Abstract
Puerarin (an isoflavone C-glucoside from kudzu root) has been the focus of several studies investigating its potential effects on health benefits. In this study, we determined single dose tissue distribution of puerarin and its metabolites in order to examine whether they undergoes selective uptake by specific organs. Puerarin was administered orally (50 mg/kg) to rats and the concentration of puerarin in tissue compartments was determined using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Puerarin was widely distributed in rat tissues with highest concentrations in lungs (799 ± 411.6 ng/gram wet tissues). In addition, we examined the excretion of puerarin into the bile. LC-MS/MS analysis of bile samples collected after infusing puerarin directly into the portal vein indicated that puerarin was excreted into the bile predominantly in the form of unconjugated puerarin. This report identifying puerarin in several organs including kidney and pancreas may explain its beneficial effects in diabetes.
Keywords: Puerarin, LC-MS/MS, tissue distribution, metabolites
Introduction
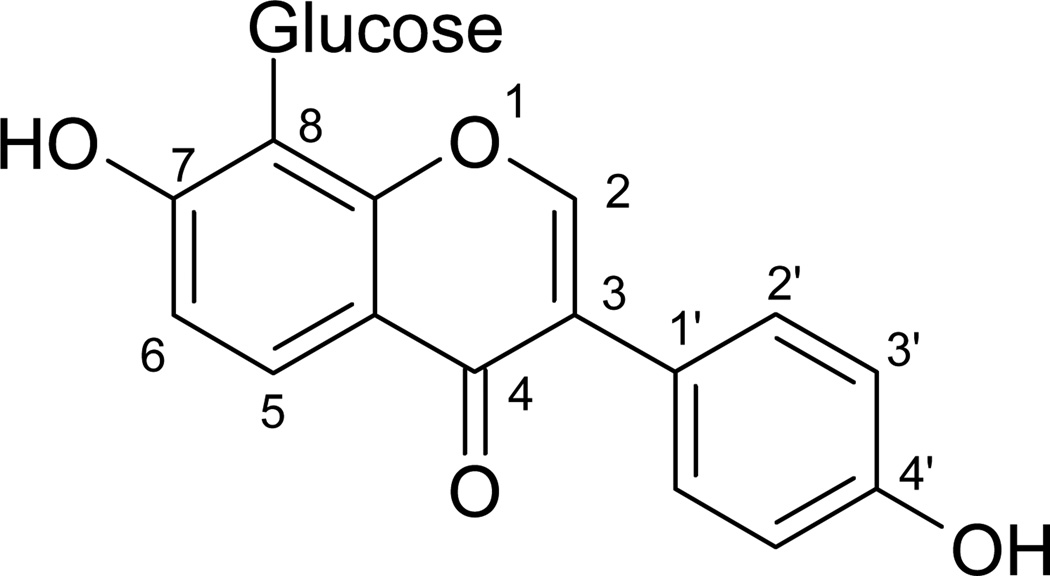
Puerarin (4′-7-dihydroxy-8-β-D-glucosylisoflavone), the chemical structure shown in Fig. 1, is the most abundant isoflavone C-glucoside isolated from traditional Chinese medicine (TCM) Pueraria Radix (the root of the kudzu Pueraria lobota). It has been shown to have beneficial effects on cardiovascular, neurological and hyperglycemic disorders (Xu and Zho, 2002; Zhu et al., 2004; Hsu et al., 2003). Kudzu is reported to be used in combination with other medicinal plants such as Danshen (Salvia miltiorrhiza) and Notoginseng (Panax notoginseng). These products have been shown to have beneficial effects on atheroscleosis (Sieveking et al., 2005) and acute ischemic myocardial injury (Wu et al., 2007). Similarly, a TCM preparation called Naodesheng which consists of five kinds of common crude drugs including puerarin and ginsenosides has been reported to be effective in the treatment of cardiovascular diseases (Yu et al., 2006). The therapeutic effectiveness of these herbal mixtures is believed to be the result of different herbs acting collectively or synergistically rather than as single active agents (Keyler et al., 2002).
Figure 1.
Chemical structure of puerarin (molecular formula C21H20O9)
We demonstrated that puerarin significantly improves glucose tolerance in C57 BL/6J ob/ob mice, an animal model of Type 2 diabetes mellitus, blunting the rise in blood glucose levels after i.p. administration of glucose (Meezan et al., 2005). In our study, puerarin administered by intraperitoneal injection was four times more bioavailable than via the oral route of administration. Puerarin was rapidly absorbed intact and the maximum blood concentration was obtained within 1 h after oral administration (Prasain et al., 2007). Interestingly, unlike the isoflavone O-glucosides, unmodified puerarin was the major component in the blood and urine, indicating that phase II metabolism is not the major metabolic pathway for puerarin excretion (Prasain et al., 2004).
