Abstract
Extensive research has documented evidence for rule learning in sequential behavior tasks in both rats and humans. We adapted the 2-choice serial multiple choice (SMC) task developed for use with rats (Fountain & Rowan, 1995a) to study sequence behavior in pigeons. Pigeons were presented with 8 disks arranged in a circular array on a touchscreen, and pecking to an illuminated disk could lead to reward. Correct responding consisted of serial patterns involving ‘run’ chunks of three elements (123 234, etc.). Some pigeons experienced a violation of the chunk rule in the final chunk. Unlike rats, pigeons made fewer errors on Violation chunks than Run chunks, suggesting the use of low-level cues to guide choices. Removal of low-level cues and increasing the number of simultaneously-illuminated disks to an 8-choice SMC task resulted in more errors on the Violation chunk. Pigeons were able to use the rule when the array of disks was contracted or expanded, and when chunk length was extended to four and five elements, but not when disks were removed from or added to the array. Pigeons were also able to abstract structure from a ‘trill’ pattern (121 232 etc.), as shown by high error rates on a Violation trial. These results suggest that pigeons, like rats and humans, can abstract sequence structure, but do so primarily in the absence of specific low-level feature-based information.
Keywords: pigeons, serial pattern learning, sequence learning, rule learning, pattern learning, associative learning
Serial pattern learning is the process of learning to organize behavior through time, typically by learning to anticipate or respond to a sequence of events or to learn to properly organize a sequence of behavior. This type of learning is likely a fundamental capacity in human and nonhuman animals (Sun & Giles, 2001a). Sequences “play a pivotal role in classical studies of instrumental conditioning, in human skill learning, and in human high-level problem solving and reasoning” (p. 2, Sun & Giles, 2001b). For example, getting behavior into the correct order is fundamental to a broad range of activities from those involving simpler rote learning (e.g., reciting the digits of your Social Security number in the correct order) to highly sophisticated rule-based cognitive processes involved in production (and perception/memory) of remarkably complex sequences (e.g., in speech, the same words in different order have different meanings). Other cognitive processes involved in serial pattern learning such as ‘chunking’, that is, cognitively breaking a serial pattern into component ‘phrases’ (e.g., cognitively breaking a musical piece into it’s component phrases or organizing words into phrases in speech production), also play an important role in facilitating our ability to learn and produce complex behavioral sequences (Fountain & Benson, Jr., 2006; Fountain, Henne, & Hulse, 1984; Fountain et al., 2007; Lashley, 1951; Simon, 1974; Terrace & McGonigle, 1994). Both humans and other animals are sensitive to ‘phrasing cues’ like pauses in sequences that strongly bias the chunking process (Fountain, 2006; Fountain et al., 1984; Fountain et al., 2007; Restle, 1972).
Despite the importance of serial pattern learning in everyday behavior, there has been disagreement regarding the nature of the behavioral and neural processes that subserve it. For example, theories of serial pattern learning have ranged from those positing simple associative learning mechanisms to those proposing that serial pattern learning depends on abstract cognitive capacities (cf. Fountain, 2006; 2008; Fountain et al., 2002). Similarly, neurobehavioral studies have suggested the involvement of frontal cortex, other cortical areas, hippocampus, and basal ganglia in serial pattern learning (e.g., Dehaene et al., 2015; Doyon et al., 1997). Not surprisingly, impairments of cognitive and motor performance in serial pattern learning tasks have been associated with neocortex, hippocampus, and subcortical structures, areas that are damaged by Alzheimer’s disease (Ferraro, Balota, & Connor, 1993), Parkinson’s disease (Ferraro et al.; Siegert et al., 2006), Huntington’s disease (Knopman & Nissen, 1991), and in obsessive-compulsive disorder (Rauch et al., 2007).
