Abstract
Cocaine addiction is often characterized by a rigid pattern of behavior in which cocaine users continue seeking and taking drug despite negative consequences associated with its use. As such, full acquisition and relapse of drug-seeking behavior may be attributed to a shift away from goal-directed responding and a shift towards the maladaptive formation of rigid and habit-like responses. This rigid nature of habitual responding can be developed with extended training and is typically characterized by insensitivity to changes in outcome value. The present study determined whether cocaine (primary reinforcer) and cocaine associated cues (secondary reinforcer) could be devalued in rats with different histories of cocaine self-administration. Specifically, rats were trained on two schedules of cocaine self-administration (long-access vs. short-access). Following training the cocaine reinforcer was devalued through three separate pairings of lithium chloride with cocaine infusions. Cocaine history did not have an impact on devaluation of cocaine-associated cues. However, the reinforcing properties of cocaine were devalued only in rats on a short-access cocaine schedule but not those trained on a long-access schedule. Taken together this pattern of findings suggests that, in short access rats, devaluation is specific to the primary reinforcer and not associative stimuli such as cues. Importantly, rats that received extended training during self-administration displayed insensitivity to outcome devaluation of the primary reinforcer as well as all associative stimuli, thus displaying rigid behavioral responding similar to behavioral patterns found in addiction. Alternatively, long access cocaine exposure may have altered the devaluation threshold.
Keywords: cocaine addiction, devaluation, habits
1.0 Introduction
Cocaine addiction is best characterized by the persistent relapsing nature of individuals who have been abstinent from the drug for a period of time. One primary theory of addiction suggests that the transition from casual drug use to chronic drug abuse and addiction requires habit formation (Belin & Everitt, 2008; Robbins, Ersche, & Everitt, 2008). During initial acquisition of drug-taking behavior, the individual is reinforced by the primary hedonic properties of drug intake, suggesting that initial behavior is driven by the relationship between action and reinforcing consequence or outcome (i.e. Action-Outcome; A-O). However, as an individual continues cocaine-use over an extended period of time, behavioral control is lost and drug intake is no longer dependent on the reinforcing properties of the drug. Rather, drug-taking and seeking is now directed by autonomic or habitual behavior, driven by a compulsive behavior in response to drug-associated stimuli (Stimulus-Response; S-R). Habits and habitual behaviors are characterized by responses that are insensitive to changes in consequence or reinforcement (Dickinson, 1985). One of the primary characteristics of substance dependence, as described by the Diagnostic and Statistical Manual of Mental Disorders V is compulsive drug-seeking/taking despite aversive consequences (American Psychiatric Association, 2013).
The operant self-administration model of addiction (de Wit and Stewart, 1981; Shaham et al., 2003) has become the gold standard for addiction research. The most common iteration used in this model, requires rats to undergo self-administration over a period of days, followed by a period of extinction. In this model, rats are trained to perform an instrumental/operant response (e.g. lever press) for contingent administration of drug (i.e. primary reinforcer/unconditioned stimulus; US). Environmental or acute stimuli associated, either spatially or temporally, with the hedonic effects of the US gain inherent salient properties through Pavlovian associations (i.e. secondary reinforcers/conditioned stimulus CS) (Everitt & Robbins, 2005). The salience of the CS is especially robust if it can be used as a predictive cue signaling the US event. While this standard model of self-administration has proven to show sufficient translatability (Epstein et al., 2006), it is still unknown whether this model fully characterizes the shift to habitual responding in drug addicts, as characterized by Belin and Everitt, (2008). Behavioral models incorporating extended-access to cocaine (6-hr/day) during the acquisition of drug-taking behavior resulted in an escalation of cocaine-intake, greater motivation for self-administered cocaine, and show an upward shift of set point for reward (Paterson & Markou, 2003; for review see Koob et al., 2004), which arguably models the development of uncontrolled drug use with greater translational relevance to human drug addiction.
