Abstract
OBJECTIVE
To evaluate kidney functional and overall survival (OS) outcomes in a cohort of patients who underwent partial nephrectomy (PN) or radical nephrectomy (RN) for tumors ≤4 cm.
MATERIALS AND METHODS
We performed a retrospective study on 2110 patients who underwent PN or RN with normal contralateral kidneys and normal serum creatinine from 1989 through 2012. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. Primary end points were baseline incidence of CKD, OS, and new onset of eGFR ≤60 and ≤45 mL/min/1.73 m2.
RESULTS
Preoperatively, 30% and 8% of the cohort had eGFR ≤60 and ≤45 mL/min/1.73 m2, respectively. Five-year freedom from eGFR ≤60 mL/min/1.73 m2 was 24% (95% confidence interval [CI], 19%-30%) and 76% (95% CI, 72%-78%) for RN and PN, respectively, and 5-year freedom from eGFR ≤45 mL/min/1.73 m2 was 51% (95% CI, 45%-56%) and 91% (95% CI, 89%-93%) for RN and PN, respectively. On multivariable analysis, hazard ratio for RN vs PN was 4.98 (95% CI, 4.11-6.04, P <.0001) for new onset of eGFR ≤60 mL/min/1.73 m2 and 9.28 (95% CI, 7.26-11.86, P <.0001) for new onset of eGFR ≤45 mL/min/1.73 m2. The RN group had a higher rate of death per year than the partial group (hazard ratio = 1.61, 95% CI, 1.24-2.08, P = .0003).
CONCLUSION
The present study confirms published works demonstrating that a significant proportion of patients have pre-existing CKD. In patients with normal kidney function, RN is associated with a significantly higher risk for developing CKD and worse OS than PN.
It is estimated that approximately 63,920 new cases of kidney cancer were diagnosed in the United States in 2014.1 Retrospective clinical studies support the oncological equivalence of radical nephrectomy (RN) and partial nephrectomy (PN) for small renal tumors.2-6 The current goals for treating small renal masses are to obtain local tumor control and prevent renal-related late morbidity, provided that the patient has a minimal competing comorbidity and sufficiently long life expectancy to benefit from treatment. PN is the treatment of choice for relatively young and healthy patients, whereas active surveillance is an effective strategy for patients who are elderly or have comorbidities.7,8
Studies have shown that patients have an increased risk of developing chronic kidney disease (CKD) after RN compared with PN.9-11 CKD is associated with increased cardiovascular morbidity and potential mortality, which is of concern for patients with pre-existing CKD who are undergoing kidney tumor surgery.12,13 In our original report, 26% of patients had pre-existing CKD despite having serum creatinine levels within normal limits.9
Our goal is to evaluate kidney functional outcomes and overall survival (OS) in an expanded cohort of patients with longer follow-up using the more accurate Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation for estimating glomerular filtration rate (GFR). We will also address controversies that have emerged in this field since our original publication in 2006.11,14,15
MATERIALS AND METHODS
Following institutional review board approval, we analyzed prospectively collected data from a total of 2320 patients who had creatinine levels within normal limits (defined as ≤1.4 mg/dL) and imaging documenting a normal contralateral kidney before undergoing PN or RN for solitary renal cortical tumors ≤4 cm at the Memorial Sloan Kettering Cancer Center from January 1989 through September 2012. This data set included patients from the 2006 Huang et al9 study who had undergone surgery before September 2005 and had extended follow-up until December 2013. These patients represent approximately a quarter of the total number of PN performed in this series. We excluded patients with metastatic disease (n = 18), insufficient follow-up (<1 month after surgery, n = 159), and those missing race information (n = 33). The final data set we analyzed included 2110 patients.
