Abstract
RATIONALE
While natural products isolated from medicinal plants can serve as a rich source of biologically active metabolites, mixtures of structurally related compounds of a polar nature are often difficult to chemically resolve by traditional separation techniques. Chemical derivatization to reduce metabolite polarity combined with liquid chromatography is the strategy presented here to resolve a mixture of structurally related natural product glycosides solvent extracted from the medicinal herb Teucrium polium for mass spectroscopy characterization.
METHODS
The partially-purified plant extract was chemical derivatization and electrospray ionization mass spectrometry (ESI-MS) fragmentation-pattern analysis allowed for structural characterization of iridoid and secoiridoid glycosides. Select ions were subjected to tandem MS analysis with a relatively higher-energy collision dissociation to assist in structural elucidation.
RESULTS
Permethylation replaced all protons from free hydroxyl and amino groups with methyls and resulted in increases in both hydrophobicity, for facilitated chromatographic separation, and proton affinity, for enhanced chemical ionization. Protonated and/or sodiated adducts were observed for the six compounds detected in positive ion mode ESI-MS with a mass accuracy of less than 2 ppm.
CONCLUSIONS
Permethylation combined with LC-MS analysis is shown here to be an effective chemical practice for separating and characterizing iridoid glucosinolates and is expected to be well suited for the chemical characterization of other polar natural-product mixtures of closely related compounds.
Keywords: Teucrium polium, iridoids, LC/MS, permethylation, fragmentation analysis
1. Introduction
Teucrium polium, a member of the Lamiaceae family, is a traditional medicinal herbal [1] with solvent extracts exhibiting antibacterial and antitumor activity [2; 3]. In addition to the expected chemical profile of flavonoids and phenylpropanoid glycosides, aerial portions of the plant contain a less common class of compounds called iridoid glycosides (IGs) [4–7]. The name originates from the ant genus Iridomyrmex, from which the original iridoid structure was identified [8]. Typically isolated as glycosides and associated with medicinal herbs, the primary role of iridoids in plants is for herbivore and/or pathogen protection [8]. IGs also exhibit a broad range of biological activity including antioxidant, anti-inflammatory and anti-cancer activities [9; 10]. Structurally, iridoids provide a biogenetic and chemotaxonomic link between terpenes and alkaloids; they can serve as intermediates in the biosynthesis of alkaloids [11]. Initially, iridoids are monoterpenoids biosynthesized from isoprene. Their basic structure is formed as a result of cis-fusion of cyclopentane and oxygen-containing hetrocyclic rings [12]; carbon skeletons include cyclopentane, cyclopentene, and epoxytane rings [13]. Iridoids can be sub-classified into IGs, bisiridoids, non glycosidic iridoids, and secoiridoids [14; 15]. Seco derivatives are generated by cyclopentane ring cleavage between C-7 and C-8 or the pyran ring between C-1 and O-2. Structural diversity, chemical instability, and high polarity are physicochemical features that complicate structural elucidation. Indeed, classic phytochemical techniques that require purified IG in sufficient quantities for NMR analysis are arduous and time-consuming and in fact, in some cases can thwart the final chemical characterization [16; 17; 18]. A variety of MS methods have been reported for investigating iridoids including chemical ionization (CI), fast atom bombardment (FAB) and ESI [19; 20; 21]; moreover, ultra-performance liquid chromatography (UPLC) coupled with ESI-MS has been effectively utilized for iridoid separation linked with chemical characterization [22]. A persistent weakness with this type of analysis is the poor resolution of these highly polar compounds combined with low ionization efficiencies that limits detection of low-abundance species. Chemical derivatization of partially purified iridoid extracts is investigated here as a possible solution for both poor chromatographic resolution and low ionization efficiencies.
Permethylation is a single-step chemical derivatization technique that replaces all protons from free hydroxyl and amino groups with methyls [23]. This structural change has been investigated with the goal to increase both hydrophobicity facilitating chromatographic separation and proton affinity enhancing chemical ionization. In addition to UPLC-ESI-MS/MSMS analysis of the permethylated compound mixture for structure analysis, MSn was achieved through higher-energy collisional dissociation. Additionally, ESI-MS in the positive ion mode was performed for underivatized extracts to observe intact ionized species, i.e. the [M+H]+ and/or [M+Na]+ ion.
2. Experiment Methods
2.1. Materials
Sodium hydroxide beads, dimethyl sulfoxide (DMSO), iodomethane, were purchased from Sigma-Aldrich (St. Louis, MO). Formic acid, high-performance liquid chromatography (HPLC)-grade acetonitrile, methanol, water; and ACS-grade dichloromethane and methanol were purchased from Fisher Scientific (Pittsburgh, PA).
