Abstract
Epithelial cells form spatially-organized adhesion complexes that establish polarity gradients, regulate cell proliferation, and direct wound healing. As cells accumulate oncogenic mutations, these key tumor suppression mechanisms are disrupted, eliminating many adhesion complexes and bypassing contact inhibition. The transcription factor Snail is often expressed in malignant cancers, where it promotes transcriptional reprogramming to drive epithelial-mesenchymal transition (EMT), which promotes a more invasive state. S-palmitoylation describes the fatty-acyl post-translational modification of cysteine residues in proteins, and is required for membrane anchoring, trafficking, localization and function of hundreds of proteins involved in cell growth, polarity, and signaling. Since Snail-expression prevents apico-basolateral cell polarity, we asked if Snail-dependent transformation induces proteome-wide changes in S-palmitoylation. MCF10A breast cancer cells were retrovirally transduced with Snail, and correlated proteome-wide changes in protein abundance and S-palmitoylation were profiled using by stable isotope labeling in cell culture with amino acids (SILAC) mass spectrometry. This analysis identified increased levels of proteins involved in migration, glycolysis, and cell junction remodeling, and decreased levels of proteins involved in cell adhesion. Overall, protein S-palmitoylation is highly correlated with protein abundance, yet for a subset of proteins, this correlation is uncoupled. These findings suggest that Snail-overexpression affects the S-palmitoylation cycle of some proteins, and may affect cell polarity and tumor suppression.
Introduction
Epithelial cells form protective cell layers that interface with the environment, forming a primary barrier to regulate nutrient uptake and pathogen defense. The integrity of the epithelial barrier formed by cell-cell adhesion complexes, which coordinate cell polarity, intercellular communication, and contact inhibition. By restricting many signaling complexes to apical or basolateral membrane domains, cells coordinate cell division with adhesion complexes to protect cells overgrowth and maintain epithelial integrity1–4. Epithelial-to-mesenchymal transition (EMT) describes the reprogramming of cells to a more motile, invasive mesenchymal state correlated with chemoresistance and metastasis2, 3. The cell-cell adhesion protein E-cadherin is a central organizer cell polarity, and is transcriptionally repressed in the majority of malignant cancers by several transcription factors, including Snail, Slug, Twist or several non-coding RNAs5–8. In cell culture models, Snail overexpression alone is sufficient to induce EMT and eliminate apical-basolateral polarity1, 2, 9. Indeed, Snail is overexpressed in more than 80% of invasive ductal carcinomas, and is highly correlated with poor clinical prognosis10. In the nucleus, Snail binds to and regulates thousands of mesenchymal-enriched genes5, 11, yet there is little known about the functional output of Snail on the cancer proteome.
Protein S-palmitoylation describes the reversible post-translational modification of select cysteine residues in proteins by long chain fatty acids12, 13. S-palmitoylation is necessary for proper trafficking and localization many signaling proteins, including Ras family small GTPases, G-proteins, and Src-family kinases. H-Ras, N-Ras, and K-Ras4A all require S-palmitoylation of cysteines in their hypervariable regions for membrane trafficking and mitogenic signaling14, 15. For most S-palmitoylated proteins, the S-acyl group has a half-life of only a few hours, much shorter than the half-life observed in other post-translational modifications16. This suggests many S-palmitoylated proteins are repeatedly de-palmitoylated and re-palmitoylated over their lifetime, likely involving multiple members of the Asp-His-His-Cys (DHHC) motif protein acyltransferase (PAT) enzyme family and acyl protein thioesterases (APTs). Indeed, pulse-chase labeling and bioorthogonal enrichment of palmitoylated proteins confirmed rapid enzymatic turnover of a subset of palmitoylated proteins, including cell polarity MAGUKs, LAP proteins, Ras-family GTPases, and certain G-proteins16.
The 23 human PATs show overlapping expression profiles across many tissues, and localize to distinct cellular compartments in cells13. For example, DHHC5 and DHHC8 are the only PATs to localize only in the lateral membranes of polarized epithelial cells. The only other plasma membrane-localized PAT is DHHC14, which distributes to both apical and basolateral domains17. Interestingly, Ankyrin-G requires DHHC5/DHHC8 palmitoylation to regulate lateral membrane polarity17. We also previously identified the caveolae protein flotillin-1 as a direct DHHC5 substrate in neuronal stem cells18, supporting further DHHC5 compartmentalization to caveolae enriched microdomains19. Other DHHC enzymes localize primarily at the Golgi apparatus and endoplasmic reticulum, and may play key roles in protein folding and transport17. Since protein S-palmitoylation contributes to the membrane compartmentalization and trafficking of many proteins, we set out to examine if S-palmitoyation is dysregulated when cells loose apico-basolateral polarization.
Since the introduction of chemoproteomic strategies for palmitoylation enrichment and profiling, over two dozen large-scale palmitoylation proteomics studies have annotated more than a thousand candidate palmitoylated proteins across many diverse cell types20, 21. There are two orthogonal methods for enrichment and proteomic analysis of protein palmitoylation. Acyl-biotin exchange (ABE) describes the most-widely adopted approach, which begins by disulfide reduction and thiol alkylation, followed by hydroxylamine treatment to hydrolyze labile thioester linkages. Any newly liberated cysteine residues are then captured with activated biotin-linked disulfide reagents for subsequent streptavidin-agarose enrichment. However, this approach detects all protein-linked thioesters, including lipoamide-linked dehydrogenases and ubiquitin processing enzymes, and only reports steady-state protein S-palmitoylation levels22, 23. The second method uses metabolic labeling with alkynyl fatty acid derivatives, such as 17-octadynoic acid (17-ODYA), and copper-catalyzed azide-alkyne cycloaddition (CuAAC) conjugation to biotin-azide for subsequent streptavidin-agarose enrichment24, 25. While some proteins found in palmitoylation databases are likely artifacts of non-specific enrichment, the combination of both enrichment strategies and reproducibility across different investigations provide a useful resource for evaluating future large-scale S-palmitoylation studies. Furthermore, since most proteomic investigations emphasize only the annotation of palmitoylated proteins, there are currently few insights into the dynamic nature of protein palmitoylation in different genetic and phenotypic models. Despite the common suggestion that S-palmitoylation changes accompany cancer and metastasis26, there have been no global analyses of malignancy-dependent perturbations in S-palmitoylation. Given the widespread role for S-palmitoylation in protein membrane anchoring and organization, we sought to explore any potential link between Snail-transformation and the global landscape of palmitoylated substrates.
After Snail overexpression, cells become more spindle-like and lose apical and basolateral markers1, 2, providing a defined system to explore any correlation between S-palmitoylation and malignancy. Using this model, we combined metabolic incorporation of the palmitate analogue 17-ODYA and SILAC quantitation to carry out pairwise quantitative mass spectrometry profiling of both S-palmitoylation and protein abundance upon Snail overexpression. Using extensive statistical analyses between SILAC replicates, we generate a pairwise profile of abundance and S-palmitoylation enrichment in nearly 500 annotated palmitoyl proteins comparing normal and Snail-transduced cells. Our unenriched SILAC analysis corroborates Snail-dependent reprogramming of metabolism, cell adhesion, and the cytoskeleton, as well as revealing a new role for Snail in antioxidant expression. Interestingly, we observe that Snail-overexpression leads to a subtle attenuation in S-palmitoylation, and in some cases this change is uncoupled from abundance. Our analysis suggests that pharmacological stabilization of S-palmitoylation could potentially restore some properties of cell polarity and tumor suppression.
