Abstract
This trial investigated the efficacy of omega-3 polyunsaturated fatty acid (n-3 PUFA) treatment for improving depressive symptoms and cognitive performance in patients with coronary artery disease (CAD) participating in cardiac rehabilitation. Patients with CAD aged 45 to 80 years were randomized to receive either 1.9-g/d n-3 PUFA treatment or placebo for 12 weeks. Depressive symptoms were measured using the Hamilton Depression Rating Scale (HAM-D, primary outcome) and the Beck Depression Inventory II (BDI-II). Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria were used to identify a depressive episode at baseline. Cognitive performance was measured using a standardized battery for vascular cognitive impairment. In 92 patients (age, 61.7 ± 8.7 y; 76% male, 40% depressed; HAM-D, 6.9 ± 5.9; BDI-II, 12.3 ± 10.9; n = 45 n-3 PUFA, n = 47 placebo), depression decreased (HAM-D, F3,91 = 2.71 and P = 0.049; BDI-II, F3,91 = 6.24 and P < 0.01), and cognitive performance improved (attention/processing speed, F1,91 = 5.57, P = 0.02; executive function, F1,91 = 14.64, P < 0.01; visuospatial memory, F1,91 = 4.01, P = 0.04) over cardiac rehabilitation. Omega-3 PUFA treatment increased plasma eicosapentaenoic acid (F1,29 = 33.29, P < 0.01) and docosahexaenoic acid (F1,29 = 15.29, P < 0.01) concentrations but did not reduce HAM-D (F3,91 = 1.59, P = 0.20) or BDI-II (F3,91 = 0.46, P = 0.50) scores compared with placebo. Treatment did not improve cognitive performance; however, n-3 PUFAs significantly increased verbal memory compared with placebo in a subgroup of nondepressed patients (F1,54 = 4.16, P = 0.04). This trial suggests that n-3 PUFAs do not improve depressive and associated cognitive symptoms in those with CAD. The possible benefits of n-3 PUFAs for verbal memory may warrant investigation in well-powered studies.
Key Words: EPA, eicosapentaenoic acid, DHA, docosahexaenoic acid, depression, mood, affect, memory, cognitive impairment, exercise, fish oil, cardiac rehabilitation, fitness, attention, executive function
Several meta-analyses of randomized controlled trials (RCTs) have detected antidepressant efficacy of omega-3 polyunsaturated fatty acid (n-3 PUFA) supplements in patients with affective disorders.1–3 Omega-3 PUFA supplements are an ideal intervention to investigate for antidepressant benefits in those with coronary artery disease (CAD) as diets rich in n-3 PUFAs may reduce cardiovascular risk.4–6 Despite this potential suitability, relatively few studies7–10 have investigated the antidepressant efficacy of n-3 PUFA supplements in CAD. Furthermore, none has used an eicosapentaenoic acid (EPA)–enriched formulation, which is now suggested to be optimal for antidepressant efficacy.1–3 Depressive symptoms can reduce adherence to secondary prevention strategies such as cardiac rehabilitation (CR)11 and double the risk of mortality.12 As available antidepressant interventions remain limited by poor response rates,13,14 clarification of potential n-3 PUFA antidepressant efficacy is needed.
In addition, n-3 PUFA supplements may benefit cognitive performance among patients with mild cognitive deficits.15,16 Mild cognitive deficits are prevalent in CAD and are associated with functional decline.17,18 Depressive symptoms and cognitive deficits can interact to quadruple the rate of dropout from CR,19 ultimately limiting its potential antidepressant20 and procognitive benefits,21 as well as increasing the risk of mortality.12,22,23 There are currently no suitable interventions for cognitive deficits in patients with CAD without dementia,24 and so, identifying potential procognitive interventions is an active area of research.
This trial investigated the efficacy of EPA-enriched n-3 PUFA treatment for reducing depressive symptoms and improving cognitive performance in patients with CAD participating in CR. As depressive symptoms are associated with poorer cognitive performance25,26 and accelerated cognitive decline,27 the influence of depression on n-3 PUFA cognitive efficacy was also explored.
MATERIALS AND METHODS
The CAD Randomized Omega-3 Trial in Depression was an RCT investigating the antidepressant efficacy of 1.9-g/d n-3 PUFA treatment compared with placebo using a 12-week parallel arm design in which patients were randomized in a 1:1 ratio. Consecutive patients were approached for recruitment at the time of entry to 1 of 2 CR programs in Toronto, Ontario—University Health Network at Toronto Rehab or Trillium Health Partners. This trial was approved by the research ethics boards of both centers, as well as by the principal trial site, Sunnybrook Research Institute, and was conducted according to the principles expressed in the Declaration of Helsinki. The clinical trial identifier (clinicaltrials.gov) is NCT00981383.
Patients
Patient eligibility was determined at a screening visit (week 2) by a trained study associate. Eligible patients were those providing written informed consent and those with evidence of stable CAD (history of: myocardial infarction, coronary artery bypass graft, percutaneous transluminal coronary angioplasty or at least a 50% stenosis in 1 or more major coronary artery) and who were aged 45 to 80 years and male or female and have the ability to speak and understand English. Excluded patients were those with a significant acute medical illness, a clinically significant cognitive impairment (Standardized Mini-Mental State Examination [sMMSE] score < 2428,29), a neurological condition, unstable angina (Canadian Cardiovascular Society class 4), ventricular tachycardia and/or an implantable cardioverter defibrillator, or a high risk of mortality (Killip class > II); who were currently abusing ethanol or other substances, women of childbearing potential, or allergic or hypersensitive to fish; or who have contraindications to soybean/corn oil or a preexisting bleeding disorder.