In rodent models, devaluation paradigms are often used to determine the habitual nature of instrumental responding (Adams & Dickinson, 1981; Yin et al., 2004). In standard devaluation paradigms, experimenters establish an aversion to a previous primary reinforcer following instrumental training. For example lithium chloride (LiCl), a compound known to create short-lasting but significant gastric malaise, when paired with a sucrose reinforcer reduces subsequent responding for that reinforcer (Dickinson et al., 1983). The reduction of instrumental response for a primary reinforcer following pairing with an aversive consequence (i.e. devaluation) implies that the representation of the reinforcer is encoded by the animal and that aversive pairings with the reinforcer alters this representation in a negative manner. However, if instrumental responding develops into habitual behavior, devaluation of the primary reinforcer do not result in a reduction of responding (Quinn et al., 2013; Yin et al., 2004; Coutureau & Killcross, 2003), suggesting that habitual responding is insensitive to devaluation of outcome. LiCl-induced illness has been used in numerous studies to devalue the reinforcing properties of gustatory stimuli (Holland et al., 1979; Adams & Dickinson, 1981; Yin et al., 2004; Mangieri et al., 2012). The present study aimed to manipulate perceived “value” of cocaine through pairings with LiCl. The use of a devaluation paradigm provides valuable insight in the study of the habitual, compulsive nature of drug addiction. In fact the LiCl devaluation paradigm has already been applied, with modest success, in various drug addiction studies (Samson et al., 2004; Root et al., 2009), while other studies have manipulated the reinforcing value of cocaine through satiety (Norman & Tsibulsky, 2006). However, studies have yet to fully elucidate the habitual, compulsive nature of addiction using the devaluation paradigm.
Given that drug addiction in humans develops over extended time and exposure to drug, the standard self-administration model (short-access; ShA) may not necessarily cultivate similar habitual responding in rats. As exposure to a long-access (LA) schedule of self-administration develops a more reliable model of drug dependence, we sought to determine whether extended access to cocaine mimicked the development of habitual responding to a greater degree. Here, we trained animals on two schedules of cocaine access (long-access vs. short-access). The reinforcing properties of cocaine were then devalued through pairings with LiCl. We hypothesized that short-access (ShA) animals would remain sensitive to cocaine devaluation and thus display decreased responding for cocaine following aversive pairings with the drug. However, long-access (LA) to cocaine during self-administration would increase habitual responding for cocaine, thus resulting in greater insensitivity to cocaine devaluation, compared to rats that received short access to cocaine.
2.0 Methods and Procedures
2.1 Subjects, Surgery, and Apparatus
Adult male (weighing 275–300 g) Sprague Dawley rats (Harlan, n=39) were single-housed on a reverse 12:12 light-dark cycle in a set temperature and humidity controlled vivarium with stable intake of food (15–30g daily) and water ad libitum. Experimental procedures were carried out in accordance with the “Guide for the Care and Use of Laboratory Rats” (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 2011) and approved by the IACUC of the Medical University of South Carolina. All rats underwent catheter surgery prior to any behavioral protocol. Catheter surgeries were carried out through standard surgical protocol (as described in Leong et al., 2015). Rats received at least 5 days of recovery from surgery prior to the beginning of cocaine self-administration. All self-administration and devaluation tests occurred in self-administration chambers (30×20×20 cm, Med Associates) containing two retractable levers, two stimulus lights, a speaker, and a house light. All self-administration chambers were housed inside sound-attenuating cubicles with ambient fans. Each chamber was equipped with tubing that extended through a spring leash attached to a swivel and a balanced metallic arm. Tubing extended outside the cubicle and attached to a 10 ml syringe, which was mounted on a pump outside the cubicle to supply infusions of drug.
2.2 Drugs
Cocaine hydrochloride (provided by the National Institute on Drug Abuse, Research Triangle Park, NC, USA) was dissolved in 0.9% sterile saline and administered at 0.2 mg cocaine per 50 ul bolus. Lithium Chloride (0.6M; LiCl; Sigma-Aldrich) was diluted in saline and injected at 5 ml/kg (i.p.).
2.3 Cocaine self-administration and devaluation
Cocaine self-administration began a week following catheter surgeries. The behavioral timeline is outlined in Figure 1. All rats received 21 days of cocaine self-administration with the first 7 days consisting of daily 1-hour sessions on an FR1 schedule of reinforcement. Starting on the 8th day, rats received one of two schedules of cocaine access (ShA vs. LA). ShA rats continued to receive daily 1-hour sessions for 14 days. LA rats received daily 6-hour sessions for 14 days. During the sessions, active lever responses resulted in a 2 s cocaine infusion and 5 s presentation of a light and tone stimulus complex, followed by a 20 s time out. Responses occurring during the time out and on the inactive lever were recorded in the absence of any scheduled consequences.
