Abstract
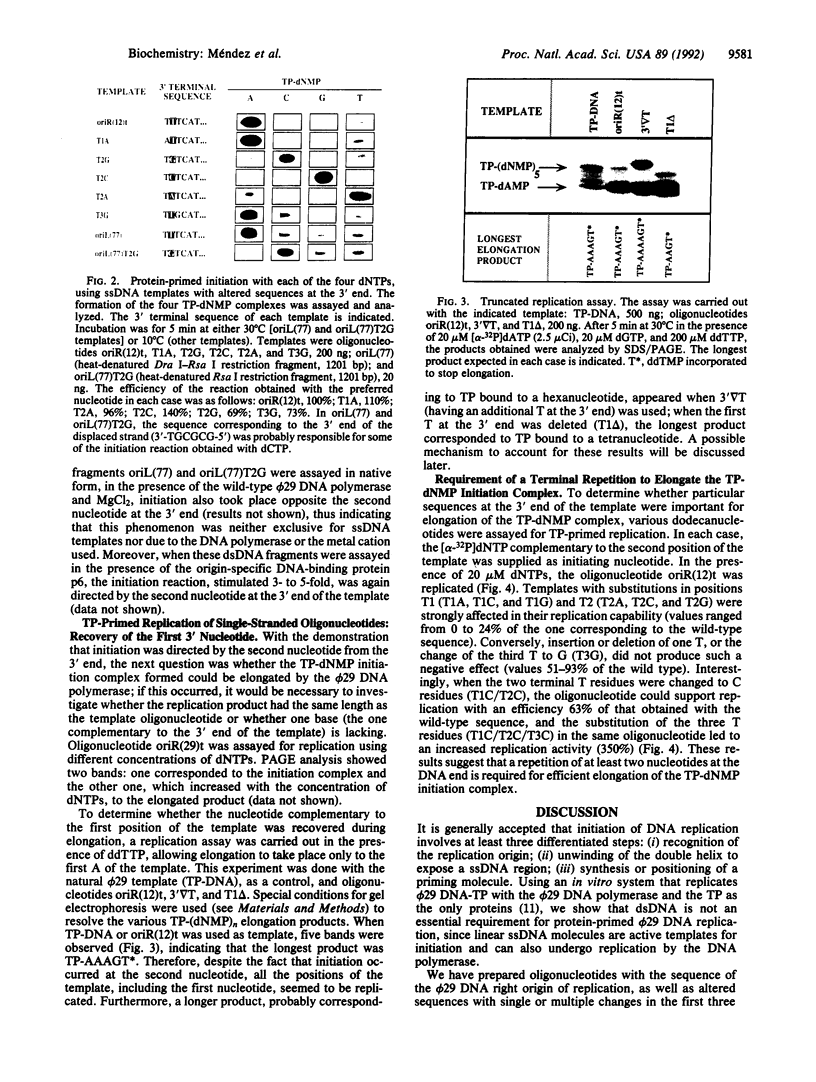
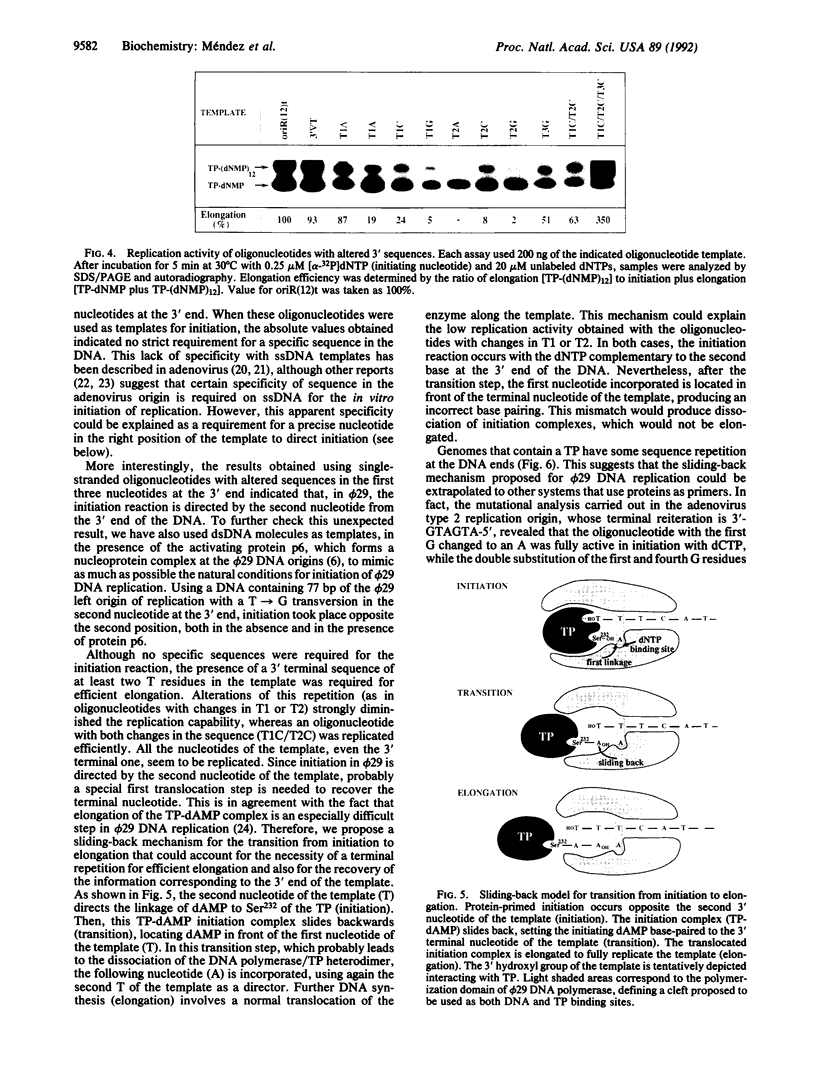
Bacteriophage phi 29 DNA replication is initiated when a molecule of dAMP is covalently linked to a free molecule of the terminal protein, in a reaction catalyzed by the viral DNA polymerase. We demonstrate that single-stranded DNA molecules are active templates for the protein-primed initiation reaction and can be replicated by phi 29 DNA polymerase. Using synthetic oligonucleotides, we carried out a mutational analysis of the phi 29 DNA right end to evaluate the effect of nucleotide changes at the replication origin and to determine the precise initiation site. The results indicate that (i) there are no strict sequence requirements for protein-primed initiation on single-stranded DNA; (ii) initiation of replication occurs opposite the second nucleotide at the 3' end of the template; (iii) a terminal repetition of at least two nucleotides is required to efficiently elongate the initiation complex; and (iv) all the nucleotides of the template, including the 3' terminal one, are replicated. A sliding-back model is proposed in which a special transition step from initiation to elongation can account for these results. The possible implication of this mechanism for the fidelity of the initiation reaction is discussed. Since all the terminal protein-containing genomes have some sequence reiteration at the DNA ends, this proposed sliding-back model could be extrapolable to other systems that use proteins as primers.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bausch J. N., Kramer F. R., Miele E. A., Dobkin C., Mills D. R. Terminal adenylation in the synthesis of RNA by Q beta replicase. J Biol Chem. 1983 Feb 10;258(3):1978–1984. [PubMed] [Google Scholar]
- Bernad A., Blanco L., Lázaro J. M., Martín G., Salas M. A conserved 3'----5' exonuclease active site in prokaryotic and eukaryotic DNA polymerases. Cell. 1989 Oct 6;59(1):219–228. doi: 10.1016/0092-8674(89)90883-0. [DOI] [PubMed] [Google Scholar]
- Blanco L., Bernad A., Lázaro J. M., Martín G., Garmendia C., Salas M. Highly efficient DNA synthesis by the phage phi 29 DNA polymerase. Symmetrical mode of DNA replication. J Biol Chem. 1989 May 25;264(15):8935–8940. [PubMed] [Google Scholar]