2.2. Extraction and Isolation
Aerial parts of T. polium were collected from North Sinai, Egypt, in June 2010. A voucher specimen SK-105 has been deposited in the herbarium of St. Katherine Protectorate, Egypt. Air-dried aerial plant tissue (2 kg) was finely crushed then extracted using solvent system CH2Cl2–MeOH (1:1) (4 L) at room temperature. The solvent was removed, and then the residue (210 g) was subjected to column chromatography (CC) silica gel eluting with n-hexanes, CH2Cl2, and MeOH in increasing the order of polarity up to 100% MeOH to afford 398 fractions 1-L each. Based on TLC similarities, methanolic fractions (250–265) were pooled (9 g), then concentrated in vacuo and subjected to silica gel CC eluting with a gradient of CH2Cl2-MeOH starting with (9:1) up to (0.5:9.5) to afford 73 sub-fractions (fractions were monitored by TLC eluting with CH2Cl2-MeOH-H2O (3:5:0.5). Sub-fractions 30–46 (3.1 g) were subjected to Sephadex LH-20 gel eluted with MeOH; 19 sub-fractions were obtained. 6–11 Sub-fractions (600 mg) were pooled and subjected to Sephadex LH-20 gel eluted with MeOH to afford 22 fractions. Sub-fractions 5–18 (210 mg) were divided into two parts: first (94.6%) was purified by RP HPLC eluting with an isocratic MeOH- H2O (acidified with 0.1% formic acid) system (17:83) to afford compounds 1–6; second part (5.4%) was permethylated for LC-MS/MS.
2.3. Permethylation
For solid phase permethylation, partially purified plant extract (7 mg) was dried in a high vacuum concentrator and re-suspended in high purity DMSO (30 µl) with H2O (1.2 µl) and methyl iodide (20 µl). Mixed sample was applied to spin columns (Harvard Apparatus, Holliston, MA) packed with sodium hydroxide beads (3 cm height) and rinsed with DMSO; the mixture was allowed to react at room temperature for 25 minutes. The column was then centrifuged at 1.6 K rpm for two minutes and the solution collected. A second aliquot of methyl iodide (20 µl) was added to the solution and the reaction mixture reapplied to the column, incubated for 15 minutes and re-centrifuged for two minutes. The column was rinsed with ACN (50 µl) centrifuged two minutes at 1.6K rpm and combined column elutions dried in vacuo.
2.4. LC-MS/MSMS
LC-MS/MSMS analysis was performed on a Dionex Ultimate 3000 UHPLC system interfaced with a LTQ Orbitrap Velos (Thermo Scientific, Pittsburgh, PA, USA) mass spectrometer. Sample trapping was achieved by C-18 cartridge column (2 cm X 75 µm, 3 µm, 100 Å) and sample separation was achieved using a C-18 nano-column (15 cm X 75 µm, 2 µm, 100 Å, Thermo Scientific, Pittsburgh, PA, USA) at a flow rate of 0.35 µl/min. Mobile phase A was 2% acetonitrile in water with 0.1% formic acid and mobile phase B was 100% acetonitrile with 0.1% formic acid. The elution gradient was set as following. Initially, 2% mobile phase B was applied for 10 min. The gradient was then increased linearly from 2% B to 55% B from 10 min to 60 min. From 60 to 61 min, the gradient increased rapidly to 80% B and then was kept at 80% B for 5 minutes. The gradient then was dropped back to 2% B in 1 min and was maintained at 2% B for 10 min.
The positive ion mode ESI-MS was operated at m/z range of 100–1000 with a 15000 resolution of FT mass analyzer. The spray voltage was set to be 1.5 KV, and the capillary temperature was 300 °C. MSMS fragmentations were conducted in HCD cell with 50% normalized collision energy. Since normalized collision energy is not a linear scale that applies a percent of the available voltage regardless of the ion mass but instead, automatically compensates for mass dependency, normalized collision energy results in greater energy for heavier than lighter ions and in turn obfuscates the determination of absolute collision energy values. Five MS2 were performed after each MS scan in a data dependent acquisition (DDA) mode. Data were processed using Xcalibur Qual Brower software (Thermo Scientific, Pittsburgh, PA, USA).
3. Result and Discussion
Six iridoid glycosides with a cyclopentenoid skeleton linked to a dihydropyran or ring-cleaved dihydropyran ring (secoiridoid) were analyzed by MS/MS and diagnostic fragments identified. ESI mass spectra in positive-ion mode exhibited protonated and/or sodiated ion peaks for permethylated IGs. While it has been previously established that LC can remove sodium from the permethylated product, the solid phase permethylation samples reported here contained sodium ions either originating from the glasswares, stainless steel, impurities in chemicals or solvents and/or picked up during purification from spin columns packed with sodium hydroxide beads. In any case, with the HCD MS/MS analysis, sodium adducts provided greater fragmentation information than the protonated adducts. The cyclopentenoids contain an olefinic C-7–C-8 methyl unit and as such are defined as 7,8-cyclopentene-type IGs (Fig. 1). As structural analysis by IR, UV and NMR have been previously reported for these IGs [5–7], this study focuses on MS characterization utilizing chemical derivatization and LC-ESI-MS/MS analysis.
Fig. 1.
Chemical structures for identified compounds.