Figure 1.
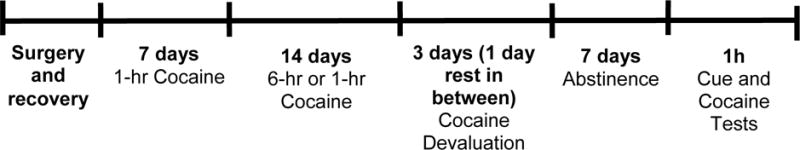
Experimental Timeline. Rats received a total of 21 days of self-administration followed by 3 days of cocaine devaluation (with a rest day between each devaluation day). Following 7 days of abstinence, rats were tested on two separate 1 hour tests.
Devaluation procedures, in which cocaine was paired with LiCl injections, began one day after the last self-administration session. The devaluation protocol was adapted from Root and colleagues (2009) that showed positive LiCl-induced devaluation of cocaine responding. During devaluation days rats were placed in a novel chamber, which contained no levers or house/cue lights, no floor grids with different bedding (different tactile stimulus), and one wall covered in red sandpaper. Rats received 30-min non-contingent cocaine infusions through a playback program based on their first 30 min infusion rate on the last day of self-administration. This was done to account for differences in behavioral output and cocaine intake between ShA and LA rats during self-administration due to session length. ShA rats received 12.18±0.74 (mean±SEM) cocaine infusions during the first 30 min of the last self-administration session while LA rats received, on average, 14.65±0.52 cocaine infusions. Immediately following the cocaine treatment, rats were injected with 0.6M LiCl (i.p.) and placed in a holding chamber for 30 minutes. Control rats (LiCl unpaired) received LiCl injection (i.p.) 6 hours prior to chamber placement to receive playback cocaine infusions. Following each devaluation day rats received one rest day. There were a total of three devaluation days followed by a seven day abstinent period in which rats remained in their home cages. Following the seventh day of abstinence, rats were returned to their self-administration chambers and tested on two devaluation tests.
Following devaluation of the US and abstinence all rats received a 1-hour cue devaluation test (Day 1) and cocaine devaluation test (Day 2). During the cue test, active lever presses resulted in a light+tone cue with no cocaine infusion. During the cocaine test, active lever presses resulted in infusion of cocaine but without any light+tone cue. All inactive lever presses had no scheduled consequence. All rats were tested on cues first because we did not want responding to the primary reinforcer (cocaine) to interfere with the secondary (reinforcer). Even though the cue test is in fact a cue-extinction session, this does not interfere with the cocaine test because the later test is conducted without the cues.
2.4 Data Analysis
To account for the differences in baseline responding following long-access and short-access cocaine self-administration, self-administration and test data were converted into a rate measure (responses/minute) to a percent change in responses/minute. Test data was also converted into a rate (responses/minute) and was then analyzed as a percent change relative to the average response/minute on the last three days of self-administration for each rat which is used as baseline rate of responding. To determine whether the LiCl pairings were successful at devaluing the US, planned comparisons (unpaired t-tests) were conducted with Welch’s correction to account for unequal standard distribution between paired and unpaired subjects. A subsequent 2 × 2 analysis of variance (ANOVA) was used to evaluate the main effects of access to cocaine and LiCl pairings on the percent change of responses/minute during both tests. All data are presented as the mean ±S.E.M. and α was set at p < 0.05.
3.0 Results
3.1 Rats receiving long-access to cocaine show escalation of cocaine-intake over time
A two-way repeated measures ANOVA revealed a significant Day x Access interaction [F(20,700) = 136.1, p < 0.01], with post-hoc Dunnett’s multiple comparisons indicating that rats receiving long-access to cocaine displayed robust escalation in cocaine infusions as self-administration progressed, while rats that received short-access to cocaine did not display any escalation (Figure 2A). A two-way repeated measures ANOVA of the first 30 minutes of self-administration following the shift to 6-hour sessions revealed a significant main effect of Day [F(13, 481) = 4.84, p < 0.01] suggests all rats increased cocaine intake during the first 30 minutes of the session over days. Additionally during the first 30 minutes LA rats took more cocaine than ShA rats (Figure 2B) demonstrated by a significant main effect of Group [F(1, 37) = 5.78, p < 0.05]. Within-group post-hoc comparisons of intake relative to Day 1 of 6-hour sessions reveal that LA rats took more cocaine on Days 3, 5–14 of 6 hour sessions, while ShA rats did not show increased intake relative to Day 1 (Figure 2B). A t-test confirmed that response/minute during the last 3 days of self-administration did not differ in rats assigned to LiCl-cocaine paired and unpaired groups in either access condition (ShA: paired: M=0.46, SD=0.2; unpaired: M=0.40, SD=0.1; LA: paired: M=0.38, SD=0.06; unpaired: M=0.37, SD=0.05).
