Abstract
Early life stress in rodents is associated with increased amygdala volume in adulthood. In humans, the amygdala develops rapidly during the first two years of life. Thus, disturbed care during this period may be particularly important to amygdala development. In the context of a 30-year longitudinal study of impoverished, highly stressed families, we assessed whether disorganization of the attachment relationship in infancy was related to amygdala volume in adulthood. Amygdala volumes were assessed among 18 low-income young adults (8M/10F, 29.33±0.49 years) first observed in infancy (8.5±5.6 months) and followed longitudinally to age 29. In infancy (18.58±1.02 mos), both disorganized infant attachment behavior and disrupted maternal communication were assessed in the standard Strange Situation Procedure (SSP). Increased left amygdala volume in adulthood was associated with both maternal and infant components of disorganized attachment interactions at 18 months of age (overall r = .679, p < .004). Later stressors, including childhood maltreatment and attachment disturbance in adolescence, were not significantly related to left amygdala volume. Left amygdala volume was further associated with dissociation and limbic irritability in adulthood. Finally, left amygdala volume mediated the prediction from attachment disturbance in infancy to limbic irritability in adulthood. Results point to the likely importance of quality of early care for amygdala development in human children as well as in rodents. The long-term prediction found here suggests that the first two years of life may be an early sensitive period for amygdala development during which clinical intervention could have particularly important consequences for later child outcomes.
Keywords: Amygdala, Attachment, Maternal Care, Early Life Stress, Limbic Irritability
1. Introduction
Early life stress is increasingly recognized as a risk factor for psychopathology. Childhood adversity has been associated with 30–70% of the population attributable risk for depression, suicide attempts, anxiety disorders and substance abuse (1). One central component of early adverse environments is impairment in parental regulation provided to the infant (2), with meta-analyses indicating that insecure and particularly disorganized attachment relationships in infancy predict internalizing and externalizing problems in childhood (2–5). Impaired regulation of early stress due to poor quality care may increase risk through excessive release of glucocorticoids and associated epigenetic modifications that alter critical developmental processes such as neurogenesis, synaptogenesis and myelination (6).
The amygdala may be particularly vulnerable to such effects of early stressors due to high glucocorticoid receptor density (7) and to a postnatal developmental trajectory characterized by rapid initial growth and gradual pruning (8, 9). In accord with this hypothesis, translational studies show that manipulating type and timing of early stressors leads to persistent alterations in amygdala development and function (10–12). Both psychological stressors and stress hormone administration stimulate dendritic arborization and formation of new spines in the amygdala, increasing volume (13, 14). This pattern is opposite to stress-induced hippocampal atrophy and less reversible when the stressor is removed (15). In particular, animal models indicate that low maternal responsiveness (LMR) during infancy is a potent stressor, associated with a host of alterations in infant development, including amygdalar effects, that persist into adulthood (10–12, 16). In rodent models, LMR has been indexed by natural or provoked variations in the responsiveness of maternal care (10, 11, 16).
Studies are now examining the relation between varieties of early life stress and amygdala volume among human children and adults. However, the consequences of early life stress on the human amygdala and the underlying causes (e.g., dendritic arborization) remain inconclusive (17).
First, a number of studies have found volumetric differences in relation to severe life events, including childhood maltreatment, institutional rearing, and poverty (18–26). However, other studies have reported no differences in amygdala volume following similar types of adversity (27–37).
Second, adult volumetric differences related to childhood maltreatment have most often involved increased hemispheric volume ((18, 19, 38); but see (20) for reduced volume). Increased volumes have also been reported among children exposed to institutional rearing or maternal depression (24, 25, 39)). However, in other studies assessing amygdala volumes in children or adolescents, effects have involved reduced volumes (21–23). In addition, smaller amygdala volumes in adulthood were reported among individuals with childhood trauma and diagnoses of Borderline Personality (40, 41) or Dissociative Identity Disorders (42, 43). Aberrant amygdala volume and function have also been reported in other psychiatric disorders marked by affective dysregulation (41, 44–46).
Third, volumetric differences have varied in whether they occurred in both hemispheres or more strongly in the left or right hemisphere of the amygdala. Adults exposed to maltreatment in childhood have shown predominately right-sided differences in amygdala volume as adults (18–20, 38). Findings based on neuroimaging in childhood among children exposed to adversity (institutional rearing, maternal depression, maltreatment) have varied, with some showing overall differences or right-sided differences (24, 25, 39) and others showing predominately left-sided effects (21–23).
Several factors may contribute to these inconsistencies. First, the timing of adversity may be critical (17, 30, 38). Given the amygdala’s developmental trajectory, it may be particularly sensitive to structural changes during early childhood when it is growing at a rapid rate, and again during preadolescence when growth peaks and pruning takes over, as observed in the hippocampus (47). Second, stress-related effects on the amygdala may be cumulative, as well as non-linear, with amygdala enlarging in response to early life stress but shrinking over time in the face of continued or overwhelming later stress (48). Third, the amygdala may show a differential response to different types of stressors, enlarging in the face of neglect or insufficient human interaction, as with prolonged institutional deprivation, or shrinking with exposure to the types of intense abuse often reported by individuals with borderline personality or dissociative identity disorders. Thus, while there is increasing literature to suggest that the human amygdala is affected by stressful developmental experiences, it is likely that factors including timing of stressor (38), age of assessment (23), development of associated psychopathology (23), genetic loading (22), and extent of stressful life events (22) will be important to the patterning of effects on the amygdala.
While most human studies have focused on stressful events after the infancy period, a few studies have examined amygdala structure or function in relation to the effect of low maternal responsiveness in the first two years of life, including prolonged institutional deprivation or rearing by depressed mothers (21, 24, 25, 39). While most studies found volumetric increases (24, 25, 39, 49) and increased activity in the amygdala (50–52), at least one study found volumetric decreases (21) and one study reported no differences (53).
To date, however, most studies have inferred low maternal responsiveness from more distal indices such as institutional care or maternal depression. One study has involved direct assessment of infant behavior, but maternal behavior was not assessed (49). To more carefully parse how the quality of maternal care during the first two years of life may affect the development of limbic stress systems, it will be important to directly assess quality of care during infancy.
For human infants, the most widely validated paradigm for direct observation of the quality of the mother-infant attachment relationship is the Strange Situation Procedure (SSP) (2, 3, 54). This paradigm includes two mildly stressful 3-minute separations of mother and infant followed by 3-minute reunions during which the degree of the infant’s security or conflict behavior in approaching the mother for care is coded. This assessment yields three classifications of attachment behavior prevalent in middle class samples that are considered organized and adaptive, including secure, ambivalent, and avoidant attachment patterns. These organized patterns are related to variations in maternal sensitivity (55, 56) but have not been consistently associated with serious family risk factors.
However, some infants do not exhibit the consistent patterning of behavior characteristic of these organized classifications, showing instead a variety of confused or conflicted behaviors upon reunion with the mother. These infants are described as disorganized in attachment behavior, and infant attachment disorganization has been meta-analytically associated with serious family risk factors, including poverty, maternal psychiatric disorder, and maltreatment, as well as later externalizing behavior problems (2, 3). Disorganized behavior on the part of the infant is also associated with directly observed disturbed maternal interaction (57). In addition, infants with disorganized attachments demonstrate a more prolonged cortisol response to stressors (58–60) and atypical patterns of diurnal cortisol secretion (61). This association between disorganized attachment and increased stress responses in infancy suggests the further hypothesis that disturbed attachment relationships may be associated with differences in amygdala volume. Given the large body of work confirming the later negative child outcomes associated with disorganized attachment, it is important to assess the aspects of brain morphology that may be affected by such early disturbed care and serve as potential mediators of later psychopathology.
To explore effects of early attachment disturbance, as well as later stressors, on amygdala volumes, we recruited subjects from a low-income cohort first seen in infancy. More than half of the longitudinal sample experienced clinically significant levels of disturbed care in infancy as judged by area service providers (see Material and Methods 2.1). Quality of mother-infant interaction was directly observed and coded with validated inventories for disrupted maternal behavior and disorganized infant behavior at 18 months of age.
A previous report assessed amygdala volume in relation to childhood maltreatment severity among the longitudinal participants studied here, as well as a group of healthy cross-sectional controls (38). Interviewed in adulthood, the longitudinal participants reported more severe exposure to maltreatment in childhood than controls and reported lower levels of maternal care on the Parental Bonding Instrument than controls (p = .002). Both left and right amygdala volumes were larger among longitudinal participants than controls, with least square mean adjusted amygdala volume 3.8% greater among the longitudinal group. Severity of childhood maltreatment was significantly related to right hemisphere volumes but not to left volumes, with severity of maltreatment at 10–11 years of age the most important predictor of right amygdala volume.