The presence of a major or minor depressive episode was assessed at baseline using standardized criteria from the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) depression scale. Both those who were experiencing a depressive episode and those who were not were eligible for the trial. Antidepressant use was permitted if used at a stable dose for at least 3 months before the trial.
Trial Design
Detailed demographic, anthropomorphic, medical, and medication information was collected from eligible, consenting patients at the screening visit. Patients were then entered into a 2-week single-bind placebo lead-in phase (starting at screening, week 2) to ensure trial eligibility and the consistency of a depressive episode (if present). At the baseline visit (week 0), patients were randomized to the double-blind phase in which they received either n-3 PUFA supplements or placebo for 12 weeks. Study outcomes were assessed at baseline and at weeks 4, 8, and 12. Adverse events and depressive symptom severity were assessed at each study visit. Cognitive performance was assessed at baseline and at week 12.
Intervention
A block randomization code was independently computer generated at Sunnybrook Health Science Centre pharmacy. Kits with study medication were consecutively prepackaged as per the randomization sequence before commencement of the trial and were administered in order by trained study personnel. All study personnel remained blind to treatment allocation until the database was “locked.” Patients randomized to receive n-3 PUFA treatment used 3 capsules daily, providing 1.9-g n-3 PUFA daily (1.2-g EPA and 0.6-g docosahexaenoic acid [DHA] with 0.1-g other n-3 PUFA) in an ethyl-ester form. The placebo was 3 capsules (3 × 1 g) of 50/50 soybean/corn oil blend containing less than 0.1-g n-3 PUFA with negligible EPA and DHA. The n-3 PUFA and placebo capsules were similar in appearance (dark brown) and taste (lemon-lime flavoring). The n-3 PUFA capsules supplied by Ocean Nutrition Canada (Dartmouth, NS) have been approved for use in Canada and are registered with the Natural Health Products Directorate of Health Canada (NPN 80000901). Patients were advised to take the study medication with their first meal of the day to minimize any possible variations in response based on time of the day. Treatment compliance was determined by the percentage of capsules used relative to those allotted during the 12-week trial.
Outcome Measures
Primary Outcome Measure
Depressive symptoms were measured using the 17-item Hamilton Depression Rating Scale (HAM-D).30 As recommended by expert consensus, the HAM-D is the gold standard for assessing antidepressant efficacy in trials with patients with CAD.31
Secondary Outcome Measure
Depressive symptoms were also reported by each patient using the self-report 21-item Beck Depression Inventory II (BDI-II),31,32 which has shown adequate sensitivity in previous antidepressant trials in patients with CAD33–35 and provided a complementary measure of depressive symptoms.
Exploratory Outcome Measures
Cognitive performance in specific domains was assessed using the National Institutes of Neurological Disorders and Stroke and Canadian Stroke Network recommended 30-minute battery for vascular cognitive impairment.36 All cognitive testing was performed at a standardized time (900 hours ± 30 minutes). Verbal memory was assessed using the composite score from performance on the immediate, short-delay, and long-delay recall lists from the California Verbal Learning Test, Second Edition. Attention and processing speed performance was measured using the composite score from performance on the Digit Symbol Substitution Test and the Trail Making Test Part A. Executive function was measured using the composite scores from performance on the Animal Naming Test, the Controlled Word Association Test, and the Trail Making Test Part B. The immediate and long-delay recall segments of the Brief Visuospatial Memory Test-Revised37 were added to measure visuospatial memory. Alternate versions of each cognitive test were used at week 12 to minimize potential practice effects.
Adverse Events
Each patient was monitored for adverse events at each trial visit using a standard adverse event checklist according to consensus guidelines.38,39 Patients demonstrating severe depression (HAM-D score > 23) or suicidal ideation at any study visit were removed from the study and received appropriate medical care.
Cardiac Rehabilitation
The CR programs at each site consisted of supervised in-class aerobic and resistance training once weekly with additional at-home sessions 4 days per week.40 Cardiopulmonary fitness at baseline and at week 12 was measured in each patient using the peak volume of oxygen (VO2) uptake during a cardiac exercise stress test adjusted to a percentage of age and sex-expected VO2 peak norms (the VO2 peak fraction).41,42 The change in VO2 peak fraction during the trial was assessed as a potential confounder of treatment efficacy.
Analysis of Plasma Fatty Acids
At the baseline and week 12 visits, 34 mL of fasting (12 hours overnight) blood was drawn from the antecubital vein. Plasma EPA and DHA concentrations were measured by gas chromatography.43 Baseline EPA + DHA concentrations (plasma “omega-3 index”) were assessed as covariate in the analyses of treatment efficacy. Changes in plasma EPA and DHA concentrations provided a complementary measure of treatment compliance, as well as any dietary intake. All analyses were performed blinded to treatment allocation and patient characteristics.