Experimental Methods
Cell lines and culture
Low passage MCF10A cells were a generous gift from Dr. Ben Margolis (University of Michigan). Cells were grown for more than 6 passages in SILAC DMEM-F12 media (Thermo) supplemented with final concentrations of 5% v/v dialyzed horse serum (Valley Biomedical), 20 ng/mL recombinant human epidermal growth factor (Shenandoah), 0.5 mg/mL hydrocortisone (Sigma), 100 ng/mL cholera toxin (Sigma), 10 μg/mL insulin (Sigma), and 0.7 mM “light” [12C6,14N4] or “heavy” [13C6,15N4] L-arginine and 0.5 mM “light” [12C6,14N2] or “heavy” [13C6,15N2] L-lysine. SILAC light and heavy labeled 293T cells were used for viral packaging of pPGS-fl.Flag-NEO (empty vector) or pPGS-hSnail-fl.Flag-NEO (Addgene plasmid no. 25695), co-transfected with pVSVG and pGAG/Pol retroviral packaging vectors. Viral supernatants were filtered using a 0.45 μm filter acetate syringe filter, and added to sub-confluent MCF10A cells with 8 μg/mL polybrene (Sigma) for viral transduction. After two days, the cells were placed under selection in 600 μg/mL G418 (Life Technologies). Surviving cells were expanded in SILAC media for large-scale experiments.
Acyl-Biotin Exchange
Cell lines were lysed in 4% (w/v) sodium dodecyl sulfate (SDS) in Dulbeccos phosphate buffered saline (DPBS, Life Technologies) and diluted to 3 mg/mL. Next, samples were alkylated with 50 mM N-ethyl maleimide (NEM) for 90 minutes at room temperature rotating end-over-end. The excess NEM was removed by chloroform-methanol extraction, washed twice with methanol, and resuspended in 4% SDS/DPBS. Samples were then incubated at 95 °C for 5 minutes in the presence of either 0.5 M hydroxylamine (+NH2OH) or 0.5 M NaCl (−NH2OH). Both +NH2OH and −NH2OH samples were chloroform-methanol extracted, washed twice with methanol, resuspended in 4% SDS/PBS, and incubated with 200 μM biotin-PEG-maleimide (Click Chemistry Tools) for 90 minutes. After chloroform-methanol precipitation, samples were resuspended in 2% SDS/DPBS, setting aside 30 μg of protein for input controls. Samples were then diluted to 0.5 mg/mL protein and 0.25% SDS in DPBS, and combined with 50 μL of streptavidin-agarose resin (Millipore) rotating for 90 minutes at room temperature. The resin was washed with 2 × 1 mL 0.25% SDS/DPBS and 3 × 1 mL DPBS. Bound proteins were eluted after boiling at 95 °C for 10 minutes in reducing Laemmli sample loading buffer and separated by SDS-PAGE for western blot analysis.
Immunoblotting
Cells were scraped and resuspended in DPBS, lysed by sonication, and quantified using the Biorad DC Protein assay. Samples were analyzed by SDS-PAGE (either 8% or 12% gels) before transferring to Immobilon-FL membrane (Millipore), blocked in LiCor Odyssey Blocking Buffer (LiCor), and incubated with the following primary antibodies in Odyssey Blocking buffer containing 0.2% (v/v) Tween 20 at the indicated concentrations: E-cadherin (1:5000, BD Biosciences cat. no. 610181), α-Tubulin (1:2500, Sigma cat. no. T6074), GAPDH (1:6000, EMD Millipore, cat. no. CB1001), β-Catenin (1:1000, Cell Signaling Technologies, 9587P), β-Actin (1:500, Cell Signaling Technologies, 4967S), PARK7 (rabbit polyclonal, 1:500, Cell Signaling Technologies, cat. no. 5560S), Ras (1:1000, EMD Millipore, cat. no. 05-516), Snail (rabbit polyclonal, 1:500, Cell Signaling Technologies, cat. no. 3879P), pyruvate carboxylase (PCX, rabbit polyclonal, 1:500, Proteintech, cat. no. 16588-1-AP), MEK1/2 (rabbit polyclonal, 1:500, Cell Signaling Technologies, cat. no. 8727S), MYH9 (rabbit polyclonal, 1:500, Cell Signaling Technologies, cat. no. 3403), FAM129B (rabbit polyclonal, 1:500, Cell Signaling Technologies, cat. no. 5122), CD44 (mouse monoclonal, 1:1000, Cell Signaling Technologies, cat. no. 3570). After primary antibody labeling, membranes were thoroughly washed with Tris-buffered saline containing 0.1% (v/v) Tween 20 (TBST), and incubated with either visible fluorescence secondary, near-IR secondary, or HRP-linked secondary antibodies for chemiluminescence detection using an Azure Biosystems c600 imager.
Unenriched proteome preparation
Light and heavy cells were harvested with a cell scraper in Dulbeccos phosphate buffered saline (DPBS, Life Technologies) and lysed by sonication (1 × 10 second pulses, Level 3, Branson Sonifier). Protein concentrations were measured using the BioRad DC assay, light and heavy samples were mixed equally (1:1). Samples were then chloroform/methanol extracted and re-solubilized in 6 M urea in 25 mM ammonium bicarbonate in DPBS, and then reduced with 10 mM dithiothreitol (Sigma) at 65° C for 15 minutes. Following equilibration back to room temperature, the samples were alkylated with 40 mM iodoacetamide (Sigma) in the dark for 1 hour. After 3-fold dilution, the samples were digested in buffer supplemented with 1 mM CaCl2 and 2 μg of mass spectrometry grade Trypsin/LysC Mix (Promega). The digestion mixture was incubated overnight at 37° C with vigorous shaking, and then acidified with 0.1% formic acid (Sigma). Digests were desalted using an Oasis 96-well plate (Waters) on a vacuum manifold, eluted with 70% acetonitrile, and transferred to a glass vial for LC-MS analysis. Solvent was removed over 2 hours at 45° C using a Thermo Savant vacuum concentrator. Peptides were resuspended at 100 ng/μL in LC-MS grade water with 3% acetonitrile, 0.1% formic acid, and 10 fmol/μL yeast aldehyde dehydrogenase (ADH, Waters Corp.).
Metabolic labeling and click chemistry enrichment
17-ODYA labeling and enrichment was performed as previously reported16, 18. Isotopically paired MCF10A Empty Vector or Snail cells were incubated with 17-ODYA (Cayman) for 6 hours, washed 3-times with DPBS, scraped, and briefly centrifuged at 500 g. Cells were lysed in the presence of 20 μM hexadecylfluorophosphonate (HDFP)16, and quantified using the BioRad DC Protein assay in DPBS. Heavy and light pairs were combined at a 1:1 ratio, and the pooled lysates were extracted with chloroform-methanol, sonicated and washed in methanol 3-times, and finally resuspended in DPBS. Click chemistry was preformed using 500 μM biotin-azide (Click Chemistry Tools), 1 mM Tris(2-carboxyethyl)phosphine (TCEP, Sigma-Aldrich), 100 μM Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (TBTA, Sigma-Aldrich), and 1 mM CuSO4 in DPBS at room temperature. After a brief centrifugation, the supernatant was discarded and the residual pellet was chloroform-methanol precipitated, sonicated in methanol, and centrifuged 3-times before re-solubilizing in 1.2% SDS with 6 M urea in DPBS. The sample was then incubated with neutralized 10 mM TCEP for 30 minutes, followed by 20 mM iodoacetamide (Sigma) for an additional 30 minutes. Next, the sample was diluted 5-fold and incubated with streptavidin-agarose beads (Millipore) for 90 minutes. The beads were then collected and washed extensively using a multi-port vacuum manifold with decreasing concentrations of urea (6 M – 1 M), before collecting the beads and incubating overnight in 2 M urea, 1 mM CaCl2, and 2 μg Trypsin/Lys C mix overnight. Tryptic peptides were concentrated and desalted using an Oasis 96-well plate connected to a vacuum manifold, and reconstituted in 3% acetonitrile, 0.1% formic acid, and 10 fmol/μL yeast aldehyde dehydrogenase (ADH) in mass spectrometry-grade water (Fisher).
