Abstract
Objectives
This study aimed to develop an inexpensive, readily available prognostic indicator in acute decompensated heart failure patients to guide management and improve outcome. Prognostic biomarkers for heart failure exist but are expensive and not routinely performed. Increasing plasma volume has been associated with worse outcomes.
Setting
UK University Teaching Hospital.
Design
Observational Cohort study.
Participants
967 patients with acute decompensated heart failure.
Methods
Haemoglobin and haematocrit were measured at admission and discharge and were used to calculate the plasma volume change using the Strauss-Davis-Rosenbaum formula.
Main outcome measures
Endpoints were death and the composite of death and/or heart failure hospitalisation. Change in plasma volume was added to ADHERE scoring to determine predictive value.
Results
During follow-up, 536 died and 626 died or were hospitalised with heart failure. Multivariable Cox models showed change in plasma volume was an independent predictor of mortality (hazard ratio (HR) [95% confidence interval (CI)]: 1.150 [1.031–1.283], p = 0.012) and death or heart failure hospitalisation (HR: 1.138 [1.029–1.259], p = 0.012). Kaplan–Meier analysis of change in plasma volume tertiles for outcome measures showed significant difference for the top tertile compared to the lower two. Multivariable analysis of change in plasma volume with ADHERE scoring showed change in plasma volume change remained an independent predictor of death (HR: 1.138 [1.026–1.261], p = 0.015) and death or heart failure hospitalisation (HR: 1.129 [1.025–1.243], p = 0.014).
Conclusions
Change in plasma volume over an admission can be used for prognostication and adds value to the ADHERE score. Change in plasma volume can be easily and inexpensively calculated from routine blood tests. Clinically, this may facilitate targeted treatment of acute decompensated heart failure patients at greatest risk.
Keywords: Plasma volume, acute heart failure, haematocrit, haemoglobin
Introduction
Heart failure affects at least 550,000 people in the UK1 with a prevalence of 7.84% in men aged over 75 years and 5.89% in women aged over 75 years.2 The incidence of heart failure is projected to increase.2 Prognosis of heart failure is poor with a quality of life worse than that of patients with chronic pulmonary disease and an estimated mortality at one year of 40%. Heart failure patients account for approximately 2% of all inpatient bed-days.2 The aim of this study was to develop an inexpensive method to guide patient management in acute decompensated heart failure patients using change in plasma volume.
In chronic heart failure increasing plasma volume has been shown to be associated with worse prognosis3 predicting mortality proportional to the increase in plasma volume independent of other factors such as left ventricular ejection fraction, urea, sodium, creatinine and albumin.3,4 In contrast, there is limited evidence evaluating the use of plasma volume to prognosticate acute decompensated heart failure. Furthermore, papers published have focused on chronic heart failure and have used plasma volume status rather than the change in plasma volume.
Accurate measurement of plasma volume is technically difficult and invasive, for example using pulmonary artery catheterisation.5–9 Clinical evaluation using signs and symptoms is known to be highly inconsistent.10–12 Of the clinical signs, jugular venous pressure and a third heart sound have been shown to be associated with morbidity.11,13 However, reliance on clinical signs is likely to lead to insufficient patient management.8 Equations have been validated to estimate plasma volume non-invasively. This includes the Strauss–Davis–Rosenbaum formula.8,14
The Strauss–Davis–Rosenbaum formula is used to estimate percentage change in plasma volume8,14
Percentage change in plasma volume = ([(Hb1/Hb2) × ((100-Hct2)/(100-Hct1))] −1) × 100
where 1 = baseline and 2 = end values. Hb = haemoglobin, Hct = haematocrit.
An easily accessible marker of prognosis such as change in plasma volume estimated from admission and discharge haemoglobin and haematocrit would enable targeted treatment of the most at-risk patients. An inexpensive, readily available method of estimating change in plasma volume to guide patient treatment may be useful in the clinical setting.
Methods
Study population
Haemoglobin and haematocrit were measured at admission and discharge in 967 patients admitted to University Hospitals of Leicester with acute decompensated heart failure between 2006 and 2011. In addition, demographic data, admission symptoms, admission observations, aetiology of heart failure, past medical history, drug history, electrocardiogram and chest X-ray findings, New York Heart Association (NYHA) functional classification, Acute Decompensated Heart Failure National Registry (ADHERE) risk score, admission and discharge blood tests and patient outcome were also recorded. Patients who were likely to have had a blood transfusion were excluded (defined as an increase in haemoglobin from admission to discharge of ≥30 g/dL. This observational cohort study complied with the Declaration of Helsinki and was approved by the local ethics committee; written informed consent was obtained from patients.