Sample Size Calculation
A sample size of 92 (46 per group) provided 80% power to detect a 3- to 4-point difference in HAM-D total score between the n-3 PUFA and placebo groups for 12 weeks at a significance level (α) of 0.05 in a population with a standard deviation (SD) in HAM-D score of 6 points. A 3- to 4-point difference in HAM-D score is consistent with a large effect size (≥0.5),44 which is in keeping with the stringency defined in previous antidepressant trials in patients with CAD.45,46
Statistical Analyses
Treatment Efficacy
An intention to treat (ITT) analysis of treatment efficacy was conducted using a repeated measures general linear regression with HAM-D total score (primary outcome) or BDI-II total score (secondary outcome) as the dependent variable with 4 observations (baseline and weeks 4, 8, and 12). Missing data from week 4, 8, and/or 12 assessments were imputed using a multiple imputation procedure (5 imputed data sets, as per Rubin47). Exploratory analyses assessed treatment efficacy on verbal memory composite z score, attention and processing speed composite z score, executive function composite z score, or visuospatial memory composite z score as the dependent variable with 2 time points (baseline and week 12).
For all analyses, covariates were selected based on significant and/or potentially clinically meaningful differenences in patient characteristics between the treatment groups at baseline.
Subgroup Analyses
Treatment efficacy in a subgroup of patients who were not using maintenance antidepressant medication at baseline was explored. In a per-protocol analysis, treatment efficacy was assessed using percentage compliance with the study intervention as a covariate.
Treatment efficacy for depression and cognition was also evaluated in the subgroup of patients meeting DSM-IV depressive episode criteria at baseline and the subgroup of patients not meeting those criteria.
Conversion of Cognitive Data
Raw scores indicating performance on each cognitive test were converted to z scores, which are standardized based on published population norms. A z score of 0 reflects the population mean performance for each test for a given age and sex category, with higher z scores indicating higher-than-average performance and lower z scores indicating lower-than-average performance. For example, a z score of 1 indicates that performance on a certain cognitive test was 1 SD higher than the population norm after adjusting for age and sex. z Scores from tests measuring similar cognitive processes were averaged, yielding a composite z score for performance on that particular cognitive domain.
RESULTS
Between August 2010 and February 2014, 645 patients were assessed for study eligibility, and 121 were entered into the screening phase. Of those, 92 patients (characteristics in Table 1) met study criteria at the baseline visit and were enrolled into the randomization phase. Randomization to treatment was not different between the study sites (χ2 = 0.03, df = 1, P = 0.87). Between treatment groups at baseline, patients randomized to n-3 PUFA treatment were older, less likely to use acetylsalicylic acid, and more likely to be diabetic compared with those randomized to placebo. Omega-3 PUFA–treated patients also had lower mean sMMSE scores and lower plasma EPA + DHA concentrations at baseline compared with those randomized to placebo. The proportion of patients meeting DSM-IV depressive episode criteria, the severity of depressive symptoms (measured using the HAM-D and the BDI-II), and history of previous depression were not different between groups at baseline. All patients were cognitively intact (mean baseline sMMSE score, 28.8 ± 1.2), despite variable performance on baseline cognitive measures (Table 2). Forty-six patients (50%) had a composite cognitive z score below standardized norms (<0). There were no differences in baseline cognitive performance between the treatment groups. At baseline, patients meeting depressive episode criteria performed significantly more poorly in all cognitive domains than those not meeting criteria.
TABLE 1.
Baseline Patient Demographic and Clinical Characteristics

TABLE 2.
Baseline Cognitive Performance by Treatment Group and Baseline Depression

Eighty-two (89%) of the randomized patients completed the trial. The dropout rate in the n-3 PUFA arm (n = 5) did not differ from that in the placebo arm (n = 5) (χ2 = 0.01, P = 0.94). Of the 10 study dropouts, two began using an antidepressant during the study (one from each group), three dropped out of CR, one was excluded because of a revascularization procedure shortly after randomization, one was excluded because of recent use of a recreational drug, two no longer wished to participate in the study because of time constraints, and one placebo user discontinued the study medication because of frequent eructation. Of the study completers, the median percentage of capsule use was 92%. Compliance was not different between treatment groups (χ2 = 0.08, P = 0.78).
Adverse events did not differ between treatment groups during the course of the trial (Table 3). The most commonly reported adverse symptoms were general pain, nasopharyngitis, and fatigue; however, none of the study dropouts cited those symptoms as the reason for withdrawal. None of the patients demonstrated severe depression or suicidal ideation at any visit, and so none were excluded for those reasons.
TABLE 3.
Postrandomization Adverse Events

Outcomes
Omega-3 PUFA treatment significantly increased plasma concentrations of EPA (pretreatment, 26.7 ± 14.2 μg/mL; posttreatment, 41.6 ± 34.4 μg/mL; F1,29 = 33.29, P < 0.01) and DHA (pretreatment, 47.3 ± 20.1 μg/mL; posttreatment, 53.6 ± 29.3 μg/mL; F1,29 = 15.29, P < 0.01) during 12 weeks. There was no significant change in the plasma concentrations of EPA (pretreatment, 28.5 ± 16.6 μg/mL; posttreatment, 23.4 ± 14.4 μg/mL; F1,39 = 3.10, P = 0.09) or DHA (pretreatment, 52.8 ± 23.8 μg/mL; posttreatment, 45.9 ± 20.4 μg/mL; F1,39 = 3.29, P = 0.08) during 12 weeks in the placebo group.