Li et al. (2006) reported the pharmacokinetics and tissue distribution following oral administration of very high doses of puerarin and a puerarin:phospholipid complex (400 mg/kg) using a reverse-phase HPLC method. However, the use of high acute dose of puerarin may have saturation effects and alters the uptake and metabolism. Furthermore, there is no evidence for bioavailability of puerarin’s metabolites in tissues using a sensitive LC-MS/MS method. It also remains unknown whether puerarin is eliminated through the bile and thereby undergoes extensive enterohepatic circulation as for other isoflavones (Sfakianos et al., 1997) or directly excreted into the urine.
It is generally accepted that the biological effects of xenobiotics are related to the forms in which they circulate and concentrate in the target cells. To assess the potential mechanism of action of puerarin and/or metabolites, it is essential to understand their bioavailability in the target tissues. As part of our continuing study on the cardiovascular and metabolic effects of puerarin in spontaneously hypertensive rats, we analyzed tissue distribution of puerarin and its metabolites in these rats and determined whether puerarin could be transported into bile using a sensitive LC-MS/MS method.
Materials and methods
Materials
Puerarin was purchased from Sigma Chemical Co. (St. Louis, MO, USA) and apigenin from Indofine (Somerville, NJ). All HPLC solvents and reagents were purchased from Fisher (Norcross, GA) and were of HPLC grade.
Puerarin administration and sample collection
Male spontaneously hypertensive rats (SHR, n = 6, body weight = 250–300 g, 8–10 weeks of age; Harlan Sprague-Dawley Inc., Indianapolis, IN) were housed in a controlled environment at 23°C and 55% relative humidity under a 12 h dark-light cycle, with free access to soy-free custom diet TD86369 (Harlan Teklad, Wisconsin, MD) and tap water for one week. All experimental procedures were conducted in accordance with Institutional Animal Care and Use Committee of the University of Alabama, Birmingham, and National Institutes of Health guidelines. After overnight fasting, puerarin was orally administered by gavage (50 mg/kg body weight) to each animal.
Following a midline incision, the bile duct was exposed and was cannulated with PE-10 tubing, tied and secured with 5-0 silk (Ethicon, Somerville, NJ) under isoflurane anesthesia. During experiments, the body temperature of rats was maintained at 37°C. Bile was collected in 10 min in tervals over 2 h. Rats were euthanized by cervical dislocation following isoflurane anesthesia and blood was collected via heart puncture using heparin as anticoagulant. Plasma was isolated by centrifugation of the blood at 3,000 × g for 5 min and stored at −20°C. After perfusion with ice-cold normal saline, the liver, kidney, spleen, pancreas, lungs, eyes and heart were dissected and immediately frozen in liquid nitrogen.
Portal vein infusion
To determine the excretion of puerarin in the bile, it was infused into the portal vein of an adult rat under isofluorethane anesthesia. The portal vein was exposed and a 27-gauge stainless steel syringe needle, connected to PE10 tubing, was inserted. Puerarin was infused into the portal vein at 0.5 µg/min for 60 min in 140 mM NaCl containing 10 mM sodium taurocholate using a Harvard syringe pump. Super glue was used immediately to seal the puncture point. Bile was collected in 5 min intervals during the first 30 min and then at 10-min intervals over the next two and half hours.
Sample preparation
The tissue samples (wet weight 1–7 g) were minced and added to (2–14 ml) methanol containing 1% acetic acid. The tissue was homogenized with a Tissue Tearor homogenizer (Biospace Product Inc., Racine, WI). The samples were vortex mixed and tumbled for 2 h at room temperature. The homogenate was centrifuged at 3,000 × g for 10 min and the supernatant was removed and dried under air and reconstituted in 80% methanol in water (200 µl).
Sample preparation and quantification of puerarin in biological samples were performed as described before (Prasain et al., 2007). Briefly, each plasma sample (20 µl) was diluted by adding 80 µl of methanol containing 1% acetic acid, vortex-mixed and centrifuged for 10 min at 3,000 × g to precipitate proteins. In the case of bile, each sample (5–20 µl) was diluted 3–4 fold with methanol containing 1% acetic acid, vortex mixed and centrifuged as described above. The supernatant solutions were directly transferred to HPLC auto samplers and injected (10 µl) into the mass spectrometer as described below to analyze the samples.
A calibration curve containing 2, 20, 50, 200, 500, 2000 and 5000 ng/ml of puerarin was generated. The correlation coefficient of each standard curve was greater than 0.99. This allowed selective and specific quantitative data to be obtained for puerarin in samples. In the case of tissue samples, the method was not validated because there was not enough material available for complete validation procedure. Thus, quantification results in tissues are approximate.
