury GMI, Zhang J, Thomas M, Banasr M, Ma X, Pittman B, Bristow L, Schaeffer E, Duman RS, Rothman DL, Behar KL, Sanacora G. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero MI, Merino JJ, Sandi C. Correlational relationship between shock intensity and corticosterone secretion on the establishment and subsequent expression of contextual fear conditioning. Behav Neurosci. 1998;112:885–891. doi: 10.1037//0735-7044.112.4.885. [DOI] [PubMed] [Google Scholar]
- Corlett PR, Cambridge V, Gardner JM, Piggot JS, Turner DC, Everitt JC, Arana FS, Morgan HL, Milton AL, Lee JL, Aitken MRF, Dickinson A, Everitt BJ, Absalom AR, Adapa R, Subramanian N, Taylor JR, Krystal JH, Fletcher PC. Ketamine effects on memory reconsolidation favor a learning model of delusions. PLoS One. 2013;8:e65088. doi: 10.1371/journal.pone.0065088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzon P, Rustay NR, Browman KE. Cued and Contextual Fear Conditioning for Rodents. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. CRC Press/Taylor & Francis; Boca Raton (FL): 2011. [PubMed] [Google Scholar]
- Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats--biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34:136–145. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- Dietz DM, Tapocik J, Gaval-Cruz M, Kabbaj M. Dopamine transporter, but not tyrosine hydroxylase, may be implicated in determining individual differences in behavioral sensitization to amphetamine. Physiol Behav. 2005;86:347–355. doi: 10.1016/j.physbeh.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Duclot F, Hollis F, Darcy MJ, Kabbaj M. Individual differences in novelty-seeking behavior in rats as a model for psychosocial stress-related mood disorders. Physiol Behav. 2011;104:296–305. doi: 10.1016/j.physbeh.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclot F, Kabbaj M. Individual Differences in Novelty Seeking Predict Subsequent Vulnerability to Social Defeat through a Differential Epigenetic Regulation of Brain-Derived Neurotrophic Factor Expression. J Neurosci. 2013;33:11048–11060. doi: 10.1523/JNEUROSCI.0199-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Paz R. Fear Generalization and Anxiety: Behavioral and Neural Mechanisms. Biol Psychiatry. 2015;78:336–343. doi: 10.1016/j.biopsych.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Eisenberg M, Kobilo T, Berman DE, Dudai Y. Stability of retrieved memory: inverse correlation with trace dominance. Science. 2003;301:1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- Evren C, Dalbudak E, Cetin R, Durkaya M, Evren B. Relationship of alexithymia and temperament and character dimensions with lifetime post-traumatic stress disorder in male alcohol-dependent inpatients. Psychiatry Clin Neurosci. 2010;64:111–119. doi: 10.1111/j.1440-1819.2009.02052.x. [DOI] [PubMed] [Google Scholar]
- Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S, Kirkwood K, Aan Het Rot M, Lapidus KAB, Wan LB, Iosifescu D, Charney DS. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71:681–688. doi: 10.1001/jamapsychiatry.2014.62. [DOI] [PubMed] [Google Scholar]
- Gil S. Pre-traumatic personality as a predictor of post-traumatic stress disorder among undergraduate students exposed to a terrorist attack: A prospective study in Israel. Pers Individ Dif. 2005;39:819–827. [Google Scholar]
- Groeber Travis CM, Altman DE, Genovese RF. Ketamine administration diminishes operant responding but does not impair conditioned fear. Pharmacol Biochem Behav. 2015;139:84–91. doi: 10.1016/j.pbb.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Hendriksen H, Olivier B, Oosting RS. From non-pharmacological treatments for post-traumatic stress disorder to novel therapeutic targets. Eur J Pharmacol. 2014;732:139–158. doi: 10.1016/j.ejphar.2014.03.031. [DOI] [PubMed] [Google Scholar]
- Hollis F, Duclot F, Gunjan A, Kabbaj M. Individual differences in the effect of social defeat on anhedonia and histone acetylation in the rat hippocampus. Horm Behav. 2011;59:331–337. doi: 10.1016/j.yhbeh.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honsberger MJ, Taylor JR, Corlett PR. Memories reactivated under ketamine are subsequently stronger: A potential pre-clinical behavioral model of psychosis. Schizophr Res. 2015;164:227–233. doi: 10.1016/j.schres.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Liem BJ, Justice JB., Jr Sensitization and individual differences to IP amphetamine, cocaine, or caffeine following repeated intracranial amphetamine infusions. Pharmacol Biochem Behav. 1992;43:815–823. doi: 10.1016/0091-3057(92)90413-a. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse. 1991;9:121–128. doi: 10.1002/syn.890090206. [DOI] [PubMed] [Google Scholar]