Mass Spectrometry
Tryptic peptides were loaded over 3 minutes using a Waters NanoAcquity UPLC system equipped with a 5 μM Symmetry C18 (180 μm × 20 mm) trap column. The peptides were then eluted to a 1.8 μm High Strength Silica (HSS-T3) analytical column (75 μm × 250 mm) and separated over a 110 minute gradient (3% acetonitrile to 40% acetonitrile over 92 minutes). The eluted peptides were delivered by electrospray ionization to a Waters Synapt G2S HDMS time-of-flight mass spectrometer using ion mobility separation and data independent fragmentation tuned using the following parameters. The quadruple mass analyzer was manually set for mass 500, 600 and 700. The sampling cone was adjusted to 32 eV and the nano flow gas was set to flow at 0.2 bar. The purge gas was set to flow at 50 L/h and the source temperature was set at 70 °C. The mass spectrometer was operated in V-mode (resolution mode) with a resolving power of at least 20,000 FWHM (full width at half maximum) in positive-mode ESI for 400 m/z. The time-of-flight analyzer of the mass spectrometer was calibrated with a 100 fmol/μL solution of [Glu1]-Fibrinopeptide B from m/z 50 to 1250 to within 0.5 ppm. The data were post-acquisition lock mass corrected using the doubly charged monoisotopic ion of [Glu1]-Fibrinopeptide B (m/z = 785.8426), which was collected every 30 seconds during the runs. For ion mobility separation (IMS), the wave height was set as 40 V and IMS wave velocity as 600 m/s. The spectral acquisition time in each mode was 0.5 s. A collision energy (CE) ramp from 16 eV to 60 eV during each 0.5 s-integration was used as standard setting for the elevated energy MS scan in the UDMSE mode. Optimal drift-time-dependent CE values were obtained by maximal number of proteins obtained using a tryptic digest human cells27.
Mass spectrometry data processing and SILAC quantification
LC-MS spectra were collected in continuum mode and searched using ProteinLynx Global SERVER version 3.0.2 (Waters) with the reviewed human reference proteome (UniProtKB downloaded on 09/14/2015). Precursor- and fragment-ion mass tolerances were determined by PLGS 3.0.2 during database searching. Identified peptides met the following search criteria: (i) digested by trypsin, (ii) no more than one missed cleavage, (iii) contains lysine (+8) or arginine (+10) defined as fixed modifier reagent groups, (iv) carbamidomethyl cysteine as a fixed modification and methionine oxidation as the variable modification. Furthermore, peptides must have a minimum of two identified fragment ions per peptide and a minimum of five fragments per protein, and at least two identified peptides per protein. The peptide false discovery rate (FDR) was set at 1% using a reversed database. Precursors and fragments that differed by more than ±10 ppm and ±20 ppm, respectively, from the theoretical mass were eliminated from further analyses. Precursor SILAC ratios were computed by PLGS 3.0.2 and in-house Python-based algorithms were used to compute y-ion (C-terminal fragments) ratios for each SILAC y-ion pair. Both precursor (MS1) and y-ion (MS2) ratios for each protein were merged (after combining data from both directions and inverting individual ratios from one direction). In-house Python scripts were used to perform a quartile test on combined MS1 and MS2 ratios for each protein, retaining only ratios within ±1.5x the inter-quartile range. Gene Ontology (GO) analysis was performed using the DAVID software (NIH) configured to assign the identified proteins with cellular function GO terms. The resulting GO categories were then manually curated such that proteins with related, similar and overlapping cellular functions were merged into a few broader categories.
Statistical analysis
An equivalence p-value metric was calculated only for proteins having SILAC (Snail/empty vector) ratio measurements from reciprocal SILAC labeling experiments, which we refer to as bi-directional SILAC ratios. To accomplish this, we applied Schuirmann’s two one-sided statistical equivalence test (TOST) to evaluate whether directional SILAC ratios were similar enough to be considered equivalent, which for a given protein reflects the true value of change between Snail-transduced and empty vector MCF10A lines. The null (H0) and alternative (HA) hypotheses were defined as follows:
Where R̄L/H and R̄H/L are the mean Snail/parent protein fold changes for the forward and reverse SILAC experiments, respectively, and Δ is a predefined ‘acceptable difference’ limit defining equivalence. Δ depends on the inherent instrument variability under a defined set of experimental conditions, and was estimated by examining the measured ratios from both the unenriched and 17-ODYA enriched proteomic data sets. We found that the absolute difference between a given set of directional ratios scales according to the average of the directional ratios with a mean proportionality constant of ~0.7 (data not shown). Thus, the Δ between directional SILAC ratios was set to 0.7 times the average of the directional ratios under evaluation. We then computed TOST statistics (tupper and tlower critical values) to determine whether the observed distance between a given set of directional SILAC ratios is sufficiently small to reject the null hypothesis:
where nL/H and nH/L are the sample sizes or the number of features used to calculate the mean fold change ratio in the forward and reverse SILAC experiments, respectively, and Sp is the pooled sample standard deviation about the means of the directional ratios defined as:
Where sL/H and sH/L are individual sample standard deviations about the mean fold changes in the forward and reverse SILAC experiments, respectively. Therefore, H0 is rejected at the 0.5 α significance level if, and only if, both:
Where is the upper-tailed, α-level, t-distribution critical value with v = nL/H + nH/L − 2 degrees of freedom.
The p-values that are presented in volcano plots were calculated using a one-sample, two-tailed Student t-test, where the null hypothesis assumes that a given protein’s population mean ratio is equal to 1 (i.e. no change between parent and Snail lines). For uni-directional SILAC ratios, Student t-statistics were calculated about the means, standard deviations and sample sizes associated with Snail/parent fold changes measured in that SILAC direction, whereas t-values for bi-directional ratios were calculated based on the weighted means, pooled standard deviations and combined sample sizes associated with Snail/parent fold changes from both SILAC directions. The resulting t-values were then converted into corresponding p-values using Excel’s two-tailed Student’s t-distribution function.