Preoperative characteristics including age, American Society of Anesthesiologists (ASA) score, ethnic origin, sex, serum creatinine, estimated glomerular filtration rate (eGFR), diabetes mellitus, and hypertension were recorded, and inclusion in the 2006 Huang et al cohort was also recorded. eGFR measurements were estimated using the CKD-EPI equation (www.qxmd.com/ renal/Calculate-CKD-EPI-GFR.php).16 Postoperative estimates of GFR were obtained ≥4 weeks after PN or RN and taken at least 3 months apart subsequently.
Main outcomes of the present study included the development of CKD as defined by the occurrence of eGFR ≤60 mL/ min/1.73 m2 on 2 consecutive follow-up visits at least 3 months apart or the occurrence of eGFR ≤45 mL/min/1.73 m2 on 2 consecutive follow-up visits at least 3 months apart, and OS.
Multivariable Cox proportional hazards regression was used to determine whether operation type (RN or PN) was associated with postoperative CKD and OS, after adjusting for age, ASA score, hypertension, diabetes mellitus, and preoperative eGFR. Patients who had no events were censored at the time of their last GFR estimate. Patients with pre-existing CKD (eGFR that was either ≤60 or ≤45 mL/min/1.73 m2) were not included in this outcome analysis. No patients were excluded from OS analysis based on preoperative eGFR measurements. When multiple eGFR measurements were performed on the same day, only the lowest daily recorded measurement was used to define CKD outcomes. All statistical analyses were conducted with Stata version 12.0 (StataCorp, College Station, TX).
RESULTS
There are several differences in baseline characteristics between patients who underwent PN and patients who underwent RN irrespective of baseline eGFR (Table 1).
Table 1.
Preoperative and pathologic characteristics of patients
| Operation Type | All Preoperative eGFR |
Preoperative eGFR >60 mL/min/1.73 m2 |
Preoperative eGFR >45 mL/min/1.73 m2 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Radical (n = 403) |
Partial (n = 1707) |
P Value | Radical (n = 284) |
Partial (n = 1184) |
P Value | Radical (n = 365) |
Partial (n = 1582) |
P Value | |
| Age at surgery (years) | 65 (56, 71) | 60 (52, 69) | <.0001 | 62 (54, 69) | 57 (48, 64) | <.0001 | 64 (56, 71) | 59 (51, 67) | <.0001 |
| Preoperative creatinine (μmol/L) | 100 (90, 120) | 110 (90, 120) | .092 | 100 (80, 110) | 100 (90, 110) | .018 | 100 (90, 110) | 110 (90, 120) | .009 |
| Preoperative eGFR (mL/min/1.73 m2) | 68 (56, 81) | 69 (57, 83) | .5 | 76 (67, 87) | 76 (68, 89) | .2 | 70 (61, 83) | 71 (60, 84) | 1 |
| ASA score of 3 or 4 | 137 (34%) | 729 (43%) | .001 | 79 (28%) | 447 (38%) | .002 | 114 (31%) | 640 (40%) | .001 |
| Black | 20 (5.0%) | 80 (4.7%) | .8 | 12 (4.2%) | 60 (5.1%) | .6 | 18 (4.9%) | 76 (4.9%) | .9 |
| Male gender | 237 (59%) | 1050 (62%) | .3 | 167 (59%) | 753 (64%) | .13 | 215 (59%) | 982 (62%) | .3 |
| Hypertension | 222 (55%) | 877 (51%) | .2 | 143 (50%) | 524 (44%) | .073 | 194 (53%) | 775 (49%) | .2 |
| Diabetes | 53 (13%) | 228 (13%) | 1 | 38 (13%) | 137 (12%) | .4 | 48 (13%) | 198 (13%) | .7 |
| Tumor size (cm) | 3.0 (2.5, 3.5) | 2.5 (1.8, 3.1) | <.0001 | 3.0 (2.5, 3.6) | 2.5 (1.8, 3.2) | <.0001 | 3.0 (2.5, 3.5) | 2.5 (1.8, 3.1) | <.0001 |
| Histology (post surgery) | |||||||||
| Conventional clear cell | 241 (60%) | 909 (53%) | .11 | 176 (62%) | 656 (55%) | .3 | 219 (60%) | 850 (54%) | .2 |
| Papillary | 51 (13%) | 246 (14%) | 34 (12%) | 155 (13%) | 47 (13%) | 218 (14%) | |||
| Chromophobe | 27 (6.7%) | 153 (9.0%) | 21 (7.4%) | 107 (9.0%) | 26 (7.1%) | 144 (9.1%) | |||
| Other | 84 (21%) | 399 (23%) | 53 (19%) | 266 (22%) | 73 (20%) | 370 (23%) | |||
| Huang et al cohort9 | 222 (55%) | 351 (21%) | <.0001 | 181 (64%) | 272 (23%) | <.0001 | 214 (59%) | 348 (22%) | <.0001 |
ASA, American Society of Anesthesiologists; eGFR, estimated glomerular filtration rate.