However, no healthy control group was available for assessing the longitudinal questions of interest in the present report. Here we examine a separate and orthogonal set of questions regarding infancy and childhood predictors of adult amygdala volumes within the longitudinal group only. Based on previous animal models, we predicted that the quality of the mother-child attachment relationship in infancy might have a unique relation to adult amygdala volumes, independent of later contributors including childhood maltreatment. To evaluate this prediction, we first assessed whether overall attachment disturbance, including both disorganization of infant attachment behavior and disruption in mother-infant interaction, was related to amygdala volume in adulthood. Second, we evaluated whether later stressors in childhood and adolescence, including maltreatment, could account for any relations between quality of early care and adult amygdala volumes. Finally, to explore whether any observed differences had functional significance in adulthood, we assessed whether adult psychiatric symptoms were related to amygdala volumes, including anxiety disorders, depressive disorders, substance dependence, and dissociative symptoms. We also assessed symptoms of limbic system irritability, which have been strongly related to childhood adversity (62–65). Limbic system irritability is assessed by the Limbic System Checklist-33 (LSCL-33; (64) which evaluates the frequency with which subjects experience symptoms often encountered as phenomena of ictaltemporal lobe epilepsy, such as brief hallucinatory events (66). Table 1 provides a listing of study measures.
Table 1.
Overview of measured variables
| Procedure | Measured variables |
|---|---|
| Infancy visit (Age 18 months) | |
| Strange Situation Procedure (SSP) | Infant attachment disorganization Maternal disrupted communication Overall attachment disturbance |
| Maternal background interviews | Family income/person/week Maternal psychosocial risk |
| Adolescent visit (Age 20) | |
| Adolescent-parent conflict discussion | Disorganized and controlling attachment interactions |
| Adult visit (Age 29) | |
| Magnetic resonance imaging | Brain gray matter volume in amygdala, hippocampus, caudate and thalamus; total grey matter volume |
| Maltreatment and Abuse Chronology of Exposure Scale (MACE) | Severity of childhood maltreatment |
| Structured Clinical Interview for DSM Axis I (SCID I) | Anxiety disorders Major depressive disorder Substance dependence |
| Dissociative Experiences Scale (DES) | Dissociative symptoms |
| Limbic System Checklist-33 (LSCL-33) | Limbic irritability |
Consistent with previous research (24), the hippocampus, caudate, and thalamus were selected a priori as contrast or control structures. The hippocampus is also highly stress susceptible, with sensitive periods in early childhood and during the pubertal period, but effects of stress are less persistent (15, 24, 47). The remaining structures are less susceptible to early stress due to their developmental trajectories and/or lower glucocorticoid receptor densities (67–69).
2. Material and Methods
2.1. Participants
Participants were 18 young adults (8M/10F, 29.33±0.49 years) first recruited as infants (8.5±5.6 months) as part of a longitudinal study of the effects of social risk factors on child development (70). The study was approved by the Harvard Medical School, Cambridge Hospital, and McLean Hospital IRBs. Subjects provided informed written consent and were reimbursed $100 for their time.
The larger study from which these participants were recruited consisted of 76 families who were at or below 200% of federal poverty levels. 52.6 % of families had been referred for clinical help in parenting their infants during the first year of life. Relations between infancy risk factors and maladaptive developmental outcomes in the larger longitudinal cohort have been well characterized from infancy to adulthood (70–73).
At age 29, 33 participants were relocated and screened for inclusion in the current study. Eighteen met inclusion criteria and participated in the MRI study. The remaining subjects did not meet MRI safety criteria or reported substance abuse in the past six months or had a significant medical or neurological condition. All participants except one were right-handed. Seventy-two percent of study participants had been referred for parent-infant clinical services during the first 18 months of life.
Participants in the MRI study were representative of the larger longitudinal cohort from which they were recruited. They did not differ from the remainder of the cohort in family demographic characteristics (effect sizes: family income η = .205, p = .130; male gender φ = − .136, p = .234; mother single parent φ = −.022, p = .867; mother high school only φ =− .026, p =.848; ethnic minority status φ = .052, p = .652; severity of childhood maltreatment (η = .100, p = .460); or extent of Axis I or Axis II psychopathology on the SCID in adulthood (η = .045–.032, p = .753 − .845). Participants were marginally more likely than non-participants to have been referred for clinical services in infancy (φ = .219, p = .093 and to have been classified in the Strange Situation Procedure as having a disorganized attachment in infancy (φ = .235, p = .084).
2.2. Attachment Assessments in Infancy
Mothers and infants were videotaped in the well-validated Strange Situation Procedure (SSP) at 18.58 (±1.02) months infant age (2, 56). In the SSP, the infant is videotaped in a playroom during a series of eight structured 3-minute episodes in which the mother leaves and rejoins the infant twice. The procedure is designed to be mildly stressful in order to activate the infant’s attachment behavior. Infant attachment behavior was reliably coded by coders naïve to all other data (70). The bivariate classification for organized versus disorganized attachment was used in this report, following earlier precedent (2). Maternal behavior was reliably coded over all episodes of the SSP using the well-validated Atypical Maternal Behavior Instrument for Assessment and Classification (AMBIANCE) by coders naïve to all other data (74) . Thee. AMBIANCE coding protocol yields a scaled score (1–7) for overall Level of Disrupted Communication that takes into account five subtypes of maternal disrupted communication: 1) affective communication errors (e.g. giving contradictory cues; non-response or inappropriate response to clear infant cues), 2) role confusion (e.g. self-referential or sexualized behavior), 3) negative-intrusive behavior (e.g. negative attributions about the infant; mocking or teasing the infant; physical intrusiveness), 4) fearful-disoriented behavior (e.g. appearing frightened by the infant; disoriented wandering in infant’s presence), and 5) withdrawal (e.g. fails to greet infant; interacts silently; backs away from infant approach). Mothers who are rated at 5 or above on the overall Level of Disruption Scale are classified as Disrupted. Validity of maternal AMBIANCE classification has been confirmed by meta-analysis in relation to infant attachment disorganization (57) and, in individual studies, in relation to adolescent psychiatric outcomes (72, 75, 76). Test-retest data indicate substantial stability in maternal behavior over periods ranging from 8 months to 5 years, meta-analytic stability coefficient t = .56 (N = 203) (57).
The above infant and maternal measures are theoretically and empirically related (57). To capture the combined effect of overall attachment disturbance, we also created a summary attachment measure as follows: 0 = No maternal or infant attachment disturbance in the Strange Situation Procedure; 1 = Disturbance on either maternal or infant assessment in the Strange Situation Procedure; 2 = Disturbance on both maternal and infant assessments in the Strange Situation Procedure.
2.3. Other Risk Factors in Infancy, Childhood, and Adolescence
Sociodemographic and psychosocial risk factors in infancy were coded from maternal interviews at study entry in infancy to yield measures of per person weekly family income and maternal psychosocial risk, which was coded positive if any of the following three risk factors were present: 1) mother had a history of psychiatric hospitalization, 2) mother had a history of state protective service involvement for maltreating a child, and 3) mother reported clinically significant depressive symptoms on the CES-Depression scale, using the established cutpoint of ≥16 (77).
Severity of exposure to childhood maltreatment was assessed at age 29 using the Maltreatment and Abuse Chronology of Exposure Scale (MACE; (1, 38, 65). The participant reports on exposure to ten types of maltreatment during each year of childhood from ages 6 to 18, including childhood sexual abuse, parental verbal abuse, parental non-verbal emotional abuse, parental physical abuse, witnessing of intra-parental physical violence or violence toward siblings, peer verbal abuse, peer physical abuse, parental emotional neglect, and parental physical neglect. Items within each category were selected using item response theory, and category scores were summed to provide a total score, which has shown excellent test-retest reliability across age (r = 0.91, n = 75).
Quality of attachment in adolescence was reliably assessed at age 20 using the validated Goal-Corrected Partnership in Adolescence Coding System (GPACS; (78)) applied to a videotaped conflict discussion task in which parent and young adult discussed a preselected topic of conflict in their relationship for 10 minutes. The GPACS coding system includes the rating of each videotape on 10 five-point scales. All coders were naïve to other study data. Coding of all scales was reliable across raters, ri = .75–.96 (N = 16). Confirmatory Factor Analysis of the ten scales yielded one factor indexing Collaborative/Organized interaction and three factors indexing disorganized forms of interaction, including Punitive, Disoriented, and Role-Confused interaction. Good validity has been demonstrated in relation to concurrent psychopathology and relations to romantic partners, as well as to disorganized attachment in infancy (78).