3.1. Compound 1
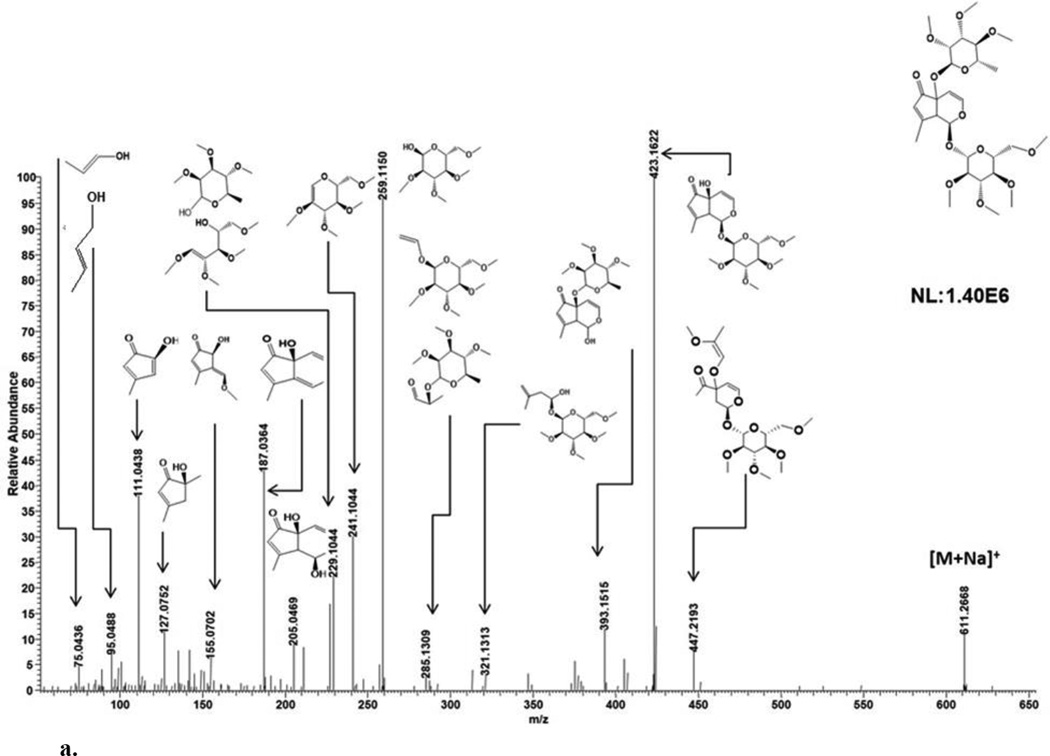
ESI-MS analysis in the positive ion mode generated cationized and protonated ions observed at m/z 611 [M+Na]+ (theoretical, 611.2674) and 589 [M+H]+ (theoretical, 589.2855), respectively (Fig. 2a; Table 1). A lack of CO2 loss for the sodiated ion by MS/MS (Fig. 3a) suggested an absence of a carboxyl group [13]. The product ion peaks at m/z 423 ([M+Na-188]+), 393 ([M+Na-218]+), 205 ([M+Na-188-218]+) and 187 ([M+Na-188-218-H2O]+), are derived from m/z 611 after losses of a permethylated rhamnose alone, a permethylated glucose alone, a permethylated rhamnose and glucose combined and a permethylated rhamnose and glucose with a water unit combined, respectively (Table 2). The sodiated molecular ion at m/z 611 cleaved the cyclopenetoid iridoid and part of the permethylated rhamnose to generate m/z 447. The m/z 423 ion additionally fragmented to yield m/z 321 (an iridoid portion with glucose). The m/z 393 ion additionally fragmented to yield m/z 285 (an iridoid portion with rhamnose). The m/z 205 ion after the neutral loss of water (m/z 187) generated 2 five membered rings at m/z 127 and 111 with dihydropyran cleavage (Fig 3a). The m/z 111 ion further fragmented within the cyclopentenoid skeleton to generate m/z 95 and 75. These observed MS/MS fragments provide chemical data on sugar and iridoid components. Based on fragmentation patterns of the sodiated molecular ion peak at m/z 611, glucose and rhamnose attachment at C-1 and C-5 respectively could also be deduced. Specifically, ion peaks at m/z 187, 155, 127 and 111 are consistent with loss of a rhamnose to generate a free OH at position C-5 (Fig. 3a). Similarly, ion peaks at m/z 393 and 205 are consistent with loss of glucose to generate a free OH at position C-1 (Fig. 3a). Diagnostic MS/MS fragments allowed for the identification of IG components as well as plausible sugar-iridoid linkages. In combination with previous NMR analyses of 7,8-cyclopentene-type IGs [5–7] for stereochemical determination, 1 was assigned as teucardoside; (1S)-5α-[(6-deoxy-α-L-mannopyranosyl)oxy]-1-(β-D-glucopyranosyloxy)-5,9α-dihydro-8-methylcyclopenta[c]pyran-5(1H)-one.
Fig. 2.
EIC and MS pattern for isolated compounds.
Table 1.
MS data for compounds 1–6.
| ID | Theoretic m/z [M+Na]+ |
Observed m/z [M+Na]+ |
Mass Accuracy (ppm) |
|---|---|---|---|
| 1 | 611.2674 | 611.2672 | −0.33 |
| 2 | 437.1782 | 437.1782 | 0.00 |
| 3 | 469.2044 | 469.2046 | 0.43 |
| 4 | 627.2987 | 627.2980 | −1.12 |
| 5 | 425.2146 | 425.2148 | 0.47 |
| 6 | 425.2146 | 425.2147 | 0.24 |
Fig. 3.
a. MSMS fragmentation patterns of compound 1.
b. MSMS fragmentation patterns of compound 2.