Models of serial pattern learning attempt to describe how humans and other animals learn to predict events or produce responses that occur in the same serial order, that is, in serial patterns. Empirical work has found that humans and other animals have much in common in terms of serial pattern learning and the processes that seem to be responsible for sequential behavior (Fountain, 2006; Fountain & Rowan, 1995a; b; Kesner, 2002; McGonigle & Chalmers, 2002; Sands & Wright, 1980; Sands & Wright, 1982; Terrace & McGonigle, 1994). Humans show the natural ability to abstract regularities from the events in our world. This has been shown through studies of statistical learning (Aslin & Newport, 2012), concept learning (Fisher, Pazzani, & Langley, 2014), and pattern learning (Brown et al., 2010). In an early study of serial pattern learning in humans in a nonverbal task, Restle and Brown (1970) required subjects to make repeated choices from a six-button array. After each choice, the subject received feedback via lights above the buttons as to whether their choice was correct. It was found that humans could be sensitive to the structure of patterns presented across successive button presses. For instance, if a part of a sequence consisted of a ‘run’ (e.g., 1 2 3 4) or a ‘trill’ (e.g., 1 2 1 2), then errors would consist of a continuation of these patterns beyond their structure.
The task described by Restle and Brown (1970) is interesting as it provides a method to study serial pattern learning – a process thought to be essential in language learning – in a non-language context. Developing a procedure to study serial pattern learning that does not involve language is especially important as it may then be adapted to other animal species as well as pre-verbal infants and patients with language disorders.
Serial Pattern Learning in Rats
Fountain and colleagues adapted a similar procedure to search for evidence of serial pattern learning in rats. In one study involving a 2-choice Serial Multiple Choice (SMC) procedure, rats were placed in an octagonal chamber with a retractable lever mounted on each wall (Fountain & Rowan, 1995a). On each trial, two levers were presented to the animal. If the correct lever in the sequence was pressed, the rat received hypothalamic brain-stimulation reward, both levers were retracted, and a delay was initiated before the next trial. If the incorrect lever was pressed, the incorrect lever was removed and reward was not given until the other, remaining lever was pressed. As with the Restle and Brown (1970) study, correct responses were defined by either ‘run’ or ‘trill’ patterns. The patterns were divided into 3-element chunks, with a 1-second pause within a chunk and a 3-second pause between chunks. Hence, for the Run group, the full sequence of correct responses was:
123 234 345 456 567 678 781 812
For the Trill group, the full sequence of correct responses was:
121 232 343 454 565 676 787 818
Each number in the sequence corresponds to one of the 8 levers in the octagonal chamber. The two levers presented at the beginning of a trial were always located on either side of the previously rewarded lever, so responses were not biased by location relative to a recently rewarded location. A number of hierarchical rules govern correct choice depending on location in the sequence. For the Run sequence, at the lowest level, there is a +1 structure that governs movement through the sequence within a chunk (implemented by Fountain and Rowan as a clockwise movement within the array of levers). At the chunk boundaries, there is a −1 rule, such that after the final selection within a chunk, the next correct lever is the one adjacent to the prior lever in a counterclockwise direction. For the trill sequence, an alternating rule governs correct choice within-chunk. At the chunk boundaries, a +1 rule governs the next correct response.
To assess the rat’s knowledge of the sequential pattern, and the rules that govern correct responses, half of the animals in each group were placed in a violation group. In the violation group, the last element in the final chunk did not follow the sequence and was instead consistent with the final chunk for the other group. Hence, for the Run Violation group, the full sequence was:
123 234 345 456 567 678 781 818
For the Trill Violation group, the full sequence of correct responses was:
121 232 343 454 565 676 787 812
If the rats were encoding the underlying sequence pattern, they should show high error rates on the final element of the final chunk (underlined above) in the violation groups alone. On the other hand, if the animals were only rote learning the serial sequence (e.g., by learning the inter-item associations, e.g., Wallace & Fountain, 2002), then error rates should be the same for both the violation and non-violation groups. Fountain and Rowan (1995a) found clear evidence of the underlying structure of the sequence influencing performance.