ADHERE score
The ADHERE risk score stratifies patients admitted with acute heart failure by mortality risk.15 In the original study, blood urea nitrogen level of ≥15.35 mmol/L, serum creatinine level of ≥243.1 µmol/L and systolic blood pressure of <115 mmHg were high-risk independent predictors of in-hospital mortality.15 In this study patients with acute decompensated heart failure were stratified into five groups ranging from low to high (1–5) risk using the ADHERE registry model.15 Groups were defined as 1 (urea < 15.35 mmol/L and systolic blood pressure ≥ 115 mmHg), 2 (urea < 15.35 mmol/L and systolic blood pressure < 115 mmHg), 3 (urea ≥ 15.35 mmol/L and systolic blood pressure ≥ 115 mmHg), 4 (urea ≥ 15.35 mmol/L, systolic blood pressure < 115 mmHg and creatinine level < 243.1 µmol/L) and 5 (urea ≥ 15.35 mmol/L, systolic blood pressure < 115 mmHg and creatinine level ≥243.1 µmol/L).
Estimation of change in plasma volume
In this study changes in plasma volume were calculated using the haematocrit and haemoglobin at admission and discharge and the difference between these values was the change in plasma volume. The Strauss–Davis–Rosenbaum formula used is shown in the ‘Introduction’ section.
Endpoints
Endpoints were death, and the composite of death and/or heart failure hospitalisation. Heart failure hospitalisation was defined as an admission for which heart failure was the primary reason, with symptoms and signs of heart failure and necessitating the use of diuretics, nitrates or inotrope therapy. Endpoints were obtained by reviewing records in the local hospital databases and the Office of National Statistics Registry. Patients were followed up for a maximum of 3168 days with most patients followed for at least 1800 days.
Statistical analysis
Statistical analyses were performed with SPSS version 22 (IBM SPSS Statistics, IBM Corporation, Armonk, New York, USA). Changes in plasma volume were Z transformed (normalised to 1 SD increment) to make values more comparable with other parameters.
Cox survival regression was used to test the prognostic ability of change in plasma volume to predict patient outcome. The multivariate analysis (model 1) included variables which are known to be significant for predicting outcome in heart failure (age, sex, admission systolic blood pressure, admission New York Heart Association functional classification, past medical history of diabetes, ischaemic heart disease, heart failure and hypertension, admission urea, sodium and creatinine). The Z transform of change in plasma volume was added to these base models and the comparative prognostic power was assessed (model 2). Another relative Cox survival regression model was used to analyse whether change in plasma volume significantly improved the value of the ADHERE risk score for prognostic value. Unless otherwise stated, results are expressed as hazard ratio (HR) (95% confidence interval), p value for statistical significance.
Kaplan–Meier survival analysis was performed to visualise the prognostic utility of change in plasma volume for survival. Patients were split into tertiles of change in plasma volume. The tertiles were plotted against death, and death or heart failure hospitalisation.
Classification and regression trees for predicting inpatient mortality were constructed using Chi-squared Automatic Interaction Detection (CHAID; performed using SPSS), which detects which biomarker has the strongest interaction with the dependent variable (death, or death and/or heart failure) in step-wise analysis. A two-sided p value of <0.05 was deemed to be statistically significant.
Results
Baseline patient characteristics
A total of 967 patients were analysed. Table 1 shows the population demographics for continuous and categorical variables. Six hundred and two (62.3%) were male gender. Thirty-six per cent had a past medical history of heart failure, 32% had a past medical history of ischaemic heart disease, 58% had a past medical history of hypertension and 33% had a past medical history of diabetes. During follow-up, 536 (55%) died and 626 (65%) died and/or were hospitalised with heart failure.
Table 1.
Characteristics of the 967 acute decompensated heart failure patients according to change in PVol tertiles.