- Isackson PJ, Huntsman MM, Murray KD, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- Ito W, Erisir A, Morozov A. Observation of Distressed Conspecific as a Model of Emotional Trauma Generates Silent Synapses in the Prefrontal-Amygdala Pathway and Enhances Fear Learning, but Ketamine Abolishes those Effects. Neuropsychopharmacology. 2015;40:2536–2545. doi: 10.1038/npp.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakšić N, Brajković L, Ivezić E, Topić R, Jakovljević M. The role of personality traits in posttraumatic stress disorder (PTSD) Psychiatr Danub. 2012;24:256–266. [PubMed] [Google Scholar]
- Juven-Wetzler A, Cohen H, Kaplan Z, Kohen A, Porat O, Zohar J. Immediate ketamine treatment does not prevent posttraumatic stress responses in an animal model for PTSD. Eur Neuropsychopharmacol. 2014;24:469–479. doi: 10.1016/j.euroneuro.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Akil H. Individual differences in novelty-seeking behavior in rats: a c-fos study. Neuroscience. 2001;106:535–545. doi: 10.1016/s0306-4522(01)00291-3. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kirtley A, Thomas KL. The exclusive induction of extinction is gated by BDNF. Learn Mem. 2010;17:612–619. doi: 10.1101/lm.1877010. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. Posttraumatic stress disorder: the role of medial prefrontal cortex and amygdala. Neuroscientist. 2009;15:540–548. doi: 10.1177/1073858409333072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal KM, Wood MA. Epigenetics and persistent memory: implications for reconsolidation and silent extinction beyond the zero. Nat Neurosci. 2013;16:124–129. doi: 10.1038/nn.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JLC, Everitt BJ. Appetitive memory reconsolidation depends upon NMDA receptor-mediated neurotransmission. Neurobiol Learn Mem. 2008;90:147–154. doi: 10.1016/j.nlm.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Lee JLC, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- Lee JLC, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten L, Vansteenwegen D, van Kuyck K, Deckers D, Nuttin B. Optimization of a contextual conditioning protocol for rats using combined measurements of startle amplitude and freezing: the effects of shock intensity and different types of conditioning. J Neurosci Methods. 2011;194:305–311. doi: 10.1016/j.jneumeth.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Matar MA, Zohar J, Cohen H. Translationally relevant modeling of PTSD in rodents. Cell Tissue Res. 2013;354:127–139. doi: 10.1007/s00441-013-1687-6. [DOI] [PubMed] [Google Scholar]
- Merlo E, Milton AL, Goozée ZY, Theobald DE, Everitt BJ. Reconsolidation and extinction are dissociable and mutually exclusive processes: behavioral and molecular evidence. J Neurosci. 2014;34:2422–2431. doi: 10.1523/JNEUROSCI.4001-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Amnesia or retrieval deficit? Implications of a molecular approach to the question of reconsolidation. Learn Mem. 2006;13:498–505. doi: 10.1101/lm.304606. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla E, Shumake J, Barrett DW, Holmes G, Sheridan EC, Gonzalez-Lima F. Novelty-evoked activity in open field predicts susceptibility to helpless behavior. Physiol Behav. 2010;101:746–754. doi: 10.1016/j.physbeh.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. A stereotaxic atlas of the rat brain. New York: Academic; 1998. [Google Scholar]
- Pedreira ME, Maldonado H. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron. 2003;38:863–869. doi: 10.1016/s0896-6273(03)00352-0. [DOI] [PubMed] [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328:1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminière JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre PJ, Vezina P. Predisposition to self-administer amphetamine: the contribution of response to novelty and prior exposure to the drug. Psychopharmacology. 1997;129:277–284. doi: 10.1007/s002130050191. [DOI] [PubMed] [Google Scholar]
- Pietersen CY, Bosker FJ, Doorduin J, Jongsma ME, Postema F, Haas JV, Johnson MP, Koch T, Vladusich T, den Boer JA. An animal model of emotional blunting in schizophrenia. PLoS One. 2007;2:e1360. doi: 10.1371/journal.pone.0001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietersen CY, Bosker FJ, Postema F, Fokkema DS, Korf J, den Boer JA. Ketamine administration disturbs behavioural and distributed neural correlates of fear conditioning in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1209–1218. doi: 10.1016/j.pnpbp.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Pitman RK. Will reconsolidation blockade offer a novel treatment for posttraumatic stress disorder? Front Behav Neurosci. 2011;5:11. doi: 10.3389/fnbeh.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikov R, Diwan M, Nobrega JN, Hamani C. Towards a better preclinical model of PTSD: characterizing animals with weak extinction, maladaptive stress responses and low plasma corticosterone. J Psychiatr Res. 2015;61:158–165. doi: 10.1016/j.jpsychires.2014.12.017. [DOI] [PubMed] [Google Scholar]