In similar research, Murphy, Mondragon, and Murphy (2008) demonstrated that rats can learn sequences when the stimuli vary. The rats were presented with sequences of stimuli that did (e.g., ABA) and did not (e.g., ABB) signal food reward. Following training on these sequences, rats were presented with novel stimuli that either did or did not correspond with the previously-rewarded sequence. It was found that the rats showed significantly more food-seeking behavior on trials that used the previously-rewarded sequence than on trials that did not, indicating that the underlying structure of the stimuli influenced behavior.
These results suggest that rats are capable of learning an underlying sequence or pattern, and use this knowledge to guide their responses. We may then ask whether other animals are also capable of being guided by underlying structure when presented with a sequence of stimuli.
Serial Pattern Learning in Pigeons
Pigeons are an attractive species to investigate given their ready availability and their highly-developed vision. Indeed, there is a long history of research examining the learning and cognitive abilities of pigeons (Colombo & Scarf, 2012; Cook, Katz, & Blaisdell, 2012; Herrnstein, Loveland & Cable, 1976; Wasserman, Kiedinger & Bhatt, 1988; Wright, 1997), including memory for items recently presented in a sequence (Cook & Blaisdell, 2006).
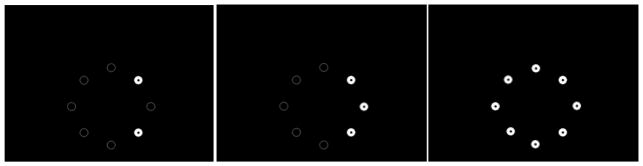
To adapt the 2-choice SMC procedure for pigeons, a touchscreen was used instead of an octagonal chamber. Eight circles in a circular arrangement were presented two at a time to the pigeon on the touchscreen (see Figure 1, left panel). The pigeon was rewarded by delivery of food in a hopper located below the screen. This procedure was similar to Fountain and Rowan (1995a), except that instead of extending levers, two circles were illuminated with a white background and central black dot. The other circles were shown as white outlines only (Figure 1, left panel). This adaptation resulted in other differences as well. For example, the procedure with rats involved the subject travelling around the octagonal apparatus to make its choices, while the pigeon procedure required the subject to stand facing the screen to make its choices. Such procedural differences could account for any species differences we may find. This issue will be discussed more fully in the General Discussion.
Figure 1.
A screen shot depicting the eight-circle display presented to the pigeons on the touchscreen. During the intertrial interval, all circles are dark with only a dim white outline of the each circle being visible. During a trial, either two circles (left panel, Experiment 1 Phases 1–3), three circles (middle panel, Experiment 1, Phase 4), or all eight circles (right panel, Experiment 1, Phase 5 and Experiments 2 and 3) were illuminated in the 2-choice, 3-choice, and 8-choice serial multiple choice (SMC) tasks, respectively.
For our initial adaptation of the procedure to pigeons, only the Run condition was investigated. Half of the birds were presented with the full Run sequence, while half of the birds were in a Violation group and were presented with the entire run sequence except for the final trial that violated this sequence (i.e., on the final chunk, the Run birds were rewarded for 812 and the Violation birds were rewarded with 818, with each number corresponding to a specific circle in the array). If pigeon behavior is similar to that of rats, it would be expected that there will be more errors on the final item of the final chunk for subjects in the Violation than in the Run group, and more errors on the final element of the final chunk than of the final element of the other chunks for subjects in the Violation group. Table 1 presents an overview of each experiment and experimental phases in this series. Experiment 1 investigated necessary task conditions that would establish behavioral control by sequence rules. Our initial 2-choice SMC procedure seemed to result in behavioral control by specific cues, rather than sequence rules. Thus, in separate phases we explored the effects of different phrasing cues (time and color), as well as increases in the number of choice options that were simultaneously available, starting with a 3-choice SMC procedure (Figure 1, middle panel), and finally an 8-chioce SMC procedure (Figure 1, right panel). The 8-choice SMC procedure seemed to break up the use of color and positional cues that had developed during training in earlier phases, especially with the addition of randomizing the start locations of the sequence across sessions.