Primary Outcome (Depression)
Hamilton Depression Rating Scale total scores changed significantly during 12 weeks of CR (F3,91 = 2.71, P = 0.049; mean change, 1.1 ± 4.6 points; range, −11 to 10 points). There was no significant difference in HAM-D total scores between the n-3 PUFA treatment group and placebo group during 12 weeks of CR (primary outcome, treatment × time interaction: F3,91 = 1.59, P = 0.20) (Fig. 1). Changes in VO2 peak fraction during 12 weeks were not correlated with changes in HAM-D total score (Pearson r = −0.04, P = 0.79).
FIGURE 1.
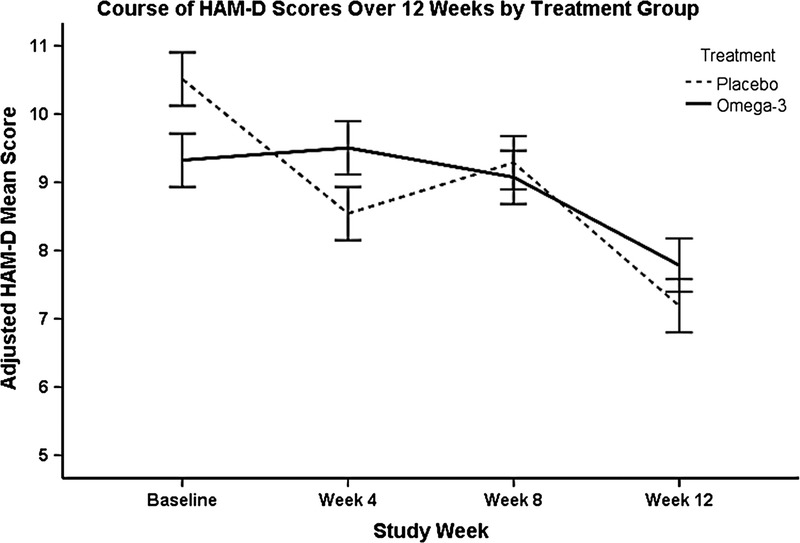
The course of depressive symptoms measured by HAM-D total scores during 12 weeks of CR in n-3 PUFA–treated and placebo-treated patients. The solid line represents the n-3 PUFA–treated group, and the dashed line represents the placebo-treated group. Error bars represent the standard error. Hamilton Depression Rating Scale scores in this figure (y-axis) are estimates adjusted for the included covariates: age, baseline sMMSE, ACE inhibitor use, acetylsalicylic acid use, and diabetes mellitus.
Secondary Outcome (Depression)
Beck Depression Inventory II total scores changed significantly during 12 weeks of CR (F3,91 = 6.24, P < 0.01; mean change, −2.5 ± 8.0 points; range, −23 to 19 points). There was no significant difference in BDI-II total scores between the n-3 PUFA treatment group and placebo group during 12 weeks of CR (secondary outcome, treatment × time interaction: F3,91 = 0.46, P = 0.50).
Exploratory Outcomes (Cognition)
Mean verbal memory composite z scores did not change during 12 weeks of CR (F1,91 = 2.16, P = 0.15), although z score changes ranged from −1.99 to 2.35 during that time. Improvements in mean composite z scores for attention and processing speed (change, 0.09 ± 0.42; range, −0.95 to 1.17; F1,91 = 5.57, P = 0.02), executive function (change, 0.16 ± 0.41; range, −0.76 to 1.08; F1,91 = 14.64, P < 0.01), and visuospatial memory (change, 0.13 ± 0.77; range, −2.33 to 2.42; F1,91 = 4.01, P = 0.04) were observed in the total sample during 12 weeks of CR. However, treatment was not efficacious for any cognitive domain (Table 4).
TABLE 4.
Exploratory Cognitive Outcomes in ITT Analysis: Treatment Efficacy Varied Based on Depressive Episode Status

Subgroup Analyses
Excluding antidepressant users from the analyses did not reveal any efficacy of n-3 PUFA treatment on depressive symptoms (HAM-D, F3,79 = 1.50 and P = 0.22; BDI-II, F3,79 = 1.12 and P = 0.35). In the subgroup of patients for whom treatment compliance data were available (n = 86), including percentage compliance as a covariate did not reveal n-3 PUFA treatment efficacy (treatment × time interaction: HAM-D, F3,85 = 1.30 and P = 0.28; BDI-II, F3,85 = 0.42 and P = 0.74). Percentage compliance was not a significant predictor of changes in depressive symptoms (HAM-D, F3,85 = 1.20 and P = 0.31; BDI-II, F3,85 = 0.74 and P = 0.53). In the subgroup of patients for whom baseline plasma EPA + DHA concentrations were available (post hoc analysis, n = 86), n-3 PUFA treatment remained inefficacious for depressive symptoms (HAM-D, F3,85 = 0.46 and P = 0.71; BDI-II, F3,85 = 0.79 and P = 0.50) and cognitive performance (verbal memory, F1,85 = 0.56 and P = 0.46; attention and processing speed, F1,85 = 0.64 and P = 0.43; executive function, F1,85 = 0.30 and P = 0.38; visuospatial memory, F1,85 = 0.41 and P = 0.52) after adjusting for baseline difference in that marker between groups.
Treatment Efficacy in Depressed Versus Nondepressed
The presence of depression (DSM-IV major or minor depressive episode) at baseline did not influence antidepressant treatment efficacy during 12 weeks of CR (depressive episode × treatment × time interaction: HAM-D, F3,91 = 1.31 and P = 0.28; BDI-II, F3,91 = 0.37 and P = 0.76).