Estimates are given as median (interquartile range) or frequency (percentage). P values were calculated using the Wilcoxon rank sum test for continuous variables and Fisher's exact test for categorical variables.
Patients who underwent RN were older at the time of surgery (P <.0001) and the majority had lower ASA scores of 1 or 2, whereas the majority of patients who underwent PN had higher ASA scores of 3 or 4 (P = .01). Patients who underwent RN had significantly larger tumors (approximately 0.5 cm larger) (P <.0001). Compared with the Huang et al 2006 cohort, a higher proportion of our current cohort underwent PN (P <.0001). Results from multivariable analyses did not meaningfully change with the addition of the original cohort indicator and were not reported. Despite having preoperative serum creatinine levels within normal limits, 642 (30%) patients had an eGFR ≤60 mL/min/1.73 m2 and 163 (8%) patients had an eGFR ≤45 mL/min/1.73 m2. The median interval between surgery and postoperative eGFR was similar for both RN at 5 months (interquartile range [IQR] 0.5-7.1) and PN at 6.2 months (IQR 2.3-11.7).
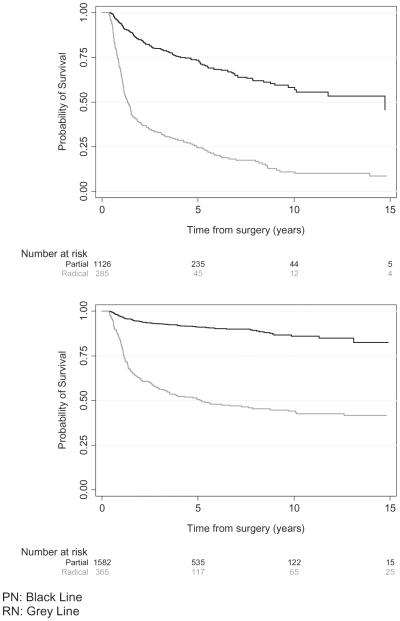
Of 1468 patients with preoperative eGFR >60 mL/min/ 1.73 m2, 455 (31%) developed postoperative eGFR <60 mL/ min/1.73 m2. Median follow-up time for patients without new onset of eGFR at this threshold was 2.8 years. Median time to development of eGFR <60 mL/min/1.73 m2 in the RN group was 1.3 years (IQR 0.8-4.8) and was not reached (lower quartile 5.0 years) in the PN group. The 3-, 5-, and 10-year probabilities of freedom from new onset of eGFR <60 mL/min/1.73 m2 were 32% (95% confidence interval [CI], 26%-38%), 24% (95% CI, 19%-30%), and 12% (95% CI, 8%-17%), respectively, for patients who underwent RN, and 81% (95% CI, 79%-84%), 76% (95% CI, 72%-78%), and 63% (95% CI, 58%-68%), respectively, for those who underwent PN. Multivariable analysis demonstrated that patients who underwent RN progressed toward this outcome faster than those who underwent PN (hazard ratio [HR] = 4.98, P <.0001, Table 2). The Kaplan-Meier curve in Figure 1 shows the probability of freedom from GFR <60 mL/min/1.73 m2 over time for patients who underwent PN compared with those who underwent RN.