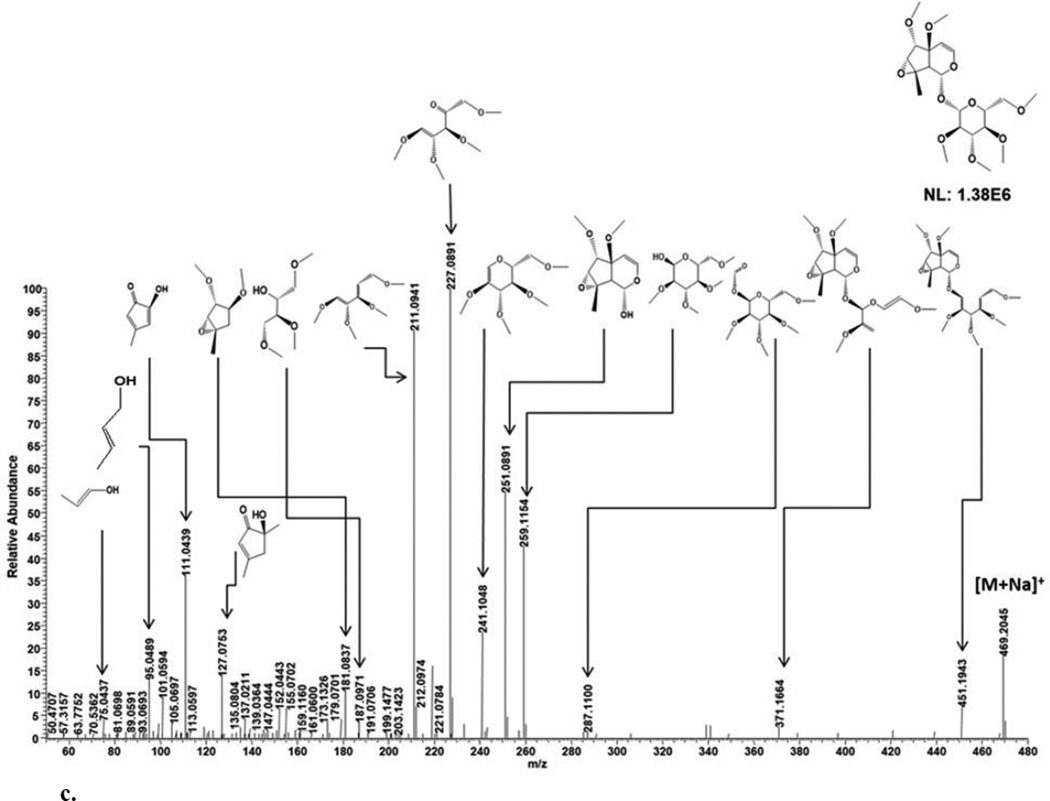
c. MSMS fragmentation patterns of compound 3.
Table 2.
Accurate mass measurements and elemental compositions of protonated or sodiated products by HCD-MS/MS analysis in positive-ion mode.
| Compound | m/z | Theoretic (m/z) |
Observed (m/z) |
Mass Accuracy (ppm) |
|---|---|---|---|---|
| 1 | 611.2667 | 447.2225 | 447.2193 | 7.2 |
| ([M+Na]+) | 423.1625 | 423.1622 | 0.7 | |
| 393.152 | 393.1515 | 1.3 | ||
| 285.1308 | 285.1309 | −0.4 | ||
| 259.1152 | 259.115 | 0.8 | ||
| 241.1046 | 241.1044 | 0.8 | ||
| 229.1046 | 229.1044 | 0.9 | ||
| 205.0471 | 205.0469 | 1.0 | ||
| 187.0365 | 187.0364 | 0.5 | ||
| 155.0703 | 155.0702 | 0.6 | ||
| 127.0754 | 127.0752 | 1.6 | ||
| 111.0441 | 111.0438 | 2.7 | ||
| 75.0441 | 75.0436 | 6.7 | ||
| 2 | 437.1781 | 259.1152 | 259.115 | 0.8 |
| ([M+Na]+) | 241.1046 | 241.1044 | 0.8 | |
| 195.0652 | 195.0652 | 0.0 | ||
| 187.0365 | 187.0364 | 0.5 | ||
| 155.0703 | 155.0702 | 0.6 | ||
| 127.0754 | 127.0752 | 1.6 | ||
| 111.0441 | 111.0438 | 2.7 | ||
| 75.0441 | 75.0436 | 6.7 | ||
| 3 | 469.2914 | 428.1938 | 428.1943 | −1.2 |
| ([M+Na]+) | 287.1101 | 287.1100 | 0.3 | |
| 259.1152 | 259.1154 | −0.8 | ||
| 251.089 | 251.0891 | −0.4 | ||
| 241.1046 | 241.1048 | −0.8 | ||
| 227.089 | 227.0891 | −0.4 | ||
| 211.0941 | 211.0941 | 0.0 | ||
| 181.0835 | 181.0837 | −1.1 | ||
| 155.0703 | 155.0702 | 0.6 | ||
| 127.0754 | 127.0753 | 0.8 | ||
| 111.0441 | 111.0439 | 1.8 | ||
| 75.0441 | 75.0437 | 5.3 | ||
| 4 | 627.2975 | 445.2068 | 445.2047 | −4.7 |
| ([M+Na]+) | 415.1963 | 415.1940 | 5.5 | |
| 385.1855 | 385.1836 | 4.9 | ||
| 329.1571 | 329.1572 | −0.3 | ||
| 259.1152 | 259.1153 | −0.4 | ||
| 227.089 | 227.0891 | −0.4 | ||
| 155.0703 | 155.0704 | −0.6 | ||
| 127.0754 | 127.0753 | 0.8 | ||
| 111.0441 | 111.0439 | 1.8 | ||
| 75.0441 | 75.0438 | 4.0 | ||
| 5 | 425.2148 | 259.1152 | 259.1161 | −3.5 |
| ([M+Na]+) | 237.1079 | 237.1102 | −9.7 | |
| 229.1046 | 229.1047 | −0.4 | ||
| 155.0703 | 155.0704 | −0.6 | ||
| 127.0754 | 127.0752 | 1.6 | ||
| 111.0441 | 111.0439 | 1.8 | ||
| 75.0441 | 75.0438 | 4.0 | ||
| 6 | 425.2147 | 259.1152 | 259.1158 | −2.3 |
| ([M+Na]+) | 111.0441 | 111.0439 | 1.8 | |
| 75.0441 | 75.0438 | 4.0 |
3.2. Compound 2
ESI-MS analysis in the positive ion mode generated cationized protonated ions observed at m/z 437.1780 [M+Na]+ (theoretical, 437.1782) and 415.1960 [M+H]+ (theoretical, 415.1963), respectively (Fig. 2b Table 1). The sodiated ion was further selected for MS/MS experiments schematized in Fig. 3b. The m/z 437 generated sodiated and protonated ion peaks at m/z 217 and m/z 195, respectively after the neutral loss of permethylated glucose followed by carbonyl formation at C-1. The sodiated permethylated glucose moiety is observed at m/z 259 which additionally generated the ion peak at m/z 241 by the loss of H2O and double bond formation. The ion peak at m/z 241 was further fragmented to form m/z 187 and 135 (partial fragments of the permethylated glucose) as shown in Fig. 3b and Table 2. The fragment ions at m/z 195 generated peak at m/z 101 after both cyclopentenoid and dihydropyran cleavage and 3 five membered rings at m/z 155, 127 and 111 after 3 successive