| Change PVol tertiles |
|||||
|---|---|---|---|---|---|
| All | 1 <5.08% | 2 −5.00 to 7.37% | 3 >7.41% | ||
| n = 967 | n = 322 | n = 323 | n = 322 | p value | |
| Change in PVol (%) | 3.06 ± 17.22 | −13.8 ± 7.68 | 1.02 ± 3.57 | 22.0 ± 13.2 | <0.001 |
| Z change PVol | −0.08 ± 0.81 | −0.86 ± 0.36 | −0.17 ± 0.17 | 0.809 ± 0.616 | <0.001 |
| Demographics | |||||
| Age (years) | 74.3 ± 12.4 | 73.5 ± 12.5 | 74.6 ± 12.5 | 74.7 ± 12.2 | NS |
| Male (%) | 602 (62) | 207 (64) | 191 (59) | 204 (63) | NS |
| Admission SBP | 133 ± 27 | 133 ± 26.1 | 133 ± 26.5 | 133 ± 29.6 | NS |
| Previous history | |||||
| Heart failure | 344 (36) | 116 (36) | 110 (34) | 118 (37) | NS |
| Ischaemic heart disease | 309 (32) | 99 (31) | 96 (30) | 114 (35) | NS |
| Hypertension | 560 (58) | 183 (57) | 193 (60) | 184 (57) | NS |
| Diabetes mellitus | 315 (33) | 102 (32) | 118 (37) | 95 (30) | NS |
| NYHA class | |||||
| ≤2 | 56 (5.8) | 20 (6.2) | 20 (6.2) | 16 (5.0) | NS |
| 3 | 394 (41) | 145 (45) | 120 (37) | 129 (40) | NS |
| 4 | 502 (52) | 152 (47) | 177 (55) | 173 (54) | NS |
| ADHERE score | |||||
| 1 | 595 (62) | 196 (61) | 201 (62) | 198 (62) | NS |
| 2 | 164 (17) | 58 (18) | 51 (16) | 55 (17) | NS |
| 3 | 100 (10) | 30 (9.3) | 40 (12) | 30 (9.3) | NS |
| 4 | 52 (5.4) | 16 (5.0) | 15 (4.6) | 21 (6.5) | NS |
| 5 | 12 (1.2) | 7 (2.2) | 3 (0.9) | 2 (0.6) | NS |
| Admission blood tests | |||||
| Creatinine (µmol/L) | 127 ± 55.2 | 126 ± 57.3 | 124 ± 52.9 | 131 ± 55.2 | NS |
| Sodium (mmol/L) | 137 ± 5.4 | 137 ± 5.1 | 137 ± 6.0 | 137 ± 5.1 | NS |
| Urea (mmol/L) | 10.9 ± 6.6 | 10.8 ± 6.6 | 10.8 ± 6.9 | 11.0 ± 6.2 | NS |
| Hb (g/dL) | 123 ± 19.9 | 115 ± 19.6 | 123 ± 18.3 | 131 ± 18.6 | <0.001 |
| Hct (L/L) | 0.370 ± 0.060 | 0.352 ± 0.054 | 0.372 ± 0.053 | 0.396 ± 0.054 | <0.001 |
| Discharge blood tests | |||||
| Hb (g/dL) | 121 ± 19.3 | 126 ± 20.2 | 122 ± 18.3 | 116 ± 18.0 | <0.001 |
| Hct (L/L) | 0.370 ± 0.056 | 0.389 ± 0.056 | 0.371 ± 0.053 | 0.353 ± 0.054 | <0.001 |
| End points | |||||
| Time to death (days) | 769 ± 653 | 777 ± 639 | 829 ± 680 | 701 ± 633 | 0.043 |
| Time to death and/or HF hospitalisation (days) | 639 ± 635 | 664 ± 634 | 693 ± 663 | 561 ± 601 | 0.022 |
| Death | 536 (55) | 160 (50) | 175 (54) | 201 (62) | 0.004 |
| Death and/or HF hospitalisation | 626 (64.7) | 193 (60) | 207 (64) | 226 (70) | 0.024 |
Numerical data are presented as n (%). The numbers (%) or mean ± SD are reported. p values are quoted for the ANOVA or Chi-squared tests for continuous or categorical variables, respectively. ADHERE: Acute Decompensated Heart Failure National Registry; ADHF: acute decompensated heart failure; HF: heart failure; NYHA: New York Heart Association; PVol: plasma volume; SBP: systolic blood pressure.
Survival analysis
Cox survival analysis revealed that change in plasma volume calculated using the Strauss–Davis–Rosenbaum formula is significant for predicting death and death and/or heart failure hospitalisation in acute decompensated heart failure patients. Table 2 shows the results of Cox survival analysis for death and Table 3 shows the results for death and/or heart failure hospitalisation. For both outcomes, a univariate analysis was completed initially. Multivariate analysis for model 1 included significant variables. Multivariate analysis for model 1 included the variables in model 1 with the addition of Z change in plasma volume as a continuous variable. New York Heart Association functional classification 1 and 2 included five and 51 cases, respectively. Individually, class 1 and 2 had too few cases for regression modelling so the two groups were combined into a ‘New York Heart Association ≤ 2’ group. Multivariable Cox regression models found that change in plasma volume was an independent predictor of mortality (HR = 1.150 [1.031–1.283], p = 0.012) and death and/or heart failure hospitalisation (HR = 1.138 [1.029–1.259], p = 0.012).
Table 2.