Table 2.
Multivariable Cox proportional hazards regression to determine factors associated with new onset of eGFR <60 and <45 mL/min/1.73 m2 3 months following surgery and overall survival
| Overall Survival |
eGFR <60 mL/min/1.73 m2 |
eGFR <45 mL/min/1.73 m2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Procedure | |||||||||
| Partial | Ref. | — | — | Ref. | — | — | Ref. | — | — |
| Radical | 1.61 | 1.24-2.08 | .0003 | 4.98 | 4.11-6.04 | <.0001 | 9.28 | 7.26-11.86 | <.0001 |
| Age per year | 1.05 | 1.04-1.07 | <.0001 | 1.03 | 1.02-1.04 | <.0001 | 1.05 | 1.04-1.07 | <.0001 |
| ASA score | |||||||||
| 1 or 2 | Ref. | — | — | Ref. | — | — | Ref. | — | — |
| 3 or 4 | 2.43 | 1.88-3.15 | <.0001 | 1.11 | 0.90-1.36 | .3 | 1.41 | 1.10-1.81 | .006 |
| Hypertension | |||||||||
| No | Ref. | — | — | Ref. | — | — | Ref. | — | — |
| Yes | 0.76 | 0.59-0.97 | .030 | 1.09 | 0.90-1.32 | .4 | 1.51 | 1.16-1.96 | .002 |
| Diabetes | |||||||||
| No | Ref. | — | — | Ref. | — | — | Ref | — | — |
| Yes | 0.97 | 0.70-1.35 | .9 | 1.27 | 0.99-1.63 | .062 | 1.54 | 1.15-2.06 | .004 |
| Preoperative eGFR (mL/min/1.73 m2) |
0.99 | 0.98-1.00 | .010 | 0.96 | 0.95-0.97 | <.0001 | 0.94 | 0.93-0.95 | <.0001 |
CI, confidence interval; HR, hazard ratio.
Figure 1.
Probability of freedom from new onset of estimated glomerular filtration rate <60 mL/min/1.73 m2 for patients who underwent partial nephrectomy vs those who underwent radical nephrectomy (top). Probability of freedom from new onset of estimated glomerular filtration rate <45 mL/min/1.73 m2 for patients who underwent partial nephrectomy vs those who underwent radical nephrectomy (bottom).
Preoperative eGFR for 1947 patients was >45 mL/min/ 1.73 m2, and 291 (15%) patients developed onset of eGFR <45 mL/min/1.73 m2. Median follow-up time for patients without onset of eGFR lower than this threshold was 43 months. Median time to development of new onset of eGFR <45 mL/min/1.73 m2 in the RN group was 5.2 years (lower quartile, 1.1; upper quartile not reached), whereas the lower quartile was not reached in the PN group. The 3-, 5-, and 10-year probabilities of freedom from new onset of eGFR <45 mL/min/1.73 m2 were 56% (95% CI, 50%-62%), 51% (95% CI, 45%-56%), and 44% (95% CI, 38%-50%), respectively, for patients who underwent RN, and 93% (95% CI, 91%-94%), 91% (95% CI, 89%-93%), and 86% (95% CI, 83%-89%), respectively, for patients who underwent PN. Multivariable analysis demonstrated that patients who underwent RN progressed toward a new onset of eGFR <45 mL/min/1.73 m2 more than those who underwent a PN (HR = 9.28, P <.001, Table 2). The Kaplan-Meier curve in Figure 1 shows the probability of freedom from eGFR <45 mL/min/1.73 m2 over time for patients who underwent PN compared with those who underwent RN.