cleavages in dihydropyran ring (Fig 3b). The m/z 111 ion further fragmented within the cyclopentenoid skeleton to generate m/z 95 and 75. These observed MS/MS fragments provided chemical data on sugar and iridoid components. Based on fragmentation patterns of the sodiated molecular ion peak at m/z 437, glucose attachment at C-1 could be deduced. Specifically, ion peaks at m/z 195 and 155 are consistent with loss of a permethylated glucose to generate a carbonyl group (after the loss of water due to free OH at position C-1) (Fig. 3b). These diagnostic MS/MS fragments allowed for the identification of IG components as well as plausible sugar-iridoid linkages. Moreover, these data revealed that 2 is similar to 1 except for an absence of the monosaccharide attached to position C-5 of the basic skeleton; the presence of a methoxy group in 2 is supported by ion peaks at m/z 195, 155 and 127. In combination with previous NMR analyses of 7,8-cyclopentene-type IGs [5–7] for stereochemical determination, the structure of 2 was assigned as teuhircoside; (1S)-5α-methoxy-1-(β-D-glucopyranosyloxy)-5,9α-dihydro-8-methylcyclopenta[c]pyran-5(1H)-one.
3.3. Compound 3
ESI-MS analysis in the positive ion mode generated cationized and protonated ions observed at m/z 469.2044 [M+Na]+ (theoretical, 469.204) and 447.222 [M+H]+ (theoretical, 447.2224), respectively (Fig. 2c, Table 1). A lack of CO2 loss for the sodiated ion by MS/MS suggested an absence of a carboxyl group [13]. The cationized ion peak at m/z 469 generated m/z 451 after the loss of H2O, which is assigned to the permethylated iridoid backbone linked to a partially fragmented glucose (Table 2). While the ion at m/z 251 ([M+Na-218]+) was derived from m/z 469 after the neutral loss of permethylated glucose which was observed as the sodiated ion at m/z 259. The permethylated glucose moiety at m/z 259 additionally generated an ion at m/z 241 by the loss of H2O and double bond formation. The ion at m/z 241 was further fragmented to form ions observed at m/z 227, 211 and 187 (partial fragments of permethylated glucose) as shown in Fig. 3c and Table 2. The fragment ions at m/z 251 generated 2 five membered rings at m/z 181, 127 and 111 after dihydropyran ring cleavage followed by cyclopentenoid ring-epoxide cleavage and demethylation (Fig 3c). The m/z 111 ion further fragmented within the cyclopentenoid skeleton to generate m/z 95 and 75. These observed MS/MS fragments provide chemical data on sugar and iridoid components. Based on fragmentation patterns of the sodiated molecular ion peak at m/z 469, glucose attachment at C-1 was deduced. Specifically, ion peaks at m/z 251 is consistent with loss of a permethylated glucose to generate a free OH at position C-1 (Fig. 3c). These data also reveal that 2 and 3 are similar except for the replacement of a C7—C8 double bond in 2 with a 7,8-epoxide in 3. In addition, the C-5 carbonyl in 2 was replaced by a free hydroxyl group in 3 (Fig. 3C). In combination with previous NMR analyses of 7,8-cyclopentene-type IGs [5–7] for stereochemical determination, 3 was assigned as 1α-(β-D-glucopyranoxy)-7α,8α-epoxy-5β,6α-dihydroxy-8-methyl-1,5,6,7,8,9β-hexahydrocyclopenta[c]pyran.