To determine whether a given protein’s fold change in S-palmitoylation was statistically equivalent to its fold-change in its abundance, a similar Schuirmann’s TOST was carried out, except that hypothesis tests were conducted with either uni-directional means, standard deviations and sample sizes or with bi-directional weighted means, pooled standard deviations and combined sample sizes from inverse SILAC labeling experiments when available. The ratios of mean fold changes (Δpalmitoylation/Δabundance) were then ranked by the absolute difference between their calculated tupper and tlower critical values as smaller differences are associated with more proportional S-palmitoylation and abundance mean ratios and hence reflect the degree of correlation between changes in palmitoylation and changes in abundance. Conversely, the ratio of Δpalmitoylation/Δabundance also reflects abundance-normalized or the relative stoichiometric changes in S-palmitoylation. Since this parameter never assumes zero values, it can also be treated as an effect size index (i.e. response ratio) and thus, the computation of +/− 95% confidence interval estimates about the ratio of the mean fold changes were carried out on a log scale and then converted back to the original metric as follows:
Where and are either the uni-directional mean or bi-directional weighted mean Snail/parent fold changes in S-palmitoylation and abundance, respectively, and are the associated standard deviations of S-palmitoylation and abundance fold changes, and npalm and nAbund are the corresponding sample sizes.
Results
Proteome-wide changes in protein abundance induced by Snail overexpression
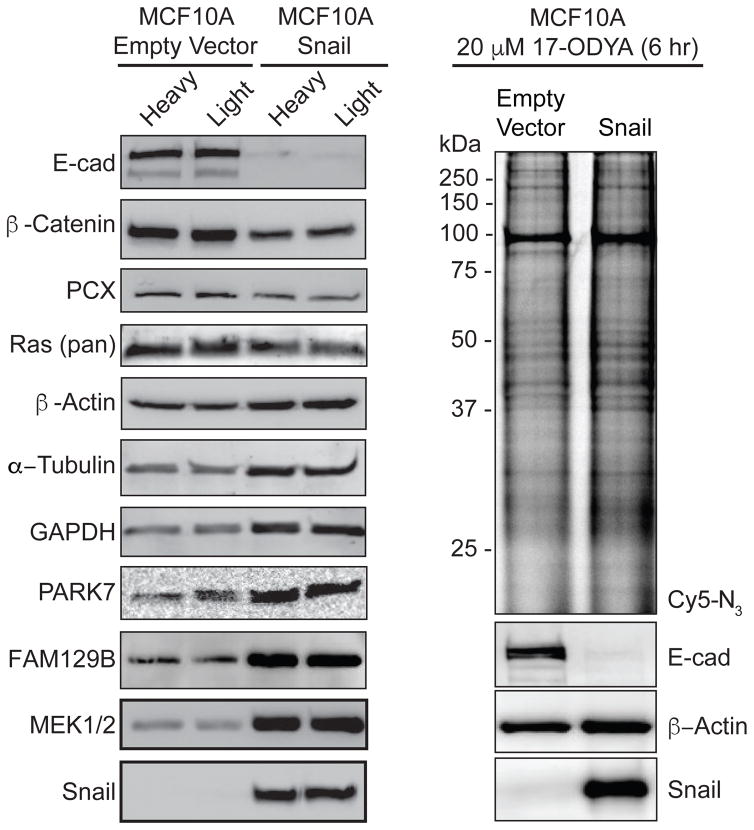
Snail over-expression promotes transcriptional reprogramming of from an epithelial to a mesenchymal state. Despite several thousand publications linking Snail expression to cancer, at the time of this submission, there has been no direct mass spectrometry-based profile of the proteome-wide consequences of Snail over-expression in epithelial cells. A recent proteomic analysis of Snail-transduced adipocyte nuclear fractions identified 463 decreased and 111 increased proteins changed by more than 150%28. Extending from this analysis, human basal epithelial MCF10A cells were retrovirally transduced with Snail and labeled in isotopic SILAC media for quantitative proteomic analysis. Western blot analysis confirmed complete repression of E-cadherin, over-expression of Snail, and reduced β-catenin levels, signifying successful reprogramming and the onset of EMT (Figure 1a). In a small search for loading control antibodies, we also observed an increase in both GAPDH and α-Tubulin, with less prominent changes in pyruvate carboxylase and β-actin. Interestingly, the redox chaperone and oncogene DJ-1/PARK729, 30, the Ras activator FAM129B31, and MEK1/2 were dramatically elevated, while total Ras levels were only slightly reduced. Since MCF10A cells do not harbor Ras mutations, Snail expression apparently amplifies downstream pathways involved in cell growth signaling.
Figure 1. Gel-based validation of Snail-dependent protein levels and S-palmitoylation evels.
(a) MCF10A-Snail cells demonstrate reduced levels of E-cadherin and β-Catenin, but increased levels of GAPDH, FAM129B, MEK1/2, and PARK7. (b) In-gel fluorescence analysis of 17-ODYA labeling in MCF10A-Snail cells is similar to MCF10A-empty vector cells.
Next, both MCF10A empty vector and Snail-transduced cells were labeled with the alkynyl fatty acid analogue 17-ODYA to confirm efficient metabolic incorporation by the cellular S-palmitoylation machinery. This analysis is necessary to rule-out if Snail-dependent transcriptional reprogramming affects the cells’ ability to convert 17-ODYA to octadecynyl-CoA or perturb other intermediates in lipid metabolism that could indirectly affect S-palmitoylation profiles. After labeling for 6 hours, cell lysates were conjugated to Cy5-azide and analyzed by in-gel fluorescence (Figure 1b). Snail over-expression had little effect on the overall 17-ODYA in-gel fluorescence labeling profile, even though Snail reduces the formation of many adhesion complexes and S-palmitoylated proteins. This analysis highlights that the most abundant S-palmitoylated proteins are unaffected by Snail overexpression.
Following this validation, isotopically labeled vector and Snail cell lysates were mixed in a 1:1 ratio, providing two directions (Light-Snail/Heavy-Vector and Light-Vector/Heavy-Snail) for triplicate mass spectrometry analysis. Using this reciprocal SILAC approach, tryptic peptides from whole cell lysates were analyzed using data-independent acquisition (DIA) methods. In-line ion mobility separation (IMS) was applied to add an orthogonal analytical dimension to reduce ion interference, and drift-time dependent collision-energy assignment was used to generate more fragment ions. In total, over 1000 proteins were quantified across replicates without multidimensional chromatographic fractionation, yielding a sufficient survey of most abundant proteins (Supplementary Table 1).
Since SILAC precursor pairs share common retention and drift times, both species co-fragment to yield multiple quantifiable isotopic y-ion peak pairs provided by the prerequisite C-terminal arginine or lysine. Furthermore, the superior dynamic range afforded by time-of-flight mass analyzers provides sufficient signal to accurately quantify lower intensity ions while remaining in the linear range of detection32. While the details of this analysis are the topic of a future publication, we found that the coefficient of variation of y-ion (MS2) ratio measurements is equal, if not better than precursor (MS1) analysis. Accordingly, both precursor (MS1) and y-ion (MS2) SILAC ratios were pooled across experiments, dramatically increasing the number of measurements and statistical representation of each peptide and protein. With increased sampling, the data presented sufficient measurements to apply a statistical quartile test to eliminate outliers and overcome interference-dependent effects on SILAC accuracy. Nonetheless, the SILAC workflow doubles the ion complexity and reduces the total number of identifications33. Despite this loss, the overall quantitative accuracy provides low coefficients of variation (~20%) for precise quantitation. With the ongoing development of IMS-DIA algorithms27, such experiments provide a repository for retroactive cross-extraction of SILAC ratios between experiments33, 34.