2.4. Adult Psychopathology
The Structured Clinical Interview for DSM Axis I was used to assess the presence of Axis I disorders (SCID;(79)). The SCID was administered in the laboratory by a trained clinician. The SCID yields reliability kappa’s around .61 for current diagnosis and .68 for lifetime diagnoses, comparable to other structured diagnostic interviews (80, 81). Diagnoses with adequate frequency of occurrence for analysis in the current sample included anxiety disorder, major depressive disorder, and substance dependence.
Dissociation was assessed with the Dissociative Experiences Scale (82). The DES is a 28-item questionnaire assessing the extent of dissociative experiences. Respondents indicate how much of the time they experience particular dissociative phenomena on a scale from 0–100%. A meta-analysis has demonstrated convergent validity with other measures of dissociation, predictive validity in relation to Dissociative Identity Disorder, and robust test-retest reliability (α = .93; (83)).
The Limbic System Checklist-33 (LSCL-33; (64)) evaluates limbic irritability, that is, the frequency with which subjects experience symptoms often encountered as phenomena of ictaltemporal lobe epilepsy (66). These items consist of paroxysmal somatic disturbances, brief hallucinatory events, visual phenomena, automatisms, and dissociative experiences. Psychometric studies have shown that the Limbic System Checklist-33 has high test-retest reliability (r = 0.92, N = 16) and scores are strongly influenced by childhood adversity (64).
2.5. Imaging Data Acquisition and Processing
Data were collected using a 3T TIM Trio scanner (Siemens AG, Erlangen, Germany) with a 32-channel head coil using a T1-weighted Magnetization Prepared Rapid Gradient Echo (MPRAGE) pulse sequence (TE = 2.25ms; TR = 2100ms; FA = 12; FOV = 256mm; slice number = 128; voxel size = 1.0 x 1.0 x 1.3mm; slice thickness = 1.33mm) in the sagittal plane (scan duration: 6 min). Gray matter volume (GMV) in amygdala, hippocampus, caudate and thalamus and total grey matter volume were assessed using FreeSurfer 5.1 (http://surfer.nmr.mgh.harvard.edu) (84). Although this program originated as a method for assessing cortical structure (85–88), it has evolved to include robust tools for subcortical volume analyses (84, 89). Voxels within subcortical regions are labeled using an elaborate process based on both a subject-independent probabilistic atlas derived from a hand-labeled training set and on subject-specific measures (89). This procedure has been found to label the brain in a manner that is statistically indistinguishable from those provided by experienced manual raters (84). Overall, FreeSurfer provides one of the most reliable automated brain segmentation methods for assessing the amygdala and these measures correlate highly with expert hand tracings (90). Automated segmentation eliminates differences between studies and facilitates replication by other investigators (19, 91). The authors (CMA, PP) visually inspected all T1-weighted and automated images. Manual adjustments were not required. Regional volumes and total GMV were extracted and exported into SPSS 19.0 and R (92) for statistical analysis.
2.6. Statistical Analysis
Regional GMV was centered and scaled for each region to provide an arbitrary mean of 100 and SD of 10, to facilitate comparisons between regions. Our primary hypothesis was that greater attachment disturbance would be associated with greater amygdala volume. Therefore our analysis strategy focused on assessing linear effects through partial Pearson and point-biserial correlations. Hypothesis testing of relations between neuroimaging measures and aspects of developmental history and adult psychopathology was conducted using partial correlations controlling for relevant covariates (SPSS 22.0). Multiple imputation was employed to estimate missing data (93), using the Markov Chain Monte Carlo procedure (94) in SPSS software. The percentages of missing data ranged from 0–11.1%, well within the recommended ranges for imputation procedures. Mediation analyses were carried out using bootstrapping techniques with INDIRECT (95).
We predicted that more severe early attachment disturbance would be associated with increased GMV in the amygdala but would not be associated with similar increase in GMV in hippocampus, caudate, or thalamus. Thus, we did not adjust the significance of amygdala measures for these planned negative control comparisons. In addition, we had hypothesized a priori that the amygdala would be most sensitive to observed quality of care in infancy, and that this relation would not be accounted for by effects of more distal risk factors in infancy, childhood, and adolescence. Therefore, we also did not adjust the significance of amygdala measures for these additional planned negative control comparisons.
3. Results
3.1. Covariates
Total grey matter volume (TGMV) was correlated strongly with amygdala volume and was controlled in all analyses to reduce error variance. Given limited degrees of freedom, we restricted additional covariates to those showing at least a moderate relationship (r > 0.25) to amygdala volume. Age, gender and race were also assessed in relation to amygdala volumes and only race (white = 0, black = 1) was retained as a covariate due to its moderate association with left amygdala volume (r = 0.34). Age and gender were eliminated due to weak relationships once TGMV was controlled. Hence, all partial correlations below are adjusted for TGMV and race.
3.2. Descriptive Data for Predictor Variables
In infancy, 67% (n=12) of infants were classified as disorganized in their attachment behavior toward mother and 61% (n=11) of mothers were classified as disrupted in their responses to their infants’ attachment cues. On the three-level variable for overall attachment disturbance, 16.7% (n=3) displayed no disturbance on either infant or maternal assessments, 38.9% (n=7) displayed disturbance on one assessment, and 44.4% (n=8) displayed disturbance on both assessments. Descriptive data on other measures are shown in Table 2.
Table 2.
Descriptive data
| Mean ± SD or N (%) | |
|---|---|
| Adult characteristics | |
| Age (yrs.) | 29.3 ± 0.5 |
| Female | 10 (57%) |
| White | 14 (78%) |
| Single | 11 (61%) |
| College degree | 2 (11%) |
| Psychosocial risk factors in infancy | |
| Family income/person/week | $45.3 ± 22.6 |
| Mother any psychosocial risk | 12 (66.7%) |
| Childhood and adolescent risk factors | |
| Childhood maltreatment | |
| Severity of childhood maltreatment | 26.8 ± 14.5 |
| Attachment disturbance in adolescence | |
| Punitive interaction | 2.2 ± .6 |
| Caregiving interaction | 2.2 ± .9 |
| Disoriented interaction | 1.5 ± .6 |
| Adult symptomatology | |
| Anxiety disorder | 4 (22.2%) |
| Major depressive disorder | 4 (22.2%) |
| Substance dependence | 7 (38.8%) |
| Dissociative symptoms | 7.1 ± 4.6 |
| Limbic irritability | 16.9 ± 9.2 |
Note. N = 18
3.3. Overall Attachment Disturbance in Infancy
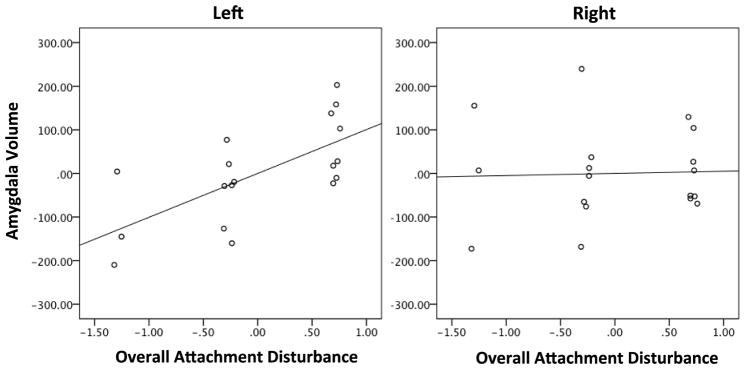
Controlling for relevant covariates, we first assessed whether greater overall attachment disturbance was associated with greater amygdala volume, as hypothesized1. Overall attachment disturbance at 18 months of age was strongly predictive of adjusted left amygdala volume in adulthood (partial r = .679, df = 14, p = .004, 95% CI = .31–.87; Figure 1). This association was significantly stronger than the correlation between overall attachment disturbance and adjusted right amygdala volume (right partial r = −.048, p = .860, 95% CI =−0.5–.43; Meng’s test for dependent correlations Z = 2.343, p = .020) (96). This asymmetry was also reflected in an increased laterality index (r = .513, df = 16, p = .030, 95% CI = .06–.79). Overall attachment disturbance accounted for 14.8% of variance in volume of the left amygdala.
Figure 1. Amygdala volume in adulthood and infant attachment disturbance.
Amygdala volume was normalized and adjusted for total grey matter volume and race. Left (r = .679, p = .004) but not right (r = −.048, p = .860) amygdala volume was related to overall attachment disturbance.
