Abstract
Fast hexavalent chromium (Cr(VI)) determination is important for environmental risk and health-related considerations. We used a microbial fuel cell-based biosensor inoculated with a facultatively anaerobic, Cr(VI)-reducing, and exoelectrogenic Ochrobactrum anthropi YC152 to determine the Cr(VI) concentration in water. The results indicated that O. anthropi YC152 exhibited high adaptability to pH, temperature, salinity, and water quality under anaerobic conditions. The stable performance of the microbial fuel cell (MFC)-based biosensor indicated its potential as a reliable biosensor system. The MFC voltage decreased as the Cr(VI) concentration in the MFC increased. Two satisfactory linear relationships were observed between the Cr(VI) concentration and voltage output for various Cr(VI) concentration ranges (0.0125–0.3 mg/L and 0.3–5 mg/L). The MFC biosensor is a simple device that can accurately measure Cr(VI) concentrations in drinking water, groundwater, and electroplating wastewater in 45 min with low deviations (<10%). The use of the biosensor can help in preventing the violation of effluent regulations and the maximum allowable concentration of Cr(VI) in water. Thus, the developed MFC biosensor has potential as an early warning detection device for Cr(VI) determination even if O. anthropi YC152 is a possible opportunistic pathogen.
Keywords: chromiun, biosensor, microbial fuel cell, wastewater
1. Introduction
Various industrial activities, such as steel production, leather tanning, agro-food production, wood preservation, and chemical manufacturing, generate wastewater containing Cr(VI) [1]. Chromium generally exists in water in the following two oxidation forms: hexavalent [Cr(VI)] and trivalent [Cr(III)]. The Cr(VI) concentration above a specific dose is toxic, mutagenic, and carcinogenic [2,3]. Compared with Cr(VI), Cr(III) is less toxic and can be more readily precipitated out of the solution in the form of Cr(OH)3; it is also impermeable to biological membranes [4]. By contrast, Cr(VI) is highly soluble and, thus, mobile and biologically available in ecosystems. Thus, authorities worldwide have stringent regulations for chromium species concentrations, especially Cr(VI). The U.S, Environmental Protection Agency identified Cr(VI) as one of the 17 chemicals posing the greatest threat to humans [5]. In Taiwan, the standard concentration of industrial and domestic effluents for Cr(VI) is 0.5 mg/L, and the maximum allowable concentration of Cr(VI) in drinking and surface water is more stringent, at 0.05 mg/L.
Environmental cleanup technologies for Cr(VI) removal from wastewater involve chemical precipitation, chemical oxidation, ion exchange, electrochemical treatment, reverse osmosis, membrane technology, and biological detoxification [4]. These processes may be ineffective, especially when the metal concentration in the solution is below 100 mg/L [6]. Moreover, the accidental release of industrial effluents without proper treatment would pose a serious threat to the environment. Thus, the determination of chromium species concentrations, especially of Cr (VI), in water is crucial for environmental risk and health-related considerations.
The currently available and widely-used analytical methods and techniques for the determination of chromium ions include atomic absorption spectroscopy (AAS), inductively coupled plasma mass spectroscopy, ion chromatography, and their combination with chromatographic techniques [7]. Although these techniques exhibit high sensitivity, accuracy, and selectivity, they are usually tedious, time consuming, expensive, and complicated. Moreover, they often require sophisticated instrumentation inadequate for use outside the laboratory; thus, they have low applicability in routine determinations [8,9]. Compared with these traditional analytical methods and techniques, a simple, inexpensive, and portable biosensor is a more feasible option. If a biosensor provides rapid measurements with acceptable accuracy, it can become a potential early warning detection device that can be used to protect ecosystems [10].
A biosensor is generally defined as a self-contained integrated device capable of providing quantitative or semi-quantitative information by using a biological recognition element and that is also specific for the compound that you want to detect [11]. Several biosensor types have been developed, including DNA-based, whole cell-based, and enzyme-based biosensors, for determining the Cr(VI) concentration [12].
Michel et al. (2006) constructed a Cyt c3-based biosensor to measure the Cr(VI) concentration in groundwater [13]. However, the response of this biosensor is affected by pH, temperature, ionic strength, oxygen content, and sulfate concentration, and the detection limit for Cr(VI) is 0.2 mg/L. In addition, Nepomuscene et al. (2007) developed a urease-based biosensor for determining the Cr(VI) concentration in wastewater [14]. Although the operational stability and reproducibility of this biosensor are satisfactory, short urease storage periods and high detection limits for Cr(VI) restrict its commercial application. Gurung et al. (2012) constructed a cell-based biosensor by using sludge containing sulfur-oxidizing bacteria (SOB) to detect the Cr(VI) concentration on the basis of the metabolic properties of SOB and obtained preliminary results [15]. The tested concentration of this system for Cr(VI) is 1 mg/L, which is not suitable to meet the requirement of effluent regulations (<0.05 mg/L). Furthermore, Bohrn et al. (2013) constructed a cell-based biosensor by using V79 hamster lung fibroblast cells as the biological recognition element. The detection limit of this biosensor is 0.026 mg/L for Cr(VI) within 6 h of exposure [16]. Although the V79 cell-based biosensor is a powerful tool for detecting Cr(VI) concentrations in the range of multinational drinking water regulations, the high cost of this biosensor limits its applicability. Panda and Sarkar (2014) developed a stable biosensor by using crude cell-free extracts of Enterobacter aerogenes immobilized with calcium alginate beads for the direct estimation of Cr(VI) in wastewater [17]. This biosensor has an excellent limit of detection of 0.0066 mg/L for Cr(VI); however, it is extremely sensitive to higher Cr(VI) concentrations (e.g., >0.04 mg/L). Moreover, the concentration range for detecting Cr(VI) concentrations in water is, reportedly, very narrow.
Calvo-Pérez et al. (2014) constructed a novel enzyme-based biosensor for measuring the Cr(VI) concentration by using glucose oxidase as the biological element and a screen-printed carbon electrode as the transduction element [9]. This biosensor exhibited a linear range for Cr(VI) concentration of 0.006–0.048 mg/L, which is suitable for measuring trace Cr(VI) in tap and drinking water. Recently, a study reported that fluorescent cell-based biosensors including pCHRGFP1 Escherichia coli and pCHRGFP2 Ochrobactrum tritici are suitable for detecting the Cr(VI) concentration in environmental waters [18]. Coelho et al. (2015) reported that the pCHRGFP1 E. coli and pCHRGFP2 O. tritici biosensors functioned within the range of 0.031–0.124 mg/L and 0.124–0.620 mg/L, respectively, for detecting Cr(VI) concentration [19]. However, the fluorescence activity of the cryopreserved cells of the pCHRGFP2 O. tritici reporter might be up to 47% lower than the fluorescence activity of these cells in fresh reporters.