However, the presence of a depressive episode seemed to influence treatment efficacy for cognition. In the nondepressed subgroup (n = 55), n-3 PUFA treatment was significantly associated with improved composite verbal memory performance during 12 weeks, particularly immediate verbal memory (F1,91 = 6.04, P = 0.02), compared with placebo (Table 4). Omega-3 PUFA cognitive benefits were not observed in the subgroup of patients who met DSM-IV depression criteria at baseline (n = 37).
DISCUSSION
Given the potential cardiovascular benefits of n-3 PUFA treatment4 and the negative impact of even minimal mood symptoms,48,49 it was important to assess whether using n-3 PUFAs would be potentially helpful in patients with CAD with any evidence of mood symptoms. This is unlike the case of using traditional antidepressants for subthreshold mood symptoms where adverse events, cost, potential drug interactions, and limited efficacy13,14 might be a concern. In this trial, n-3 PUFA treatment did not reduce depressive symptom severity during 12 weeks of CR compared with placebo, despite n-3 PUFA treatment significantly increasing plasma EPA and DHA concentrations. This finding is in keeping with several previous trials (reviewed in Grosso et al1). However, it is in contrast to the meta-analytic findings that EPA-enriched formulations, such as the one used in this trial, demonstrate efficacy for reducing depressive symptoms.2,3
The lack of antidepressant efficacy observed in this trial may be related to the wide range of depressive symptoms among included patients. Patients with and without depressive symptoms were included in this study to generate a sample of patients with CAD representative of the CR population. Although meta-analytic evidence supports treatment efficacy in patients with depressive symptoms but no diagnosis of major depression,1 efficacy is most consistently observed in studies including patients with major depression uncomplicated by comorbid medical and/or psychiatric conditions.1 Although our trial did not observe any treatment efficacy in patients with CAD meeting DSM-IV depressive episode criteria at baseline, it is still possible that n-3 PUFA treatment may be most relevant to that subgroup and that this study was underpowered to detect that association.
In exploratory analyses, n-3 PUFA treatment was not associated with improvements in cognitive performance, although attention and processing speed, executive function, and visuospatial memory all improved during 12 weeks of CR. These findings are consistent with those of several meta-analyses and large RCTs suggesting that, in general, n-3 PUFA treatment is not efficacious for improving cognitive performance.15,16,50–53 However, previous trials have shown that n-3 PUFA treatment may be suitable for particularly responsive patient subgroups, such as those with mild cognitive deficits,15,16 or particularly responsive cognitive domains, such as verbal memory.15 In line with those findings, this trial found that n-3 PUFA treatment improved verbal memory performance, particularly immediate verbal recall, among patients with CAD who were not depressed at baseline.
It is interesting that treatment benefits were observed among patients who were not depressed and demonstrated higher-than-average verbal memory, rather than those who were depressed and demonstrated lower-than-average verbal memory. In a previously published meta-analysis,21 we found that n-3 PUFA treatment improved verbal memory performance among adults with mild cognitive deficits, although the included studies did not assess patients with depression or those with CAD. Depression is consistently associated with cognitive deficits cross-sectionally54 and can accelerate cognitive decline even in patient populations already at risk for decline, such as CAD.10 It may therefore be possible that the presence of a depressive episode, despite possibly contributing to cognitive deficits, conferred a resistance to cognitive change despite n-3 PUFA treatment. Pathophysiology associated with depression may shed light on this lack of treatment efficacy. Aberrant activity of inflammatory cytokine, oxidative stress, and lipid homeostasis mechanisms are commonly observed in depressed patients and may collectively mediate neurodegenerative mechanisms leading to cognitive deficits and decline.55 Those processes can also result in increased peroxidation of n-3 PUFA supplements,64 which may limit their metabolism into beneficial, anti-inflammatory products with potential cognitive effects.65,66 Among patients who are not depressed, the activity of those pathways may not be as great, and n-3 PUFA treatment efficacy may therefore be more likely.
The lack of n-3 PUFA treatment efficacy for executive function and visuospatial recall is also consistent with meta-analytic findings.15 Surprisingly, attention and processing speed was not improved with n-3 PUFA treatment despite previous data supporting its benefits to that domain.15
Measurement of efficacy for cognitive outcomes was limited by several factors. Foremost, these results are limited by a small sample size, particularly the findings in the subgroup of nondepressed patients. As such, the clinical efficacy of n-3 PUFA treatment for improving verbal memory should be interpreted with caution. Replication of this finding in a larger trial is warranted.
Despite half of the included patients demonstrating a composite cognitive z score lower than population norms, none met criteria for clinically significant cognitive impairment. It is possible that changes in cognitive performance during the course of CR represented normal fluctuations rather than pathophysiological processes and that n-3 PUFA treatment did not mechanistically improve verbal memory. Potential practice effects may have also contributed to improvements in cognitive performance during the study, despite our use of alternate tests at follow-up. However, it is unlikely that these results were influenced by ceiling effects of the cognitive measures. These tests provided a wide range of potential scores,36 and few patients approached the maximum. Furthermore, the mean cognitive performance on each test other than those measuring verbal memory was lower than population norms, supporting the potential for improvement with treatment.