As expected, overall attachment disturbance was not significantly related to hippocampal (left partial r = .177, p = .512, 95% CI = −.32–.59; right partial r = .400, p = 0.125, 95% CI = −.08–.73), caudate (left partial r = .410, p = .115; right partial r = .400, p = .125, 95% CI = −.07–.74), or thalamic (left partial r = .210, p = .435, 95% CI = −.29–.62; right partial r = .052, p = .848, 95% CI = −.43–.51) volumes. However, the size of the relations to right hippocampus and left and right caudate suggests that further work may be warranted in larger samples.
Given these initial findings, overall attachment disturbance was decomposed into the separate variables for maternal disrupted interaction and infant disorganization. Both maternal (partial r = .595, p = .015, 95% CI = .18–.83) and infant (partial r = .543, p = .030, 95% CI = .1 –.81) assessments were strongly predictive of left amygdala volume. Relations to right amygdala volume were not significant (maternal partial r = −.272, p = .308, 95% CI = −.66–.22; infant partial r = .146, p = .590, 95% CI = −.34–.57). 1
Given the significant prediction from maternal disrupted communication found above, we also examined the five component scores for types of maternal disrupted interaction. Consistent with animal data on low maternal responsiveness, maternal withdrawing behavior and contradictory communications made the strongest contributions to left amygdala volume (withdrawing partial r = .397, p = .128, 95% CI = −.09–.73; contradictory communications partial r = .405, p = .120, 95% CI = −.08–.73). Though not reaching significance with this sample size, these moderate effect sizes suggest that further work on maternal withdrawal and contradictory cues to the infant would be fruitful. Other types of disturbed interaction were negligible or negative in effect, including negative intrusive behavior (partial r = .111, p = .682, 95% CI = −.38–.55), role confusion (partial r = −.280, p = .294, 95% CI = −.66–.22), and disorientation (partial r = −.190, p = .481, 95% CI = −.60–.30).
3.4. Other Risk Factors in Infancy, Childhood, and Adolescence
We then assessed whether the obtained relation between overall attachment disturbance and left amygdala volume could be explained by other risk factors in infancy, childhood, and adolescence. In infancy, risk factors such as low family income or maternal psychosocial risk might serve as third variables explaining the above relations. However, neither left or right amygdala volumes were significantly related to family income (left partial r = −.054, p = .843, 95% CI = −.51–.42; right partial r = −.374, p = .154, 95% CI = −.72–.11) or presence of serious maternal psychosocial risk (history of psychiatric hospitalization, involvement with state protective services, or high depressive symptoms; left partial r = −.003, p = .991, 95% CI = −.47–.46; right partial r = −.218, p = .417, 95% CI = −.62–.28). In addition, controlling for these risk factors did not alter the significant relation of overall attachment disturbance to left amygdala volume, Fchg (1,10) = 6.22, p = 0.032, R2chg = 0.090.
Because later difficulties in family relationships might also serve as third variables accounting for prediction from overall attachment disturbance to later amygdala volume, we then assessed whether severity of childhood maltreatment might account for the obtained association between amygdala volume and attachment disturbance. Severity of maltreatment was unrelated to left amygdala volume (left partial r = −.006, p = .982, 95% CI = −.47–.46). In addition, controlling for severity of maltreatment did not alter the relation of overall attachment disturbance to left amygdala volume, Fchg(1,12) = 7.07, p = .020, R2chg = .090.
In relation to right amygdala, however, and consistent with other literature, severity of maltreatment was moderately associated with increased volume (partial r = .377, p = .150, 95% CI = −.11–.72). The effect did not reach significance with this sample size, but the effect size was similar to those that did reach significance in studies with larger samples (38).
Finally, we assessed whether later attachment disturbance in adolescence might account for the link between early attachment disturbance and left amygdala volume. However, none of the three forms of disturbed attachment in adolescence were significantly related to left or right amygdala volume (punitive left partial r = .151, p = .577, 95% CI = −.34–.58; right partial r = −.179, p = .507, 95% CI = −.6–.31; disoriented left partial r = −.006, p = .982, 95% CI = −.47–.46; right partial r = −.237, p =.377, 95% CI = −.63–.26; role-confused left partial r = .209, p = .437, 95% CI = −.29–.62; right partial r = −.293, p = .271, 95% CI = −.20–.67). In addition, controlling for all three forms of attachment disturbance in adolescence did not alter the relation of early attachment disturbance to left amygdala volume, Fchg(1, 11) = 12.50, p = .005, R2chg = .15.
3.5. Psychiatric Symptoms in Adulthood
The last set of analyses assessed whether enlarged left amygdala volume had functional significance for psychiatric outcomes in adulthood. The three psychiatric diagnoses with sufficient frequency for analysis in this cohort (anxiety disorder, major depressive disorder, lifetime substance dependence) were not significantly related to left amygdala volume (anxiety partial r = .428, p = .098, 95% CI = −.05–.75; major depression, partial r = −.111, p = .682. 95% CI = −.55–.38; substance dependence, partial r = .404, p =.121, 95% CI = −.08–.73). However, again, the effect sizes for anxiety disorders and substance dependence were moderate and warrant further investigation in larger samples. Right amygdala volume was unrelated to these disorders (anxiety disorder: partial r = −.090, p = .740, 95% CI = −.39–.53; major depression: partial r = −.031, p = .909, 95% CI = −.49–.44; substance dependence: partial r = −.154, p = .569, 95% CI = −.58–.34).
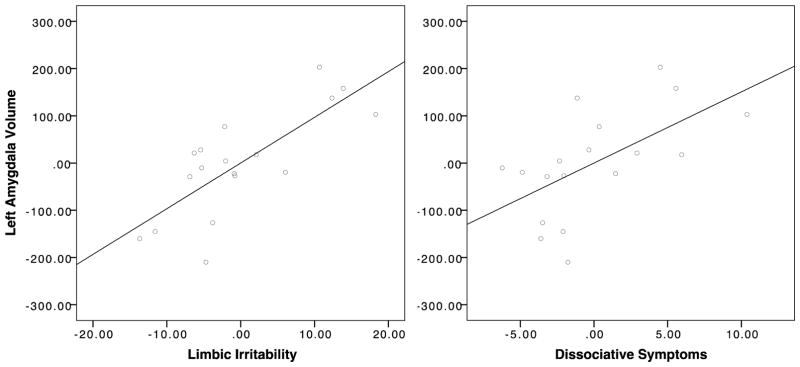
In contrast, left amygdala volume was strongly related to both dissociative symptoms (partial r = .586, p = .017, 95% CI = .16–.83) and limbic irritability (partial r = .770, p = .001, 95% CI = .47–.91). There were no corresponding associations between right amygdala volume and either limbic irritability (partial r = .032, p = .906, 95% CI = −.44–.49) or dissociative symptoms (partial r = −.310, p = .242, 95% CI = −.68–.18) (Figure 2).
Figure 2. Amygdala volume and adulthood psychopathology.
Amygdala volume was normalized and adjusted for total grey matter volume and race. Left amygdala volume was strongly related to both limbic irritability (r = .770, p = .001) and dissociative symptoms (r = .586, p = .017) in adulthood, controlling for total grey matter volume and race.
Similar to left amygdala volume, early attachment disturbance was also strongly related to both dissociation and limbic irritability but did not reach significance for the three psychiatric disorders above (Table 3). However, the moderately strong relation between attachment disturbance and substance dependence (r = .422) indicates a fruitful direction for further work.
Table 3.
Relations between Overall Attachment Disturbance and Psychiatric Symptoms in Adulthood
| Overall attachment disturbance | ||
|---|---|---|
| Pearson partial r | (95% CI) | |
| Anxiety disorder | .111 | (−.38–.55) |
| Major depressive disorder | −.119 | (−.56–.37) |
| Substance dependence | .422x | (−.06–.74) |
| Dissociative symptoms | .586** | (.16–.83) |
| Limbic Irritability | .606** | (.19–.84) |
Note.
p <.10,
p < .01.
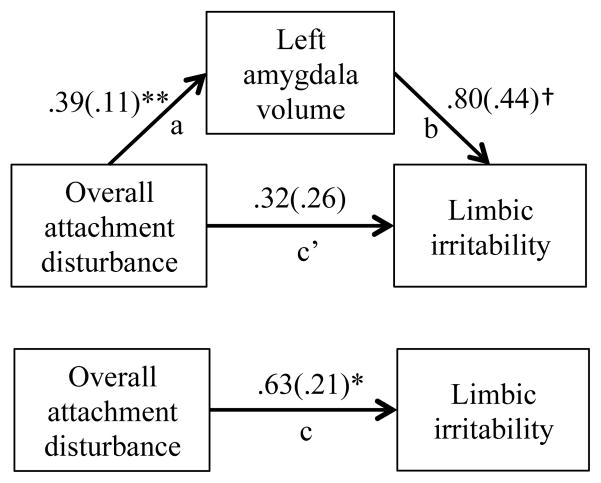
Finally, we assessed whether left amygdala volume might mediate the relation between early attachment disturbance and later dissociation and limbic irritability. Mediation analyses, using recommended bootstrapping methods (95), indicated that the prediction from overall attachment disturbance in infancy to limbic irritability in adulthood was mediated by increased volume of the left amygdala (bias-corrected confidence interval did not contain zero (CI 2.05–13.27); Figure 3). In regards to dissociation, mediation by left amygdala volume of the relation between early attachment disturbance and later dissociation could not be confirmed with this modest sample size (CI −.25–4.52).