A microbial fuel cell (MFC) is a device using microorganisms as catalysts to generate electricity from chemical compounds. It has become commonly cited as a potential alternative for energy production because of its lower pollution levels, low cost, and wide applicability [20]. A typical MFC design consists of two compartments: one is anaerobic (i.e., the anode) and the other is aerobic (i.e., the cathode). Bacteria oxidize the substrate, generating electrons and protons in the anaerobic compartment. The electrons transfer to the anode either by the mediator, an exogenous electron carrier, or directly from the bacterial enzymes to the electrode. The protons transfer to the cathode compartment [21]. In addition, MFCs can produce a signal for practical applications such as powering electronic sensors to analyze pollutants and monitoring state variables for system control [22,23]. At present, MFC biosensors are applied to detect biochemical oxygen demand (BOD), toxicity, (volatile fatty acid) VFA, and Nickel (Ni) in wastewater and are validated to minimize the time and the cost [23,24,25,26]. Theoretically, most microbes can potentially be used as a biocatalyst in MFCs. However, anaerobic bacteria are often used in the anode compartment of MFCs because this compartment is designed under anaerobic conditions. Chromate reduction by bacteria occurs under aerobic or anaerobic conditions, and anaerobic reductions proceed through the use of Cr(VI) as a terminal electron acceptor [5]. Chromate-reducing bacteria include Arthrobacter aurescens, Arthrobacter sp., Bacillus cereus S-6, B. subtilis, Micrococcus sp. SDCr-4, O. anthropi CTS-325, Oscillatoria sp. BJ2, Providencia sp., Streptomyces griseus, and Synechocystis sp. and can remove Cr(VI); however, the strains nearly belong to aerobic strains [27], which are not used in MFCs. Other major players, like Shewanella oneidensis MR-1; a facultative anaerobe, Cr6+ reducer and exoelectrogen, has been used as a biocathode in MFCs to reduce Cr(VI) [28]. The possible mechanism for inoculating chromate-reducing bacteria in the anode compartment of MFC is as follows:
| Anode: Organics → CO2 + H+ + e− (by chromate-reducing bacteria) |
| Cr6+ + e− → Cr3+ (by chromate-reducing bacteria) |
| Cathode: O2 + H+ + e− → H2O (by chemical reaction) |
The higher the Cr6+ concentration exists in the anode, the fewer electrons are transferred to the cathode if organic concentrations remain constant. Thus, the potential output will decrease with the increasing Cr6+ concentration.
In this study, O. anthropi YC152, a facultatively anaerobic, Cr(VI)-reducing, and exoelectrogenic bacterium, was isolated from wastewater containing Cr(VI). It was inoculated in an MFC to evaluate its feasibility as a biosensor or an early warning device for the detection of Cr(VI). Crucial operating parameters were established to optimize the performance of the MFC. The relationship between the voltage output and Cr(VI) concentration was investigated.
2. Materials and Methods
2.1. Bacterial Strains, Cultivation, and Identification
Sludge samples were collected from an electroplating wastewater treatment plant in Taoyuan City, Taiwan, and then centrifuged at 8000× g for 40 min. Precipitates were inoculated in a 3-L working volume of a chemostat and mixed with Luria Bertani (LB) broth supplemented with Na2Cr2O7, (LBCr medium). Subsequently, the LBCr medium containing 5–100 mg/L of Cr(VI) was progressively added into the chemostat to acclimate Cr(VI)-resistant or -reducing bacteria under the anaerobic conditions at 35 °C and a liquid retention time (LRT) of 24 h. A dominant strain (YC152) was isolated from the chemostat by using the spread plate method after a 36-d acclimation period. To identify the isolated YC152 bacterium, cell lysis, DNA extraction, 16S rRNA gene amplification, and sequencing were performed as described previously [29]. The genomic DNA of YC152 bacterium was extracted using a DNeasy® Blood and Tissue Kit (QIAGEN, Hilden, Germany). The DNA sample was stored at −20 °C in ddH2O. This DNA was used as a template to amplify the 16S rRNA gene with primers 27f (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1522r (5′-AAGGAGGTGATCCAGCCGCA-3′) [29]. Each reaction mixture (final volume, 50 μL) consisted of 20 mM Tris-HCl (pH 8.4), 3 mM MgCl2, each deoxynucleoside triphosphate at a concentration of 0.2 mM, each primer at a concentration of 0.2 μM, 1.25 U of Taq polymerase, and 1 μL of appropriately-diluted template DNA. The PCR program comprised of: predenaturation at 94 °C for 5 min; 35 cycles of denaturation 94 °C for 30 s, annealing at 56 °C for 20 s, extension at 72 °C for 40 s, and a final extension at 72 °C for 10 min. The sequences representing the YC152 bacterium were compared with the NCBI database by using BLASTN, and the closest match to the bacterial isolate was retrieved.
2.2. Factors Affecting the Cr(VI) Removal Efficiency of Isolate YC152
To obtain the growth curve of the isolate YC152, YC152 was cultured in 300-mL LB broth supplemented with 0.25 mM Na2Cr2O7 (26 mg/L of Cr(VI)) and incubated at 35 °C at pH 7.0 under anaerobic conditions. Samples were obtained every 2 h during the culture period. Each sample was immediately measured at 600 nm by using a UV-VIS spectrophotometer. At the same time, cell numbers in samples were determined using the plate count method after 24 h of cultivation at 35 °C.