Liquid chromatography-mass spectrometry
LC-MS/MS analyses of rat plasma, bile and tissue extracts were performed using a system consisting of a model SIL-HT refrigerated Shimadzu autosampler and HPLC instrument (Shimadzu Scientific Instruments, Inc. Columbia, MD), and an API 4000 mass spectrometer (Applied Biosystems/MDS Sciex, Concord, Ontario, Canada). Chromatography was carried out on a reversed-phase Phenomenex Synergi 4 micron Fusion-RP80 column (150 × 2.0 mm i.d.) pre-equilibrated with 10 mM ammonium acetate (NH4OAc). The mobile phase consisted of acetonitrile-water (10:90, v/v) in 10 mM NH4OAc with a flow rate of 0.2 ml/min. Multiple reaction monitoring (MRM) was used to perform mass spectrometric quantification of puerarin. The column effluent was introduced into the mass spectrometer using atmospheric pressure chemical ionization (APCI) and electrospray ionization (ESI) in the negative mode for puerarin and conjugated metabolites of puerarin, respectively. Nitrogen was used as nebulizer, gas 1 and curtain gas. The nebulizer current and temperature were 5 amps and 500°C, respectively. The collision gas (N2) was set at high and collision energy was −45 eV. The MRM analysis for puerarin was conducted by monitoring the precursor ion to product ion transition m/z 415/267. Puerarin’s phase I and phase II metabolites were also characterized by MRM of anticipated mass transitions (Q1/Q3 transitions) m/z 591/415 (puerarin glucuronide), 495/415 (puerarin sulfate), 431/283 (monhydroxylated puerarin), 429/415 (methyl puerarin), and 253/223 (daidzein). The LC-MS-MS system was controlled by BioAnalyst 1.4.1. software (Applied Biosystems/MDS Sciex).
Results
Tissue distribution of puerarin and its metabolites
LC-MS/MS with ESI turbo ionspray analysis was performed on rat tissues, bile and plasma samples collected following oral administration of puerarin (50 mg/kg). Multiple MS/MS experiments (MRM, MS/MS and neutral loss scan) were carried out to identify the metabolites of puerarin (e.g., glucuronidated, sulfated and hydroxylated). This enabled rapid identification, characterization and confirmation of Phase I (functionalization reactions such as oxidation and hydrolysis) and Phase II (conjugation reactions such as glucuronidation and sulfation) metabolites in a single LC-MS/MS run.
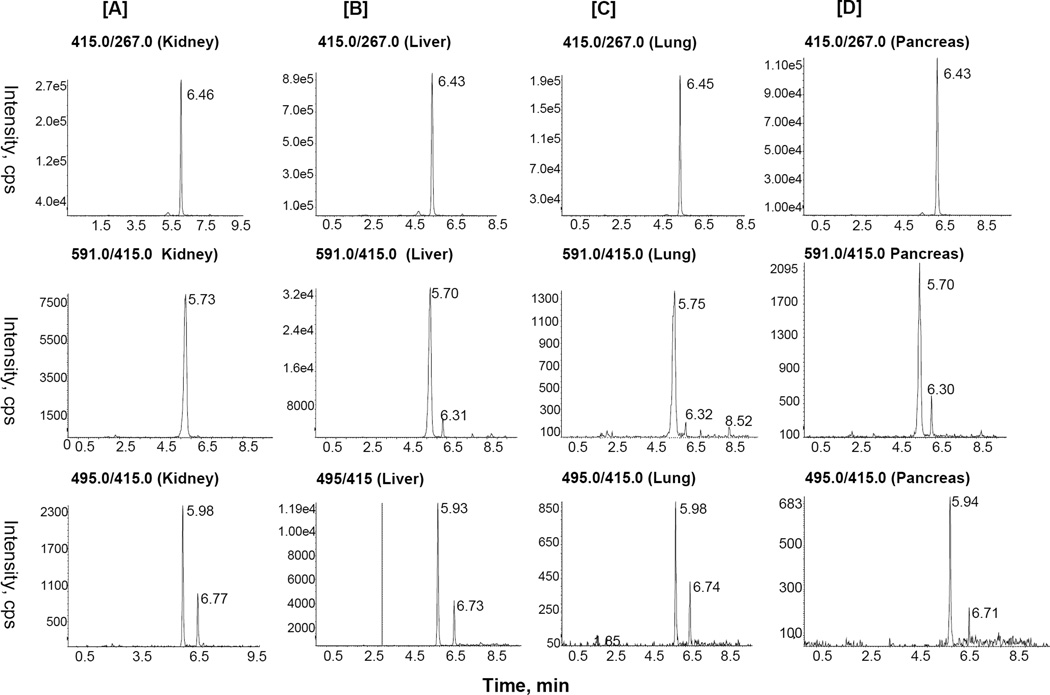
As can be seen in MRM chromatogram of puerarin-treated kidney sample (Fig. 2A), a prominent peak eluting at tR 6.46 min corresponded to intact puerarin (Prasain et al., 2007; Prasain at al., 2004). For the detection of puerarin’s phase I and phase II metabolites, the MRM mass transitions m/z 591/415 (puerarin glucuronide), 495/415 (puerarin sulfate), 431/283 (monhydroxylated puerarin), 429/415 (methyl puerarin), and 253/223 (daidzein) were used. A broad peak appearing at tR 5.73 min corresponded to puerarin glucuronide (Fig. 2A). Two well separated sharp peaks with mass transition m/z 495/415 at tR 5.98 and 6.77 min indicated the presence of two isomers of puerarin sulfates. We also detected monohydroxylated derivatives of puerarin with mass transition m/z 431/311 at tR 6.40 and 6.92 min (data not shown). No methyl puerarin was detected in the kidney. The detection of aglycone daidzein was inconclusive.