Figure 3. Left amygdala volume mediating the relation between infant attachment disturbance and limbic irritability in adulthood.
The association between attachment disturbance and limbic irritability in adulthood was mediated by adjusted left amygdala volume. The values in the figure are r coefficients (bootstrap standard error) for each path. Path a1, direct effect of attachment disturbance on left amygdala volume. Path b1, direct effect of left amygdala volume on limbic irritability. Path c’, direct effect of attachment disturbance on limbic irritability. Path c, total effect of attachment disturbance on limbic irritability. Indirect effect ab = .481 [95% CI = .175–0.977], p < 0.01. * p < .05, ** p < .01.
4. Discussion
Congruent with animal studies on poor maternal care (10, 11) and human data on institutional care (21, 24, 39), the present findings underscore the potential importance of the quality of early maternal care for the development of the amygdala. In addition, these findings add to the evidence base that human amygdala volume is sensitive not only to very atypical rearing conditions, such as institutional care, but also to serious disturbances in maternal care observed among infants reared at home (21, 25).
The high rate of infant attachment disorganization in this sample (67%) points to a high level of disturbance, compared to a rate of 15% among infants in middle-income samples (2), 25 % of infants in low-income families (2), and up to 85–90% of maltreated infants (97–100). Cyr et al. (100) also found that families with multiple social risk factors, but no reported maltreatment, were as likely to have disorganized infants as maltreating families. As noted earlier, this was a degree of risk that was evident to pediatric nurses and other service providers in the community and, in many cases, resulted in referral to infant mental health services.
Notably, attachment assessments are among the best validated assessments of early risk in developmental science. Supporting large scale studies and meta-analyses have confirmed their relation to concurrent risk factors in infancy and their predictive value for behavior problems in childhood (2–4, 54, 101, 102). In addition, these studies have consistently pointed to disorganized attachments as the forms of insecure attachment that carry the greatest risk for later psychopathology (2–4, 101, 102). Thus, these results suggest that further work exploring the neural mechanisms associated with deviations in early attachment relationships may be particularly fruitful for understanding developmental trajectories toward pathology.
The specificity of the effect of early disturbance on regional brain volumes was also important. We hypothesized that the amygdala would be most affected by quality of early care compared to hippocampus, thalamus, or caudate, based on evidence that the amygdala has high glucocorticoid receptor density (7) and is developing more rapidly shortly after birth (9). In accord with expectations, these other regions were not strongly or significantly related to early attachment disturbance. However, as noted, the medium effect sizes for left and right caudate (103) and right hippocampus, while not as strong as those for the amygdala, may warrant further study in larger samples.
In addition, the effect of early care on amygdala volume in adulthood was not explained by intervening family disturbances in childhood and adolescence, including both severity of childhood maltreatment and observed disturbances in attachment in adolescence. Thus, this work adds to the evidence from animal models that early stress-related dendritic growth in the amygdala may be resistant to change (10–12, 15).
The results also contribute additional intriguing evidence regarding the possibility of differences in left versus right amygdala sensitivity to the types and timing of critical stressors in infancy and childhood. While both left and right amygdala develop shortly after birth, the left amygdala develops more rapidly, while the right amygdala has a more prolonged developmental period (9). In addition, left amygdala has been shown to be particularly responsive to maternal stimuli in childhood (104), as well as to the discrimination of family faces more generally (105). Such findings are consistent with the motivational hypothesis emerging from EEG studies that the left hemisphere is more involved in motivating approach behavior and the right hemisphere is more involved in motivating avoidance behavior (106). Thus, right amygdala may be differentially responsive to negative or threatening stimuli (107–109), and alterations in structure (38, 109, 110) of the right amygdala in adulthood have been particularly related to exposure to childhood maltreatment. Also notably, compared to cross-sectional controls, the same participants studied here exhibited right amygdala enlargement in relation to the severity of maltreatment in middle childhood, with peak effect from maltreatment between 10 and 11 years of age (38), but show left amygdala enlargement here in relation to quality of early care. These differential effects within the same subjects, using well-validated assessments of both early care and later maltreatment, suggest some differential hemispheric sensitivity to type and timing of childhood stressors.
The profile of maternal behavior associated with left amygdala enlargement was similar to that described in the translational literature on low responsive mothers, with mothers’ withdrawing behaviors (e.g. failure to greet, backing away from the infant, interacting from a distance, interacting silently) and contradictory communications (failure to respond appropriately to clear infant cues; mixed communications such as sweet voice but negative message) showing the largest associations with amygdala volume (r = .397 and .405, respectively). In contrast, mother’s negative-intrusive, disoriented, and role-confused behaviors showed negligible to negative associations to left amygdala volume (r = .11, −.19, and −.28, respectively). Behavioral studies have confirmed that, even in the context of overall disorganization, maternal withdrawing behavior is associated with higher levels of infant approach behavior, while maternal negative- intrusive, disoriented, and role-confused behaviors are associated with increased infant avoidance of the mother (74). Thus, the mother’s relative unavailability to the infant may be important in stimulating infant hypervigilance to the mother’s whereabouts and infant approach motivation, mediated in part by the left amygdala.
Consistent with this thinking, the infant’s disorganized behavior is thought to indicate that the infant cannot organize a strategy of consistently avoiding or consistently approaching the caregiver and, instead, exhibits confused, contradictory or aborted attempts to approach the mother for comfort. While consistent avoidance of the mother at reunion has been associated with maternal irritation, anger, rejection, and interfering behavior (56, 74, 111–113), infant disorganized behavior has been associated with mixed signs of both maternal hostility and withdrawal (57, 74, 114). The patterning of maternal data here suggests that the mother’s withdrawal and contradictory communications may be particularly important to the left amygdalar effects related here to disorganized attachment, in contrast to the right hemisphere effects recently associated with infant organized avoidant behavior (49).
Finally, intriguing findings from rodent models indicate that low maternal responsiveness (LMR) is related to differential left hemisphere responding in medial prefrontal cortex (mPFC). Animals exposed to LMR develop excessive hemispheric asymmetry in mPFC DA stress responsivity, such that peak DA stress responses in left mPFC of LMR animals are two to three times greater than those of controls (115). Although mPFC and amygdala have extensive bidirectional connections and often function in tandem (110, 116, 117), these mPFC findings on greater left responding under conditions of deprived care are suggestive but have yet to be extended to amygdala structure or function.
In relation to the current findings, one potential hypothesis is that low levels of maternal care differentially activate the left amygdala and promote infant hypervigilance to the whereabouts of the mother, possibly also dampening early right amygdala activity (115). In this view, the amygdala may be lateralized to respond differentially to different evolutionary threats to survival, and this laterality may be enhanced under threat of abandonment. Models of prototypic threat have focused on threat of attack, with prototypic responses of fight, flee, or freeze. An equally potent survival threat to the infant is the threat of neglect or abandonment by the caregiver. Fight, flight, or freeze responses would be counterproductive in response to maternal withdrawal and might decrease survival. When the mother is withdrawn or inattentive, the adaptive response is to increase the intensity of the distress signal to the parent and to pursue proximity and contact with the parent, that is, to seek and squeak rather than to fight or flee.
The consequences of increased left amygdala volume for adult symptomatology further suggest the potential clinical implications of the present findings. The Limbic System Checklist-33 (LSCL-33) was created to test the hypothesis that childhood adversity kindled the amygdala and associated limbic system and increased the occurrence of symptoms characteristically observed in individuals with temporal lobe epilepsy (64). In previous work, these ‘limbic irritability’ symptoms were dramatically elevated among adults with maltreatment histories (33, 63, 65, 118). In addition, a significant inverse correlation has been found between LSCL-33 ratings and integrity of left fornix (119), integrity of left inferior longitudinal fasciculus (62), and T2-relaxation time (an indirect measure of relative cerebral blood flow) in the cerebellar vermis (120). These fiber tracts and regions all have specific connections to components of the limbic system (121). Current findings using a prospective design extend this work to indicate that limbic irritability is also predicted by serious disturbances in the attachment relationship in infancy. Notably, this prediction is mediated here by increased volume of the left amygdala.