To determine the effects of different environmental factors on the Cr(VI) removal efficiency of the isolate YC152, different pH values (5–10), culture temperatures (20–40 °C), and NaCl concentrations (5–25 g/L), and four simulated wastewater types containing Cr(VI) were tested. Simulated Wastewater I contained diluted LBCr medium (1/1000 LB supplemented with 26 mg/L of Cr(VI)). Simulated Wastewater II contained diluted LBCr medium, 15 mg/L Cu2+, 25 mg/L Zn2+, 10 mg/L Ni2+, 5 mg/L Na+, and 5 mg/L SO42−. Simulated Wastewater III contained diluted LBCr medium, 30 mg/L Cu2+, 50 mg/L Zn2+, 20 mg/L Ni2+, 10 mg/L Na+, and 10 mg/L SO42−. Simulated Wastewater IV contained diluted LBCr medium, 60 mg/L Cu2+, 100 mg/L Zn2+, 40 mg/L Ni2+, 20 mg/L Na+, and 20 mg/L SO42−. In these batch experiments, the diluted LBCr medium was used. The shaker culture was maintained at 35 °C and run at 200 rpm, and the cell number of inoculated YC152 was 2.0 × 108 cfu/mL, unless stated otherwise. The Cr(VI) removal efficiency of the isolate was analyzed after 48-h cultivation periods. All of the experiments were conducted at least in triplicate.
2.3. MFC Construction
The structure of a MFC biosensor was a typical two-chamber unit. The rectangular anode and cathode compartments were constructed from polyacrylic plastic (working volume: 170 mL each) and physically separated using a proton exchange membrane (PEM; Nafion 117, DuPont Co., Fayetteville, NC, USA) with a surface area of 49 cm2. The plain porous carbon paper (30.25 cm2 surface area) was used as electrodes with an OK line or wire connecting them through a variable resistor. Four pores were located at the top of the MFC for in and out of the electrode wire, addition and sampling of solutions, and online detection of ORP and pH. The anolyte of the MFC contained the diluted LBCr medium, unless stated otherwise, and the catholyte of the MFC contained 50 mM phosphate buffer (pH 7) and 100 mM NaCl solutions. The anode compartment was maintained anoxic by purging with nitrogen gas.
2.4. Cell Immobilization and Biosensor Operation
The anode compartment of the MFC biosensor with a 500-Ω resistor was continuously introduced with the LB medium containing the isolate YC152 (2.0 × 108 cfu/mL) for cell immobilization at 10-d LRT. The feed solution and anode compartment were kept anoxic by purging with nitrogen gas. When the voltage of the MFC reached a steady state (approximately 572 mV) after 20-d operation, the biofilm in the anode of the MFC was considered stable or mature. Thus, the effects of operation parameters on MFC characteristics were evaluated.
The 1/1000 LB medium was used as the anolyte to evaluate the performance of the MFC biosensor. The circuit was adjusted using variable resistance (50–3900 Ω) to obtain a polarization curve of the MFC. Subsequently, one ml of Cr(VI) with a final concentration (0.0125–5 mg/L) was added to the 1/1000 LB medium (original anolyte) to establish the relationship between the Cr(VI) concentration and voltage output of the MFC biosensor. To examine the stability of the MFC, 2/3 of the 1/1000 LB medium in the MFC was replaced by a fresh medium when the voltage decreased to approximately 1/10 of the maximal value over a 27-d period. The MFC biosensor was operated in a batch mode for at least 12 h for each operation parameter to obtain a stable voltage production. The MFC biosensor was placed in a temperature-controlled chamber maintained at 35 °C.
2.5. Cr(VI) Measurement in Artificial and Real Wastewater
Cr(VI) concentrations in artificial and real water samples (including drinking water, groundwater, domestic wastewater, and electroplating wastewater) were measured using the MFC biosensor, modified AAS technique, or/and colorimetric method. To evaluate the feasibility of the MFC biosensor, the original anolyte in the MFC was completely replaced by artificial and real water samples. Artificial wastewater contained 1/1000 of the LB medium supplemented with different Cr(VI) concentrations (0.05–3.5 mg/L). Furthermore, 169 mL of real wastewater was supplemented with 1 mL of the 17/100 LB medium to maintain the LB concentration in the anolyte of the MFC. According to the relationship between the Cr(VI) concentration and voltage output of the MFC biosensor (described in Section 2.4), the Cr(VI) concentration in the water sample was easily obtained. In this study, the reaction time was set at 45 min. All experiments were conducted using five separate MFCs, and all analyses were conducted in triplicate.
2.6. Analysis
Na2Cr2O7 of special grade chemicals was dried at 200 °C for 1 h and left in a desiccator. Subsequently, 25.2 mg of Na2Cr2O7 was weighed and dissolved in water and diluted to 100 mL. The diluted solution was used as a standard stock solution of 100 mg/L of Cr (VI). The Cr(VI) was first chelated with ammonium pyrrolidine dithiocarbamate (APDC) and then extracted with methyl isobutyl ketone (MIBK) [30]. The extract was aspirated into the flame of the atomic absorption spectrophotometer (Hitachi, Tokyo, Japan). The colorimetric method for Cr(VI) measurement was performed as described previously [31].
The MFC potential or voltage was measured using a multimeter (Model 2700, Keithley Instruments, Inc., Solon, OH, USA). Data were digitally recorded every minute on a computer by using an interface card (Model PCI-488, Keithley Instruments, Inc.). The measured voltage (V) was converted to current (I) according to the following relationship: voltage (V, volt) = current (I, amp) × resistance (R, ohm). The power (P, watt) was calculated as P = I × V and then normalized using the surface area of the anode. All experiments were conducted using five separate MFCs, and all analyses were conducted at least in triplicate.
3. Results and Discussion
3.1. Identification and Characterization of Isolate YC152
The 16S rRNA of the isolate YC152 exhibited the highest sequence similarity (97.8%) to the 16S rRNA of O. anthropi. The strain was Gram-negative, rod-shaped, facultatively anaerobic, and motile by means of peritrichous flagella. After growth on nutrient agar for 24 h, the colonies had an average diameter of 1.2 μm and were circular, smooth, low convex, and non-pigmented. O. anthropi YC152 belonged to the α subclass of Proteobacteria.
The growth curve of O. anthropi YC152 exhibited the lag phase in the first 6 h, followed by the log phase from 10 to 22 h, and then entered the stationary phase under the anaerobic condition. According to the growth curve, the inoculation time was set at 20 h for further experiments. In addition, the OD600 value of cell growth was proportional to the log cell number, and the regression equation was determined to be y (log cell number) = 2.26x (OD600) + 4.263 (r2 = 0.991). We calculated the cell number of O. anthropi YC152 by using this equation. According to the growth curve, the specific growth rate was determined to be 0.482 h−1.