Table 1.
| Exp. (Phase) | Procedure | Rule | Manipulation |
|---|---|---|---|
| 1 (1) | 2-choice SMC | Run | Replicate Fountain & Rowan (1995a) Run versus Violation. |
| 1 (2) | 2-choice SMC | Run | Use color as phrasing cues. |
| 1 (3) | 2-choice SMC | Run | Test role of color-position associations by randomizing trial order. |
| 1 (4) | 3-choice SMC | Run | Use reinforcement as a phrasing cue. |
| 1 (5) | 8-choice SMC | Run | All 8 choices available and randomized start locations across session. |
| 2 (1) | 8-choice SMC | Run | Test contractions and expansions of 8-circle array. |
| 2 (2) | 8-choice SMC | Run | Test removal and addition of elements with 4-circle and 16-circle arrays. |
| 2 (3) | 8-choice SMC | Run | Test within-chunk rule with 4-element and 5-element chunks. |
| 3 (1) | 8-choice SMC | Trill | Replicate Fountain & Rowan (1995a) Trill versus Violation. |
| 3 (2) | 8-choice SMC | Trill | Test immediate effects of randomized chunk order. |
| 3 (3) | 8-choice SMC | Trill | Test long-term effects of randomized chunk order. |
Note: SMC = serial multiple choice. Run = run rule of trial structure (123-234-345-456-567-678-812). Trill = trill rule structure (121-232-343-454-565-676-787-818). Violation = change in final chunk from run to trill, or from trill to run.
Having finally established behavioral control by sequence rules in Experiment 1, in Experiment 2 we then interrogated the generality of rule use by testing the pigeons on contractions and expansions of the array, the removal or addition of elements to the array, and extensions of chunk length.
In Experiment 3, a separate group of pigeons was trained on a Trill pattern using the 8-choice SMC procedure (Figure 1, right panel). The three subjects in the Trill group were trained on a perfect Trill sequence, while the three subjects in the Violation group had a violation trial as the last element of the final chunk. After task acquisition, we investigated the effects of immediate and extended presentations of chunks in randomized order, in which the starting position of each chunk within the array was randomized—thereby breaking the hierarchical sequence while leaving the within-chunk trill sequence intact. This manipulation served to break up control by the hierarchical rule and interfere with behavioral control by the within-chunk trill rule.
Experiment 1
Methods
Subjects
Six experimentally-naïve adult White Carneaux pigeons (Columba livia) participated in the experiment. Pigeons were individually housed in steel home-cages with metal-wire mesh floors in a vivarium, and a 12-hr light-dark cycle was maintained. Testing was conducted 5 days a week during the light cycle. The pigeons were maintained at approximately 85% of their free-feeding weights, and were given free access to grit and water while in their home-cages.
Apparatus
Testing was conducted in a flat-black Plexiglas chamber (38 cm wide × 36 cm deep × 38 cm high). All stimuli were presented by computer on a color LCD monitor (NEC MultiSync LCD1550M) visible through a 23.2 × 30.5 cm viewing window in the middle of the front panel of the chamber. Pecks to the monitor were detected by an infrared touchscreen (Carroll Touch, Elotouch Systems, Fremont, CA) mounted on the front panel. A 28-V house-light located in the ceiling of the box was used for illumination, except during time outs. A food hopper (Coulbourn Instruments, Allentown, PA) was located below the monitor with an access hole situated flush with the floor. When in the raised position, the hopper provided access to pigeon pellets. All experimental events were controlled and data recorded with a Pentium III-class computer (Dell, Austin, TX). A video card controlled the monitor using the SVGA graphics mode (800 × 600 pixels).
