Abstract
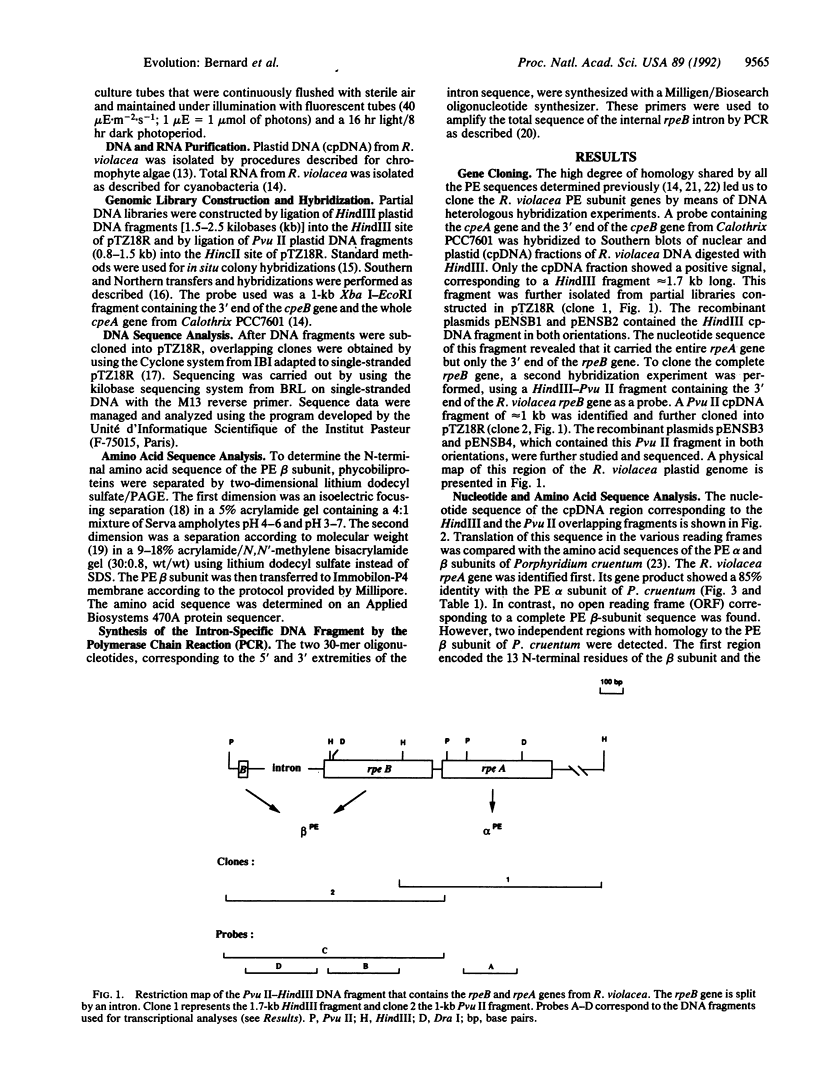
The phycobilisome of the eukaryotic unicellular red alga Rhodella violacea presents in some respects an organization that is intermediate between those of the homologous counterparts found in cyanobacteria (the putative chloroplast progenitor) and more advanced, pluricellular red algae. This suggests evolutionary relationships that we investigated at the genome level. The present work describes the sequences of two rhodophytan phycobilisome genes, rpeA and rpeB. These chloroplast genes encode the alpha and beta subunits of phycoerythrin, the major component of the light-harvesting antennae and one of the most abundant cellular proteins in these algae. The amino acid sequences deduced from both rpeA and rpeB present strong homologies with those previously reported for phycoerythrin subunits of cyanobacteria, rhodophyta, and cryptomonads. The main difference with the corresponding cyanobacterial genes was the unexpected occurrence of an intervening sequence that split rpeB into two exons. This intervening sequence presents characteristics of group II introns but lacks several structural domains. Transcriptional analyses showed that the two rpe genes are cotranscribed and that the major RNA species detected corresponds to a mature mRNA lacking the intron. As the phycobiliproteins form a group of closely related polypeptides in cyanobacteria and rhodophyta, the molecular events affecting the corresponding genes, such as the rpeB intron, may be a clue to elucidate some aspects of the molecular processes involved in the evolution of plastid genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. K., Grossman A. R. Structure and light-regulated expression of phycoerythrin genes in wild-type and phycobilisome assembly mutants of Synechocystis sp. strain PCC 6701. J Bacteriol. 1990 Mar;172(3):1297–1305. doi: 10.1128/jb.172.3.1297-1305.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G. Determinants of messenger RNA stability. Cell. 1987 Jan 16;48(1):5–6. doi: 10.1016/0092-8674(87)90346-1. [DOI] [PubMed] [Google Scholar]
- Choquet Y., Goldschmidt-Clermont M., Girard-Bascou J., Kück U., Bennoun P., Rochaix J. D. Mutant phenotypes support a trans-splicing mechanism for the expression of the tripartite psaA gene in the C. reinhardtii chloroplast. Cell. 1988 Mar 25;52(6):903–913. doi: 10.1016/0092-8674(88)90432-1. [DOI] [PubMed] [Google Scholar]
- Damerval T., Castets A. M., Guglielmi G., Houmard J., Tandeau de Marsac N. Occurrence and distribution of gas vesicle genes among cyanobacteria. J Bacteriol. 1989 Mar;171(3):1445–1452. doi: 10.1128/jb.171.3.1445-1452.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelhoff T., Grossman A. Cytoplasmic and chloroplast synthesis of phycobilisome polypeptides. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3339–3343. doi: 10.1073/pnas.80.11.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N. Light guides. Directional energy transfer in a photosynthetic antenna. J Biol Chem. 1989 Jan 5;264(1):1–4. [PubMed] [Google Scholar]
- Goldschmidt-Clermont M., Choquet Y., Girard-Bascou J., Michel F., Schirmer-Rahire M., Rochaix J. D. A small chloroplast RNA may be required for trans-splicing in Chlamydomonas reinhardtii. Cell. 1991 Apr 5;65(1):135–143. doi: 10.1016/0092-8674(91)90415-u. [DOI] [PubMed] [Google Scholar]