Figure 2.
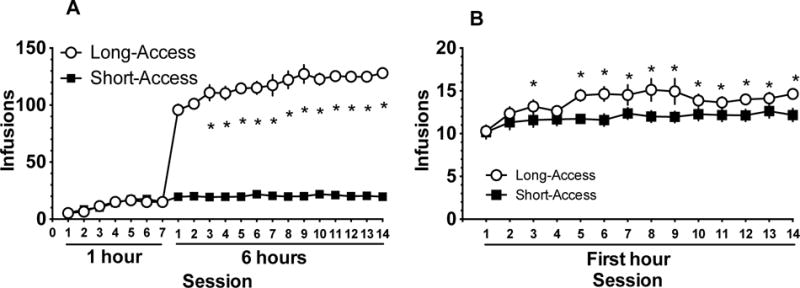
Rats given long access, but not short access, to cocaine escalated the number of infusions per day over 14 days (A) and during the first 30 minutes of the session (B). *Significant difference from the first long-access session on Day 8 in (A) and Day 1 in (B) (p<0.05).
3.2 Schedule of cocaine-access or LiCl pairing does not impact responding to associative stimuli
When rats were returned to the drug-paired context and were tested in the presence of conditioned cues, all rats had an increase in responses/minute relative to the self-administration baseline. Change in responses/minute between LiCl-paired and unpaired rats in the short-access condition [t(19.88) = 0.72, n.s.; Figure 3A] and long-access condition [t(15) = 0.73, n.s.; Figure 3B] did not differ on this test. Additionally, access condition did not interact with LiCl pairing, nor were there any main effects.
Figure 3.
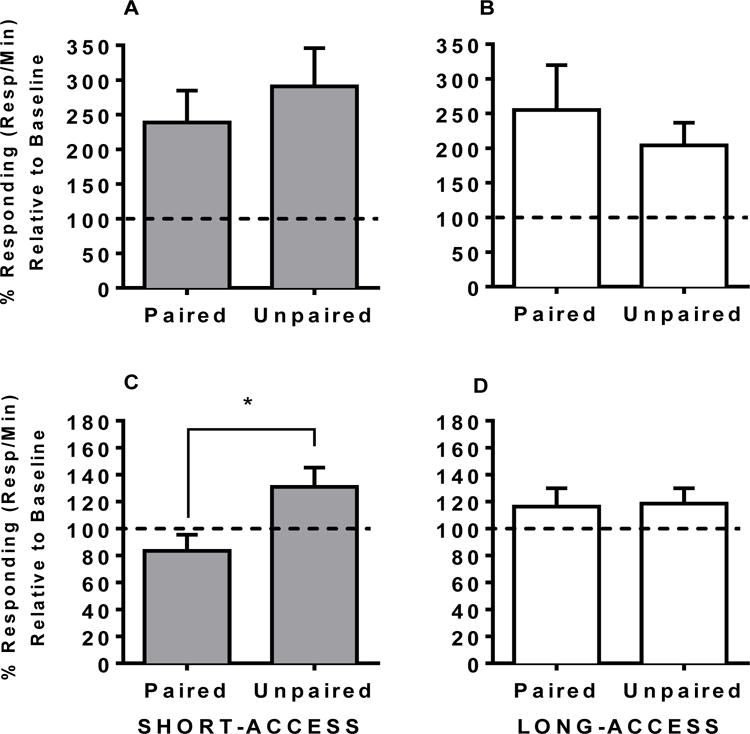
Percent responding relative to last 3 days of self-administration during cue test (upper panel) and cocaine test (lower panel). Short-access (A) or long-access (B) to cocaine does not impact devaluation to the secondary reinforcer of cue+light stimuli following cocaine-LiCl pairings relative to unpaired rats. During cocaine test, rats that received short-access to cocaine (C) display devaluation following cocaine-LiCl pairings relative to unpaired rats. Long-access to cocaine (D) does not impact devaluation to the primary reinforcer of cocaine following cocaine-LiCl pairings. Dashed line indicates 100% responding relative to baseline; no change. *Significant difference of p < 0.05.