2.4. Compound 4
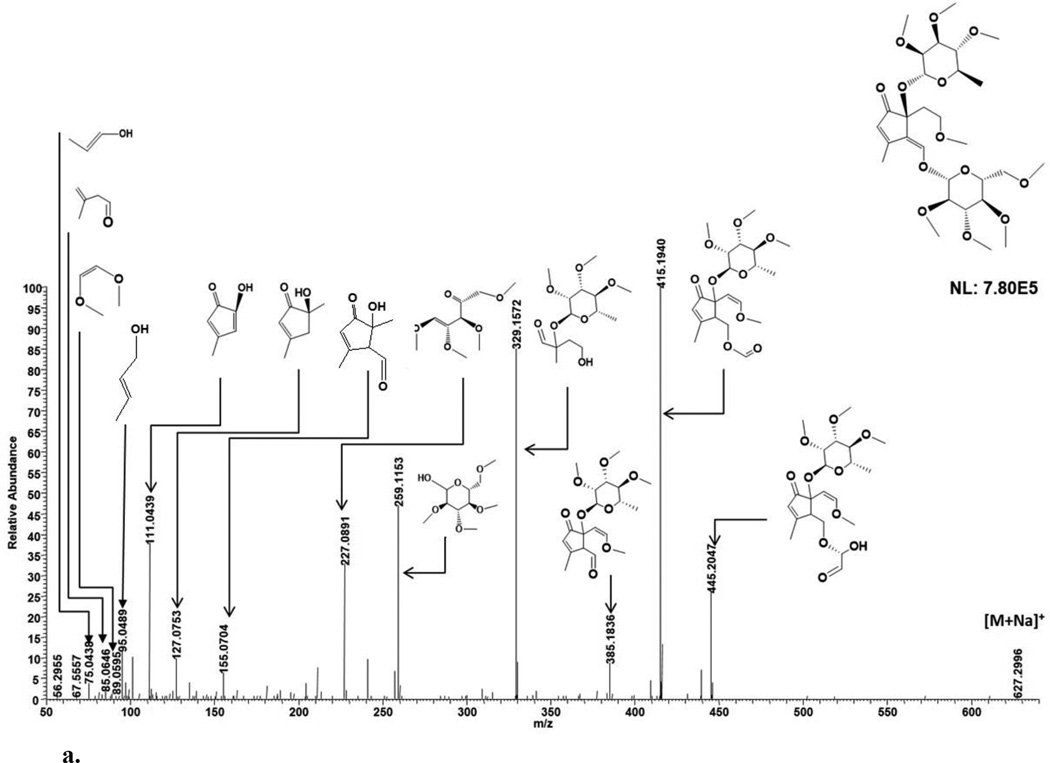
ESI-MS analysis in the positive ion mode generated cationized and protonated ions observed at m/z 627.2996 [M+Na]+ (theoretical, 627.2987) and 605.3168 [M+H]+ (theoretical, 605.3168), respectively (Fig. 2d; Table 2). A lack of CO2 loss for the sodiated ion by MS/MS suggested an absence of a carboxyl group [13]. The sodiated molecular ion at m/z 627 generated the ion peak at m/z 445 and 415 (a glucose portion linked to iridoid backbone with rhamnose) after partial cleavage of permethylated glucose. The m/z 627 also generated the ion peak m/z 385 after the neutral loss of permethylated glucose (m/z 259) which additionally generated the ion peak at m/z 227 (Fig. 4a; Table 1). The m/z 385 ion additionally fragmented to yield m/z 329 (an iridoid backbone portion with rhamnose). The m/z 385 ion generated 3 five membered rings at m/z 155, 127 and 111 with dihydropyran cleavage (Fig 4a). The m/z 111 ion further fragmented within the cyclopentenoid skeleton to generate m/z 95 and 75. These observed MS/MS fragments provide chemical data on sugar and iridoid components. Consistent with these fragmentation patterns of the sodiated molecular ion peak at m/z 627, glucose and rhamnose attachment at C-1 and C-5, respectively, could be deduced. Specifically, ion peaks at m/z 385 is consistent with loss of a permethylated glucose to generate a carbonyl group (after the loss of water due to the free OH at position C-1) (Fig. 4a). Similarly, ion peaks at m/z 155 and 127 are consistent with loss of rhamnose to generate a free OH at position C-5 (Fig. 4a). These diagnostic MS/MS fragments allowed for the identification of IG components as well as plausible sugar-iridoid linkages. Based on the fragmentation pattern and previously reported NMR data, 4 is similar to 1 except for a dihydropyran unit opening at an ether linkage and unsaturation of C9—C1 bond. In combination with previous NMR analyses of 7,8-cyclopentene-type IGs [5–7] for stereochemical determination, 4 was assigned as 1α-(β-D-glucopyranoxy)-7α,8α-epoxy-5β,6α-dihydroxy-8-methyl-1,5,6,7,8,9β-hexahydrocyclopenta[c]pyran.