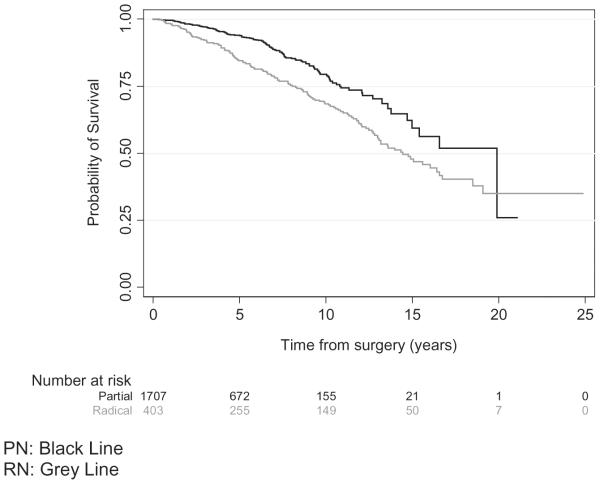
Of 2110 patients included in OS analysis, 279 patients (13%) died. Median follow-up time for patients who were alive was 49 months. Median time to death for the RN group was 14 years (lower quantile, 8 years; upper quantile not reached) and median time to death for the PN group was 20 years (lower quantile, 11 years; upper quantile not reached). Patients who underwent RN had a higher rate of death per year compared with those who underwent PN (HR = 1.61, P = .0003, Table 2). The probability of survival was greater for those undergoing PN from the time of surgery to roughly 20 years after surgery (Fig. 2). The 3-, 5-, and 10-year probabilities of survival for patients who underwent RN were 92% (95% CI, 89%-95%), 84% (95% CI, 80%-88%), and 68% (95% CI, 63%-74%), respectively, and 97% (95% CI, 96%-98%), 94% (95% CI, 92%-95%), and 79% (95% CI, 75%-83%) for those who underwent PN, respectively.
Figure 2.
Probability of overall survival for patients who underwent partial nephrectomy vs those who underwent radical nephrectomy.
DISCUSSION
CKD describes a level of renal function ≤60 mL/min/ 1.73 m2 but above end-stage renal disease of 15 mL/min/ 1.73 m2 and is classified into stages based on eGFR with each increase in stage associated with higher risk for hospitalization events, cardiovascular disease, and death.12,13 To simplify and facilitate earlier identification of CKD and initiate medical interventions to delay CKD progression, equations have been developed to estimate GFR.17,18 There have been concerns regarding the general applicability and accuracy of these equations for patient populations for which they were not originally developed.19 A newer eGFR equation, the CKD Epidemiology Collaboration (CKD-EPI) equation, was introduced in 2010 and validated in larger and more diverse patient populations. The CKD-EPI equation was reported to be more accurate for patients with eGFR >60 mL/min/1.73 m2 than the Modification of Diet in Renal Disease (MDRD) equation.20 Another study found the MDRD equation to overestimate by 7% newly developed stage III CKD (eGFR ≤60 mL/min/1.73 m2) in patients who underwent PN when compared with the CKD-EPI equation.21
In 2006, using the MDRD equation, we reported that 26% of patients who underwent PN or RN for a small kidney tumor had underlying stage III CKD (eGFR <60 mL/ min/1.73 m2), despite serum creatinine levels within normal limits.9 Using the CKD-EPI equation for the same cohort, we now found that 21% of those with normal baseline serum creatinine levels had pre-existing stage III CKD, which is consistent with comparison reports described above. This finding underlines the importance of now using more accurate estimates of kidney function to avoid overdiagnosis of CKD both at the baseline and as a result of treatment. Furthermore, using the CKD-EPI equation in the entire data set, we demonstrated that 31% of patients presenting with a serum creatinine within normal limits had an eGFR <60 mL/min/1.73 m2. Unlike carefully selected kidney donors, patients with renal tumors have more baseline comorbidities, including hypertension, advanced age, diabetes, and vascular disease that can adversely impact baseline renal function.