- Blanco L., Gutiérrez J., Lázaro J. M., Bernad A., Salas M. Replication of phage phi 29 DNA in vitro: role of the viral protein p6 in initiation and elongation. Nucleic Acids Res. 1986 Jun 25;14(12):4923–4937. doi: 10.1093/nar/14.12.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Prieto I., Gutiérrez J., Bernad A., Lázaro J. M., Hermoso J. M., Salas M. Effect of NH4+ ions on phi 29 DNA-protein p3 replication: formation of a complex between the terminal protein and the DNA polymerase. J Virol. 1987 Dec;61(12):3983–3991. doi: 10.1128/jvi.61.12.3983-3991.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Salas M. Characterization and purification of a phage phi 29-encoded DNA polymerase required for the initiation of replication. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5325–5329. doi: 10.1073/pnas.81.17.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Salas M. Replication of phage phi 29 DNA with purified terminal protein and DNA polymerase: synthesis of full-length phi 29 DNA. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6404–6408. doi: 10.1073/pnas.82.19.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Rawlins D. R. Template requirements for the initiation of adenovirus DNA replication. Proc Natl Acad Sci U S A. 1984 Jan;81(1):100–104. doi: 10.1073/pnas.81.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs L., Zhao L. J., Sripad G., Padmanabhan R. Mutational analysis of single-stranded DNA templates active in the in vitro initiation assay for adenovirus DNA replication. Virology. 1990 Sep;178(1):43–51. doi: 10.1016/0042-6822(90)90377-4. [DOI] [PubMed] [Google Scholar]
- Escarmís C., Salas M. Nucleotide sequence at the termini of the DNA of Bacillus subtilis phage phi 29. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1446–1450. doi: 10.1073/pnas.78.3.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban J. A., Bernad A., Salas M., Blanco L. Metal activation of synthetic and degradative activities of phi 29 DNA polymerase, a model enzyme for protein-primed DNA replication. Biochemistry. 1992 Jan 21;31(2):350–359. doi: 10.1021/bi00117a006. [DOI] [PubMed] [Google Scholar]
- Garcin D., Kolakofsky D. Tacaribe arenavirus RNA synthesis in vitro is primer dependent and suggests an unusual model for the initiation of genome replication. J Virol. 1992 Mar;66(3):1370–1376. doi: 10.1128/jvi.66.3.1370-1376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmendia C., Bernad A., Esteban J. A., Blanco L., Salas M. The bacteriophage phi 29 DNA polymerase, a proofreading enzyme. J Biol Chem. 1992 Feb 5;267(4):2594–2599. [PubMed] [Google Scholar]