3.3 LiCl pairing following long-access, but not short-access, to cocaine results in devaluation of primary reinforcer
When rats were given contingent cocaine in the self administration context following devaluation, LiCl-paired ShA animals had lower rate of responding relative to baseline [M = 83.47; SEM = 11.97] compared to unpaired ShA animals [M = 131.0; SEM = 14.26; t(19.89) = 2.55, p < 0.05; Figure 3C], In contrast, in the long-access condition, LiCl-paired and unpaired rats did not differ [t(14.19) = 0.12, n.s. Figure 3D]. Additionally, access condition did not interact with LiCl pairing, nor were there any main effects. Taken together, cocaine paired with LiCl was sufficient to reduce rate of responding in the cocaine test in animals that received short-access, but not long-access, to cocaine during self-administration.
4.0 Discussion
The present experiment shows rats that received short-access daily cocaine self-administration and subsequent LiCl-pairings displayed a reduction in responses compared to LiCl-unpaired rats during the cocaine test (i.e., post probe) but not cue test (i.e., probe test). Rats that received long-access to cocaine continued to show stable responding in both cue and cocaine tests regardless of whether LiCl was paired with cocaine. LiCl paired with cocaine was sufficient to reduce active lever responding for the primary reinforcer (i.e. cocaine) in ShA rats but LA rats showed insensitivity to devaluation following LiCl-cocaine pairings. As such, interpretations of these results indicate that extended access to cocaine results in a more habitual behavioral profile or an increased threshold for the devaluation of cocaine.
The shift from initial drug use to drug abuse can be thought of as a shift from initial goal-directed behavior to habitual and compulsive drug taking (Dickinson & Balleine, 1994), and is driven by studies showing that a shift from cognitive to habitual behaviors follow extended training (Dickinson, 1985; Packard & McGaugh, 1996). Initially, “cognitive” processes based on the relationship between action (i.e. lever presses) and outcome (i.e. reinforcing properties of cocaine) drive responses. With increased training, the reinforcing properties of the drug become associated with the various stimuli to a point where the presence of the stimuli is sufficient to evoke a response regardless of the value of the reinforcer. Habitual behavior in addiction is characterized by loss of control in which drug seeking is no longer dependent on the reinforcing properties of the drug but rather done automatically regardless of the consequence (Robbins, Ersche, & Everitt, 2008).
In this study, LA rats were impervious to devaluation of the US a cocaine test (i.e., postprobe) in which active lever presses resulted in an infusion of cocaine. These results complement previous work showing that extended drug history increased resistance to unconditioned (Pelloux et al., 2007) and conditioned (Vanderschuren & Everitt, 2004) punishment associated with seeking behavior, in which rats with extended cocaine history did not suppress seeking of cocaine even if seeking behavior was paired with shock or shock- associated cue. Similarly, Deroche-Gamonet et al. (2004) found modest effects of extended training on rats’ resistance to contingent punishment during self-administration. There is also a rich literature showing that stimulant exposure (e.g. cocaine or amphetamine) devalues a natural reinforcer (Grigson & Twining, 2002; Schoenbaum & Setlow, 2005) or facilitates formation of habitual responding for natural reinforcers (Nordquist et al., 2007; Nelson & Killcross, 2006). The present study, to our knowledge, is one of a few studies that have attempted to use cocaine as the reinforcer to be devalued whilst examining different histories of cocaine exposure on habitual responding.