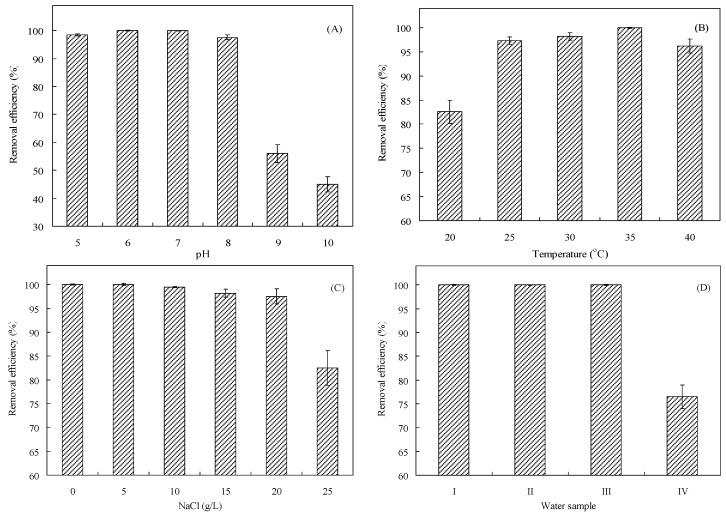
Since the pH of wastewater containing Cr(VI) might be weakly acidic, the effect of pH on the Cr(VI) removal efficiency of O. anthropi YC152 was evaluated. As presented in Figure 1A, the weak acidic condition (i.e., pH 5 and 6) did not affect the Cr(VI) removal efficiency of O. anthropi YC152 (98.5%–100%); however, the basic condition (i.e., pH 9 and 10) reduced the Cr(VI) removal efficiency to 45%–56%. Thus, the MFC biosensor was used for measuring the Cr(VI) concentration when the pH value of the solution was pH 5–8. This characteristic can favor the operation of the MFC biosensor for measuring the Cr(VI) concentration. Figure 1B illustrates the effect of temperature on the Cr(VI) removal efficiency of O. anthropi YC152. The results revealed that high Cr(VI) removal efficiencies were observed in the range of 25–40 °C, with the highest removal efficiency of 100% observed at 35 °C. However, the psychrophilic temperature (e.g., 20 °C) was not beneficial to the Cr(VI) removal efficiency of O. anthropi YC152. The optimal pH and temperature for Cr(VI) removal by using O. anthropi observed in this study were consistent with those reported by Sultan and Hasnain (2012) [32]. Electrolytes, such as Na+ and Cl−, exist at a high concentration in wastewater and can potentially affect the activity of microbes and the function of ion-exchange membranes in MFCs [23]. Thus, the effect of NaCl on the Cr(VI) removal efficiency of O. anthropi YC152 was investigated. The average Cl− concentration in seawater is approximately 20 g/L [33]. As presented in Figure 1C, the Cr(VI) removal efficiency of O. anthropi YC152 was not significantly affected by increasing Cl− concentrations (0–20 g/L); the removal efficiency was 97.5%–100%. In addition, the halotolerant characteristics have been reported by Wang et al. [34]. These results suggest that the MFC biosensor inoculated with O. anthropi YC152 has potential for measuring Cr(VI) concentrations in seawater. To evaluate the effect of coexisting ions on the Cr(VI) removal efficiency of O. anthropi YC152, different simulated wastewaters containing Cr(VI) were investigated. Figure 1D indicates that the Cr(VI) removal efficiency of O. anthropi YC152 was 100% when the wastewater contained 0–30 mg/L Cu2+, 0–50 mg/L Zn2+, 0–20 mg/L Ni2+, 0–10 mg/L Na+, and 5–10 mg/L SO42− (water samples I–III). However, the relatively low Cr(VI) removal efficiency (76.5%) of O. anthropi YC152 was observed when the wastewater contained 60 mg/L Cu2+, 100 mg/L Zn2+, 40 mg/L Ni2+, 20 mg/L Na+, and 20 mg/L SO42− (water samples IV). These results suggest that the MFC biosensor inoculated with O. anthropi YC152 has a high potential for Cr(VI) removal and measurement in different water bodies.
Figure 1.
Effects of (A) pH (temperature: 30 °C, NaCl concentration: 0 M); (B) temperature (pH: 7, NaCl concentration: 0 M); (C) NaCl concentration (temperature: 30 °C, pH: 7); and (D) various water quality on the Cr(VI) removal efficiency of O. anthropi YC152 (Cr(VI) concentration was: 26 mg/L).
3.2. MFC Operation
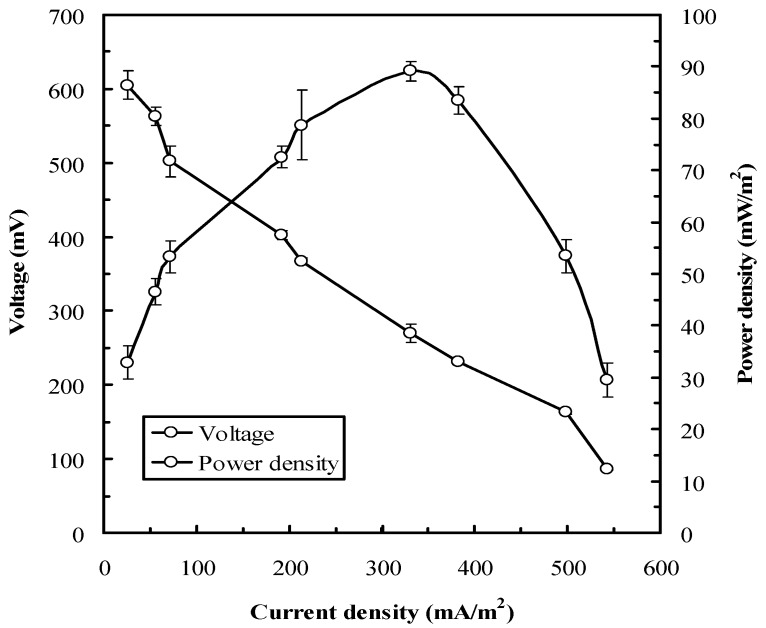
Since the outer layers of most bacteria are composed of nonconductive compounds that hinder the electron transfer to the anode, exoelectrogens in the anodic chamber of an MFC are crucial. Zuo et al. (2008) first reported the exoelectrogenic property of Ochrobactrum sp. and demonstrated its potential in MFC applications [35]. We observed that the voltage and power density of the MFC biosensor inoculated with O. anthropi YC152 were a function of the current density under various external resistances (50–3900 Ω). As presented in Figure 2, the MFC potential decreased as the current density increased, and the open circuit voltage was approximately 630 mV. Under such conditions, the maximum power density was 89.1 ± 1.2 mW/m2 and the optimal external resistance was 270 Ω. In addition, our results indicated that the decrease in MFC voltage had three different slopes because of the activation loss, ohmic loss, and mass transport loss. This result is in accordance with the characteristic of the MFC type [36]. Hence, the external resistance was set at 270 Ω for subsequent experiments.