Fig. 4.
a. MSMS fragmentation patterns of compound 4.
b. MSMS fragmentation patterns of compound 5.
c. MSMS fragmentation patterns of compound 6.
2.5. Compound 5
ESI-MS analysis in the positive ion mode generated cationized and protonated ions observed at m/z 425.2148 [M+Na]+ (theoretical, 425.2146) and 403.2327 [M+H]+ (theoretical, 403.2327), respectively (Fig. 2e; Table 1). A lack of CO2 loss for the sodiated ion by MS/MS (Fig. 4b) suggested an absence of a carboxyl group [13]. The sodiated molecular ion at m/z 425, after partial cleavage of permethylated glucose, generated the ion peaks at m/z 237 and 219, assigned to a permethylated C-3 iridoid backbone linked to glucose fragment CH2OH and a demethylated C-3 iridoid backbone linked to glucose fragment CH2=CH, respectively. The m/z 237 ion generated m/z 175 after partial cleavage of both the dihydropyran and permethylated glucose. The glucose was observed at m/z 259 which additionally generated ion fragments at m/z 229 and 135 (Fig. 4b; Table 2). The m/z 237 ion also fragmented to yield m/z 127 (dihydropyran ring-cleaved iridoid backbone) and m/z 111 (both cyclopentenoid and dihydropyran ring cleavage) (Fig 4b). The m/z 127 ion further fragmented within the cyclopentenoid skeleton to generate m/z 95. These observed MS/MS fragments provide chemical data on sugar and iridoid components. Based on fragmentation patterns of the sodiated molecular ion peak at m/z 425, glucose attached at C-1 could be deduced. Specifically, ion peak at m/z 127 is consistent with loss of a permethylated glucose to generate the free OH at position C-1 (Fig. 4b). Consistent with these assignments 5 is similar to 4 except for the absence of a 5-O-rhamnopyranosyl moiety and saturation at C9—C1. In combination with previously reported NMR data of 7,8-cyclopentene-type IGs [5–7] for stereochemical determination, 5 was assigned as 4-[(β-D-glucopyranosyloxy)methylene]-5α-(2-hydroxyethyl)-5-(α-L-rhamnopyranosyloxy)-3-methylcyclopent-2-en-1-one.
3.6. Compound 6
ESI-MS analysis in the positive ion mode generated a cationized ion observed at m/z 425.2147 [M+Na]+ (theoretical, 425.2146) (Fig. 2e; Table 2). Similar to 5, the m/z 425 generated ion peaks at m/z 259, 219, 191, 135 for 6. Also similar to 5, ions at m/z 111, 95 and 75 generated from m/z 219 were observed. Based on fragmentation patterns of the sodiated molecular ion peak at m/z 425 and similarity to 5, glucose attached at C-3 or C1 could be proposed. The ion peak at m/z 219 support the loss of permethylated glucose portion and generation of free OH at position C-1 or C-3 after demethylation (Fig. 4c). Comparing the fragmentation behavior and the abundance of daughter ion peaks of 6 with 5 MSMS spectra (Fig. 4c), the structural similarity of 5 and 6 was evident. Consistent with these assignments and previous NMR analyses of 7,8-cyclopentene-type IGs [5–7] for stereochemical determination, glucose linkage assignment is specified to C-3. This result was supported by molecular mechanics modeling study as shown in Fig. S1a and b (supplementary data). Based on these assignments, the structure of 6 was assigned as 5α-[2-(β-D-glucopyranosyloxy)ethyl]-4α-hydroxymethyl-3-methylcyclopent-2-en-1-one.
4. Conclusions
A mixture of iridoid glycosides was isolated from a methanolic fraction of T. polium using chromatographic techniques. MS fragmentation patterns were characterized for permethylated derivatives based on positive ion mode ESI-MS.
Supplementary Material
Acknowledgments
Research was supported in part by the Robert Welch Foundation (D-1478, PWP) and a by an NIH grant (1R01GM112490-02, YM).
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Supplementary data
Supplementary data associated with this article can be found, in the online version.