Figure 2.
Representative MRM ion chromatograms with transitions m/z 415/267, 591/415, and 495/415 obtained from rat [A] kidney, [B] liver, [C] lungs, [D] pancreas samples 2h after oral administration of puerarin.
Similarly, MRM chromatograms of liver, lungs and pancreas indicated the presence of glucuronides, sulfates and monohydroxylated metabolites of puerarin (Figs. 2B–D). In addition to two puerarin sulfate conjugates, two glucuronide conjugates of puerarin (a major peak at tR 5.70 min and a minor peak at tR 6.31 min) were detected in these samples. The different conjugation sites (7 and 4’ positions of puerarin) of the sulfate or glucuronic acid group result in the different retention behaviors under these HPLC conditions.
The major peak eluting earlier was considered to be puerarin 7-O-β-glucuronide and the latter puerarin 4’-O-β-glucuronide on the basis of comparison of the relative retention times of daidzein glucuronides (Clarke et al., 2002). This is also in accord with the detection of puerarin 7-O-β-D-glucuronide as a biliary metabolite of puerarin (Yasuda et al., 1994). Similarly, the major MRM peak (m/z 495/415) eluting earlier in the tissue samples was assigned to be puerarin 4’-O-β-sulfate which is supported by the previous detection of this metabolite in puerarin-treated rat bile and the relative retention time behavior of daidzein sulfates (Yasuda et al., 1994; Fang et al., 2002; Clarke et al., 2002). However, there could be an opposite scenario for glucuronidation at the 7-position due to the presence of sterically hindered C-glucoside at the 8-position of puerarin. Therefore, further characterization of these conjugates based on NMR interpretation of standards is needed to fully establish their structures. Puerarin was detected by MRM in the heart and eye samples, but was not quantified due to its very low concentration. No puerarin and its metabolites were detected in the spleen.
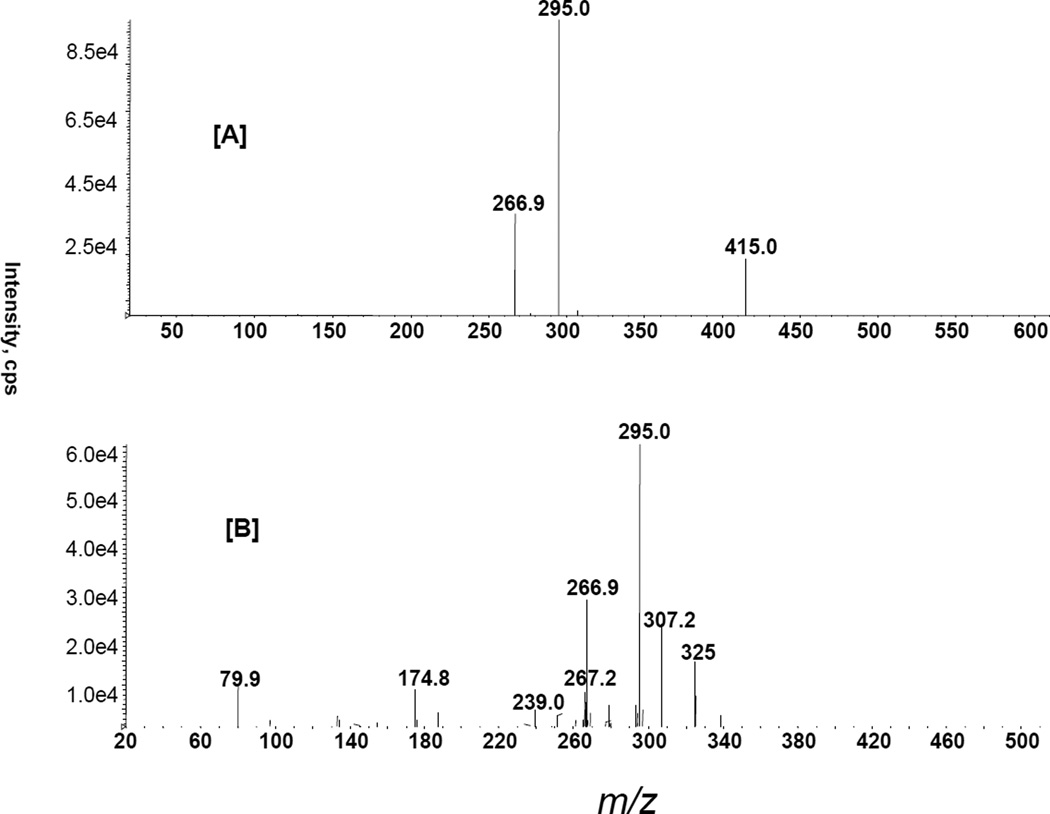
For further identification of puerarin’s conjugated metabolites, we performed MS/MS of the m/z 591 and 495 ions. MS/MS of the m/z 591 ion yielded characteristic product ions m/z 415 and 295 due to the subsequent losses of 176 (glucuronic acid moiety) and 120 amu (Fig. 3A). Interestingly, in the case of puerarin, the product ion m/z 267 is the base peak, whereas the conjugated puerarin showed m/z 295 as the base peak. The neutral losses of 176 and 120 amu are indicative of glucuronide and C-glucoside moieties, respectively (Prasain et al., 2004; Brownsill at al., 1994; Zhang et al., 2000; Lafaye et al., 2004; Clarke et al., 2002). Thus, this metabolite was proposed to be a glucuronide conjugate of puerarin. MS/MS of m/z 495 showed characteristic product ions obtained from the subsequent neutral losses of 80 and 120 amu from this ion (Fig. 3B). The product ion at m/z 80 is a typical fragment for aromatic sulfate conjugate and ions at m/z 295 and 267 are formed from a puerarin moiety.