Treatment efficacy for cognitive outcomes may have been limited by the n-3 PUFA formulation used in this trial, which was optimized for antidepressant effects. Docosahexaenoic acid has been previously investigated as the n-3 PUFA possibly responsible for changes in cognition. Dietary deficits of DHA, as well as low blood concentrations of DHA, have been related to cognitive decline,56 and most RCTs using n-3 PUFA to treat cognition have optimized their formulation for DHA (summarized in Yurko-Mauro et al16). The supplements used in this trial contained 600 mg/d of DHA, which is greater than the mean DHA dose used by previous trials investigating cognitive outcomes (580 mg/d).16 As such, the DHA dose used in this trial is consistent with previous trials, despite the supplement consisting mainly of EPA. The potential cognitive benefits of EPA have been supported by a more recent literature. For example, lower serum concentrations of EPA have been correlated with poorer cognitive performance in patients with CAD,57 and EPA may enhance the cognitive benefits of exercise in older patients.58 Eicosapentaenoic acid may be relevant to cognitive performance on its own,59 or it may be relevant due to its conversion to DHA, reflected in the plasma,60 and subsequent uptake by the brain61 during periods of increased brain DHA utilization. Regardless of which n-3 PUFA is optimal, emerging evidence supports combined EPA + DHA doses greater than 1 g/d for improving cognitive performance among patients with cognitive deficits and those who are cognitively healthy.16 Additional research may be needed to determine the most suitable formulation and dose of n-3 PUFA supplements for antidepressant and procognitive efficacy in those with CAD. To this end, doses greater than 3 or 5 g/d have recently been deemed safe by the Food and Drug Administration61 and the European Food Safety Authority,62 respectively.
Duration of treatment may have also limited detection of cognitive efficacy. Omega-3 PUFA–associated cognitive changes during 12 weeks or less have been previously reported,62,63 although studies of cognition typically measure outcomes during periods of 18 to 24 weeks or longer.16
Finally, it is possible that treatment efficacy on depressive symptoms or cognitive performance may have been overshadowed by the antidepressant and procognitive efficacy of CR,21,54,55 although this is unlikely as several patients experienced worsening of depressive symptoms and cognitive performance despite compliance with CR. Because CR is the standard of care for CAD, it is important to evaluate any interventions against this background, despite this approach limiting the generalizability of any findings. Our results highlight the persisting clinical need for efficacious antidepressant interventions for a range of depressive symptom severity in this population.
CONCLUSIONS
Omega-3 PUFA treatment was not efficacious for improving depressive symptoms or cognitive performance during 12 weeks in patients with CAD participating in CR, despite increasing plasma EPA and DHA concentrations. However, n-3 PUFAs significantly improved verbal memory performance in a subgroup of patients not meeting depressive episode criteria, suggesting that this may be an optimal subgroup to target. The detection of efficacy in this trial may have been limited by low mean baseline depressive symptom severity and a small sample size. Replication of these findings in a larger sample may clarify the clinical potential of n-3 PUFA treatment for improving depressive symptoms and cognition in those with CAD.
AUTHOR DISCLOSURE INFORMATION
This study was funded by the Ontario Mental Health Foundation, Canadian Institutes of Health Research (MOP 114913).
The authors declare no conflicts of interest.
Footnotes
This study was funded by the Ontario Mental Health Foundation, Canadian Institutes of Health Research (MOP 114913).
REFERENCES
- 1.Grosso G, Pajak A, Marventano S, et al. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PLoS One. 2014;9:e96905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martins JG, Bentsen H, Puri BK. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Mol Psychiatry. 2012;17:1144–1149. [DOI] [PubMed] [Google Scholar]
- 3.Sublette ME, Ellis SP, Geant AL, et al. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry. 2011;72:1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nestel P, Clifton P, Colquhoun D, et al. Indications for omega-3 long chain polyunsaturated fatty acid in the prevention and treatment of cardiovascular disease. Heart Lung Circ. 2015;24:769–779. [DOI] [PubMed] [Google Scholar]
- 5.Franzese CJ, Bliden KP, Gesheff MG, et al. Relation of fish oil supplementation to markers of atherothrombotic risk in patients with cardiovascular disease not receiving lipid-lowering therapy. Am J Cardiol. 2015;115:1204–1211. [DOI] [PubMed] [Google Scholar]
- 6.Balk EM, Lichtenstein AH, Chung M, et al. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis. 2006;189:19–30. [DOI] [PubMed] [Google Scholar]
- 7.Carney RM, Freedland KE, Rubin EH, et al. Omega-3 augmentation of sertraline in treatment of depression in patients with coronary heart disease: a randomized controlled trial. JAMA. 2009;302:1651–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmer R, Riemer T, Rauch B, et al. Effects of 1-year treatment with highly purified omega-3 fatty acids on depression after myocardial infarction: results from the OMEGA trial. J Clin Psychiatry. 2013;74:e1037–e1045. [DOI] [PubMed] [Google Scholar]