References
- 1.Aburjai T, Hudaib M, Cavrini V. Composition of the essential oil from Jordanian germander (Teucrium polium L.) J. Essent. Oil Res. 2006;18:97–99. [Google Scholar]
- 2.Djabou N, Lorenzi V, Guinoiseau E, Andreani S, Giuliani M-C, Desjobert J-M, Bolla J-M, Costa J, Berti L, Luciani A, Muselli A. Phytochemical composition of Corsican Teucrium essential oils and antibacterial activity against foodborne or toxi-infectious pathogens. Food Control. 2013;30:354–363. [Google Scholar]
- 3.Elmasri WA, Hegazy M-EF, Mechref Y, Paré PW. Cytotoxic saponin poliusaposide from Teucrium polium. RSC Advances. 2015;5:27126–27133. [Google Scholar]
- 4.De Marino S, Festa C, Zollo F, Incollingo F, Raimo G, Evangelista G, Iorizzi M. Antioxidant activity of phenolic and phenylethanoid glycosides from Teucrium polium L. Food Chem. 2012;133:21–28. [Google Scholar]
- 5.Elmasri WA, Hegazy ME, Aziz M, Koksal E, Amor W, Mechref Y, Hamood AN, Cordes DB, Paré PW. Biofilm blocking sesquiterpenes from Teucrium polium. Phytochemistry. 2014;103:107–113. doi: 10.1016/j.phytochem.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 6.Elmasri WA, Yang T, Tran P, Hegazy ME, Hamood AN, Mechref Y, Paré PW. Teucrium polium phenylethanol and iridoid glycoside characterization and flavonoid inhibition of biofilm-forming Staphylococcus aureus. J. Nat. Prod. 2015;78:2–9. doi: 10.1021/np5004092. [DOI] [PubMed] [Google Scholar]
- 7.Elmasri WA, Hegazy M-EF, Mechref Y, Paré PW. Structure-antioxidant and anti-tumor activity of Teucrium polium phytochemicals. Phytochemistry Lett. 2016;15:81–87. [Google Scholar]
- 8.Avasthi P, Gupta N, Sapra S, Dhar KL. Iridoids - a review. Int. J. Pharm. Sci. Lett. 2013;3:183–189. [Google Scholar]
- 9.Ghisalberti EL. Biological and pharmacological activity of naturally occurring iridoids and secoiridoids. Phytomedicine. 1998;5:147–163. doi: 10.1016/S0944-7113(98)80012-3. [DOI] [PubMed] [Google Scholar]
- 10.Singh GB, Bani S, Singh S, Khajuria A, Sharma ML, Gupta BD, Banerjee SK. Antiinflammatory activity of the iridoids kutkin, picroside-1 and kutkoside from Picrorhiza kurrooa. Phytother. Res. 1993;7:402–407. [Google Scholar]
- 11.Es-Safi N-E, Kerhoas L, Ducrot P-H. Fragmentation study of iridoid glucosides through positive and negative electrospray ionization, collision-induced dissociation and tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007;21:1165–1175. doi: 10.1002/rcm.2930. [DOI] [PubMed] [Google Scholar]
- 12.Nangia A, Prasuna G, Rao PB. Synthesis of cyclopenta[c]pyran skeleton of iridoid lactones. Tetrahedron. 1997;53:14507–14545. [Google Scholar]
- 13.Li C-m, Zhang X-l, Xue X-y, Zhang F-f, Xu Q, Liang X-m. Structural characterization of iridoid glucosides by ultra-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2008;22:1941–1954. doi: 10.1002/rcm.3579. [DOI] [PubMed] [Google Scholar]
- 14.Bianco A. The chemistry of iridoids. Stud. Nat. Prod. Chem. 1990;7:439–497. [Google Scholar]
- 15.Bianco A. Recent developments in iridoids chemistry. Pure Appl. Chem. 1994;66:2335–2338. [Google Scholar]
- 16.Wang H, Wu J-J, Liu G, Ye W-C, Zhao S-X. Chemical studies on iridoids from Picrorhiza scrophulariiflora. Zhongguo Tianran Yaowu. 2006;4:36–39. [Google Scholar]
- 17.Cao X, Qiao J, Wang L, Ye X, Zheng L, Jiang N, Mo W. Screening of glycoside isomers in P. scrophulariiflora using ionic liquid-based ultrasonic-assisted extraction and ultra-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2012;26:740–748. doi: 10.1002/rcm.6158. [DOI] [PubMed] [Google Scholar]
- 18.Huang SX, Zhou Y, Nie QJ, Ding LS, Peng SL. Two new iridoid glucosides from Picrorhiza scrophulariiflora. J. Asian Nat. Prod. Res. 2006;8:259–263. doi: 10.1080/10286020500034543. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen AT, Fontaine J, Malonne H, Claeys M, Luhmer M, Duez P. A sugar ester and an iridoid glycoside from Scrophularia ningpoensis. Phytochemistry. 2005;66:1186–1191. doi: 10.1016/j.phytochem.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 20.Suomi J, Wiedmer SK, Jussila M, Riekkola ML. Analysis of eleven iridoid glycosides by micellar electrokinetic capillary chromatography (MECC) and screening of plant samples by partial filling (MECC)-electrospray ionisation mass spectrometry. J. Chromatogr. 2002;A 970:287–296. doi: 10.1016/s0021-9673(02)00381-3. [DOI] [PubMed] [Google Scholar]
- 21.Madhusudanan KP, Chattopadhyay SK, Srivastava S. Elimination of 118 Da: a characteristic fragmentation in the tandem mass spectra of 11(15 -->1)-abeo-taxanes. J. Mass Spectrom. 2002;37:91–98. doi: 10.1002/jms.265. [DOI] [PubMed] [Google Scholar]
- 22.Cao X, Qiao J, Wang L, Ye X, Zheng L, Jiang N, Mo W. Screening of glycoside isomers in P. scrophulariiflora using ionic liquid-based ultrasonic-assisted extraction and ultra-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2012;26:740–748. doi: 10.1002/rcm.6158. [DOI] [PubMed] [Google Scholar]
- 23.Harvey DJ. Derivatization of carbohydrates for analysis by chromatography; electrophoresis and mass spectrometry. J. Chromatogr. 2011;B 879:1196–1225. doi: 10.1016/j.jchromb.2010.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.