Retrospective studies have shown that there is an increased risk of developing new onset of CKD after RN when compared with PN.9-11 The European Organisation for Research and Treatment of Cancer (EORTC) randomized trial 30904 confirmed the negative renal functional impact of RN compared with PN for small renal masses on postoperative kidney function; however, they reported worse OS for the PN cohort.11 Although widely cited as the only level-1 evidence for comparing RN with PN, significant limitations exist in the EORTC 30904 trial. The study began in 1992, designed as an intention to treat noninferiority study, at a time when all renal tumors, regardless of size, were resected by RN if the contralateral kidney appeared normal. PN was used sparingly for bilateral renal tumors or tumor in the solitary kidney, and few surgeons world-wide were skilled at PN. Skepticism existed on whether PN was a safe oncological procedure; elements of PN that have evolved since (renoprotection, regional ischemia, careful case selection) were likely not widely employed then. There was little concern that adverse health events would occur following RN because this clinical situation was felt to be similar to that of living kidney donors undergoing surgery for transplantation, despite the fact that even then donors were carefully selected, on average 25 years younger than patients with renal tumors, and by definition, devoid of all serious medical comorbidities. Accrual was poor; in 1998, the EORTC invited the Eastern Cooperative Oncology Group and Southwest Oncology Group from the United States and the National Cancer Institute of Canada to participate, with the goal of randomizing 1300 patients to detect a 3% difference in survival. The study closed after 11 years with only 541 patients randomized from a total of 45 centers in Europe and North America. For various reasons (including missing pathology, higher stage pathology, tumor multifocality), 81 patients randomized to RN and 73 patients randomized to PN were excluded, leaving 71% of patients for analysis.
Survival outcomes from the EORTC 30904 trial were published in 2011.15 Median follow-up was 9.3 years, with 12 of 117 (10.3%) deaths attributed to renal cancer. OS was compared utilizing intention-to-treat analysis; however, 55 (14.1%) patients switched treatment: 39 patients randomized to PN underwent RN (14.9%) and 16 patients randomized to RN underwent PN (5.9%). A total of 53 patients (13.5%) were lost to follow-up with statistical analysis conducted under the assumption that they were dead from unknown causes. There was a significant 10-year OS advantage of 81% for RN vs 75.7% for PN. This difference was not seen when only the patients with renal cancer were analyzed and hence there was a conclusion that PN was not inferior to RN in the surgical management of small renal cancers. The authors of the EORTC 30904 trial provided no details for the number and complexity of operations performed at each center, estimated residual preserved kidney in patients undergoing PN, whether renoprotective measures were employed, or what the ischemia times were.
In 2013, using the MDRD equation to estimate kidney function, the authors found that at a median follow-up of almost 7 years, 86% of patients who had RN developed stage III CKD, compared with 65% of patients who had PN.11 Approximately 93% of all patients had a serum creatinine level ≤1.25 mg/dL; however, baseline eGFR was not calculated and the number of patients with pre-existing CKD was unknown, which could account for some who developed CKD or advanced to a greater CKD stage. There was no significant difference in the number of patients with stage IV or V CKD and end-stage renal disease between the groups, although the numbers were very small. There was a significant difference in postoperative eGFR, until about 10 years postoperatively, favoring PN.
Our current study confirms the conclusion from our previous study that PN decreases the risk of developing CKD in comparison with RN. With a median follow-up of 2.2 and 3.1 years, RN leads to an eGFR <60 and <45 mL/min/ 1.73 m2, which is 5 and 9 times the rate corresponding to PN, respectively. This difference persisted >10 years even when using the more accurate CKD-EPI equation to estimate kidney function.