Cox regression analysis for death.
| Univariable HR (95% CI) | p | Multivariable model 1 HR (95% CI) | p | Multivariable model 2 HR (95% CI) | p | |
|---|---|---|---|---|---|---|
| Age (years) | 1.046 (1.037–1.055) | 0.000 | 1.044 (1.034–1.055) | 0.000 | 1.044 (1.034–1.055) | 0.000 |
| Male sex | 0.974 (0.819–1.158) | NS | 1.050 (0.869–1.269) | NS | 1.061 (0.878–1.282) | NS |
| Admission creatinine (µmol/L) | 1.006 (1.005–1.008) | 0.000 | 1.000 (0.998–1.003) | NS | 1.000 (0.998–1.003) | NS |
| Admission urea (mmol/L) | 1.064 (1.052–1.076) | 0.000 | 1.039 (1.018–1.061) | 0.000 | 1.041 (1.020–1.063) | 0.000 |
| Admission sodium (mmol/L) | 0.980 (0.966–0.994) | 0.005 | 0.974 (0.959–0.989) | 0.000 | 0.972 (0.957–0.987) | 0.000 |
| Admission systolic BP (mmHg) | 0.993 (−.990–0.997) | 0.000 | 0.995 (0.991–0.998) | 0.005 | 0.995 (0.992–0.999) | 0.008 |
| Past history | ||||||
| Ischaemic heart disease | 1.362 (1.146–1.619) | 0.000 | 1.168 (0.971–1.404) | NS | 1.139 (0.946–1.371) | NS |
| Hypertension | 1.078 (0.907–1.281) | NS | 0.845 (0.699–1.021) | NS | 0.866 (0.716–1.047) | NS |
| HF | 1.561 (1.313–1.855) | 0.000 | 1.096 (0.908–1.323) | NS | 1.115 (0.923–1.347) | NS |
| Diabetes | 1.219 (1.021–1.456) | 0.029 | 1.245 (1.027–1.508) | 0.025 | 1.244 (1.027–1.507) | 0.026 |
| NYHA functional classification | ||||||
| ≤2 (Reference) | 1 | 1 | 1 | |||
| 3 | 3.500(1.955–6.264) | 0.000 | 2.598 (1.411–4.785) | 0.002 | 2.574 (1.398–4.740) | 0.002 |
| 4 | 4.989 (2.797–8.898) | 0.000 | 3.283 (1.783–6.043) | 0.000 | 3.225 (1.751–5.937) | 0.000 |
| Z change PVol | 1.151 (1.039–1.274) | 0.007 | Excluded | 1.150 (1.031–1.283) | 0.012 | |
Multivariable analysis results are reported for model 1 which included variables which were significant on univariable analysis. Multivariable model 2 used the variables in model 1 with the addition of change in PVol as a continuous variable. BP: blood pressure; CI: confidence interval; HF: heart failure; NYHA: New York Heart Association; PVol: plasma volume.
Table 3.
Cox regression analysis for death or HF hospitalisation.
| Univariable HR (95% CI) | p | Multivariable model 1 HR (95% CI) | p | Multivariable model 2 HR (95% CI) | p | |
|---|---|---|---|---|---|---|
| Age (years) | 1.031 (1.023–1.038) | 0.000 | 1.027 (1.018–1.035) | 0.000 | 1.027 (1.018–1.035) | 0.000 |
| Male sex | 1.003 (0.854–1.179) | NS | 1.008 (0.846–1.202) | NS | 1.017 (0.853–1.212) | NS |
| Admission creatinine (µmol/L) | 1.005 (1.004–1.006) | 0.000 | 1.000 (0.998–1.002) | NS | 1.000 (0.997–1.002) | NS |
| Admission urea (mmol/L) | 1.051 (1.040–1.063) | 0.000 | 1.030 (1.010–1.050) | 0.003 | 1.032 (1.012–1.052)) | 0.002 |
| Admission sodium (mmol/L) | 0.986 (0.972–0.999) | 0.033 | 0.985 (0.971–0.999) | 0.034 | 0.984 (0.970–0.998) | 0.025 |
| Admission systolic BP (mmHg) | 0.994 (0.991–0.997) | 0.000 | 0.996 (0.993–0.999) | 0.012 | 0.996 (0.993–0.999) | 0.015 |
| Past history | ||||||
| HF | 1.647 (1.404–1.932) | 0.000 | 1.217 (1.021–1.450) | 0.028 | 1.230 (1.032–1.466) | 0.021 |
| Ischaemic heart disease | 1.446 (1.232–1.697) | 0.000 | 1.244 (1.049–1.476) | 0.012 | 1.215 (1.023–1.443) | 0.026 |
| Hypertension | 1.145 (0.975–1.344) | NS | 0.922 (0.775–1.097) | NS | 0.941 (0.790–1.121) | NS |
| Diabetes | 1.368 (1.161–1.611) | 0.000 | 1.346 (1.129–1.605) | 0.001 | 1.343 (1.126–1.601) | 0.001 |
| NYHA functional classification | ||||||
| ≤2 (Reference) | 1 | 1 | 1 | |||
| 3 | 2.368 (1.516–3.700) | 0.000 | 1.816 (1.145–2.881) | 0.011 | 1.824 (1.150–2.894) | 0.011 |
| 4 | 3.263 (2.096–5.080) | 0.000 | 2.245 (1.414–3.564) | 0.001 | 2.234 (1.407–3.547) | 0.001 |
| Z change PVol | 1.139 (1.036–1.252) | 0.007 | Excluded | 1.138 (1.029–1.259) | 0.012 | |
Multivariable analysis results are reported for model 1 which included variables which were significant on univariable analysis. Multivariable model 2 used the variables in model 1 with the addition of change in PVol as a continuous variable. BP: blood pressure; CI: confidence interval; HF: heart failure; NYHA: New York Heart Association; PVol: plasma volume.