Figure 3.
Product ion spectra obtained from LC-MS/MS analysis of [A] m/z 591 and [B] m/z 495 in puerarin treated kidney and liver samples, respectively.
Quantification of intact puerarin in tissues
Because tissues of rats had been perfused with saline, they contained no residual blood containing puerarin. The highest concentration of puerarin was found in the lungs (799 ± 411 ng/gram). The puerarin concentrations in kidney, liver and pancreas were 270 ± 74, 45 ± 11 and 66.09 ± 47.69 ng/gram tissues, respectively. The method was not validated for tissue samples, since there was not enough material available for complete validation procedure. Thus, quantification results in tissues are approximate.
Biliary excretion of puerarin
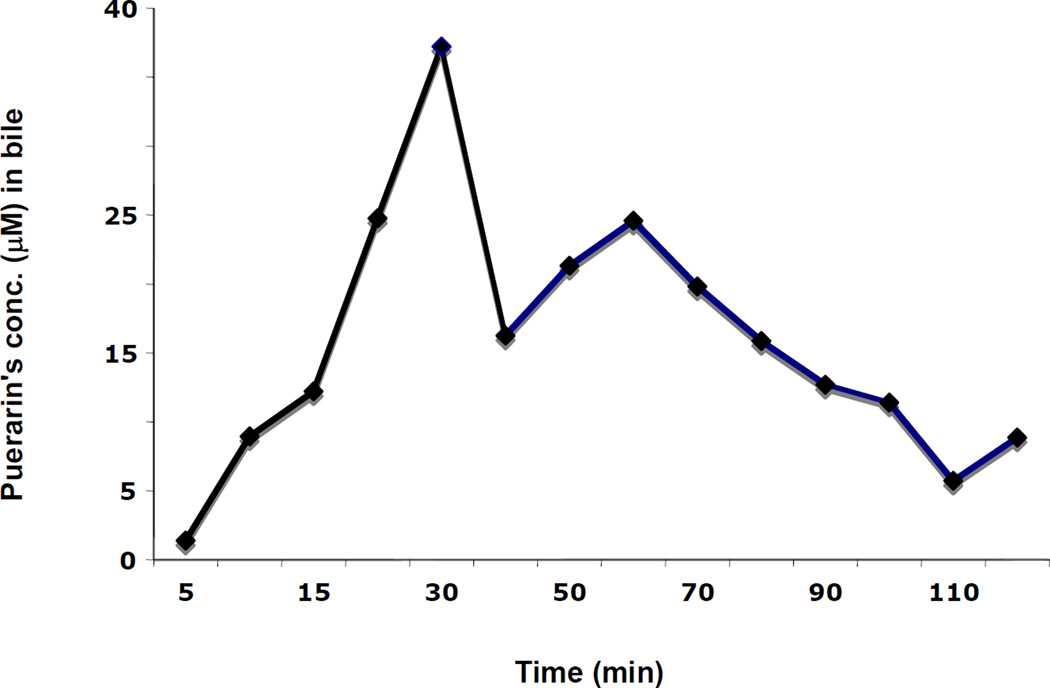
We performed a pilot study to understand whether puerarin undergoes an efficient enterohepatic circulation. Time-dependent biliary excretion of puerarin in a rat following portal vein infusion of puerarin is shown in Fig. 4. Integration of the total biliary output recovered at the end of the experiment revealed that only 18.1% of infused puerarin could be accounted for in the bile (infused amount = 30.3 µg; recovered amount = 5.5 µg). The highest amount of puerarin was excreted in the 30 min bile sample. Interestingly, only 0.17% of total unmetabolized puerarin was excreted into bile during 2 h after oral administration. As can be seen in Fig. 5, puerarin was excreted into the bile mostly as unmetabolized puerarin with small amounts of conjugated metabolites during 2 h after oral administration of puerarin. This suggests that biliary excretion of puerarin does not represent a major route of elimination, although a small amount of the material is excreted as metabolites. A neutral loss scan of 176 was performed to profile all glucuronides and only m/z 591 and its sodiated ion m/z 613 were detected. The plasma concentration of puerarin was 1035 ± 232 ng/ml.
Figure 4.
Time-dependent biliary excretion of puerarin following infusion of puerarin into the portal vein. Experimental conditions are described in the Materials and Methods.
Figure 5.