- Richman H, Frueh BC. Personality and PTSD II: Personality assessment of PTSD-diagnosed Vietnam veterans using the Cloninger Tridimensional Personality Questionnaire (TPQ) Depress Anxiety. 1997;6:70–77. doi: 10.1002/(sici)1520-6394(1997)6:2<70::aid-da3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Rosas-Vidal LE, Do-Monte FH, Sotres-Bayon F, Quirk GJ. Hippocampal--prefrontal BDNF and memory for fear extinction. Neuropsychopharmacology. 2014;39:2161–2169. doi: 10.1038/npp.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Touyarot K. Mid-life stress and cognitive deficits during early aging in rats: individual differences and hippocampal correlates. Neurobiol Aging. 2006;27:128–140. doi: 10.1016/j.neurobiolaging.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Sangha S, Scheibenstock A, Lukowiak K. Reconsolidation of a long-term memory in Lymnaea requires new protein and RNA synthesis and the soma of right pedal dorsal 1. J Neurosci. 2003;23:8034–8040. doi: 10.1523/JNEUROSCI.23-22-08034.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumake J, Barrett D, Gonzalez-Lima F. Behavioral characteristics of rats predisposed to learned helplessness: reduced reward sensitivity, increased novelty seeking, and persistent fear memories. Behav Brain Res. 2005;164:222–230. doi: 10.1016/j.bbr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Siegmund A, Wotjak CT. Toward an animal model of posttraumatic stress disorder. Ann N Y Acad Sci. 2006;1071:324–334. doi: 10.1196/annals.1364.025. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron. 2012;76:804–812. doi: 10.1016/j.neuron.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack A, Carrier N, Dietz D, Hollis F, Sorenson J, Kabbaj M. Sex differences in social interaction in rats: role of the immediate-early gene zif268. Neuropsychopharmacology. 2010;35:570–580. doi: 10.1038/npp.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckler T, Risbrough V. Pharmacological treatment of PTSD - established and new approaches. Neuropharmacology. 2012;62:617–627. doi: 10.1016/j.neuropharm.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for post traumatic stress disorder (PTSD) Cochrane Database Syst Rev. 2006:CD002795. doi: 10.1002/14651858.CD002795.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touyarot K, Venero C, Sandi C. Spatial learning impairment induced by chronic stress is related to individual differences in novelty reactivity: search for neurobiological correlates. Psychoneuroendocrinology. 2004;29:290–305. doi: 10.1016/s0306-4530(03)00031-3. [DOI] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM. From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem. 2014;113:3–18. doi: 10.1016/j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veyrac A, Besnard A, Caboche J, Davis S, Laroche S. The transcription factor Zif268/Egr1, brain plasticity, and memory. Prog Mol Biol Transl Sci. 2014;122:89–129. doi: 10.1016/B978-0-12-420170-5.00004-0. [DOI] [PubMed] [Google Scholar]
- Wang S, Mason J, Charney D, Yehuda R, Riney S, Southwick S. Relationships between hormonal profile and novelty seeking in combat-related posttraumatic stress disorder. Biol Psychiatry. 1997;41:145–151. doi: 10.1016/S0006-3223(95)00648-6. [DOI] [PubMed] [Google Scholar]
- Whitaker AM, Gilpin NW, Edwards S. Animal models of post-traumatic stress disorder and recent neurobiological insights. Behav Pharmacol. 2014;25:398–409. doi: 10.1097/FBP.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Hu YM, Zhou ZQ, Zhang GF, Yang JJ. Acute administration of ketamine in rats increases hippocampal BDNF and mTOR levels during forced swimming test. Ups J Med Sci. 2013;118:3–8. doi: 10.3109/03009734.2012.724118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Antelman SM. Criteria for rationally evaluating animal models of posttraumatic stress disorder. Biol Psychiatry. 1993;33:479–486. doi: 10.1016/0006-3223(93)90001-t. [DOI] [PubMed] [Google Scholar]
- Zhang LM, Zhou WW, Ji YJ, Li Y, Zhao N, Chen HX, Xue R, Mei XG, Zhang YZ, Wang HL, Li YF. Anxiolytic effects of ketamine in animal models of posttraumatic stress disorder. Psychopharmacology. 2015;232:663–672. doi: 10.1007/s00213-014-3697-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.