- Kaminski P. A., Elmerich C. Involvement of fixLJ in the regulation of nitrogen fixation in Azorhizobium caulinodans. Mol Microbiol. 1991 Mar;5(3):665–673. doi: 10.1111/j.1365-2958.1991.tb00738.x. [DOI] [PubMed] [Google Scholar]
- Klotz A. V., Glazer A. N. Characterization of the bilin attachment sites in R-phycoerythrin. J Biol Chem. 1985 Apr 25;260(8):4856–4863. [PubMed] [Google Scholar]
- Kuhsel M. G., Strickland R., Palmer J. D. An ancient group I intron shared by eubacteria and chloroplasts. Science. 1990 Dec 14;250(4987):1570–1573. doi: 10.1126/science.2125748. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mazel D., Guglielmi G., Houmard J., Sidler W., Bryant D. A., Tandeau de Marsac N. Green light induces transcription of the phycoerythrin operon in the cyanobacterium Calothrix 7601. Nucleic Acids Res. 1986 Nov 11;14(21):8279–8290. doi: 10.1093/nar/14.21.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel F., Umesono K., Ozeki H. Comparative and functional anatomy of group II catalytic introns--a review. Gene. 1989 Oct 15;82(1):5–30. doi: 10.1016/0378-1119(89)90026-7. [DOI] [PubMed] [Google Scholar]
- Mörschel E., Wehrmeyer W., Koller K. P. Biliprotein assembly in the disc-shaped phycobilisomes of Rhodella violacea. Electron microscopical and biochemical analysis of B-phycoerythrin and B-phycoerythrin--C-phycocyanin aggregates. Eur J Cell Biol. 1980 Aug;21(3):319–327. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Peebles C. L., Perlman P. S., Mecklenburg K. L., Petrillo M. L., Tabor J. H., Jarrell K. A., Cheng H. L. A self-splicing RNA excises an intron lariat. Cell. 1986 Jan 31;44(2):213–223. doi: 10.1016/0092-8674(86)90755-5. [DOI] [PubMed] [Google Scholar]
- Reith M., Douglas S. Localization of beta-phycoerythrin to the thylakoid lumen of Cryptomonas phi does not involve a signal peptide. Plant Mol Biol. 1990 Oct;15(4):585–592. doi: 10.1007/BF00017833. [DOI] [PubMed] [Google Scholar]
- Schmelzer C., Schweyen R. J. Self-splicing of group II introns in vitro: mapping of the branch point and mutational inhibition of lariat formation. Cell. 1986 Aug 15;46(4):557–565. doi: 10.1016/0092-8674(86)90881-0. [DOI] [PubMed] [Google Scholar]
- Sidler W., Kumpf B., Suter F., Klotz A. V., Glazer A. N., Zuber H. The complete amino-acid sequence of the alpha and beta subunits of B-phycoerythrin from the rhodophytan alga Porphyridium cruentum. Biol Chem Hoppe Seyler. 1989 Feb;370(2):115–124. doi: 10.1515/bchm3.1989.370.1.115. [DOI] [PubMed] [Google Scholar]
- Turner S., Burger-Wiersma T., Giovannoni S. J., Mur L. R., Pace N. R. The relationship of a prochlorophyte Prochlorothrix hollandica to green chloroplasts. Nature. 1989 Jan 26;337(6205):380–382. doi: 10.1038/337380a0. [DOI] [PubMed] [Google Scholar]
- Wilbanks S. M., de Lorimier R., Glazer A. N. Phycoerythrins of marine unicellular cyanobacteria. III. Sequence of a class II phycoerythrin. J Biol Chem. 1991 May 25;266(15):9535–9539. [PubMed] [Google Scholar]
- Xu M. Q., Kathe S. D., Goodrich-Blair H., Nierzwicki-Bauer S. A., Shub D. A. Bacterial origin of a chloroplast intron: conserved self-splicing group I introns in cyanobacteria. Science. 1990 Dec 14;250(4987):1566–1570. doi: 10.1126/science.2125747. [DOI] [PubMed] [Google Scholar]
- van der Veen R., Arnberg A. C., van der Horst G., Bonen L., Tabak H. F., Grivell L. A. Excised group II introns in yeast mitochondria are lariats and can be formed by self-splicing in vitro. Cell. 1986 Jan 31;44(2):225–234. doi: 10.1016/0092-8674(86)90756-7. [DOI] [PubMed] [Google Scholar]