Figure 2.
Polarization and power curves obtained from the MFC biosensor inoculated with O. anthropi YC152 during the stable phase of power generation (operating temperature: 35 °C, anolyte: 1/1000 LB, catholyte: 50 mM phosphate and 100 mM NaCl).
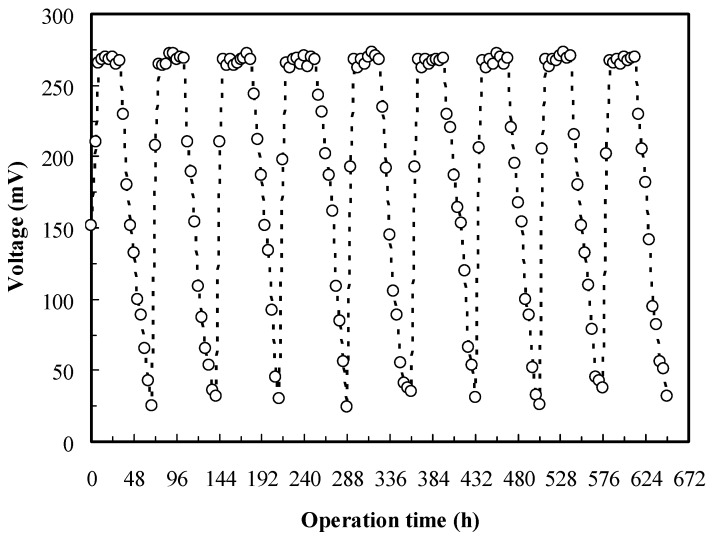
The stable performance of the MFC biosensor during a desired operational period is essential for a reliable biosensor system. Figure 3 reveals that the complete voltage cycle (25–268–25 mV) was approximately 72 h for the O. anthropi YC152 MFC. When two-thirds of the anolyte was replaced with the fresh medium, the MFC reached the stable maximum voltage (268.0 ± 2.8 mV) in 8 h and continued until 32 h. The results of nine similar cycles in the 27-d operation demonstrated the operational stability of the MFC biosensor. Theoretically, when the anolyte in the MFC was periodically refreshed, the MFC biosensor could be continuously maintained.
Figure 3.
The stability of MFC biosensor inoculated with O. anthropi YC152 (operational temperature: 35 °C, resistance of external circuit: 270 Ω, anolyte: 1/1000 LB, catholyte: 50 mM phosphate and 25 mM NaCl).
3.3. Relationship between Cr(VI) Concentration and Voltage Output
Under optimal operation conditions, the relationship between the Cr(VI) concentration and voltage output of the MFC biosensor was determined. Since the MFC was designed to function as a Cr(VI) warning device to conform with water quality regulations, the 1/1000 LB medium supplemented with different Cr(VI) final concentrations (0.0125–5 mg/L) was introduced into the anode compartment of the MFC biosensor in a batch mode to establish their relationships. In the anode compartment of the MFC, Cr(VI) acted as an electron acceptor; thus, the MFC voltage was expected to decrease with an increasing Cr(VI) concentration. The results revealed that the MFC voltage decreased with reaction time. Higher Cr(VI) concentrations required longer reaction times to achieve a stable voltage output. However, only 15–45 min of reaction time was required for various Cr(VI) concentrations for stable voltage production. Moreover, compared with the original anolyte without Cr(VI), the anolyte supplemented with 1 mg/L of Cr(VI) significantly reduced the MFC voltage by 45%. Compared with the extent of voltage decrease in the cube MFC developed by Liu et al. (2014) and the flat microliter membrane-based MFC developed by Xu et al. (2015), the Cr(VI) MFC biosensor was more sensitive [37,38]. The signal amplification in our system would facilitate measuring the Cr(VI) concentration in water samplers. In this study, a stable voltage production in the MFC was observed in 15–45 min when different Cr(VI) concentrations (0.0125–5 mg/L) were introduced. The 5–15 min of recovery time of this biosensor was required in accordance with the Cr(VI) concentrations.
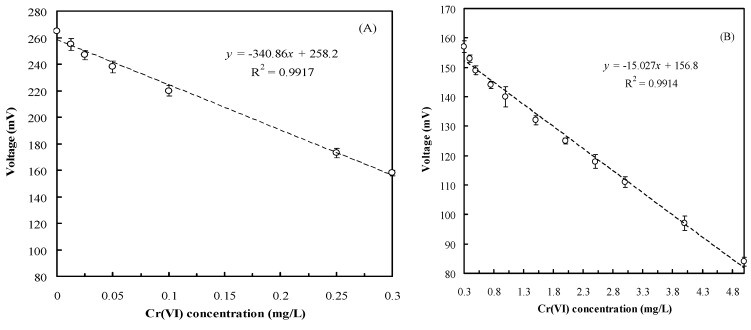
Figure 4 presents the relationship between the Cr(VI) concentration and voltage output of the MFC biosensor. Two satisfactory linear relationships were observed between the Cr(VI) concentration and voltage output for various Cr(VI) concentration ranges. Figure 4A indicates that the regression equation for the Cr(VI) concentration and the voltage output of the MFC biosensor was determined to be y = −340.86x + 258.2 (r2 = 0.9917) when Cr(VI) concentrations ranged from 0.0125 to 0.3 mg/L. Furthermore, Figure 4B indicates that the other regression equation was determined to be y = −15.027x + 156.8 (r2 = 0.9914) when the Cr(VI) concentration ranged from 0.3 to 5 mg/L. The different relationships observed in different Cr(VI) concentrations may be because of Cr(VI) reduction rates and bacterial characteristics [32]. Hence, on the basis of these two equations, the Cr(VI) concentration in the water samples can be rapidly determined using the developed MFC biosensor. To prevent the MFC voltage decreasing because of the toxicity of Cr(VI) to microbes, the cell numbers of O. anthropi YC152 were evaluated by using the plate count method after experiments. The results revealed that the changes in the cell numbers (2.8–6.5 × 107 cfu/mL) after the addition of different Cr(VI) concentrations were not significant.