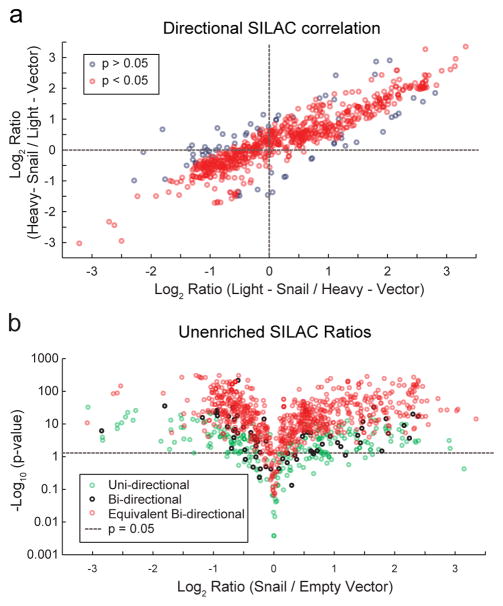
Since a quartile test primarily controls for instrument error associated with SILAC ratio calculations, we sought to leverage the correlation between ratios from reciprocal SILAC labeling experiments as a measure of confidence in the final SILAC ratio value. We reasoned that if a biological change in protein abundance is related to Snail expression, then the calculated ratio is independent of the SILAC labeling order. Hence, SILAC ratios that are not equal in both forward and reverse SILAC experiments are likely less accurate. We previously employed a similar strategy to annotate S-palmitoylated proteins using MS1 ion quantitation16, 18. Here, we added statistical equivalence tests to evaluate the mean, quartile-filtered, pooled MS1 and MS2 ratios to quantify the degree of closeness between ratios from reciprocal SILAC experiments. Using this approach, we were able to confidently assign equivalency to about 89% of SILAC ratios computed from inverse labeling directions (bi-directional measurements), corresponding to 65% of all SILAC measurements. This yields a Pearson product-moment correlation coefficient (r) of 0.86 for equivalent bi-directional ratios and 0.32 for non-equivalent bi-directional ratios. Since non-specific epithelial proteins (i.e. keratins) are often inadvertently introduced during sample preparation, this bi-directional statistical equivalence test validates biological changes across a range of SILAC ratios, eliminating contributions from extrinsic contamination and biological drift (Figure 2a). An additional one-sided t-test determined that 93% of equivalent bi-directional SILAC ratios were statistically different (p < 0.05) than the expected ratio of 1. Of the proteins only quantified in a single SILAC direction (uni-directional measurements), 74% were statistically different than the expected ratio of 1, yet most are associated with overall smaller effect sizes (Figure 2b). Of all the measured SILAC ratios combined, 88% of proteins showed significant deviation from the expected ratio of 1, with more than 75% exhibiting a 2-fold increase or decrease in abundance. Across these directionally equivalent proteins, 8% decreased > 2-fold and 24% increased > 2-fold, demonstrating an accurate quantitative measure across a broad profile of abundant proteins affected by Snail-dependent reprogramming.
Figure 2. Quantitative profiling of the Snail-reprogrammed proteome.
(a) SILAC directional correlation between inverse isotopic labeling mass spectrometry experiments comparing empty vector and Snail-transduced MCF10A cells. Grey circles represent proteins that are not statistically equivalent in both SILAC labeling experiments, while red circles represent equivalent bi-directional SILAC ratios (p < 0.05). (b) Unenriched mass spectrometry profiling of Snail-transduced cells reveals widespread, statistically significant changes in protein abundance. Uni-directional proteins (green circles) were identified in only one SILAC direction, while bi-directional proteins were identified in both directions (black circles) and are statistically equivalent (red circles).
This subset of reduced proteins (>2-fold) includes β4- and α6-integrins, β- and δ-catenin, and other cytoskeletal and adhesion proteins (plakoglobin, desmoplakin, and a number of keratins) (Supplementary Table 1). Conversely, Snail increases the abundance of antioxidant enzymes (aldose reductase, peroxiredoxins, DJ-1/PARK7, superoxide dismutase, thioredoxin), glycolytic enzymes (enolase, triose phosphate isomerase, phosphoglycerate mutase, phosphoglycerate kinase), nitrogen metabolism (argininosuccinate synthase, glutamate dehydrogenase), and cytoskeletal remodeling enzymes (tubulins, cofilin, profilin). Gene ontology analysis identified several significantly altered pathways (Supplementary Figure 1), including a major induction of anti-apoptotic proteins, antioxidant proteins, and a considerable reprogramming away from mitochondrial oxidative metabolism. Based on this proteomic analysis, we identify broad changes in protein abundance induced by Snail overexpression, providing a roadmap for biomarker and target discovery in malignant cells.
Quantitative profiling of Snail-dependent changes in palmitoylation
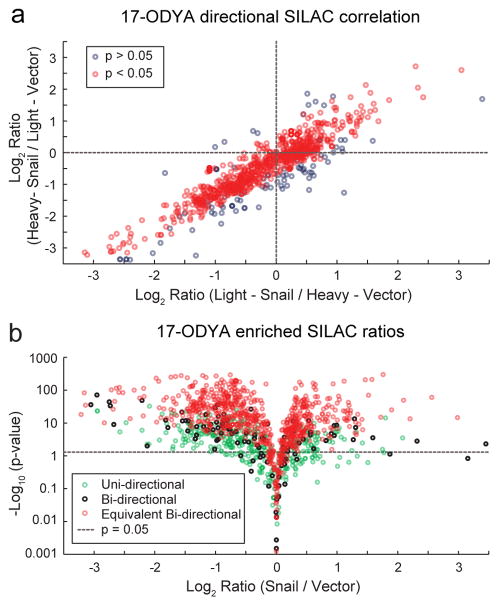
We next sought to examine if there is a link between Snail-driven EMT reprogramming and levels of protein S-palmitoylation. SILAC MCF10A-Snail and control cells were labeled with the alkynyl fatty acid analogue 17-ODYA for 6 hours. After mixing forward and reverse SILAC paired membrane lysates, samples were conjugated to biotin-azide by copper catalyzed click chemistry for streptavidin enrichment, proteolytic digestion, and quantitative mass spectrometry analysis. More than 70% of the 17-ODYA enriched proteins were identified in both SILAC directions, and 88% of these were statistically equivalent, presenting bi-directional SILAC filtering as a useful approach to remove extrinsic contaminants, poorly quantified events, and biological variation (Figure 3a). Rather than provide extensive validation of putative palmitoylated proteins, we chose to leverage the archive of 26 existing palmitoylation proteomics studies20 to assign candidate palmitoylated proteins. This approach avoids eliminating any proteins from our dataset (Supplementary Table 2), as such filters often hide many putative palmitoylated proteins from further outside analysis. Thus, our dataset reports the total profile of 17-ODYA enriched proteins, and their differential enrichment in Snail-transduced cells. Due to the reduced sample complexity following 17-ODYA enrichment, this palmitoylation-dependent analysis surpassed the previous unenriched analysis, identifying and quantifying 30% more proteins in only two bi-directional SILAC replicates.
Figure 3. Quantitative S-palmitoylation profiling of Snail-expressing cells.
(a) SILAC directional correlation between inverse isotopic labeling mass spectrometry experiments comparing empty vector and Snail-transduced MCF10A cells. Following metabolic labeling and click chemistry enrichment with 17-ODYA Grey circles represent proteins that are not statistically equivalent in both SILAC labeling experiments, while red circles represent equivalent bi-directional SILAC ratios (p < 0.05). (b) 17-ODYA enriched mass spectrometry profiling of Snail-transduced cells reveals widespread statistically significant changes in protein palmitoylation enrichment. Uni-directional proteins (green circles) were identified in only one SILAC direction, while bi-directional proteins were identified in both directions (black circles) and are statistically equivalent (red circles).