Procedure
The pigeons were first trained to peck a white circle that was presented in the center of the screen. A single peck to the circle resulted in the circle disappearing and the hopper rising for 3 seconds before lowering again. This was followed by a 60-second intertrial interval (ITI) before the next circle was displayed. Once the pigeon was consistently responding to the circle, the entire array of eight circles was presented simultaneously (Figure 1, right panel). Each circle was 12 mm in diameter, and had a black 3 mm dot at the center. The bottom of the bottom circle was located 22 mm from the bottom of the screen, and each circle was 42 mm from each adjacent circle. Now, a single peck to an illuminated circle led to the circle disappearing to a white outline, with the hopper again rising for 3 seconds. There was no ITI during this phase of training. Pecking to a non-illuminated circle (i.e., the blank circle with the white outline) had no effect. Once the eighth and final illuminated circle was pecked, the pigeon was reinforced, and all eight circles would again be illuminated simultaneously, allowing the bird to continue instrumental circle pecking. Each session lasted 60 minutes.
Phase 1: 2-choice SMC procedure with timing cues defining chunks
After each pigeon had been rewarded for pecking to 960 total circles in the eight circle arrangement, they were allocated to a Run or Violation group. The 3 pigeons in the Run group were rewarded for choosing circles that corresponded to the consistent sequence:
123 234 345 456 567 678 781 812
The 3 pigeons in the Violation group were rewarded for choosing the same circles except the final circle differed (indicated by the underline):
123 234 345 456 567 678 781 818
The pigeons were rewarded immediately after choosing the correct circle with both circles disappearing and the hopper rising for 3 seconds. When the next trial was within a chunk, the two circles for the next trial would appear immediately after the hopper had again lowered. When the next trial was the start of a new chunk (i.e., after every set of three trials), there was a three-second ITI following the lowering of the hopper before the next trial commenced. Initially, when the pigeons pecked on an illuminated circle that was incorrect, it would simply disappear leaving only the correct circle illuminated. Pecking this circle would then lead to reward. However, the birds showed poor performance on this version of the task even after 25 sessions. To remedy this, we implemented a correction procedure when an incorrect circle was pecked. This consisted initially of both circles remaining illuminated and no reward delivery. Once the pigeon subsequently pecked on the correct circle, the incorrect circle disappeared, and the correct circle remained illuminated for a fixed-interval of 8 seconds. Once the correct circle was pecked again after this fixed interval, the circle disappeared and the hopper was raised for 3 seconds. This correction procedure led to improved acquisition across the trials. The start position (i.e., the location in the array of Circle 1) was counterbalanced across birds but was always the same across sessions within a bird. In this and all experiments in which a correction procedure was active, correction trials were not included in statistical analyses. Data analysis was performed on performance pooled across the last five sessions of Phase 1.
Phase 2: 2-choice SMC procedure with color cues defining chunks
The procedure was the same as in Phase 1, except that each chunk had a different color for the illuminated circles, counterbalanced across birds. For instance, when presented with trials from the 123 chunk (trials 1 to 3), the illuminated circles could be red. When presented with trials from the 234 chunk (trials 4 to 6), blue circles could be used. In addition, we used a consistent ITI between all trials, both within and between chunk boundaries. The trial would begin immediately after the hopper was lowered following the 3-second reward period, irrespective of whether the next trial was a continuation of the current chunk or the beginning of a new chunk. The correction procedure was discontinued in all sessions of Phase 2. Phase 2 training commenced at the completion of data collection from the last 5 sessions of Phase 1. Training continued for 35 sessions. Data analysis was performed on performance pooled across the last five sessions of Phase 2.
Phase 3: Test for behavioral control by color cues
The procedure was the same as in Phase 2, except that the order of trials was randomized such that all 24 trial types were presented in each successive block of 24 trials, but the specific order within each block varied across blocks. Only 5 sessions of Phase 3 were conducted, and data analysis was pooled across these sessions.
Phase 4: 3-choice SMC procedure
Prior to the manipulations described here, pigeons received a few other manipulations involving circle color and the number of white circles presented on each trial. None of these manipulations had any noticeable effect on performance and so we do not report further on them.