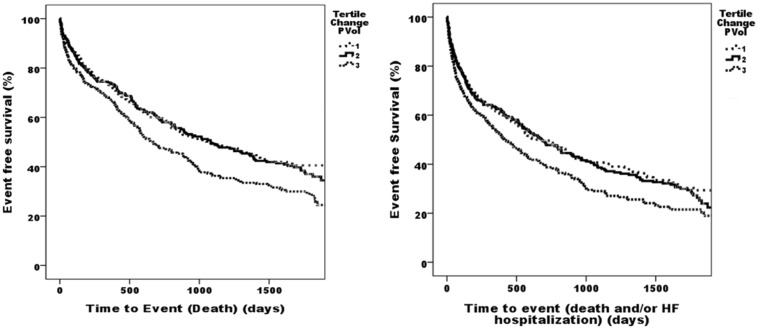
Kaplan–Meier survival analysis was performed with patients split into tertiles by change in plasma volume. Survival was plotted against their tertile group. Tertiles for change in plasma volume were found to be significant for predicting death and for predicting death of heart failure. Kaplan–Meier survival analysis comparing the highest tertile of change in plasma volume to the two lowest for death was significant for log rank (Mantel–Cox) analysis (p = 0.001). Analysis for death and/or heart failure hospitalisation was significant for log rank (Mantel–Cox) analysis (p = 0.002) (Figure 1).
Figure 1.
Kaplan–Meier curves showing death and death and/or HF hospitalisation of ADHF patients HF per tertile for Z change PVol. ADHF: acute decompensated heart failure; HF: heart failure; PVol: plasma volume.
Comparison with ADHERE risk scores
Cox survival analysis was carried out to determine whether change in plasma volume added value to the ADHERE risk score. Table 4 shows the Cox survival analysis of change in plasma volume and ADHERE risk score with death and Table 5 shows the Cox survival analysis of change in plasma volume and ADHERE risk score with death and/or heart failure hospitalisation. Change in plasma volume was found to add to the prognostic value for death of the ADHERE risk score (p = 0.015, Table 4) and the prognostic value for death and/or heart failure (p = 0.014, Table 5). Multivariate analysis of change in plasma volume with ADHERE scoring showed that change in plasma volume improved the ability of the ADHERE score to predict death (HR = 1.138 [1.026–1.261], p = 0.015) and death or heart failure hospitalisation (HR = 1.129 [1.025–1.243], p = 0.014).
Table 4.
Cox survival analysis for ADHERE score and Z change PVol for death.
| Model of ADHERE score with Z change PVol HR (95% CI) | p | |
|---|---|---|
| ADHERE 1 (Reference) | 1 | |
| ADHERE 2 | 1.150 (0.907–1.458) | NS |
| ADHERE 3 | 2.272 (1.746–2.954) | 0.000 |
| ADHERE 4 | 2.800 (2.021–3.879) | 0.000 |
| ADHERE 5 | 4.541 (2.406–8.568) | 0.000 |
| Z change PVol | 1.138 (1.026–1.261) | 0.015 |
ADHERE: Acute Decompensated Heart Failure National Registry; CI: confidence interval; PVol: plasma volume.
Table 5.
Cox survival analysis for ADHERE score and Z change PVol for death and/or HF hospitalisation.
| Model of ADHERE score with Z change PVol HR (95% CI) | p | |
|---|---|---|
| ADHERE 1 (Reference) | 1 | |
| ADHERE 2 | 1.122 (0.902–1.397) | NS |
| ADHERE 3 | 1.908 (1.485–2.452) | 0.000 |
| ADHERE 4 | 2.436 (1.783–3.328) | 0.000 |
| ADHERE 5 | 3.434 (1.826–6.458) | 0.000 |
| Z change PVol | 1.129 (1.025–1.243) | 0.014 |
ADHERE: Acute Decompensated Heart Failure National Registry; CI: confidence interval; HF: heart failure; PVol: plasma volume.