This palmitoylation analysis identified 630 proteins previously confirmed in 2 or more palmitoylation proteomics studies, and another 262 proteins identified in a single study. Approximately 1/3 of the 17-ODYA enriched proteins are not represented in the reported 26 palmitoylation proteomics studies from mouse and human derived cells, yet demonstrate 17-ODYA enrichment and warrant further analysis. This Swiss-Palm validated list includes many established palmitoylated proteins, including Ras family small GTPases, G-proteins, Src-family kinases, and dozens of polarity and junctional proteins. Interestingly, 13% of annotated palmitoylated proteins (≥2 studies) and 20% of all 17-ODYA enriched proteins were reduced more than 2-fold in enrichment in Snail transduced cells. Only 6% of 17-ODYA enriched proteins increased their palmitoylation enrichment more than 2-fold in Snail-transduced cells. This result highlights a significant discrepancy between predominantly Snail-dependent increases in abundance, yet an overall trend towards reduced protein palmitoylation (Figure 3b).
In order to validate a panel of putative palmitoylated proteins, we performed acyl biotin exchange on several proteins (Supplementary Figure 2). Both Ras and CD44 S-palmitoylation and abundance were equally unaffected by Snail expression. We were unable to confirm myosin IIa, α-tubulin, β-catenin, or FAM129B palmitoylation by ABE due to either the absence of a hydroxylamine-dependent linkage or high background enrichment. This suggests any palmitoylation is likely sub-stoichiometric, affecting only a small fraction of each protein. Interestingly, the myosin IIa holoenzyme consists of two heavy (MYH9) and two light (MYL6) chains, which have both only been annotated as a palmitoylated protein using alkynyl fatty acids. MYL6 encodes a cysteine immediately after the initiator methionine that is predicted as a high confidence site of palmitoylation35. This site likely undergoes S-N acyl transfer after cleavage of the initiator methionine, suggesting MYL6 may also be fractionally N-palmitoylated. FAM129B encodes a myristoylation consensus site (Met-Gly), which explains its potential hydroxylamine-insensitive linkage and exclusive annotation from studies using alkynyl fatty acid enrichment. Finally, GAPDH S-palmitoylation is enhanced in Snail-expressing cells, which is reported to occur through enzymatic thioester exchange36.
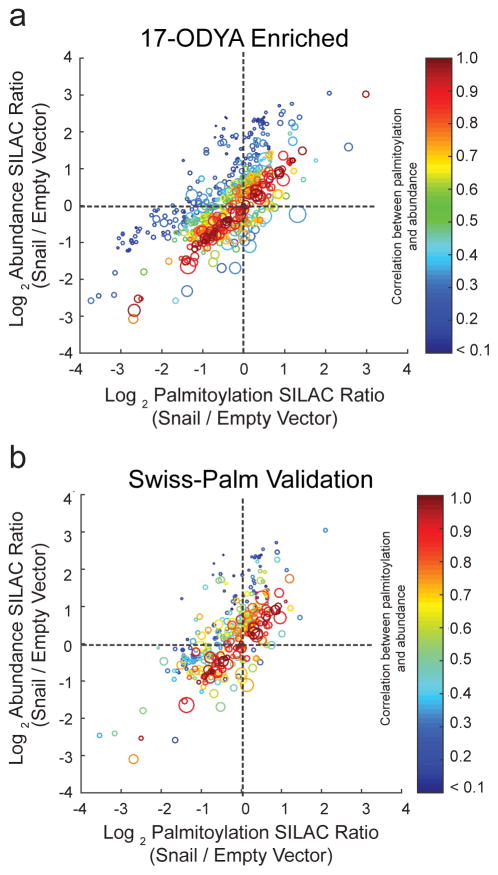
If Snail transcriptionally represses the gene of a S-palmitoylated protein, there will be less protein present to be labeled by 17-ODYA, and will report a reduced 17-ODYA enrichment ratio. In this situation, S-palmitoylation is tightly coupled to abundance, and both proteomics experiments should report equivalent values. Conversely, since protein palmitoylation is likely to affect protein stability, Snail-expression could reduce S-palmitoylation occupancy and lower steady-state protein levels. In neuronal stem cells with reduced DHHC5 expression, the abundance of many putative S-palmitoylated substrates was significantly reduced, potentially due to protein mislocalization and destabilization18. Therefore, when the change in a protein’s abundance co-varies with change in its palmitoylation, it is not straightforward to classify the causative factor leading to reduced protein palmitoylation. To measure extent of palmitoylation and abundance co-variation in our data, 17-ODYA enriched and unenriched SILAC proteomics datasets were cross-referenced, providing correlated palmitoylation enrichment and abundance for 59% of the 17-ODYA-enriched, Swiss-Palm-validated dataset. Statistical analysis of the paired correlation revealed that 71% of proteins exhibited statistically equivalent fold changes in both their palmitoylation and abundance upon Snail expression. As discussed above, this does not rule out post-translational regulation of steady-state protein levels by palmitoylation, or vice versa. However, a two-tailed t-test comparing proteins that were not deemed equivalent revealed a subset of 17-ODYA enriched, Swiss-Palm-validated proteins with uncoupled S-palmitoylation and abundance (Figure 4). This pool of palmitoylated proteins (~28%) exhibited Snail-dependent palmitoylation changes that did not mirror the corresponding changes in their abundance, suggesting that while most palmitoylated proteins likely require this modification for their cellular stability, the stability of this set of proteins may be unaffected by palmitoylation. Moreover, since these palmitoylation ratios are statistically different than the corresponding abundance ratios, the quotient of these two terms may reflect stoichiometric changes in palmitoylation accompanying the changes in protein abundance. Comparative analysis of the correlated palmitoylation and abundance SILAC ratios highlights many proteins with uncoupled palmitoylation and abundance, including several cytoskeletal proteins, metabolic enzymes, and signaling proteins (Figure 5). Overall, 22% of all of the correlated ratios demonstrate a >2-fold reduction in normalized palmitoylation, while less than 1% of proteins demonstrate a corresponding increase in palmitoylation. In addition, many proteins were identified exclusively in the Empty Vector or Snail line, yielding infinite ratios (Supplementary Table 3). Based on this analysis, a subset of proteins demonstrate selective attenuation of protein palmitoylation after Snail overexpression. More importantly, for the majority of palmitoylated proteins, S-palmitoylation is tightly coupled with abundance. Accordingly, Snail not only disorganizes cell adhesion complexes and cell polarity, but it also may contribute to a cellular program that attenuates S-palmitoylation for a number of proteins.
Figure 4. Correlated palmitoylation enrichment and abundance changes in Snail-expressing cells.
Both abundance and palmitoylation SILAC ratios are plotted using a heat map to signify statistical correlation, with red circles representing high correlation, and blue circles representing weak correlation. Effectively, blue circles signify uncoupled palmitoylation and abundance. The circle diameter represents the size of the 95% confidence interval, reflecting the statistical significance of each measurement. Smaller circles are more statistically significant than bigger circles. (a) Correlated S-palmitoylation and abundance analysis of all 17-ODYA enriched proteins. (b) Filtered correlation analysis for proteins reported in 2 or more palmitoylation proteomics studies reported in Swiss-Palm.