Figure 4.
Relationship between Cr(VI) concentration and voltage output of the MFC biosensor: (A) Cr(VI) concentration: 0.0125 to 0.3 mg/L; (B) Cr(VI) concentration: 0.3 to 5 mg/L (operational temperature: 35 °C, resistance of external circuit: 270 Ω, anolyte: 1/1000 LB supplemented with different Cr(VI) concentration, catholyte: 50 mM phosphate and 25 mM NaCl, response time: 45 min).
3.4. Cr(VI) Measurement in Artificial and Real Wastewater
To evaluate the feasibility of using the MFC biosensor to measure Cr(VI) concentrations in wastewater, different artificial wastewaters containing Cr(VI) were synthesized, and the Cr(VI) concentrations of the samples were measured using the MFC biosensor and through AAS (Table 1). As listed in Table 1, compared with values determined using the MFC biosensor, those determined through AAS were slightly more accurate. Furthermore, compared with the standard Cr(VI) concentration, the deviation of the MFC biosensor was less than 10% when the Cr(VI) concentration in water samples was <0.5 mg/L. The precise MFC biosensor can be used as an early warning device for determining Cr(VI) effluents. Moreover, when values determined using the MFC biosensor and AAS were compared, <10% deviation (−3.8% to 9.8%) was observed for all tested concentrations. These results indicate that the accuracies of the developed Cr(VI) MFC biosensor and the standard test method employing AAS are similar for measuring the Cr(VI) concentration, ranging from 0 to 3.5 mg/L.
Table 1.
Cr(VI) measurement from artificial wastewater by atomic absorption spectroscopy and MFC biosensor.
| Standard Cr(VI) Concentration of (mg/L) | ||||||
|---|---|---|---|---|---|---|
| 0.05 | 0.1 | 0.25 | 0.5 | 1.5 | 3.5 | |
| AAS 1 | 0.051 ± 0.01 | 0.112 ± 0.01 | 0.25 ± 0.02 | 0.53 ± 0.03 | 1.58 ± 0.02 | 3.46 ± 0.05 |
| MFC biosensor | 0.053 ± 0.01 | 0.109 ± 0.02 | 0.26 ± 0.06 | 0.51 ± 0.02 | 1.72 ± 0.01 | 3.80 ± 0.03 |
| Deviation (%) 2 | 2 | 12 | 0 | 6 | 5.3 | −1.1 |
| Deviation (%) 3 | 6 | 9 | 4 | 2 | 14.7 | 8.6 |
| Deviation (%) 4 | 3.9 | −1.8 | 4 | −3.8 | 8.9 | 9.8 |
1 Modified atomic absorption spectroscopy technique; 2 The determined value by atomic absorption spectroscopy compared to standard Cr(VI) concentration; 3 The determined value by MFC biosensor compared to standard Cr(VI) concentration; 4 The determined value by MFC biosensor compared to that by modified atomic absorption spectroscopy technique.
Different water samples usually contain different types and concentrations of organic compounds, metal ions, and pH. Thus, the possible effects of these coexisting compounds on the Cr(VI) concentration measured using the MFC biosensor were evaluated. Table 2 lists Cr(VI) concentrations in various real water samples measured through a modified atomic absorption spectroscopy technique, by using the MFC biosensor, or using colorimetric method. Compared with Cr(VI) concentrations measured through AAS, those measured using the MFC biosensor in drinking water, groundwater, and electroplating wastewater were more accurate and had low deviations (−7.7% to 9.2%). Furthermore, Cr(VI) concentrations in domestic wastewater measured using the MFC biosensor exhibited higher deviations (−17.7% to −18.4%) than did those measured through AAS. Similar results were also found when those measurements obtained by the MFC biosensor were compared with Cr(VI) concentrations measured by using colorimetric method. These results seem to conflict with those in Table 1, which indicate that the deviation was <10% when Cr(VI) concentrations in water samples were <5 mg/L. The high adaptability of O. anthropi YC152 to pH, temperature, salinity, and water quality is presented in Figure 1. Thus, this inconsistency in the results may be attributed to the content of organic compounds in water samples. Since organic concentrations in domestic wastewater were higher than those in other tested waters or wastewaters, a high initial voltage would be produced because of the high electron donors (organic compounds), followed by a decrease in voltage. According to the estimated equation of the Cr(VI) concentration presented in Section 3.3, the Cr(VI) concentration would be underestimated. Compared with previous studies [9,13,15,17], our results demonstrate that the developed Cr(VI) biosensor has an appropriate detection limit range for Cr(VI) (0.0125–5 mg/L) and can measure the concentration in the short time of 45 min. Thus, the developed MFC biosensor has the potential to be used as an early warning device to protect ecosystems.
Table 2.
Cr(VI) measurement from real wastewater by atomic absorption spectroscopy, MFC biosensor, and colorimetric method.
| Drinking Water | Groundwater | Domestic Wastewater | Electroplating Wastewater | |||||
|---|---|---|---|---|---|---|---|---|
| A | B | A | B | A | B | A | B | |
| AAS 1 | 0.015 ± 0.001 | 0.036 ± 0.002 | 0.052 ± 0.009 | 0.120 ± 0.018 | 0.49 ± 0.031 | 0.62 ± 0.052 | 2.06 ± 0.082 | 4.31 ± 0.069 |
| MFC biosensor | 0.016 ± 0.002 | 0.038 ± 0.003 | 0.048 ± 0.010 | 0.131 ± 0.015 | 0.40 ± 0.026 | 0.51 ± 0.026 | 2.19 ± 0.051 | 4.65 ± 0.031 |
| Colorimetric method | 0.017 ± 0.005 | 0.037 ± 0.005 | 0.050 ± 0.012 | 0.128 ± 0.026 | 0.46 ± 0.051 | 0.58 ± 0.041 | 2.13 ± 0.062 | 4.38 ± 0.064 |
| Deviation (%) 2 | 6.7 | 5.6 | −7.7 | 9.2 | −18.4 | −17.7 | 6.3 | 7.9 |
| Deviation (%) 3 | −5.9 | 2.7 | −4.0 | 2.3 | −13.0 | −12.1 | 2.8 | 6.2 |
1 modified atomic absorption spectroscopy technique; 2 compared to the value measuring by AAS; 3 compared to the value measuring by colorimetric method.