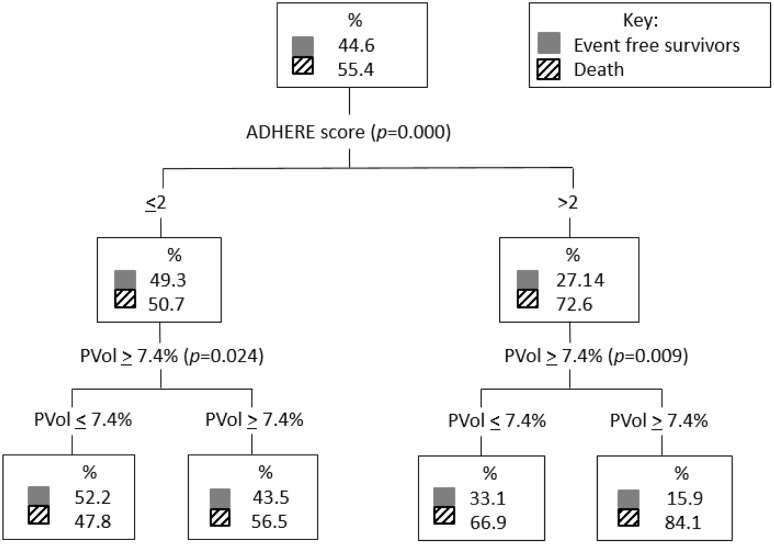
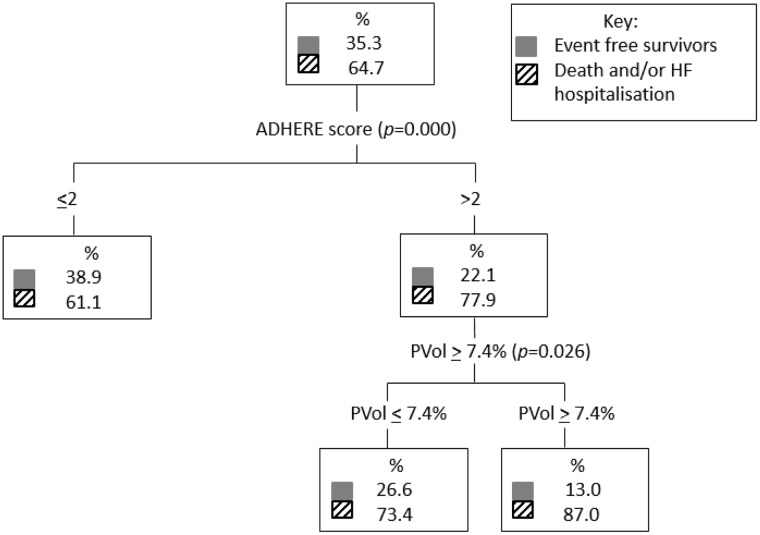
A classification and regression trees analysis comparing ADHERE score 1–5 and plasma volume lower tertiles (tertiles 1 and 2) and upper tertile (tertile 3) which corresponded to change in plasma volume <7.4% versus ≥7.4%, respectively, found that plasma volume further stratified the risk in both the low and high ADHERE risk groups for the death endpoint (Figure 2). For the death and/or heart failure hospitalisation outcome, plasma volume further stratifies higher-risk patients (Figure 3).
Figure 2.
Classification and regression trees analysis comparing ADHERE score 1–5 and PVol lower tertiles (PVol ≤ 7.4%) and upper tertile (PVol ≥ 7.4%) for the death endpoint. ADHERE: Acute Decompensated Heart Failure National Registry; CART: classification and regression trees; PVol: plasma volume.
Figure 3.
Classification and regression trees analysis comparing ADHERE score 1–5 and PVol lower tertiles (PVol ≤ 7.4%) and upper tertile (PVol ≥ 7.4%) for the dead and/or HF hospitalisation endpoint. ADHERE: Acute Decompensated Heart Failure National Registry; CART: classification and regression trees; HF: heart failure; PVol: plasma volume.
Discussion
In this observational cohort study of acute decompensated heart failure patients, we found change in plasma volume to be prognostic for death and/or heart failure hospitalisation and all-cause mortality in acute decompensated heart failure. This was independent of other covariates such as age, sex, systolic blood pressure, past history of hypertension, diabetes, heart failure, hypertension or ischaemic heart disease, serum creatinine, urea and sodium and New York Heart Association functional score. Unlike other molecular biomarkers of heart failure, change in plasma volume is calculated from routine blood test results without additional expense.
Change in plasma volume augments the value of the ADHERE risk score in predicting death or heart failure hospitalisation and all-cause mortality, as seen in Figures 2 and 3 with additional risk stratification at both higher and lower ADHERE risk groups.