Despite these suggestive findings, the interpretation of morphometric change remains challenging, as multiple factors contribute to structural volume (e.g., size of neurons and glial cells, density, vascularity) (122). Based on randomized animal models, possible neurobiological mechanisms relating early attachment disturbance to increased left amygdala volume include both early stress-related overproduction of new spines in the amygdala and later inadequate pruning. In addition, because amygdala volume was assessed in adulthood, non-linear developmental change in regional volumes and intervening stressful events that were not measured here may have influenced the relation between quality of early care and left amygdala volume. Although we examined the intervening factors most prominent in current literature, other factors may have been involved in mediating the observed relation. In addition, the small sample size makes this a ‘proof of construct’ preliminary study that needs to be followed up by larger longitudinal studies of limbic development in relation to quality of care in infancy. We are currently conducting one such study.
Although there is current concern regarding replicability of small sample findings in neuroscience (123) we would argue that small-scale, carefully done exploratory studies play an important role in emerging areas of science, by exploring a broad range of possible influences and assessment paradigms to identify those that warrant more expensive and long-range studies. Committing to large-scale studies too soon in the development of a field runs the risk of foreclosing this exploratory phase and weakening rather than strengthening future work.
5. Conclusions
In conclusion, the results suggest that disorganized early attachment relationships may have long-term effects on later adaptation by promoting increased volume in the left amygdala that contributes to increased irritability in limbic pathways. These findings also suggest that the first two years of life may be an early sensitive period during which clinical intervention could preempt long-term consequences of early attachment disturbance.
Research Highlights.
Attachment disturbance in infancy predicts larger left amygdala volume in adulthood.
Prediction is independent of later maltreatment and later attachment quality.
Attachment disturbance also predicts adult dissociation and limbic irritability.
Left volume mediates the relation between early attachment and limbic irritability.
Disturbed infant attachment may affect adult amygdala volume and psychopathology.
Acknowledgments
This research was supported by funding received from the Harvard Catalyst/Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758) and the Frederick Leonhardt Foundation awarded to KLR, MHT, and PP, R01 MH091391 awarded to MHT, and R01 MH062030 awarded to KLR. The authors would like to thank Nancy Hall Brooks, Sarah Richardt, and Cynthia McGreenery for their invaluable contributions to participant recruitment and data collection.
Footnotes
Given low power, our primary analysis strategy focused on assessing a linear effect through Pearson and point biserial partial correlations. However, we further tested the robustness of these effects by treating overall attachment disturbance, maternal disrupted communication, and infant disorganization as categorical variables in an ANCOVA, with TGMV and race as covariates. Consistent with the previous analyses, there was a significant between-group effect of overall attachment disturbance on left amygdala volume, F (2, 13) = 5.97, p = .031. Post-hoc comparisons revealed that the group with both maternal and infant attachment disturbance (95% CI 1461.8–1604.3) had greater left amygdala volume compared to the groups with no attachment disturbance (95% CI 1363.3–1575.3; p = .008) and with either maternal or infant attachment disturbance alone (95% CI 1591.0–1729.6; p = .029). The group with either maternal or infant attachment disturbance alone did not differ significantly from the group with no attachment disturbance (p = .314). However, the very low power for examining subgroup differences should be taken into account in interpreting the post-hoc comparisons. Similarly, for maternal disrupted interaction there was a significant between-group effect of disrupted interaction on later left amygdala volume, F (1,14) = 7.72, p = .017 (not-disrupted 95% CI = 1414.6–1567.7; disrupted 95% CI 1566.1–1685.3), as well as a significant between-group effect of infant attachment disorganization on left amygdala volume, F (1, 14) = 5.95, p = .031 (organized 95% CI = 1391.3–1574.3; disorganized 95% CI = 1554.2–1669.6). For right amygdala volume, there were no between-group effects of overall attachment disturbance (F (2, 13) = .06, p = .940), maternal disrupted interactions (F (1, 14) = 1.22, p = .328), or infant disorganization (F (1, 14) = .36, p = .597).
Authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Teicher MH, Samson JA. Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. American Journal of Psychiatry. 2013;170(10):1114–1133. doi: 10.1176/appi.ajp.2013.12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van IJzendoorn MH, Schuengel C, Bakermans-Kranenburg MJ. Disorganized attachment in early childhood: meta-analysis of precursors, concomitants, and sequelae. Development and Psychopathology. 1999;11(2):225–249. doi: 10.1017/s0954579499002035. [DOI] [PubMed] [Google Scholar]
- 3.Fearon RP, Bakersman-Kranenberg MJ, Van IJzendoorn MH, Lapsley A, Roisman GI. The significance of insecure attachment and disorganizarion in the development of children's externalizing behavior: A meta-analytic study. Child Development. 2010;81(2):435–456. doi: 10.1111/j.1467-8624.2009.01405.x. [DOI] [PubMed] [Google Scholar]
- 4.Madigan S, Brumariu L, Villani V, Atkinson L, Lyons-Ruth K. Representational and questionnaire measures of attachment after early childhood: A meta-analysis of relations to child internalizing and externalizing problems. Psychological Bulletin. doi: 10.1037/bul0000029. in press. [DOI] [PubMed] [Google Scholar]
- 5.Madigan S, Atkinson L, Laurin K, Benoit D. Attachment and internalizing behavior in early childhood: A meta-analysis. Developmental Psychology. 2013;49(4):672–689. doi: 10.1037/a0028793. [DOI] [PubMed] [Google Scholar]
- 6.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 7.Peiffer A, Barden N, Meaney MJ. Age-related changes in glucocorticoid receptor binding and mRNA levels in the rat brain and pituitary. Neurobiol Aging. 1991;12(5):475–479. doi: 10.1016/0197-4580(91)90076-v. [DOI] [PubMed] [Google Scholar]
- 8.Payne C, Machado CJ, Bliwise NG, Bachevalier J. Maturation of the hippocampal formation and amygdala in Macaca mulatta: a volumetric magnetic resonance imaging study. Hippocampus. 2010;20(8):922–935. doi: 10.1002/hipo.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uematsu A, et al. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS One. 2012;7(10):e46970. doi: 10.1371/journal.pone.0046970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plotsky PM, et al. Long term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30(12):2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- 11.Cohen MM, et al. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc Natl Acad Sci. 2013;110(45):18274–18278. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raineki C, Moriceau S, Sullivan R. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biological Psychiatry. 2010;67:1137–1145. doi: 10.1016/j.biopsych.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci. 2005;102(26):9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143(2):387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128(4):667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Caldji C, et al. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci. 1998;95(9):5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weems CF, Klabunde M, Russell JD, Reiss AL, Carrion VG. Post-traumatic stress and age variation in amygdala volumes among youth exposed to trauma. Social Cognitive and Affective Neuroscience. 2015;10(12):1661–1667. doi: 10.1093/scan/nsv053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morey RA, et al. Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Archives of General Psychiatry. 2012;69(11):1169–1178. doi: 10.1001/archgenpsychiatry.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veer IM, et al. Evidence for smaller right amygdala volumes in posttraumatic stress disorder following childhood trauma. Psychiatry Research-Neuroimaging. 2015;233(3):436–442. doi: 10.1016/j.pscychresns.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Hanson JL, et al. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biological Psychiatry. 2015;77(4):314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagliaccio D, et al. Stress-system genes and life stress predict cortisol levels and amygdala and hippocampal volumes in children. Neuropsychopharmacology. 2014;39:1245–1253. doi: 10.1038/npp.2013.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whittle S, et al. Childhood maltreatment and psychopathology affect brain ddvelopment during adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(9):940–952. doi: 10.1016/j.jaac.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Tottenham N, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lupien SJ, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci. 2011;108(34):14324–14329. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH. Sensitive periods of amygdala development: The role of maltreatment in preadolescence. NeuroImage. 2015;97:236–244. doi: 10.1016/j.neuroimage.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bremner JD, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse--a preliminary report. Biological Psychiatry. 