4. Conclusions
In this study, we developed an MFC biosensor inoculated with O. anthropi YC152 to rapidly determine Cr(VI) at trace concentrations. Our results revealed that the MFC biosensor can be used as a reliable warning device for Cr(VI) determination in different water samples with low deviation and high sensitivity, which had previously not been fully studied. The physiological characteristics of O. anthropi YC152, including facultative anaerobe, Cr(VI) reduction, exoelectrogen, and high adaptability to pH, temperature, salinity, and water quality, demonstrated that it was suitable for application in an MFC to determine Cr(VI). The developed MFC biosensor can, in only 45 min, evaluate whether Cr(VI) concentrations in water samples conform with the effluent regulations and the maximum allowable concentration in water. Thus, the application of the MFC biosensor as an early warning device for Cr(VI) determination is promising.
Acknowledgments
The authors thank Y.J. Chen, S.W. Chou and C.H. Lin for help with partially analytical measurements. This research was supported by NSC Grant NSC 102-2313-B-157-002 and MOST 104-2313-B-157-001-MY3
Author Contributions
All authors collaborated to carry out the work presented here. Ying-Chien Chung and Man-Hai Liu conceived and designed the experiments; Guey-Horng Wang and Chiu-Yu Cheng performed the experiments; Tzu-Yu Chen and Min-Chi Hsieh analyzed the data; Chiu-Yu Cheng wrote the paper; Ying-Chien Chung and Guey-Horng Wang reviewed and edited the manuscript. All authors read and approved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mishra R.R., Dhal B., Dutta S.K., Dangar T.K., Das N.N., Thatoi H.N. Optimization and characterization of chromium(VI) reduction in saline condition by moderately halophilic Vigribacillus sp. isolated from mangrove soil of Bhitarkanika, India. J. Hazard. Mater. 2012;15:219–226. doi: 10.1016/j.jhazmat.2012.05.063. [DOI] [PubMed] [Google Scholar]
- 2.Shrivastava R., Upreti R.K., Chaturvedi U.C. Various cells of the immune system and intestine differ in their capacity to reduce hexavalent chromium. FEMS Immun. Med. Microbiol. 2003;38:65–70. doi: 10.1016/S0928-8244(03)00107-X. [DOI] [PubMed] [Google Scholar]
- 3.Das K.K., Dhundasi S.A., Das S.N. Hexavalent chromium and its effect on health: Possible protective role of garlic (Allium sativum Linn) J. Basic Clin. Physiol. Pharmacol. 2011;22:3–10. doi: 10.1515/jbcpp.2011.008. [DOI] [PubMed] [Google Scholar]
- 4.Barrera-Diaza C.E., Lugo-Lugo V., Bilyeu B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater. 2012;223–224:1–12. doi: 10.1016/j.jhazmat.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 5.Cheung K.H., Gu J.D. Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: A review. Int. Biodeter. Biodegrad. 2007;59:8–15. doi: 10.1016/j.ibiod.2006.05.002. [DOI] [Google Scholar]
- 6.Hirpara P., Nikhil B., Murty D.S. Bacterial treatment for removal of chromium(VI) containing electroplating waste waters. Indian J. Appl. Res. 2014;4:436–438. doi: 10.15373/2249555X/June2014/136. [DOI] [Google Scholar]
- 7.Turdean G.L. Design and development of biosensors for the detection of heavy metal toxicity. Int. J. Electrochem. 2011;2011:1–15. doi: 10.4061/2011/343125. [DOI] [Google Scholar]
- 8.Han S., Zhu M., Yuan Z., Li X. A methylene blue-mediated enzyme electrode for the determination of trace mercury(II), mercury(I), methylmercury, and mercuryglutathione complex. Biosens. Bioelectron. 2001;16:9–16. doi: 10.1016/S0956-5663(00)00114-7. [DOI] [PubMed] [Google Scholar]
- 9.Calvo-Pérez A., Domínguez-Renedo O., Alonso-Lomillo M., Arcos-Martínez M. Speciation of chromium using chronoamperometric biosensors based on screen-printed electrodes. Anal. Chim. Acta. 2014;833:15–21. doi: 10.1016/j.aca.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Dai C., Choi S. Technology and applications of microbial biosensor. Open J. Appl. Biosens. 2013;2:83–93. doi: 10.4236/ojab.2013.23011. [DOI] [Google Scholar]
- 11.Pearson J.E., Gill A., Vadgama P. Analytical aspects of biosensors. Ann. Clin. Biochem. 2000;37:119–145. doi: 10.1258/0004563001899131. [DOI] [PubMed] [Google Scholar]
- 12.De-Bashan L.E., Bashan Y. Immobilized microalgae for removing pollutants: Review of practical aspects. Bioresour. Technol. 2010;101:1611–1627. doi: 10.1016/j.biortech.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 13.Michel C., Ouerd A., Battaglia-Brunet F., Guigues N., Grasa J.P., Bruschi M., Ignatiadis I. Cr(VI) quantification using an amperometric enzyme-based sensor: Interference and physical and chemical factors controlling the biosensor response in ground waters. Biosens. Bioelectron. 2006;22:285–290. doi: 10.1016/j.bios.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Nepomuscene N.J., Daniel D., Krastanov A. Biosensor to detect chromium in wastewater. Biotechnol. Biotechnol. Equip. 2007;21:377–381. doi: 10.1080/13102818.2007.10817477. [DOI] [Google Scholar]
- 15.Gurung A., Oh S., Kim K.D., Shin B. Semi-continuous detection of toxic hexavalent chromium using a sulfur-oxidizing bacteria biosensor. J. Environ. Manag. 2012;106:110–112. doi: 10.1016/j.jenvman.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Bohrn U., Mucha A., Werner C.F., Trattner B., Backer M., Krumbe C., Schienle M., Stutz E., Schmitt-Landsiedele D., Fleischer M., et al. A critical comparison of cell-based sensor systems for the detection of Cr(VI) in aquatic environment. Sens. Actuator B Chem. 2013;182:58–65. doi: 10.1016/j.snb.2013.02.105. [DOI] [Google Scholar]
- 17.Panda J., Sarkar P. Biosensing and bioremediation of Cr(VI) by cell free extract of Enterobacter aerogenes T2. J. Environ. Sci. Health Part A. 2014;49:600–608. doi: 10.1080/10934529.2014.859466. [DOI] [PubMed] [Google Scholar]