- 9.Giltay EJ, Geleijnse JM, Kromhout D. Effects of n-3 fatty acids on depressive symptoms and dispositional optimism after myocardial infarction. Am J Clin Nutr. 2011;94:1442–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haberka M, Mizia-Stec K, Mizia M, et al. Effects of n-3 polyunsaturated fatty acids on depressive symptoms, anxiety and emotional state in patients with acute myocardial infarction. Pharmacol Rep. 2013;65:59–68. [DOI] [PubMed] [Google Scholar]
- 11.Swardfager W, Herrmann N, Marzolini S, et al. Major depressive disorder predicts completion, adherence, and outcomes in cardiac rehabilitation:a prospective cohort study of 195 patients with coronary artery disease. J Clin Psychiatry. 2011;72:1181–1188. [DOI] [PubMed] [Google Scholar]
- 12.Penninx BW, Beekman AT, Honig A, et al. Depression and cardiac mortality: results from a community-based longitudinal study. Arch Gen Psychiatry. 2001;58:221–227. [DOI] [PubMed] [Google Scholar]
- 13.Dowlati Y, Herrmann N, Swardfager WL, et al. Efficacy and tolerability of antidepressants for treatment of depression in coronary artery disease: a meta-analysis. Can J Psychiatry. 2010;55:91–99. [DOI] [PubMed] [Google Scholar]
- 14.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. [DOI] [PubMed] [Google Scholar]
- 15.Mazereeuw G, Lanctôt KL, Chau SA, et al. Effects of ω-3 fatty acids on cognitive performance: a meta-analysis. Neurobiol Aging. 2012;33:1482.e17–1482.e1429. [DOI] [PubMed] [Google Scholar]
- 16.Yurko-Mauro K, Alexander DD, Van Elswyk ME. Docosahexaenoic acid and adult memory: a systematic review and meta-analysis. PLoS One. 2015;10:e0120391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jefferson AL, Cahn-Weiner D, Boyle P, et al. Cognitive predictors of functional decline in vascular dementia. Int J Geriatr Psychiatry. 2006;21:752–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyle PA, Paul RH, Moser DJ. Executive impairments predict functional declines in vascular dementia. Clin Neuropsychol. 2004;18:75–82. [DOI] [PubMed] [Google Scholar]
- 19.Swardfager W, Herrmann N, Marzolini S, et al. Verbal memory performance and completion of cardiac rehabilitation in patients with coronary artery disease. Psychosom Med. 2011;73:580–587. [DOI] [PubMed] [Google Scholar]
- 20.Rimer J, Dwan K, Lawlor DA, et al. Exercise for depression. Cochrane Database Syst Rev. 2012;7:CD004366. [DOI] [PubMed] [Google Scholar]
- 21.Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72:239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alter DA, Zagorski B, Marzolini S, et al. On-site programmatic attendance to cardiac rehabilitation and the healthy-adherer effect. Eur J Prev Cardiol. 2015;22:1232–1246. [DOI] [PubMed] [Google Scholar]
- 23.Lawler PR, Filion KB, Eisenberg MJ. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Am Heart J. 2011;162:571.e2–584.e2. [DOI] [PubMed] [Google Scholar]
- 24.Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312:2551–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bora E, Harrison BJ, Yucel M, et al. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol Med. 2013;43:2017–2026. [DOI] [PubMed] [Google Scholar]
- 26.McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009;119:1–8. [DOI] [PubMed] [Google Scholar]
- 27.Freiheit EA, Hogan DB, Eliasziw M, et al. A dynamic view of depressive symptoms and neurocognitive change among patients with coronary artery disease. Arch Gen Psychiatry. 2012;69:244–255. [DOI] [PubMed] [Google Scholar]
- 28.Molloy DW. Standardized Mini Mental State Examination. Troy, Ontario: New Grange Press; 1999. [Google Scholar]
- 29.Perry RJ, Watson P, Hodges JR. The nature and staging of attention dysfunction in early (minimal and mild) Alzheimer's disease: relationship to episodic and semantic memory impairment. Neuropsychologia. 2000;38:252–271. [DOI] [PubMed] [Google Scholar]
- 30.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–747. [DOI] [PubMed] [Google Scholar]
- 31.Davidson KW, Kupfer DJ, Bigger JT, et al. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute Working Group Report. Psychosom Med. 2006;68:645–650. [DOI] [PubMed] [Google Scholar]
- 32.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. [DOI] [PubMed] [Google Scholar]
- 33.Lespérance F, Frasure-Smith N, Koszycki D, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA. 2007;297:367–379. [DOI] [PubMed] [Google Scholar]
- 34.Honig A, Kuyper AM, Schene AH, et al. Treatment of post-myocardial infarction depressive disorder: a randomized, placebo-controlled trial with mirtazapine. Psychosom Med. 2007;69:606–613. [DOI] [PubMed] [Google Scholar]
- 35.Glassman AH, O'Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701–709. [DOI] [PubMed] [Google Scholar]
- 36.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. [DOI] [PubMed] [Google Scholar]
- 37.Benedict R. Brief Visuospatial Memory Test-Revised: Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc; 1997. [Google Scholar]
- 38.Bays HE. Safety considerations with omega-3 fatty acid therapy. Am J Cardiol. 2007;99:35C–43C. [DOI] [PubMed] [Google Scholar]
- 39.Harris WS. Expert opinion: omega-3 fatty acids and bleeding-cause for concern? Am J Cardiol. 2007;99:44C–46C. [DOI] [PubMed] [Google Scholar]