1997;41(1):23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brambilla P, et al. Anatomical MRI study of borderline personality disorder patients. Psychiatry Research. 2004;131(2):125–133. doi: 10.1016/j.pscychresns.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 29.van Harmelen AL, et al. Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biological Psychiatry. 2010;68(9):832–838. doi: 10.1016/j.biopsych.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Andersen SL, et al. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20(3):292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biological Psychiatry. 2012;72(1):57–64. doi: 10.1016/j.biopsych.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen RA, et al. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biological Psychiatry. 2006;59(10):975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 33.Dannlowski U, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry. 2012;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 34.De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biological Psychiatry. 2001;50(4):305–309. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- 35.De Bellis MD, et al. Developmental traumatology. Part II: Brain development. Biological Psychiatry. 1999;45(10):1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 36.De Bellis MD, et al. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biological Psychiatry. 2002;52(11):1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- 37.Carrion VG, et al. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biological Psychiatry. 2001;50(12):943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- 38.Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH. Sensitive periods of amygdala development: The role of maltreatment in preadolescence. NeuroImage. 2015;97:236–244. doi: 10.1016/j.neuroimage.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta MA, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. Journal of Child Psychology and Psychiatry. 2009;50(8):943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 40.Driessen M, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Archives of General Psychiatry. 2000;57(12):1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- 41.Schmahl CG, Vermetten E, Elzinga BM, Bremner JD. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Research-Neuroimaging. 2003;122(3):193–198. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- 42.Vermetten E, Schmahl C, Lindner S, Loewenstein RJ, Bremner JD. Hippocampal and amygdalar volumes in dissociative identity disorder. American Journal of Psychiatry. 2006;163(4):630–636. doi: 10.1176/appi.ajp.163.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weniger G, Lange C, Sachsse U, Irle E. Reduced amygdala and hippocampus size in trauma-exposed women with borderline personality disorder and without posttraumatic stress disorder. J Psychiatry Neurosci. 2009;34(5):383–388. [PMC free article] [PubMed] [Google Scholar]
- 44.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biological Psychiatry. 2006;60(4):376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Saleh K, et al. Impact of family history and depression on amygdala volume. Psychiatry Research. 2012;203(1):24–30. doi: 10.1016/j.pscychresns.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Lange C, Irle E. Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychological Medicine. 2004;34(6):1059–1064. doi: 10.1017/s0033291703001806. [DOI] [PubMed] [Google Scholar]
- 47.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31(4):183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 48.Teicher MH, Samson JA. Annual research review: Enduring neurobiological effects of childhood abuse and neglect. Journal of Child Psychology and Psychiatry. 2016;57(3):241–266. doi: 10.1111/jcpp.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moutsiana C, et al. Insecure attachment during infancy predicts greater amygdala volumes in early adulthood. Journal of Child Psychology and Psychiatry. 2015;56(5):540–548. doi: 10.1111/jcpp.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maheu F, et al. A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cognitive, Affective, & Behavioral Neurscience. 2010;10(1):34–49. doi: 10.3758/CABN.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCrory EJ, et al. Heightened neural reactivity to threat in child victims of family violence. Current Biology. 2011;21(23):947–948. doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 52.Tottenham N, et al. Elevated amygdala response to faces following early deprivation. Developmental Science. 2011;15(3):307–319. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheridan MA, Fox ME, Zeanah CH, McLaughlin KA, Nelson CA. Variation in neural development as a result of exposure to institutionalization early in childhood. Proc Natl Acad Sci. 2012;109(32):12927–12932. doi: 10.1073/pnas.1200041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madigan S, Atkinson L, Laurin K, Benoit D. Attachment and internalizing behavior in early childhood: A meta-analysis. Developmental Psychology. 2013;49(4):672–689. doi: 10.1037/a0028793. [DOI] [PubMed] [Google Scholar]
- 55.van IJzendoorn M. Adult attachment representations, parental responsiveness, and infant attachment: a meta-analysis on the predictive validity of the Adult Attachment Interview. Psychological Bulletin. 1995;117(3):387–403. doi: 10.1037/0033-2909.117.3.387. [DOI] [PubMed] [Google Scholar]
- 56.Ainsworth MDS, Blehar MC, Waters E, Wall S. Patterns of attachment: A psychological study of the strange situation. Lawrence Erlbaum; Oxford England: 1978. [Google Scholar]
- 57.Madigan S, et al. Unresolved states of mind, anomalous parental behavior, and disorganized attachment: a review and meta-analysis of a transmission gap. Attachment & Human Development. 2006;8(2):89–111. doi: 10.1080/14616730600774458. [DOI] [PubMed] [Google Scholar]
- 58.Nachmias M, Gunnar M, Mangelsdorf S, Parritz RH, Buss K. Behavioral inhibition and stress reactivity: the moderating role of attachment security. Child Development. 1996;67(2):508–522. [PubMed] [Google Scholar]
- 59.Spangler G, Grossman KE. Biobehavioral organization in securely and insecurely attached infants. Child Development. 1993;64:1439–1450. doi: 10.1111/j.1467-8624.1993.tb02962.x. [DOI] [PubMed] [Google Scholar]
- 60.Bernard K, Dozier M. Examining infants' cortisol responses to laboratory tasks among children varying in attachment disorganization: stress reactivity or return to baseline? Developmental Psychology. 2010;46(6):1771–1778. doi: 10.1037/a0020660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luijk MP, et al. Attachment, depression, and cortisol: Deviant patterns in insecure-resistant and disorganized infants. Developmental Psychobiology. 2010;52(5):441–452. doi: 10.1002/dev.20446. [DOI] [PubMed] [Google Scholar]
- 62.Choi J, Jeong B, Polcari A, Rohan ML, Teicher MH. Reduced fractional anisotropy in the visual limbic pathway of young adults witnessing domestic violence in childhood. Neuroimage. 2012;59(2):1071–1079. doi: 10.1016/j.neuroimage.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dackis MN, Rogosch FA, Oshri A, Cicchetti D. The role of limbic system irritability in linking history of childhood maltreatment and psychiatric outcomes in low-income, high-risk women: moderation by FK506 binding protein 5 haplotype. Development and Psychopathology. 2012;24(4):1237–1252. doi: 10.1017/S0954579412000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teicher MH, Glod CA, Surrey J, Swett C., Jr Early childhood abuse and limbic system ratings in adult psychiatric outpatients. J Neuropsychiatry Clin Neurosci. 1993;5(3):301–306. doi: 10.1176/jnp.5.3.301. [DOI] [PubMed] [Google Scholar]
- 65.Teicher MH, Samson JA, Polcari A, McGreenery CE. Sticks, stones, and hurtful words: Relative effects of various forms of childhood maltreatment. American Journal of Psychiatry. 2006;163:993–1000. doi: 10.1176/ajp.2006.163.6.993. [DOI] [PubMed] [Google Scholar]
- 66.Spiers PA, Schomer DL, Blume HW, Mesulam MM. Principles of Behavioral Neurology. Philadelphia: FA Davis; 1985. Temporolimbic epilepsy and behavior; pp. 289–326. [Google Scholar]
- 67.Patel PD, et al. Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. Journal of Psychiatric Research. 2000;34(6):383–392. doi: 10.1016/s0022-3956(00)00035-2. [DOI] [PubMed] [Google Scholar]
- 68.Giedd JN, et al. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cerebral Cortex. 1996;6(4):551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 69.Hasan KM, et al. Multimodal quantitative magnetic resonance imaging of thalamic development and aging across the human lifespan: implications to neurodegeneration in multiple sclerosis. J Neurosci. 2011;31(46):16826–16832. doi: 10.1523/JNEUROSCI.4184-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lyons-Ruth K, Connell DB, Grunebaum HU, Botein S. Infants at social risk: maternal depression and family support services as mediators of infant development and security of attachment. Child Development. 1990;61(1):85–98. doi: 10.1111/j.1467-8624.1990.tb02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lyons-Ruth K, Easterbrooks MA, Cibelli CD. Infant attachment strategies, infant mental lag, and maternal depressive symptoms: predictors of internalizing and externalizing problems at age 7. Developmental Psychology. 1997;33(4):681–692. doi: 10.1037//0012-1649.33.4.681. [DOI] [PubMed] [Google Scholar]
- 72.Lyons-Ruth K, Bureau JF, Holmes B, Easterbrooks A, Brooks NH. Borderline symptoms and suicidality/self-injury in late adolescence: prospectively observed relationship correlates in infancy and childhood. Psychiatry Research. 2013;206(2–3):273–281. doi: 10.1016/j.psychres.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lyons-Ruth K, Alpern L, Repacholi B. Disorganized infant attachment classification and maternal psychosocial problems as predictors of hostile-aggressive behavior in the preschool classroom. Child Development. 1993;64:572–585. doi: 10.1111/j.1467-8624.1993.tb02929.x. [DOI] [PubMed] [Google Scholar]