This is a unique study due to its large size and application in acute decompensated heart failure. There is limited evidence (in the form of abstracts) evaluating change in plasma volume (utilising the Strauss–Davis–Rosenbaum formula) and prognosis in acute decompensated heart failure.4,16 Other studies looking at plasma volume and its prognostic capability have evaluated either interval change in haemoglobin, haematocrit, weight or a combination of haematocrit and weight in acute decompensated heart failure and chronic heart failure.16,17 The largest studies have been post hoc studies in chronic heart failure populations and are not directly comparable due to differences in the definition plasma volume change or status.7,16–18
A similar study by Norrington et al. found a mortality rate with a HR of 1.11 to predict death at six months and 12 months which is comparable to our study where a HR of 1.15 was found.4 More robust data from post hoc analysis of the EVEREST study (also conducted in acute decompensated heart failure) found that haemoconcentration was associated with improved congestion and decreased mortality.17 Dilutional anaemia was more likely in HFREF while a red-cell deficit was more likely in HFPEF.19
The mechanism by which changes in plasma volume affect prognosis is not well understood. One recent explanation has been that plasma volume expansion results in reduction in intracellular volume that contributes to worsening renal function (WRF) in heart failure patients.20,21 Worsening renal function is used to describe a decline in renal function without renal injury in patients with heart failure.22 It appears to have prognostic value. The most widely accepted definition of worsening renal function is a rise in creatinine of at least 26.5 µg/mol (0.3 mg/dL) from baseline.20,22–24 Our study found that change in plasma volume remains independently predictive of mortality despite including admission urea and creatinine as covariates.
Molecular biomarkers for prediction of outcome in heart failure exist such as NT-proBNP but are costly and are not routinely performed,25,26 especially with an ever-increasing pressure on existing finite healthcare resources. Active monitoring of change in plasma volume (estimated using serum haemoglobin and haematocrit values) during an acute decompensated heart failure hospital episode could guide in hospital patient management.3,4,16,18 For instance, clinicians could optimise diuresis, aiming for a low–medium-risk change in plasma volume in order to avoid worsening renal function which has been associated with poor prognosis.27 In addition, change in plasma volume from admission to discharge could be used at discharge by clinicians without access to routine in-hospital BNP/NTproBNP testing to prioritise earlier postdischarge follow-up visits, which may allow for intensified heart failure therapy in those at highest risk, although the actual benefit from either intervention will require testing in a prospective controlled study.
Study limitations
The study was based on data collected at a single centre with two admitting hospitals and extrapolation to the wider population requires verification. plasma volume was estimated using a formula. It would have been ideal to compare the estimated plasma volume to the actual measured plasma volume to further validate the formula but measured plasma volume was not available in this patient sample and is invasive. The formula used has been validated in chronic, not acute heart failure.3,4
Patients may have received a blood transfusion during their admission. Information on transfusion was not available. To account for patients who were likely to have had a blood transfusion, patients were excluded if their haemoglobin increased by 30 g/dL or more during the admission. Clinical effectiveness studies are required to assess the use of change in plasma volume for management strategies in patients identified to have differing prognostic risk.
Conclusion
Change in plasma volume over an admission can be used for prognostication in acute decompensated heart failure and adds value to an existing estimator of risk, the ADHERE score. It can be easily and inexpensively calculated from routine blood tests. Clinically, this may facilitate risk stratification with targeted treatment of heart failure patients at greatest risk of an adverse outcome.
Declarations
Competing interests
None declared.
Funding
This work was supported by the John and Lucille Van Geest Foundation and the National Institute of Health Research, Leicester Cardiovascular Biomedical Research Unit.
Ethical approval
This study complied with the Declaration of Helsinki and was approved by the Derbyshire research ethics committee (ethics approval number 06/Q2401/112); written informed consent was obtained from patients.
Guarantor
LLN.
Contributorship
SRH and LLN were responsible for study design and conduct. SRH collected and analysed the data and wrote the paper. DC and LLN analysed the data and made substantial contributions to the manuscript.
Acknowledgements
The authors wish to thank Florence Lai for assistance with the statistical analysis.
Provenance
Not commissioned; peer-reviewed by Edward Absoud and Michal Polguj.
References
- 1.Townsend N, Williams J, Bhatnagar P, Wickramasinghe K and Rayner M. Cardiovascular Disease Statistics 2014. British Heart Foundation Centre on Population Approaches for Non-Communicable diseases. London: University of Oxford. British Heart Foundation, 2014.