Numerous studies have shown increased risk of CKD-related morbidity after RN when compared with PN,22-25 and improved OS.26 Despite limitations of the EORTC 30904 study, some began to question the nononcological benefits of PN. An explanation for the findings from the EORTC trial may be that there is an innate difference between medical renal disease, low eGFR due to comorbid conditions (CKD-M), and low eGFR caused by surgical removal of nephrons (CKD-S). To investigate this, Cleveland Clinic investigators analyzed the data of >4000 patients with a median follow-up of 6.6 years; OS was only significantly associated with a decline in eGFR for patients with pre-existing CKD.14 The CKD-EPI equation was used to compare preoperative eGFR to postoperative nadir, the highest and most recent eGFR. Preoperative eGFR ≤60 mL/min/1.73 m2 was present in 28% of patients. Post-operatively, 47% of patients developed CKD, with 25% of patients having preoperative CKD-M and 22% of patients having preoperative eGFR >60 mL/min/1.73 m2 and developed CKD-S. Notably, preoperative CKD in 145 patients resolved after surgery. For patients with normal preoperative eGFR, OS was not related to postoperative eGFR but was related to RN (odds ratio = 3.56, 95% CI, 2.87-4.44, P <.0001) and absence of hypertension. These are counterintuitive as RN compared with PN is associated with development of CKD and hypertension was protective. Multivariable analysis demonstrated this finding with hypertension in our study as well (HR = 0.76, P = .030). The mechanism by which hypertension is protective in this setting is unclear and worth further study. Despite the distinction of medical vs surgical as made by the Cleveland Clinic investigators, eGFR <60 mL/min/1.73 m2 is still considered CKD and further work needs to be done to confirm or refute if this distinction is clinically meaningful. In addition, the degree to which the healthy contralateral kidney can recover by physiological compensation after RN is not currently well understood. It is conceivable that the healthier kidneys (CKD-S) retain this capacity, whereas medically impaired contralateral kidney (CKD-M) cannot recover.
In our study, >2000 patients underwent PN or RN for more than 23 years; 13% died with an overall median follow-up of 6.3 years. On multivariate analysis, RN has a 61% greater risk rate of death per year than PN. We did not directly assess the relationship of OS to the development of eGFR <60 or <45 mL/min/1.73 m2 (CKD stage III).
Both our previous and present studies are limited by being nonrandomized, retrospective studies at a single center. Further bias is present, as our center has been dedicated to nephron-sparing surgery, even in complex cases, and we have adopted the practice of active surveillance in elderly patients with small renal masses and comorbidities. Lastly, regarding the limitation of OS as an end point, we are looking at cardiovascular-related death and disease-specific survival in ongoing studies. Future studies need to evaluate the possible differences in outcome in patients with surgical vs patients with medically induced CKD, which may in fact be a significant bias. Ultimately, prospective randomized trials would be best, although the numbers, as well as the longevity and coordination needed for such a study would be substantial, forcing us to rely on large retrospective cohort studies until then.
CONCLUSION
New-onset CKD as estimated by the more accurate CKD-EPI equation is more likely in patients who underwent RN than in patients who underwent PN. Longer follow-up has shown that OS benefits exist for patients who underwent PN and supports our continued dedication to performing PN for patients with small renal masses who are fit for surgery, whenever technically possible.
Acknowledgments
Funding Support: This study was supported by grants from theSteven Hanson Family Renal Cancer Research Fund and the Sidney Kimmel Center for Prostate and Urologic Cancers.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.American Cancer Society: Cancer Facts & Figures 2014 Available at: http://www.cancer.org/research/cancerfactsstatistics/ cancerfactsfigures2014/. Accessed March 10, 2015.