- Goodman H. M., Billeter M. A., Hindley J., Weissmann C. The nucleotide sequence at the 5'-terminus of the Q RNA minus trand. Proc Natl Acad Sci U S A. 1970 Oct;67(2):921–928. doi: 10.1073/pnas.67.2.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Rudy J., Brinkley P. Infectious circular DNA of human adenovirus type 5: regeneration of viral DNA termini from molecules lacking terminal sequences. EMBO J. 1989 Jul;8(7):2077–2085. doi: 10.1002/j.1460-2075.1989.tb03616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenheimer R. A., Stillman B. W., Nagata K., Tamanoi F., Hurwitz J. DNA sequences required for the in vitro replication of adenovirus DNA. Proc Natl Acad Sci U S A. 1984 May;81(10):3069–3073. doi: 10.1073/pnas.81.10.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez J., García J. A., Blanco L., Salas M. Cloning and template activity of the origins of replication of phage phi 29 DNA. Gene. 1986;43(1-2):1–11. doi: 10.1016/0378-1119(86)90002-8. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J., Garmendia C., Salas M. Characterization of the origins of replication of bacteriophage phi 29 DNA. Nucleic Acids Res. 1988 Jul 11;16(13):5895–5914. doi: 10.1093/nar/16.13.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. P., Hay R. T. DNA sequences required for the initiation of adenovirus type 4 DNA replication in vitro. J Mol Biol. 1988 May 5;201(1):57–67. doi: 10.1016/0022-2836(88)90438-x. [DOI] [PubMed] [Google Scholar]
- Hermoso J. M., Méndez E., Soriano F., Salas M. Location of the serine residue involved in the linkage between the terminal protein and the DNA of phage phi 29. Nucleic Acids Res. 1985 Nov 11;13(21):7715–7728. doi: 10.1093/nar/13.21.7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. A., Bujarski J. J., Dreher T. W., Hall T. C. Minus-strand initiation by brome mosaic virus replicase within the 3' tRNA-like structure of native and modified RNA templates. J Mol Biol. 1986 Feb 20;187(4):537–546. doi: 10.1016/0022-2836(86)90332-3. [DOI] [PubMed] [Google Scholar]
- Pastrana R., Lázaro J. M., Blanco L., García J. A., Méndez E., Salas M. Overproduction and purification of protein P6 of Bacillus subtilis phage phi 29: role in the initiation of DNA replication. Nucleic Acids Res. 1985 May 10;13(9):3083–3100. doi: 10.1093/nar/13.9.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñalva M. A., Salas M. Initiation of phage phi 29 DNA replication in vitro: formation of a covalent complex between the terminal protein, p3, and 5'-dAMP. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5522–5526. doi: 10.1073/pnas.79.18.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing U., August J. T. The 3'-terminus and the replication of phage RNA. Nature. 1969 Nov 29;224(5222):853–856. doi: 10.1038/224853a0. [DOI] [PubMed] [Google Scholar]