- 74.Lyons-Ruth K, Bronfman E, Parsons E. Atypical attachment in infancy and early childhood among children at developmental risk. IV. Maternal frightened, frightening, or atypical behavior and disorganized infant attachment patterns. Monographs of the Society for Research in Child Development. 1999;64(3):67–96. doi: 10.1111/1540-5834.00034. [DOI] [PubMed] [Google Scholar]
- 75.Shi Z, Bureau J-F, Easterbrooks MA, Zhao X, Lyons-Ruth K. Childhood maltreatment and prospectively observed quality of early care as predictors of antisocial personality disorder features. Infant Mental Health Journal. 2012;33:55–69. doi: 10.1002/imhj.20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pechtel P, Woodman A, Lyons-Ruth K. Early maternal withdrawal and non-verbal childhood IQ as precursors for substance abuse diagnosis in young adulthood: Results of a 20 year prospective study. International Journal of Cognitive Therapy. 2012;5:316–329. doi: 10.1521/ijct.2012.5.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Radloff LS, Locke BZ. The community mental health assessment survey and the CES-D scale. In: Weismann MM, Myers JK, Ross CE, editors. Surveys in Psychological Epidemiology. Vol. 4. 1986. pp. 177–189. Community surveys of psychoatric disorders. [Google Scholar]
- 78.Obsuth I, Hennighausen K, Brumariu LE, Lyons-Ruth K. Disorganized behavior in adolescent-parent interaction: relations to attachment state of mind, partner abuse, and psychopathology. Child Development. 2014;85(1):370–387. doi: 10.1111/cdev.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- 80.Segal DL, Hersen M, Van Hasselt VB. Reliability of the structured clinical interview for DSM-III-R: an evaluative review. Comprehensive Psychiatry. 1994;35(4):316–327. doi: 10.1016/0010-440x(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 81.Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Research. 1998;79(2):163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- 82.Bernstein EM, Putnam FW. DevelopmMent, reliability, and validity of a dissociation scale. The Journal of Nervous and Mental Disease. 1986;174(12):727–735. doi: 10.1097/00005053-198612000-00004. [DOI] [PubMed] [Google Scholar]
- 83.van IJzendoorn MH, Schuengel C. The measurement of dissociation in normal and clinical populations: Meta-analytic validation of the Dissociative Experiences Scale (DES) Clinical Psychology Review. 1996;16:365–382. [Google Scholar]
- 84.Fischl B, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 85.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 86.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 87.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 88.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fischl B, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 90.Morey RA, et al. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45(3):855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bergouignan L, et al. Can voxel based morphometry, manual segmentation and automated segmentation equally detect hippocampal volume differences in acute depression? Neuroimage. 2009;45(1):29–37. doi: 10.1016/j.neuroimage.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 92.R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2010. [Google Scholar]
- 93.Schafer J, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- 94.Gilks W, Richardson S, Spiegelhalter D. Markov Chain Monte Carlo in Practice. Chapman, Hall; London: 1996. [Google Scholar]
- 95.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40(3):691–879. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 96.Meng X-l, Rosenthal R, Rubin DB. Comparing Correlated Correlation Coefficients. Psychological Bulletin. 1992;111(1):172–175. [Google Scholar]
- 97.Carlson V, Cicchetti D, Barnett D, Braunwald K. Disorganized/disoriented attachment relationships in maltreated infants. Developmental Psychology. 1989;25(4):525–531. [Google Scholar]
- 98.Barnett D, Ganiban J, Cicchetti D. Maltreatment, negative expressivity, and the development of Type D attachments from 12 to 24 months of age. Monographs of the Society for Research in Child Development. 1999;64(3):97–118. doi: 10.1111/1540-5834.00035. [DOI] [PubMed] [Google Scholar]
- 99.Cicchetti D, Rogosch FA, Toth SL. Fostering secure attachment in infants in maltreating families through preventive interventions. Development and Psychopathology. 2006;18(3):623–649. doi: 10.1017/s0954579406060329. [DOI] [PubMed] [Google Scholar]
- 100.Cyr C, Euser EM, Bakermans-Kranenburg MJ, Van IJzendoorn MH. Attachment security and disorganization in maltreating and high-risk families: a series of meta-analyses. Development and Psychopathology. 2010;22(1):87–108. doi: 10.1017/S0954579409990289. [DOI] [PubMed] [Google Scholar]
- 101.Fearon P, Belsky J. Infant–mother attachment and the growth of externalizing problems across the primary-school years. Journal of Child Psychology and Psychiatry. 2011;52(7):782–791. doi: 10.1111/j.1469-7610.2010.02350.x. [DOI] [PubMed] [Google Scholar]
- 102.NICHD Early Child Care Research Network. Child-care and family predictors of preschool attachment and stability from infancy. Developmental Psychology. 2001;37:847–862. [PubMed] [Google Scholar]
- 103.Villablanca JR. Why do we have a caudate nucleus? Acta Neurobiologiae Experimentalis. 2010;70(1):95–105. doi: 10.55782/ane-2010-1778. [DOI] [PubMed] [Google Scholar]
- 104.Tottenham N, Shapiro M, Telzer EH, Humphreys KL. Amygdala response to mother. Developmental Science. 2012;15(3):307–319. doi: 10.1111/j.1467-7687.2011.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Platek SM, Kemp SM. Is family special to the brain? An event-related fMRI study of familiar, familial, and self-face recognition. Neuropsychologia. 2009;47(3):849–858. doi: 10.1016/j.neuropsychologia.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 106.Fetterman AK, Ode S, Robinson MD. For which side the bell tolls: The laterality of approach-avoidance associative networks. Motiv Emot. 2013;37(1):33–38. doi: 10.1007/s11031-012-9306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schneider S, et al. Boys do it the right way: sex-dependent amygdala lateralization during face processing in adolescents. Neuroimage. 2011;56(3):1847–1853. doi: 10.1016/j.neuroimage.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 108.Yoshimura S, et al. Self-referential processing of negative stimuli within the ventral anterior cingulate gyrus and right amygdala. Brain Cogn. 2009;69(1):218–225. doi: 10.1016/j.bandc.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 109.Dannlowski U, et al. Childhood maltreatment is associated with an antomatic negative emotion processing bias in the amygdala. Human Brain Mapping. 2013;34:2899–2909. doi: 10.1002/hbm.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Herringa RJ, et al. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci. 2013;110(47):19119–19124. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Belsky J, Rovine M, Taylor D. The Pennsylvania infant and family development project III: The origins of individual differences in infant-mother attachment: Maternal and infant contributions. Child Development. 1984;55:718–728. [PubMed] [Google Scholar]
- 112.Lyons-Ruth K, Connell D, Zoll D, Stahl J. Infants at social risk: Relationships among infant maltreatment, maternal behavior, and infant attachment behavior. Developmental Psychology. 1987;23:223–232. [Google Scholar]
- 113.Main M, Tomasini L, Tolan W. Differences among mothers of infants judged to differ in security of attachment. Developmental Psychology. 1979;15:472–473. [Google Scholar]
- 114.Main M, Hesse E. Parents’ unresolved traumatic experiences are related to infant disorganized attachment status: Is frightened and/or frightening parental behavior the linking mechanism? In: Greenberg MT, Cicchetti D, Cummings EM, editors. Attachment in the preschool years: Theory, research and intervention. University of Chicago Press; Chicago, IL: 1990. pp. 161–182. [Google Scholar]
- 115.Zhang TY, Chretien P, Meaney MJ, Gratton A. Influence of naturally occurring variations in maternal care on prepulse inhibition of acoustic startle and the medial prefrontal cortical dopamine response to stress in adult rats. Journal of Neuroscience. 2005;25:1493–1502. doi: 10.1523/JNEUROSCI.3293-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gabard-Durnam LJ, et al. The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. NeuroImage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Burghy CA, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience. 2012;15(12):1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ito Y, et al. Increased prevalence of electrophysiological abnormalities in children with psychological, physical, and sexual abuse. J Neuropsychiatry Clin Neurosci. 1993;5(4):401–408. doi: 10.1176/jnp.5.4.401. [DOI] [PubMed] [Google Scholar]
- 119.Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biological Psychiatry. 2009;65(3):227–234. doi: 10.1016/j.biopsych.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Anderson CM, Teicher MH, Polcari A, Renshaw PF. Abnormal T2 relaxation time in the cerebellar vermis of adults sexually abused in childhood: potential role of the vermis in stress-enhanced risk for drug abuse. Psychoneuroendocrinology. 2002;27:231–244. doi: 10.1016/s0306-4530(01)00047-6. [DOI] [PubMed] [Google Scholar]
- 121.Glascher J, Adolphs R. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. J Neurosci. 2003;23(32):10274–10282. doi: 10.1523/JNEUROSCI.23-32-10274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Giedd JN, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366(2):223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 123.Button KS, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]