- 18.Branco R., Cristovao A., Morais P.V. Highly sensitive, highly specific whole cell bioreporters for the detection of chromate in environmental samples. PLoS ONE. 2013;8:1272. doi: 10.1371/journal.pone.0054005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coelho C., Branco R., Natal-da-Luz T., Sousa J.P., Morais P.V. Evaluation of bacterial biosensors to determine chromate bioavailability and to assess ecotoxicity of soils. Chemosphere. 2015;128:62–69. doi: 10.1016/j.chemosphere.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh M.C., Chung Y.C. Measurement of biochemical oxygen demand from different wastewater samples using a mediator-less microbial fuel cell biosensor. Environ. Technol. 2014;35:2204–2211. doi: 10.1080/09593330.2014.898700. [DOI] [PubMed] [Google Scholar]
- 21.Chen C.Y., Chen T.Y., Chung Y.C. A comparison of bioelectricity in microbial fuel cells with aerobic and anaerobic anodes. Environ. Technol. 2014;35:286–293. doi: 10.1080/09593330.2013.826254. [DOI] [PubMed] [Google Scholar]
- 22.Du Z., Li H., Gu T. A state of the art review on microbial fuel cells: A promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 2007;25:464–482. doi: 10.1016/j.biotechadv.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh M.C., Cheng C.Y., Liu M.H., Chung Y.C. Effects of operating parameters on measurements of biochemical oxygen demand using a mediatorless microbial fuel cell biosensor. Sensors. 2016;16:35. doi: 10.3390/s16010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dávila D., Esquivel J.P., Sabate N., Mas J. Silicon-based microfabricated microbial fuel cell toxicity sensor. Biosens. Bioelectron. 2011;26:2426–2430. doi: 10.1016/j.bios.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z., Liu J., Zhang S., Xing X., Su Z. Microbial fuel cell based biosensor for in situ monitoring of anaerobic digestion process. Bioresour. Technol. 2011;102:10221–10229. doi: 10.1016/j.biortech.2011.08.053. [DOI] [PubMed] [Google Scholar]
- 26.Stein N.E., Hamelers H.V.M., Straten G., Keesman K.J. On-line detection of toxic components using a microbial fuel cell-based biosensor. J. Process Control. 2012;22:1755–1761. doi: 10.1016/j.jprocont.2012.07.009. [DOI] [Google Scholar]
- 27.Joutey N.T., Sayel H., Bahafid W., El Ghachtouli N. Mechanisms of hexavalent chromium resistance and removal by microorganisms. Rev. Environ. Contam. Toxicol. 2015;233:45–69. doi: 10.1007/978-3-319-10479-9_2. [DOI] [PubMed] [Google Scholar]
- 28.Xafenias N., Zhang Y., Banks C.J. Enhanced performance of hexavalent chromium reducing cathodes in the presence of Shewanella oneidensis MR-1 and lactate. Environ. Sci. Technol. 2013;47:4512–4520. doi: 10.1021/es304606u. [DOI] [PubMed] [Google Scholar]
- 29.Corby-Harris V., Snyder L.A., Schwan M.R., Maes P., McFrederick Q.S., Anderson K.E. Origin and effect of alpha 2.2 Acetobacteraceae in honey bee larvae and description of Parasaccharibacter apium gen. nov., sp. nov. Appl. Environ. Microbiol. 2014;80:7460–7472. doi: 10.1128/AEM.02043-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.USEPA . Methods for Chemical Analysis of Water and Wastes. EPA; Cincinnati, OH, USA: 1983. [(accessed on 9 August 2016)]. Available online: http://www.state.in.us/dnr/fishwild/files/Methods_Analysis_Water_Wastes_USEPA_March1983.pdf. [Google Scholar]
- 31.Chen C.Y., Cheng C.Y., Chen C.K., Hsieh M.C., Lin S.T., Ho K.Y., Li J.W., Lin C.P., Chung Y.C. Hexavalent chromium removal and bioelectricity generation by Ochrobactrum sp. YC211 under different oxygen conditions. J. Environ. Sci. Health Part A. 2014;51:502–508. doi: 10.1080/10934529.2015.1128731. [DOI] [PubMed] [Google Scholar]
- 32.Sultan S., Hasnain S. Chromium (VI) reduction by cell free extract of Ochrobactrum anthropi isolated from tannery effluent. Bull. Environ. Contam. Toxicol. 2012;89:152–157. doi: 10.1007/s00128-012-0648-1. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura H., Suzuki K., Ishikuro H., Kinoshita S., Koizumi R., Okuma S., Gotoh M., Karube I. A new BOD estimation method employing a double-mediator system by ferricyanide and menadione using the eukaryote Saccharomyces cerevisiae. Talanta. 2007;72:210–216. doi: 10.1016/j.talanta.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Wang X., Jin D., Zhou L., Zhang Z. Draft genome sequence of Ochrobactrum anthropi strain W13P3, a halotolerant polycyclic aromatic hydrocarbon-degrading bacterium. Genome Announc. 2015;3:e00867-15. doi: 10.1128/genomeA.00867-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuo Y., Xing D., Regan J.M., Logan B.E. Isolation of the exoelectrogenic bacterium Ochrobactrum anthropi YZ-1 by using a U-tube microbial fuel cell. Appl. Environ. Microbiol. 2008;74:3130–3137. doi: 10.1128/AEM.02732-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rinaldi A., Mecheri B., Garavaglia V., Licoccia S., Nardo P.D., Traversa E. Engineering materials and biology to boost performance of microbial fuel cells: A critical review. Energy Environ. Sci. 2008;1:417–429. doi: 10.1039/b806498a. [DOI] [Google Scholar]
- 37.Liu B., Lei Y., Li B. A batch-mode cube microbial fuel cell based “shock” biosensor for wastewater quality monitoring. Biosens. Bioelectron. 2014;62:308–314. doi: 10.1016/j.bios.2014.06.051. [DOI] [PubMed] [Google Scholar]
- 38.Xu Z., Liu B., Dong Q., Lei Y., Li Y., Ren J., McCutcheon J., Li B. Flat microliter membrane-based microbial fuel cell as on-line sticker sensor for self-supported in situ monitoring of wastewater shocks. Bioresour. Technol. 2015;197:244–251. doi: 10.1016/j.biortech.2015.08.081. [DOI] [PubMed] [Google Scholar]