- Salas M., Mellado R. P., Viñuela E. Characterization of a protein covalently linked to the 5' termini of the DNA of Bacillus subtilis phage phi29. J Mol Biol. 1978 Feb 25;119(2):269–291. doi: 10.1016/0022-2836(78)90438-2. [DOI] [PubMed] [Google Scholar]
- Salas M. Protein-priming of DNA replication. Annu Rev Biochem. 1991;60:39–71. doi: 10.1146/annurev.bi.60.070191.000351. [DOI] [PubMed] [Google Scholar]
- Serrano M., Salas M., Hermoso J. M. A novel nucleoprotein complex at a replication origin. Science. 1990 May 25;248(4958):1012–1016. doi: 10.1126/science.2111580. [DOI] [PubMed] [Google Scholar]
- Strauss E. C., Kobori J. A., Siu G., Hood L. E. Specific-primer-directed DNA sequencing. Anal Biochem. 1986 Apr;154(1):353–360. doi: 10.1016/0003-2697(86)90536-1. [DOI] [PubMed] [Google Scholar]
- Vlcek C., Paces V. Nucleotide sequence of the late region of Bacillus phage phi 29 completes the 19,285-bp sequence of phi 29 genome. Comparison with the homologous sequence of phage PZA. Gene. 1986;46(2-3):215–225. doi: 10.1016/0378-1119(86)90406-3. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wengler G., Gross H. J. Terminal sequences of Sindbis virus-specific nucleic acids: identity in molecules synthesized in vertebrate and insect cells and characteristic properties of the replicative form RNA. Virology. 1982 Dec;123(2):273–283. doi: 10.1016/0042-6822(82)90261-6. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wengler G., Gross H. S. Replicative form of Semliki Forest virus RNA contains an unpaired guanosine. Nature. 1979 Dec 13;282(5740):754–756. doi: 10.1038/282754a0. [DOI] [PubMed] [Google Scholar]
- Yoo S. K., Ito J. Sequence requirements for protein-primed DNA replication of bacteriophage PRD1. J Mol Biol. 1991 Apr 20;218(4):779–789. doi: 10.1016/0022-2836(91)90266-9. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H., Friedmann T., Ito J. Nucleotide sequences at the termini of phi 29 DNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1336–1340. doi: 10.1073/pnas.78.3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaballos A., Salas M. Functional domains in the bacteriophage phi 29 terminal protein for interaction with the phi 29 DNA polymerase and with DNA. Nucleic Acids Res. 1989 Dec 25;17(24):10353–10366. doi: 10.1093/nar/17.24.10353. [DOI] [PMC free article] [PubMed] [Google Scholar]