For the manipulation of Phase 4, rather than only two circles being illuminated on each trial as in prior phases, all 3 circles for a given chunk were simultaneously illuminated white (Figure 1, middle panel). For instance, for the first chunk, circles 1, 2 and 3 were illuminated. Pecking to the correct circle in the correct order caused the circle to darken for half a second, before becoming white again. Hence, the birds needed to peck to, for instance, circle 1, circle 2, and finally circle 3. Pecking to incorrect circles had no effect on the display and did not influence the required sequence. Thus, birds were not punished for pecking circles out of order or repeatedly. Once the final circle was pecked, all 3 circles darkened and the hopper was raised for 3 seconds. Following the hopper lowering again, there was an ITI of 3 seconds before the three circles required for the next chunk were shown. The correction procedure was implemented for all sessions of Phase 4. Training continued for 50 sessions. Data analysis was performed on performance pooled across the last five sessions of Phase 4.
Phase 5: 8-choice SMC procedure with random start locations
In prior phases, the start location within the array of circles was always the same across sessions. In Phase 5, the start location was randomly chosen at the beginning of each session. This meant that there was a 7 out of 8 chance that the violation trial was located at a different physical location on the screen relative to the previous session. In addition, all 8 circles were illuminated on each trial to further reduce discriminative cues based on the location of illuminated circles on a trial-by-trial basis (Figure 1, right panel). The same randomly-determined start location for each session was given to all the birds. As in Phase 4, pecking to incorrect circles had no effect on the display and did not influence the required sequence, and pecking to a correct circle resulted in it darkening for half a second. Once the final circle in a chunk was pecked, all 8 circles darkened and the hopper was raised for 3 seconds. Following the hopper lowering again, there was an ITI of 3 seconds before the eight circles were again illuminated for the next chunk. The correction procedure was active during all sessions in Phase 5. Training continued for 60 sessions. Data analysis was performed on performance pooled across the last five sessions of Phase 5.
Results & Discussion
Phase 1
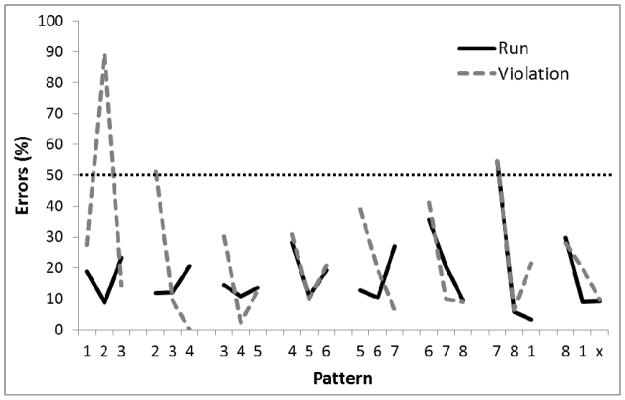
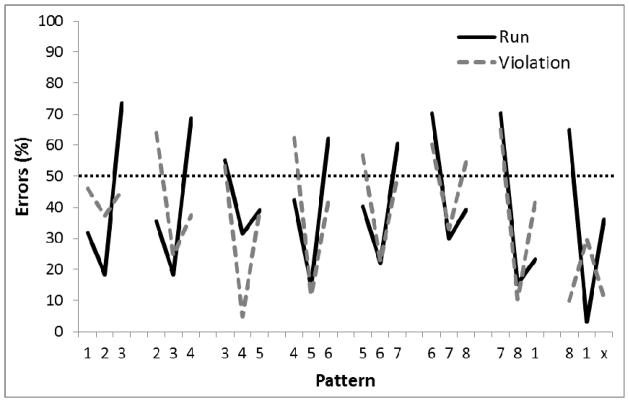
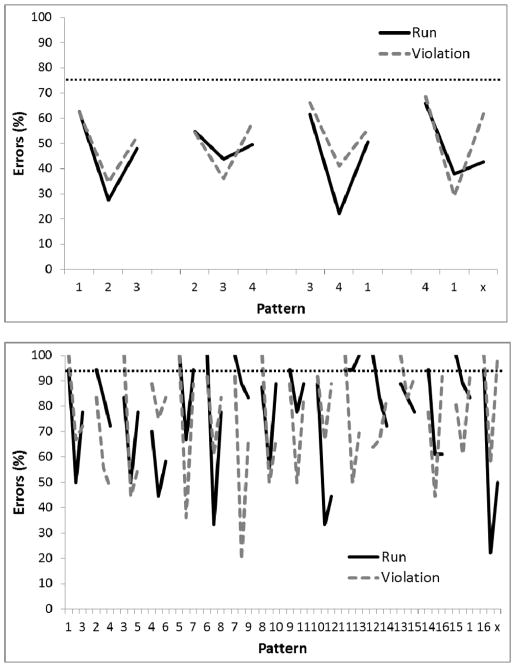