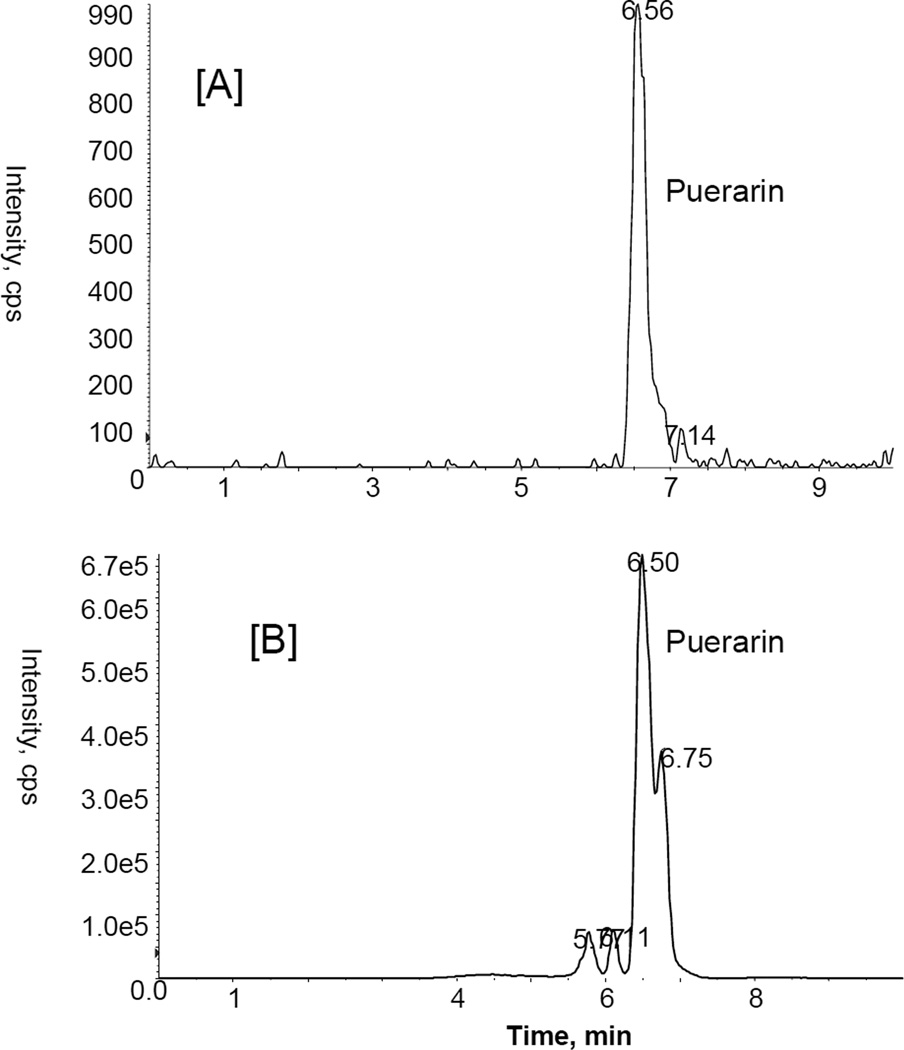
MRM ion chromatograms for puerarin with mass transition m/z 416/267 at LLOQ 2 ng/ml [A]; bile sample collected at 120 min after oral administration of puerarin [B].
Discussion
Current interest in puerarin is based on emerging evidence suggesting its health beneficial effects (Xu and Zho, 2002; Zhu et al., 2004; Hsu et al., 2003; Meezan et al., 2005). Pharmacokinetic studies from this laboratory have shown that puerarin is rapidly absorbed into the blood, reaching a maximum and then declining within 1 h (Prasain et al., 2007). In an attempt to establish whether puerarin is distributed in rat tissues after single oral dose of puerarin and in what form(s), sensitive LC-MS/MS techniques were used to detect and directly measure the concentration of puerarin.
The present study shows that orally administered puerarin is rapidly absorbed from the intestinal compartment to the blood and widely distributed to various organs such as liver, kidney, lungs, pancreas, heart and eyes. Of the organs tested, the highest concentration of puerarin was in lungs, exceeding even that in kidney. A previous report indicates that quercetin and its metabolites also distribute in the highest concentrations in the lungs (de Boer et al., 2005). The significance of puerarin accumulation in these organs is not known yet; however, the lung may be a site for discovery of biomarkers of puerarin effects.
Previous study from our laboratory revealed that puerarin is absorbed as the intact glucoside and blunts the rise in blood glucose concentration after oral and i.p. administration of glucose (Meezan et al., 2005). We propose that the mechanism for this action involves two sodium-dependent glucose cotransporters (SGLT1 and/or SGLT2). It is also possible that puerarin suppresses renal tubular glucose reabsorption by inhibiting renal SGTL2, thereby increasing urinary glucose excretion. To improve our understanding of the mechanism of puerarin’s action on glucose metabolism, it is necessary to know whether puerarin or its metabolites are accessible to the major metabolizing organs. In the present study, accumulation of puerarin in kidney tissues was found, which supports our hypothesis that puerarin could be a substrate for SGLT2 in kidney.