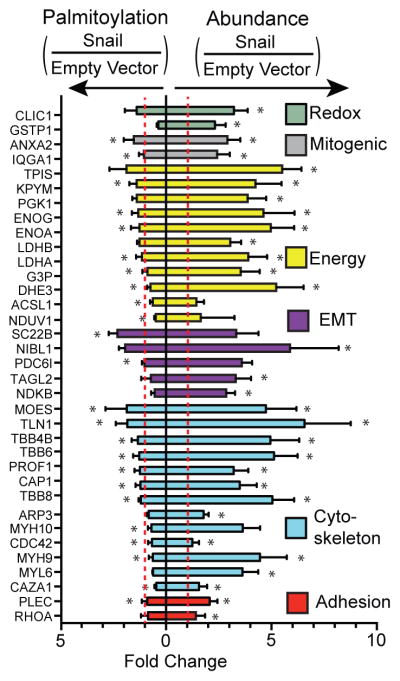
Figure 5. Discordant palmitoylation and protein abundance.
Select proteins are shown with increased abundance and little of no change in palmitoylation levels, indicating decreased fractional palmitoylation. Asterisks indicate statistically equivalent ratios in bi-directional SILAC analysis (p < 0.05).
Conclusions
Snail over-expression is highly correlated with poor clinical outcomes, recurrence, and chemoresistance2. Here we present a quantitative proteomic profile of proteins affected by Snail overexpression in MCF10A cells, highlighting new pathways contributing to malignancy. This includes Snail repression of adhesion and polarity proteins, a shift to glycolytic metabolism, and enhanced antioxidant protection consistent with a more malignant state.
In combination with palmitoylation proteomic profiling, we present evidence that supports Snail-dependent uncoupling of S-palmitoylation from protein abundance for a select profile of S-palmitoylated proteins. There are several potential mechanisms supporting this phenomenon, including Snail-dependent reduction in DHHC protein acyl transferase activity, increased expression or activity of acyl protein thioesterases, or reduction in palmitoyl-CoA levels. Several DHHC enzymes are reported to safeguard cells from malignancy, including DHHC2, DHHC14, and DHHC1737–39, altering the membrane organization and localization of key signaling proteins. This repression is unlikely due to redox regulation of DHHC activity, since we report Snail expression leads to a broad induction of antioxidant enzymes. Alternatively, Snail could induce expression of APT enzymes, accelerating palmitoylation hydrolysis to reduce steady-state palmitoylation. The protein thioesterases APT1 and APT2 are both significantly elevated as cells increase their cancer aggressiveness, as determined by serine hydrolase activity-based profiling40. Finally, Snail may also affect the cellular pool of palmitoyl-CoA, or affect the metabolism of 17-ODYA to reduce protein acyl transferase substrate pools. Since 82% of palmitoylated proteins have less than a 2-fold change upon Snail expression, reduced palmitoyl-CoA levels are unlikely to mediate this attenuation, which would be predicted to be significantly more dramatic. Rather, an imbalance in PAT and APT activities is more likely in effect, shifting cells to a less palmitoylated equilibrium. Therefore, those proteins with reduced palmitoylation are particularly sensitive to palmitoylation cycles, or are dedicated substrates of a particular subset of Snail-dependent palmitoylation regulators.
Regardless of the mechanism of attenuated palmitoylation, this study provides the first direct systems-level analysis of palmitoylation in a defined model of malignancy. While this study largely confirms that palmitoylation enrichment is directly correlated with protein abundance, there is also a subset of proteins where the two are uncoupled, suggesting post-translational regulation. This is particularly striking for myosin-9 and myosin light chain 6, which interact to form an actin-dependent motor protein critical for malignant cell invasion and metastasis41. Reminiscent of cell polarity pathways in drosophila regulated by the protein acyl transferase approximated42, the drosophila myosin variant dachs may indeed be a direct palmitoylated substrate, tethering the motor protein to membranes in polarized cells. RhoA is also less palmitoylated following Snail-expression, suggesting a broader role in post-translational modulation of cytoskeletal remodeling and invasion. Furthermore, protein palmitoylation inhibits glutamate dehydrogenase, yet is specifically de-palmitoylated in Snail-expressing cells43. Reactivation of glutamate dehydrogenase shifts cells towards glutaminolysis, which leads to elevated α-ketoglutarate and activation of antioxidant pathways to support proliferation and tumor growth44. Interestingly, we observe no steady-state changes in Ras abundance or palmitoylation, yet a dramatic increase in MEK and FAM129B levels suggesting an overall increase in mitogenic signaling.
In summary, this study provides evidence that Snail expression reduces S-palmitoylation levels on a number of proteins. Future studies will explore the mechanism of attenuation, evaluate other models of EMT, and provide more detailed quantitative profiling of oncogene-induced regulation of palmitoylation in cancer. This approach will be explored further to examine if pharmacological manipulation of palmitoylation enzymes can rescue membrane localization and tumor suppression by cell polarity complexes. Finally, the experimental framework presented is readily transferable to other systems for in-depth statistical analysis of IMS-DIA datasets.
Supplementary Material
Acknowledgments
Support for these studies was provided by the National Institutes of Health R00 CA151460, DP2 GM114848, the American Cancer Society PF-13-177-01 (J.L.H.), the American Heart Association 14POST20420040 (J.D.M.), and the University of Michigan.
References
- 1.Halaoui R, McCaffrey L. Oncogene. 2015;34:939–950. doi: 10.1038/onc.2014.59. [DOI] [PubMed] [Google Scholar]
- 2.Ye X, Weinberg RA. Trends in Cell Biology. 2015;25:675–686. doi: 10.1016/j.tcb.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg Robert A. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Zheng H, Kang Y. Oncogene. 2014;33:1755–1763. doi: 10.1038/onc.2013.128. [DOI] [PubMed] [Google Scholar]
- 5.Ye X, Tam WL, Shibue T, Kaygusuz Y, Reinhardt F, Ng Eaton E, Weinberg RA. Nature. 2015;525:256–260. doi: 10.1038/nature14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemler R. Trends in Genetics. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- 7.Moreno-Bueno G, Portillo F, Cano A. Oncogene. 2008;27:6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 8.Martin-Belmonte F, Perez-Moreno M. Nat Rev Cancer. 2012;12:23–38. doi: 10.1038/nrc3169. [DOI] [PubMed] [Google Scholar]
- 9.Whiteman EL, Liu CJ, Fearon ER, Margolis B. Oncogene. 2008;27:3875–3879. doi: 10.1038/onc.2008.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Natrajan R, Weigelt B, Mackay A, Geyer F, Grigoriadis A, Tan DP, Jones C, Lord C, Vatcheva R, Rodriguez-Pinilla S, Palacios J, Ashworth A, Reis-Filho J. Breast Cancer Res Treat. 2010;121:575–589. doi: 10.1007/s10549-009-0501-3. [DOI] [PubMed] [Google Scholar]