Not surprisingly, LiCl did not devalue cocaine-associated cues regardless of cocaine access condition. The devaluation procedure we used paired LiCl with cocaine; thereby only the primary reinforcer (i.e. cocaine) was devalued. This methodology left the secondary reinforce (i.e. drug-associated discrete cues) void of manipulation. During cocaine self-administration, rats acquire an instrumental response (A-O) through a relationship between active lever pressing and cocaine infusion (US). This exposure to the US or primary reinforcer was paired with a CS or secondary reinforcer (light+tone). There are two theoretical relationships driving behavior: an instrumental relationship between lever press and drug reinforcer, and a Pavlovian association pairing the light+tone with the drug. In the present study, rats were given non-contingent cocaine infusions immediately before LiCl injections in order to specifically devalue the physiological effects of cocaine independent of the light+tone stimulus complex. We also devalued cocaine in a novel context to prevent an inadvertent pairing of LiCl with the original drug-taking context. Pairing LiCl with cocaine in the drug-paired context may have interfered with the devaluation of cocaine itself. Combined, omission of discrete cues and use of a novel context to devalue cocaine allowed us to establish that LiCl specifically impacted the primary reinforcing effect of cocaine.
An alternative interpretation for the lack of cue devaluation posits that rats in both access conditions displayed habitual behavior, albeit on a continuum. Specifically, the LiCl-cocaine paired ShA rats responded robustly during the cue test but then decreased responding during the cocaine test. Based on classic devaluation procedures in which a food reinforcer is devalued, this response pattern could be interpreted as an inability to use the value of the secondary reinforcer (i.e. cues) to guide responding. Thus, these rats are exhibiting a habitual response pattern (Smith et al., 2012; Smith & Graybiel, 2013), evidenced by continued responding during the cue test (i.e. probe test) but not during cocaine test (i.e. postprobe test). Given that LA rats responded in the presence of both primary and secondary reinforcers conclusion motivating these responses are unclear. It is a possibility that the lack of change in responding in LA rats indicates a difference in “devaluation thresholds” that is determined by the schedule of cocaine self-administration and subject to further investigation.
Indeed, inherent differences between SA and LA groups can render direct comparison subject to multiple interpretations based on differences in cocaine history. However, the discrepancy between cocaine histories is exactly why this model is so translationally relevant, as it models differences in “causal” and “long-term” drug users in the human population. However, it is possible that greater exposure to cocaine in the LA group (i.e., latent inhibition) would be expected to weaken associative learning during devaluation. We did our best to equate the devaluation paradigm between groups. As described in the methods, during devaluation rats received cocaine exposure from a 30 minute playback program based on the first 30 minutes of their last day of self-administration. Through this, rats would receive a “regular” amount of cocaine relative to their normal intake during self-administration over the 30 minute devaluation session. Although this does not fully account for differences in cocaine history, these results continue to highlight a fundamental difference in rats that receive either ShA or LA cocaine histories. In this report, all rats receive non-contingent cocaine exposure during the devaluation procedure. Non-contingent cocaine can be aversive (Dworkin, Mirkis, & Smith, 1995) and increase sensitization (Lecca et al., 2007) towards cocaine. However, it is unlikely that this aversion impacted responding during testing because rats in this study were had cocaine histories. Further, robust responding of LiCl unpaired control groups in both access conditions suggests that attenuation in responding during test was due to LiCl-cocaine pairing, not due to the potential aversion of non-contingent cocaine.
Rats received two separate devaluation tests in these experiments. This testing paradigm was adapted from food reward devaluation paradigms in which the probe test of the secondary reinforcer is subsequently followed by a post-probe test which consists of presentation of the primary reinforcer (Smith et al., 2012; Smith & Graybiel, 2013). Here the cue test (i.e. probe test) occurred in the presence of cocaine-associated cues but no cocaine. The subsequent cocaine test (i.e. post-probe test) occurred in the absence of cocaine-associated cues but cocaine was again available upon active lever presses. Although the cocaine test is carried out in the absence of cues, it is conceivable that the cue test may result in a degree of extinction of the cocaine-associated cues or context, which could affect subsequent responding for the primary reinforcer during the cocaine test. It should be taken into consideration that behavioral responding may differ following abstinence vs. abstinence+extinction, as previous work shows that varying combinations of extinction and abstinence (either alone or combined at different lengths) can impact cocaine-seeking (Di Ciano & Everitt, 2002). With this in mind, it is important to consider that behavioral responding might differ during cocaine test if rats did not receive an initial cue test. Future studies should determine whether a counterbalanced testing schedule would yield different patterns of responding between LA and ShA rats.