LC-MS/MS analysis of pancreatic tissue of rats treated with puerarin showed the presence. This indicated that puerarin penetrates into the pancrease and may be effective in preventing islet cells from the toxic action of reactive oxygen species in diabetes (Xiong et al., 2006). In our previous study, we have demonstrated that puerarin is bioavailable in brain tissues (Prasain et al., 2004). It is assumed that blood-brain barrier-specific influx transporters may be involved for the transport of puerarin into the brain.
The data from this study demonstrate that small amount of puerarin is excreted in bile along with puerarin glucuronide and puerarin sulfate. These data suggest that hydrolysis of puerarin in this model does not take place before it is excreted into bile. This is in contrast to the isoflavone O-glucoside genistin which undergoes intestinal hydrolysis before its absorption (Sfakianos et al., 1997; Prasain et al., 2006); puerarin is β-glucosidase-resistant. It is interesting to note that isoflavones like genistein and daidzein undergo extensive metabolism in the gut and liver, which might affect their biological properties. It has been reported that sulfation of genistein, with the associated loss of hydroxyl groups, decreases its antioxidant activity and its effect on platelet aggregation, inflammation, cell adhesion and chemotaxis (Rimbach et al., 2003; Turner et al., 2004). In this context, the presence of low levels of phase I and phase II metabolites of puerarin in body fluids and tissues may have special relevance to its health beneficial effects.
In summary, the present study demonstrated that a single oral administration of puerarin to rats results in a wide distribution of puerarin to the most of the organs, being highest in lungs. In addition, it provides preliminary evidence on biliary excretion of puerarin and its metabolites in rats which may be useful for designing toxicology and pharmacology studies and for interpreting the results of these experiments. Tissue distribution of puerarin requires further examination, particularly, the long-term tissue distribution of puerarin after chronic exposure in animals to different concentrations of puerarin.
Acknowledgments
These studies were supported in part by grants from the National Center for Complementary and Alternative Medicine and the National Institutes of Health Office of Dietary Supplements (5P50 AT-00477 – to the Purdue University-UAB Botanicals Center for Age-Related Disease, Connie Weaver, PI). The mass spectrometer was purchased by funds from a NIH/NCRR Shared Instrumentation Grant (S10 RR19231) and from this institution. Operation of the UAB Comprehensive Cancer Center Mass Spectrometry Shared Facility has been supported in part by a NCI Core Research Support Grant to the UAB Comprehensive Cancer (P30 CA13148).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brownsill R, Combal JP, Taylor A, Bertrand M, Luijten W, Walther B. The application of electrospray and neutral-loss mass spectrometry to the identification of the metabolites of S12813 in rat liver slices. Rapid Commun. Mass Spectrom. 1994;8:361–365. doi: 10.1002/rcm.1290080504. [DOI] [PubMed] [Google Scholar]
- Clarke DB, Lloyd AS, Botting NP, Oldfield MF, Needs PW, Wiseman H. Measurement of intact sulfate and glucuronide phytoestrogen conjugates in human urine using isotope dilution liquid chromatography-tandem mass spectrometry with [13C(3)]isoflavone internal standards. Anal. Biochem. 2002;309:158–172. doi: 10.1016/s0003-2697(02)00275-0. [DOI] [PubMed] [Google Scholar]
- de Boer VC, Dihal AA, van der Woude H, Arts IC, Wolffram S, Alink GM, Rietjens IM, Keijer J, Hollman PC. Tissue distribution of quercetin in rats and pigs. J. Nutr. 2005;135:1718–1725. doi: 10.1093/jn/135.7.1718. [DOI] [PubMed] [Google Scholar]
- Fang N, Yu S, Badger TM. Characterization of Isoflavones and Their Conjugates in Female Rat Urine Using LC/MS/MS. J. Agric. Food Chem. 2002;50:2700–2707. doi: 10.1021/jf011384v. [DOI] [PubMed] [Google Scholar]
- Hsu FL, Liu IM, Kuo DH, Chen WC, Su HC, Cheng JT. Antihyperglycemic effect of puerarin in streptozotocin-induced diabetic rats. J. Nat. Prod. 2003;66:788–792. doi: 10.1021/np0203887. [DOI] [PubMed] [Google Scholar]
- Keyler DE, Baker JI, Lee DY, Overstreet DH, Boucher TA, Lenz SK. Toxicity study of an antidipsotropic Chinese herbal mixture in rats: NPI-028. J. Altern. Complement. Med. 2002;8:175–183. doi: 10.1089/107555302317371460. [DOI] [PubMed] [Google Scholar]