- 2.Petersen S, Rayner M, Wolstenholme J. Coronary Heart Disease Statistics: Heart Failure Supplement, London: British Heart Foundation, 2002. [Google Scholar]
- 3.Papalia F, Aung N, Ling HZ, Aggarwal S, Flint J, Connell A, et al. Prognostic utility of the hemoglobin/hematocrit equation for estimating plasma volume changes over time in chronic heart failure. Circulation 2011; 124: A16398–A16398. [Google Scholar]
- 4.Norrington KD, Turner HK, Barakat MF, Konstantinou K, Kelshikir M, O’Driscoll S, et al. Prognostic utility of the hemoglobin/hematocrit equation for estimating plasma volume changes during hospitalization for acute decompensated heart failure. Circulation 2012; 126: A17469–A17469. [Google Scholar]
- 5.Anand IS, Ferrari R, Kalra GS, Wahi PL, Poole-Wilson PA, Harris PC. Edema of cardiac origin. Studies of body water and sodium, renal function, hemodynamic indexes, and plasma hormones in untreated congestive cardiac failure. Circulation 1989; 80: 299–305. [DOI] [PubMed] [Google Scholar]
- 6.Binanay C, Califf RM, Hasselblad V, O’Connor CM, Shah MR, Sopko G, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. J Am Med Assoc 2005; 294: 1625–1633. [DOI] [PubMed] [Google Scholar]
- 7.Dupont M, Mullens W, Tang WHW. Impact of systemic venous congestion in heart failure. Curr Heart Failure Rep 2011; 8: 233–241. [DOI] [PubMed] [Google Scholar]
- 8.Kalra PR, Anagnostopoulos C, Bolger AP, Coats AJ, Anker SD. The regulation and measurement of plasma volume in heart failure. J Am College Cardiol 2002; 39: 1901–1908. [DOI] [PubMed] [Google Scholar]
- 9.Pettit JE and Lewis SM. Recommended methods for measurement of red-cell and plasma volume: International Committee for Standardization in Haematology. J Nucl Med 1980; 21: 793–800. [PubMed]
- 10.Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JEA, Cleland JG, et al. Assessing and grading congestion in acute heart failure: a scientific statement from the Acute Heart Failure Committee of the Heart Failure Association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail 2010; 12: 423–433. [DOI] [PubMed] [Google Scholar]
- 11.Rame JE, Dries DL, Drazner MH. The prognostic value of the physical examination in patients with chronic heart failure. Congest Heart Fail 2003; 9: 170–178. [DOI] [PubMed] [Google Scholar]
- 12.Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart-failure. J Am Med Assoc 1989; 261: 884–888. [PubMed] [Google Scholar]
- 13.Drazner MH, Rame JE, Stevenson LW, Dries DL. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. N Engl J Med 2001; 345: 574–581. [DOI] [PubMed] [Google Scholar]
- 14.Strauss M, Davis R, Rosenbaum J, Rossmeisl EC. Water diuresis produced during recumbency by the intravernous infusion of isotonic saline solution. J Clin Invest 1951; 30: 862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fonarow GC, Adams KF, Abraham WT, Yancy CW, Boscardin WJ, ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure – classification and regression tree analysis. J Am Med Assoc 2005; 293: 572–580. [DOI] [PubMed] [Google Scholar]
- 16.Ling HZ, Aung N, Cheng A, Flint J, Aggarwal S, Mendonca M, et al. Plasma volume status calculated instantaneously from weight and hematocrit relates to objectively measured plasma volumes and powerfully predicts death and first morbid events in chronic heart failure. Circulation 2012; 126: A18594–A18594. [Google Scholar]
- 17.Greene SJ, Gheorghiade M, Vaduganathan M, Ambrosy AP, Mentz RJ, Subacius H, et al. Haemoconcentration, renal function, and post-discharge outcomes among patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Eur J Heart Fail 2013; 15: 1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ling HZ, Flint J, Damgaard M, Bonfils PK, Cheng AS, Aggarwal S, et al. Calculated plasma volume status and prognosis in chronic heart failure. Eur J Heart Fail 2015; 17: 35–43. [DOI] [PubMed] [Google Scholar]
- 19.Abramov D, Cohen RS, Katz SD, Mancini D, Maurer MS. Comparison of blood volume characteristics in anemic patients with low versus preserved left ventricular ejection fractions. Am J Cardiol 2008; 102: 1069–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation 2010; 122: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davila C, Reyentovich A, Katz SD. Clinical correlates of hemoconcentration during hospitalization for acute decompensated heart failure. J Card Fail 2011; 17: 1018–1022. [DOI] [PubMed] [Google Scholar]
- 22.Damman K, Tang WHW, Testani JM, McMurray JJV. Terminology and definition of changes renal function in heart failure. Eur Heart J 2014; 35: 3413–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am College Cardiol 2000; 35: 681–689. [DOI] [PubMed] [Google Scholar]
- 24.Wattad M, Darawsha W, Solomonica A, Hijazi M, Kaplan M, Makhoul BF, et al. Interaction between worsening renal function and persistent congestion in acute decompensated heart failure. Am J Cardiol 2015; 115: 932–937. [DOI] [PubMed] [Google Scholar]
- 25.Januzzi JL, Camargo CA, Anwaruddin S, Baggish AL, Chen AA, Krauser DG, et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol 2005; 95: 948–954. [DOI] [PubMed] [Google Scholar]
- 26.Januzzi JL, van Kimmenade R, Lainchbury J, Bayes-Genis A, Ordonez-Llanos J, Santalo-Bel M, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients. Eur Heart J 2006; 27: 330–337. [DOI] [PubMed] [Google Scholar]
- 27.Abo-Salem E, Sherif K, Dunlap S, Prabhakar S. Potential aetiologies and prognostic implications of worsening renal function in acute decompensated heart failure. Acta Cardiol 2014; 69: 657–663. [DOI] [PubMed] [Google Scholar]