The pairing of LiCl and cocaine in our devaluation paradigm is similar to that used by Root et al. (2009) in which they show that LiCl-induced sickness can be associated with a cocaine reinforcer to inhibit responding for cocaine during the presence of a discriminative stimulus, but not in the absence of it. LiCl unpaired groups in both the study presented here and in Root et al. (2009) show no attenuation of responding during test, suggesting that LiCl-induced illness was associated with cocaine exposure only in paired rats. However, our study differs in that cocaine, but not cocaine-cues, impacted responding in ShA LiCl-cocaine paired rats. This difference between the results found in Root et al. (2009) and our present study may be attributed to differences in self-administration paradigms. In Root et al., (2009), the experimenters used a discriminative stimulus to signal availability of the drug, while in our paradigm we used a tone+light complex as an associative stimulus paired with drug infusion. However, this does not explain why ShA rats did “update” their Pavlovian association between cocaine-associated cues and cocaine now paired with illness, although devaluation has been found to not affect second order conditioning responding (Holland and Rescorla, 1975). Similar to the conclusions drawn by Holland and Rescorla (1975), the instrumental association (i.e. lever press) may have acted as a first-order association while the Pavlovian association (i.e. tone+light presentation) may have acted as a second-order association. To account for the differences between our results and those of Root et al., (2009), we posit that our devaluation paradigm affects responding through instrumental conditioning more readily than through associative conditioning.
4.1 Conclusions
The present findings indicate differences in cocaine-seeking following either LA or ShA to cocaine. A possible interpretation of these results is that LA rats displayed habitual responding as extended access to cocaine in these rats were insensitive to devaluation through cocaine-LiCl pairings regardless of presentation of the primary and secondary reinforcers. ShA rats decreased responding during the cocaine, but not cue, test when receiving LiCl-cocaine pairings. Alternatively, it can be argued that this nature of responding in ShA rats could also be indicative of habitual responding, as response behavior was no longer guided by the conditioned reinforcer despite devaluation of the primary reinforcer (cocaine), while LA conditions resulted in a shift in “devaluation threshold”. Regardless, LA or ShA cocaine exposure result in marked differences in cocaine-responding following LiCl-induced devaluation which may provide translational value when considering differences in cocaine history of patient populations.
Highlights.
Extended cocaine-self administration may serve as a more translatable model of cocaine addiction as it results in escalation of intake and greater motivation for cocaine-seeking.
Devaluation paradigms have previously been used to determine whether responding goal-directed behavior or habitual.
Here we determined that extended cocaine-self administration results in insensitivity to sickness-induced devaluation during a cocaine test, indicating that extended cocaine self-administration may develop more rigid patterns of behavior characteristic in cocaine addicts. Other interpretations are also explored.
Responding differed following devaluation to the primary or secondary reinforcer, suggesting possible differences in processes that mediate cocaine-associated memories.
Acknowledgments
This research was supported by NIDA grants DA016511 (CMR) and T32 DA728823 (KCL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors contributed in a significant way to the manuscript and all authors have read and approved the final manuscript.
The authors declare that there are no conflicts of interest to disclose.
References
- Adams CD, Dickinson A. Instrumental responding following reinforcer devaluation. The Quarterly journal of experimental psychology. 1981;33(2):109–121. [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57(3):432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Coutureau E, Killcross S. Inactivation of the infralimbic prefrontal cortex reinstates goal-directed responding in overtrained rats. Behavioural brain research. 2003;146(1):167–174. doi: 10.1016/j.bbr.2003.09.025. [DOI] [PubMed] [Google Scholar]
- De Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75(2):134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305(5686):1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Reinstatement and spontaneous recovery of cocaine seeking following extinction and different durations of withdrawal. Behavioural pharmacology. 2002;13(5–6):397–405. doi: 10.1097/00008877-200209000-00013. [DOI] [PubMed] [Google Scholar]
- Dickinson A. Actions and habits: the development of behavioural autonomy. Philosophical Transactions of the Royal Society B: Biological Sciences. 1985;308(1135):67–78. [Google Scholar]
- Dickinson A, Balleine B. Motivational control of goal-directed action. Animal Learning & Behavior. 1994;22(1):1–18. [Google Scholar]