- Li Y, Pan WS, Chen SL, Xu HX, Yang DJ, Chan AS. Pharmacokinetic, tissue distribution, and excretion of puerarin and puerarin-phospholipid complex in rats. Drug Dev. Ind. Pharm. 2006;32:413–422. doi: 10.1080/03639040600559123. [DOI] [PubMed] [Google Scholar]
- Lafaye A, Junot C, Ramounet-Le Gall B, Fritsch P, Ezan E, Tabet JC. Profiling of sulfoconjugates in urine by using precursor ion and neutral loss scans in tandem mass spectrometry. Application to the investigation of heavy metal toxicity in rats. J. Mass Spectrom. 2004;39:655–664. doi: 10.1002/jms.635. [DOI] [PubMed] [Google Scholar]
- Meezan E, Meezan EM, Jones K, Moore R, Barnes S, Prasain JK. Contrasting effects of puerarin and daidzin on glucose homeostasis in mice. J. Agric. Food Chem. 2005;53:8760–8767. doi: 10.1021/jf058105e. [DOI] [PubMed] [Google Scholar]
- Prasain JK, Xu J, Kirk M, Smith Johnson M, Sfakianos J, Barnes S. Differential biliary excretion of genistein metabolites following intraduodenal and intravenous infusion of genistin in female rats. J. Nutr. 2006;136:2975–2979. doi: 10.1093/jn/136.12.2975. [DOI] [PubMed] [Google Scholar]
- Prasain J, Peng N, Acosta E, Moore R, Arabshahi A, Meezan E, Barnes S, Wyss JM. Pharmacokinetic study of puerarin in rat serum by liquid chromatography tandem mass spectrometry. Biomed. Chromatogr. 2007;21:410–414. doi: 10.1002/bmc.772. [DOI] [PubMed] [Google Scholar]
- Prasain JK, Jones K, Brissie N, Moore R, Wyss JM, Barnes S. Identification of puerarin and its metabolites in rats by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2004;52:3708–3712. doi: 10.1021/jf040037t. [DOI] [PubMed] [Google Scholar]
- Rimbach G, De Pascual-Teresa S, Ewins BA, Matsugo S, Uchida Y, Minihane AM, Turner R, VafeiAdou K, Weinberg PD. Antioxidant and free radical scavenging activity of isoflavone metabolites. Xenobiotica. 2003;33:913–925. doi: 10.1080/0049825031000150444. [DOI] [PubMed] [Google Scholar]
- Sieveking DP, Woo KS, Fung KP, Lundman P, Nakhla S, Celermajer DS. Chinese herbs Danshen and Gegen modulate key early atherogenic events in vitro. Int J Cardiol. 2005;105:40–45. doi: 10.1016/j.ijcard.2004.10.052. [DOI] [PubMed] [Google Scholar]
- Sfakianos J, Coward L, Kirk M, Barnes S. Intestinal uptake and biliary excretion of the isoflavone genistein in rats. J. Nutr. 1997;127:1260–1268. doi: 10.1093/jn/127.7.1260. [DOI] [PubMed] [Google Scholar]
- Turner R, Baron T, Wolffram S, Minihane AM, Cassidy A, Rimbach G, Weinberg PD. Effect of circulating forms of soy isoflavones on the oxidation of low density lipoprotein. Free Radic. Res. 2004;38:209–216. doi: 10.1080/10715760310001641854. [DOI] [PubMed] [Google Scholar]
- Wu L, Qiao H, Li Y, Li L. Protective roles of puerarin and Danshensu on acute ischemic myocardial injury in rats. Phytomedicine. 2007;14:652–658. doi: 10.1016/j.phymed.2007.07.060. [DOI] [PubMed] [Google Scholar]
- Xiong FL, Sun XH, Gan L, Yang XL, Xu HB. Puerarin protects rat pancreatic islets from damage by hydrogen peroxide. Eur. J. Pharmacol. 2006;529:1–7. doi: 10.1016/j.ejphar.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Xu XH, Zhao TQ. Effects of puerarin on D-galactose-induced memory deficits in mice. Acta Pharmacol Sin. 2002;23:587–590. [PubMed] [Google Scholar]
- Yasuda T, Kano Y, Saito K, Ohsawa K. Urinary and biliary metabolites of daidzin and daidzein in rats. Biol Pharm Bull. 1994;17:1369–1374. doi: 10.1248/bpb.17.1369. [DOI] [PubMed] [Google Scholar]
- Yu Z, Gao X, Zhao Y, Bi K. Determination of safflor yellow A, puerarin, ferulic acid, ginsenoside Rg1, and Rb1 in the Traditional Chinese Medicinal preparation Naodesheng injection by high-performance liquid chromatography. J. Chromatogr. Sci. 2006;44:272–275. doi: 10.1093/chromsci/44.5.272. [DOI] [PubMed] [Google Scholar]
- Zhu JH, Wang XX, Chen JZ, Shang YP, Zhu JH, Guo XG, Sun J. Effects of puerarin on number and activity of endothelial progenitor cells from peripheral blood. Acta Pharmacol. Sin. 2004;25:1045–1051. [PubMed] [Google Scholar]
- Zhang JY, Wang Y, Dudkowski C, Yang D, Chang M, Yuan J, Paulson SK, Breau AP. Characterization of metabolites of Celecoxib in rabbits by liquid chromatography/tandem mass spectrometry. J. Mass Spectrom. 2000;35:1259–1270. doi: 10.1002/1096-9888(200011)35:11<1259::AID-JMS57>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]