- 40.Hamm LF, Kavanagh T. The Toronto Cardiac Rehabilitation and Secondary Prevention Program: 1968 into the new millennium. J Cardiopulm Rehabil. 2000;20:16–22. [DOI] [PubMed] [Google Scholar]
- 41.Jones NL, Campbell EJM. Clinical exercise testing. 2nd ed Philadelphia, PA: W.B. Saunders; 1982. [Google Scholar]
- 42.Milani RV, Lavie CJ, Mehra MR, et al. Understanding the basics of cardiopulmonary exercise testing. Mayo Clin Proc. 2006;81:1603–1611. [DOI] [PubMed] [Google Scholar]
- 43.Abdelmagid SA, Clarke SE, Nielsen DE, et al. Comprehensive profiling of plasma fatty acid concentrations in young healthy Canadian adults. PLoS One. 2015;10:e0116195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen J. Statistical Power Analysis for the Behavioural Sciences. 2nd ed Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 45.Strik JJ, Honig A, Lousberg R, et al. Efficacy and safety of fluoxetine in the treatment of patients with major depression after first myocardial infarction: findings from a double-blind, placebo-controlled trial. Psychosom Med. 2000;62:783–789. [DOI] [PubMed] [Google Scholar]
- 46.Frasure-Smith N, Koszycki D, Swenson JR, et al. Design and rationale for a randomized, controlled trial of interpersonal psychotherapy and citalopram for depression in coronary artery disease (CREATE). Psychosom Med. 2006;68:87–93. [DOI] [PubMed] [Google Scholar]
- 47.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 48.Patten SB, Williams JV, Lavorato DH, et al. Depressive episode characteristics and subsequent recurrence risk. J Affect Disord. 2012;140:277–284. [DOI] [PubMed] [Google Scholar]
- 49.White J, Zaninotto P, Walters K, et al. Severity of depressive symptoms as a predictor of mortality: the English longitudinal study of ageing. Psychol Med. 2015;45:2771–2779. [DOI] [PubMed] [Google Scholar]
- 50.Cooper RE, Tye C, Kuntsi J, et al. Omega-3 polyunsaturated fatty acid supplementation and cognition: a systematic review and meta-analysis. J Psychopharmacol. 2015;29:753–763. [DOI] [PubMed] [Google Scholar]
- 51.Jiao J, Li Q, Chu J, et al. Effect of n-3 PUFA supplementation on cognitive function throughout the life span from infancy to old age: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;100:1422–1436. [DOI] [PubMed] [Google Scholar]
- 52.Sydenham E, Dangour AD, Lim WS. Omega 3 fatty acid for the prevention of cognitive decline and dementia. Cochrane Database Syst Rev. 2012;6:CD005379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chew EY, Clemons TE, Agron E, et al. Effect of omega-3 fatty acids, lutein/zeaxanthin, or other nutrient supplementation on cognitive function: the AREDS2 randomized clinical trial. JAMA. 2015;314:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rutledge T, Redwine LS, Linke SE, et al. A meta-analysis of mental health treatments and cardiac rehabilitation for improving clinical outcomes and depression among patients with coronary heart disease. Psychosom Med. 2013;75:335–349. [DOI] [PubMed] [Google Scholar]
- 55.Yohannes AM, Doherty P, Bundy C, et al. The long-term benefits of cardiac rehabilitation on depression, anxiety, physical activity and quality of life. J Clin Nurs. 2010;19:2806–2813. [DOI] [PubMed] [Google Scholar]
- 56.Beydoun MA, Beydoun HA, Gamaldo AA, et al. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health. 2014;14:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yagi S, Hara T, Ueno R, et al. Serum concentration of eicosapentaenoic acid is associated with cognitive function in patients with coronary artery disease. Nutr J. 2014;13:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Street SJ, Parletta N, Milte C, et al. Interaction of erythrocyte eicosapentaenoic acid and physical activity predicts reduced risk of mild cognitive impairment. Aging Ment Health. 2015;19:885–891. [DOI] [PubMed] [Google Scholar]
- 59.Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013;75:645–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008;47:147–155. [DOI] [PubMed] [Google Scholar]
- 61.Chen CT, Green JT, Orr SK, et al. Regulation of brain polyunsaturated fatty acid uptake and turnover. Prostaglandins Leukot Essent Fatty Acids. 2008;79:85–91. [DOI] [PubMed] [Google Scholar]
- 62.Richter Y, Herzog Y, Cohen T, et al. The effect of phosphatidylserine-containing omega-3 fatty acids on memory abilities in subjects with subjective memory complaints: a pilot study. Clin Interv Aging. 2010;5:313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jackson PA, Deary ME, Reay JL, et al. No effect of 12 weeks' supplementation with 1 g DHA-rich or EPA-rich fish oil on cognitive function or mood in healthy young adults aged 18-35 years. Br J Nutr. 2012;107:1232–1243. [DOI] [PubMed] [Google Scholar]
- 64.Assies J, Mocking RJT, Lok A, et al. Effects of oxidative stress on fatty acid- and one-carbon-metabolism in psychiatric and cardiovascular disease comorbidity. Acta Psychiatr Scand. 2014;130:163–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crupi R, Marino A, Cuzzocrea S. n-3 fatty acids: role in neurogenesis and neuroplasticity. Curr Med Chem. 2013;20:2953–2963. [DOI] [PubMed] [Google Scholar]
- 66.Orr SK, Trepanier MO, Bazinet RP. n-3 Polyunsaturated fatty acids in animal models with neuroinflammation. Prostaglandins Leukot Essent Fatty Acids. 2013;88:97–103. [DOI] [PubMed] [Google Scholar]