- Dickinson A, Nicholas DJ, Adams CD. The effect of the instrumental training contingency on susceptibility to reinforcer devaluation. The Quarterly Journal of Experimental Psychology. 1983;35(1):35–51. [Google Scholar]
- DSM-5 American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- Dworkin SI, Mirkis S, Smith JE. Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology. 1995;117(3):262–266. doi: 10.1007/BF02246100. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189(1):1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature neuroscience. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behavioral neuroscience. 2002;116(2):321. [PubMed] [Google Scholar]
- Holland PC, Rescorla RA. The effect of two ways of devaluing the unconditioned stimulus after first-and second-order appetitive conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1975;1(4):355. doi: 10.1037//0097-7403.1.4.355. [DOI] [PubMed] [Google Scholar]
- Holland PC, Straub JJ. Differential effects of two ways of devaluing the unconditioned stimulus after Pavlovian appetitive conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1979;5(1):65. doi: 10.1037//0097-7403.5.1.65. [DOI] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, … &, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neuroscience & Biobehavioral Reviews. 2004;27(8):739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Lecca D, Cacciapaglia F, Valentini V, Acquas E, Di Chiara G. Differential neurochemical and behavioral adaptation to cocaine after response contingent and noncontingent exposure in the rat. Psychopharmacology. 2007;191(3):653–667. doi: 10.1007/s00213-006-0496-y. [DOI] [PubMed] [Google Scholar]
- Leong KC, Zhou L, Ghee SM, See RE, Reichel CM. Oxytocin Decreases Cocaine Taking, Cocaine Seeking, and Locomotor Activity in Female Rats. Experimental and Clinical Psychopharmacology. 2015 doi: 10.1037/pha0000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangieri RA, Cofresí RU, Gonzales RA. Ethanol seeking by Long Evans rats is not always a goal-directed behavior. PloS one. 2012;7(8) doi: 10.1371/journal.pone.0042886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson A, Killcross S. Amphetamine exposure enhances habit formation. The Journal of neuroscience. 2006;26(14):3805–3812. doi: 10.1523/JNEUROSCI.4305-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordquist RE, Voorn P, De Mooij-van Malsen JG, Joosten RNJMA, Pennartz CMA, Vanderschuren LJMJ. Augmented reinforcer value and accelerated habit formation after repeated amphetamine treatment. European neuropsychopharmacology. 2007;17(8):532–540. doi: 10.1016/j.euroneuro.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Norman AB, Tsibulsky VL. The compulsion zone: a pharmacological theory of acquired cocaine self-administration. Brain research. 2006;1116(1):143–152. doi: 10.1016/j.brainres.2006.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiology of learning and memory. 1996;65(1):65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport. 2003;14(17):2229–2232. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Everitt BJ, Dickinson A. Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology. 2007;194(1):127–137. doi: 10.1007/s00213-007-0805-0. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Pittenger C, Lee AS, Pierson JL, Taylor JR. Striatum-dependent habits are insensitive to both increases and decreases in reinforcer value in mice. European Journal of Neuroscience. 2013;37(6):1012–1021. doi: 10.1111/ejn.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Annals of the New York Academy of Sciences. 2008;1141(1):1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Root DH, Fabbricatore AT, Barker DJ, Ma S, Pawlak AP, West MO. Evidence for habitual and goal-directed behavior following devaluation of cocaine: a multifaceted interpretation of relapse. PLoS One. 2009;4(9):e7170. doi: 10.1371/journal.pone.0007170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH, Cunningham CL, Czachowski CL, Chappell A, Legg B, Shannon E. Devaluation of ethanol reinforcement. Alcohol. 2004;32(3):203–212. doi: 10.1016/j.alcohol.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B. Cocaine makes actions insensitive to outcomes but not extinction: implications for altered orbitofrontal–amygdalar function. Cerebral Cortex. 2005;15(8):1162–1169. doi: 10.1093/cercor/bhh216. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. 2003 doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Smith KS, Graybiel AM. A dual operator view of habitual behavior reflecting cortical and striatal dynamics. Neuron. 2013;79(2):361–374. doi: 10.1016/j.neuron.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Virkud A, Deisseroth K, Graybiel AM. Reversible online control of habitual behavior by optogenetic perturbation of medial prefrontal cortex. Proceedings of the National Academy of Sciences. 2012;109(46):18932–18937. doi: 10.1073/pnas.1216264109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305(5686):1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. European Journal of Neuroscience. 2004;19(1):181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]