- 11.Dong C, Yuan T, Wu Y, Wang Y, Fan Teresa WM, Miriyala S, Lin Y, Yao J, Shi J, Kang T, Lorkiewicz P, St Clair D, Hung M-C, Evers BM, Zhou Binhua P. Cancer Cell. 2013;23:316–331. doi: 10.1016/j.ccr.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez JL, Majmudar JD, Martin BR. Current opinion in chemical biology. 2013;17:20–26. doi: 10.1016/j.cbpa.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tom CT, Martin BR. ACS chemical biology. 2013;8:46–57. doi: 10.1021/cb300607e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox AD, Der CJ, Philips MR. Clinical Cancer Research. 2015;21:1819–1827. doi: 10.1158/1078-0432.CCR-14-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai FD, Lopes MS, Zhou M, Court H, Ponce O, Fiordalisi JJ, Gierut JJ, Cox AD, Haigis KM, Philips MR. Proceedings of the National Academy of Sciences. 2015;112:779–784. doi: 10.1073/pnas.1412811112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin BR, Wang C, Adibekian A, Tully SE, Cravatt BF. Nat Methods. 2012;9:84–89. doi: 10.1038/nmeth.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He M, Abdi KM, Bennett V. The Journal of Cell Biology. 2014;206:273–288. doi: 10.1083/jcb.201401016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Martin BR, Cravatt BF, Hofmann SL. The Journal of biological chemistry. 2012;287:523–530. doi: 10.1074/jbc.M111.306183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howie J, Reilly L, Fraser NJ, Vlachaki Walker JM, Wypijewski KJ, Ashford MLJ, Calaghan SC, McClafferty H, Tian L, Shipston MJ, Boguslavskyi A, Shattock MJ, Fuller W. Proceedings of the National Academy of Sciences. 2014;111:17534–17539. doi: 10.1073/pnas.1413627111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanc M, David F, Abrami L, Migliozzi D, Armand F, Bürgi J, van der Goot F. SwissPalm: Protein Palmitoylation database. 2015 doi: 10.12688/f1000research.6464.1. [DOI] [PMC free article] [PubMed]
- 21.Sanders SS, Martin DDO, Butland SL, Lavallée-Adam M, Calzolari D, Kay C, Yates JR, III, Hayden MR. PLoS Comput Biol. 2015;11:e1004405. doi: 10.1371/journal.pcbi.1004405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roth AF, Wan J, Bailey AO, Sun B, Kuchar JA, Green WN, Phinney BS, Yates JR, III, Davis NG. Cell. 125:1003–1013. doi: 10.1016/j.cell.2006.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang R, Wan J, Arstikaitis P, Takahashi H, Huang K, Bailey AO, Thompson JX, Roth AF, Drisdel RC, Mastro R, Green WN, Yates JR, III, Davis NG, El-Husseini A. Nature. 2008;456:904–909. doi: 10.1038/nature07605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hang HC, Geutjes EJ, Grotenbreg G, Pollington AM, Bijlmakers MJ, Ploegh HL. Journal of the American Chemical Society. 2007;129:2744–2745. doi: 10.1021/ja0685001. [DOI] [PubMed] [Google Scholar]
- 25.Martin BR, Cravatt BF. Nat Methods. 2009;6:135–138. doi: 10.1038/nmeth.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeste-Velasco M, Linder ME, Lu YJ. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2015;1856:107–120. doi: 10.1016/j.bbcan.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Distler U, Kuharev J, Navarro P, Levin Y, Schild H, Tenzer S. Nat Meth. 2014;11:167–170. doi: 10.1038/nmeth.2767. [DOI] [PubMed] [Google Scholar]
- 28.Peláez-García A, Barderas R, Batlle R, Viñas-Castells R, Bartolomé RA, Torres S, Mendes M, Lopez-Lucendo M, Mazzolini R, Bonilla F, de Herreros AG, Casal JI. Molecular & Cellular Proteomics. 2015;14:303–315. doi: 10.1074/mcp.M114.045328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, Iguchi-Ariga SMM, Ariga H. Biochemical and Biophysical Research Communications. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- 30.Kim RH, Peters M, Jang Y, Shi W, Pintilie M, Fletcher GC, DeLuca C, Liepa J, Zhou L, Snow B, Binari RC, Manoukian AS, Bray MR, Liu F-F, Tsao M-S, Mak TW. Cancer Cell. 7:263–273. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Ji H, Lee JH, Wang Y, Pang Y, Zhang T, Xia Y, Zhong L, Lyu J, Lu Z. Proceedings of the National Academy of Sciences. 2016;113:644–649. doi: 10.1073/pnas.1517112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daly CE, Ng LL, Hakimi A, Willingale R, Jones DJL. Analytical Chemistry. 2014;86:1972–1979. doi: 10.1021/ac403901t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geromanos S, Hughes C, Ciavarini S, Vissers JC, Langridge J. Anal Bioanal Chem. 2012;404:1127–1139. doi: 10.1007/s00216-012-6197-y. [DOI] [PubMed] [Google Scholar]
- 34.Thalassinos K, Vissers JC, Tenzer S, Levin Y, Thompson JW, Daniel D, Mann D, DeLong M, Moseley MA, America A, Ottens A, Cavey G, Efstathiou G, Scrivens J, Langridge J, Geromanos S. J Am Soc Mass Spectrom. 2012;23:1808–1820. doi: 10.1007/s13361-012-0416-9. [DOI] [PubMed] [Google Scholar]
- 35.Ren J, Wen L, Gao X, Jin C, Xue Y, Yao X. Protein Engineering Design and Selection. 2008;21:639–644. doi: 10.1093/protein/gzn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J, Gibson B, Snider J, Jenkins CM, Han X, Gross RW. Biochemistry. 2005;44:11903–11912. doi: 10.1021/bi0508082. [DOI] [PubMed] [Google Scholar]
- 37.Perez CJ, Mecklenburg L, Jaubert J, Martinez-Santamaria L, Iritani BM, Espejo A, Napoli E, Song G, Del Rio M, DiGiovanni J, Giulivi C, Bedford MT, Dent SY, Wood RD, Kusewitt DF, Guenet JL, Conti CJ, Benavides F. J Invest Dermatol. 2015;135:3133–3143. doi: 10.1038/jid.2015.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeste-Velasco M, Mao X, Grose R, Kudahetti SC, Lin D, Marzec J, Vasiljevic N, Chaplin T, Xue L, Xu M, Foster JM, Karnam SS, James SY, Chioni AM, Gould D, Lorincz AT, Oliver RT, Chelala C, Thomas GM, Shipley JM, Mather SJ, Berney DM, Young BD, Lu YJ. The Journal of pathology. 2014;232:566–577. doi: 10.1002/path.4327. [DOI] [PubMed] [Google Scholar]
- 39.Yan SM, Tang JJ, Huang CY, Xi SY, Huang MY, Liang JZ, Jiang YX, Li YH, Zhou ZW, Ernberg I, Wu QL, Du ZM. PloS one. 2013;8:e56366. doi: 10.1371/journal.pone.0056366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nomura DK, Long JZ, Niessen S, Hoover HS, Ng SW, Cravatt BF. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medjkane S, Perez-Sanchez C, Gaggioli C, Sahai E, Treisman R. Nat Cell Biol. 2009;11:257–268. doi: 10.1038/ncb1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matakatsu H, Blair SS. Current biology: CB. 2008;18:1390–1395. doi: 10.1016/j.cub.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Son HJ, Ha SC, Hwang EY, Kim EA, Ahn JY, Choi SY, Cho SW. BMB Reports. 2012;45:707–712. doi: 10.5483/BMBRep.2012.45.12.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin L, Li D, Alesi Gina N, Fan J, Kang H-B, Lu Z, Boggon Titus J, Jin P, Yi H, Wright Elizabeth R, Duong D, Seyfried Nicholas T, Egnatchik R, DeBerardinis Ralph J, Magliocca Kelly R, He C, Arellano Martha L, Khoury Hanna J, Shin Dong M, Khuri Fadlo R, Kang S. Cancer Cell. 27:257–270. doi: 10.1016/j.ccell.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.