- 2.Lau WK, Blute ML, Weaver AL, et al. Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc. 2000;75:1236–1242. doi: 10.4065/75.12.1236. [DOI] [PubMed] [Google Scholar]
- 3.Herr HW. Partial nephrectomy for unilateral renal carcinoma and a normal contralateral kidney: 10-year followup. J Urol. 1999;161:33–34. doi: 10.1016/s0022-5347(01)62052-4. [DOI] [PubMed] [Google Scholar]
- 4.Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol. 2000;163:442–445. [PubMed] [Google Scholar]
- 5.Mitchell RE, Gilbert SM, Murphy AM, et al. Partial nephrectomy and radical nephrectomy offer similar cancer outcomes in renal cortical tumors 4 cm or larger. Urology. 2006;67:260–264. doi: 10.1016/j.urology.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 6.Badalato GM, Kates M, Wisnivesky JP, et al. Survival after partial and radical nephrectomy for the treatment of stage T1bN0M0 renal cell carcinoma (RCC) in the USA: a propensity scoring approach. BJU Int. 2012;109:1457–1462. doi: 10.1111/j.1464-410X.2011.10597.x. [DOI] [PubMed] [Google Scholar]
- 7.Smaldone MC, Kutikov A, Egleston BL, et al. Small renal masses progressing to metastases under active surveillance: a systematic review and pooled analysis. Cancer. 2012;118:997–1006. doi: 10.1002/cncr.26369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jewett MA, Mattar K, Basiuk J, et al. Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol. 2011;60:39–44. doi: 10.1016/j.eururo.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 9.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735–740. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun M, Bianchi M, Hansen J, et al. Chronic kidney disease after nephrectomy in patients with small renal masses: a retrospective observational analysis. Eur Urol. 2012;62:696–703. doi: 10.1016/j.eururo.2012.03.051. [DOI] [PubMed] [Google Scholar]
- 11.Scosyrev E, Messing EM, Sylvester R, et al. Renal function after nephron-sparing surgery versus radical nephrectomy: results from EORTC randomized trial 30904. Eur Urol. 2014;65:372–377. doi: 10.1016/j.eururo.2013.06.044. [DOI] [PubMed] [Google Scholar]
- 12.Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293:1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 13.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 14.Lane BR, Campbell SC, Demirjian S, et al. Surgically induced chronic kidney disease may be associated with a lower risk of progression and mortality than medical chronic kidney disease. J Urol. 2013;189:1649–1655. doi: 10.1016/j.juro.2012.11.121. [DOI] [PubMed] [Google Scholar]
- 15.Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011;59:543–552. doi: 10.1016/j.eururo.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.Delanaye P, Mariat C. The applicability of eGFR equations to different populations. Nat Rev Nephrol. 2013;9:513–522. doi: 10.1038/nrneph.2013.143. [DOI] [PubMed] [Google Scholar]
- 20.Stevens LA, Schmid CH, Greene T, et al. Comparative performance of the CKD epidemiology collaboration (CKD-EPI) and the modification of diet in renal disease (MDRD) study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis. 2010;56:486–495. doi: 10.1053/j.ajkd.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shikanov S, Clark MA, Raman JD, et al. Chronic kidney disease epidemiology collaboration versus Modification of diet in renal disease equations for renal function evaluation in patients undergoing partial nephrectomy. J Urol. 2010;184:1867–1871. doi: 10.1016/j.juro.2010.06.104. [DOI] [PubMed] [Google Scholar]
- 22.Huang WC, Elkin EB, Levey AS, et al. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors—is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181:55–61. doi: 10.1016/j.juro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malcolm JB, Bagrodia A, Derweesh IH, et al. Comparison of rates and risk factors for developing chronic renal insufficiency, proteinuria and metabolic acidosis after radical or partial nephrectomy. BJU Int. 2009;104:476–481. doi: 10.1111/j.1464-410X.2009.08376.x. [DOI] [PubMed] [Google Scholar]
- 24.Tan HJ, Norton EC, Ye Z, et al. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA. 2012;307:1629–1635. doi: 10.1001/jama.2012.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaushik D, Kim SP, Childs MA, et al. Overall survival and development of stage IV chronic kidney disease in patients undergoing partial and radical nephrectomy for benign renal tumors. Eur Urol. 2013;64:600–606. doi: 10.1016/j.eururo.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 26.Kim SP, Thompson RH, Boorjian SA, et al. Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: a systematic review and metaanalysis. J Urol. 2012;188:51–57. doi: 10.1016/j.juro.2012.03.006. [DOI] [PubMed] [Google Scholar]