After about 60 sessions, performance reached a stable asymptote for all birds. Performance across the trials in the last five sessions of training is shown in Figure 2. A two-way analysis of variance (ANOVA) was conducted on percent errors with Group (Run versus Violation) as a between-group factor and sequence Position as a repeated measure. There was no main effect of Group, F(1,4)=3.37, p=.14, but, there was a main effect of position in sequence, F(23,92)=5.96, p<.01, ηp2 = .60, and a Group by Position interaction, F(23,92)=3.88, p<.01, ηp2 = .49. Post-hoc Bonferroni analyses revealed significantly more errors in Group Violation than in Group run at Position 2 in the first chunk, p < .01, as well as a more errors at location 7 than at location 8 of the 7th chunk for Group Run, p<.01, and more errors at location 7 than at locations 8 and 1 of the 7th chunk for Group Violation, ps < .01. Single-sample t-tests revealed that performance was better than chance (50%) on all three chunk elements (first, second, and third) for Run birds, but only for the second and third chunk elements for Violation birds (p<.05).
Figure 2.
Mean percentage of error rates for Experiment 1, Phase 1 where two white circles were illuminated simultaneously. Performance is shown collapsed across 5 sessions following 60 sessions of training. Digits on the abscissa indicate the correct circles for successive trials of the pattern. The terminal elements (indicated by ‘x’) were the only trials in which the Run and Violation patterns differed. The ‘x’ represents Circle 2 for Run group and Circle 8 for the Violation group. The horizontal dotted line indicates chance performance (50%).
A mixed ANOVA collapsing across chunks with Group (Run versus Violation) as the between-group factor and chunk Element (1, 2, and 3) as the repeated measure was conducted on percent errors. There was no main effect of Group, F(1, 4)=3.38, p=.14, but there was a main effect of Element, F(2, 8)=52.76, p<.001, ηp2 = .93, and a Group by Element interaction, F(2, 8)=10.22, p=.006; ηp2 = .72. Post-hoc Bonferroni analyses revealed significantly more errors to Element 1 than Element 2 for Group Run, p<.01, and more errors to Element 1 than Elements 2 and 3 and to Element 2 than to Element 3 for Group Violation, ps<.01.
In contrast to Fountain and Rowan (1995a), pigeons’ performance on the final element of the final chunk did not differ between the Run and Violation groups (Bayes Factor=17.41). Indeed, pigeon performance on the violation trial by the Violation birds had one of the lowest error rates, reflecting a failure to find evidence of rule-learning. Furthermore, we found an unanticipatedly high error rate by the Violation birds on the second trial of the first chunk. This might have occurred if the pigeons were perseverating on a repeating pattern that was consistent with the violation trial in the final chunk. In particular, the Violation birds were rewarded for 8 1 8. If they continued this pattern, they would again respond with 1 8. It becomes especially clear if we view the sequence without chunk boundaries: 123234345456567678181812… The first response was rewarded, but the second response was then incorrect. This perseveration of behavior suggested that the temporal cue providing information about chunk boundary was not salient enough to the pigeons to be effective. They were then missing the repeating pattern that was defined by the chunks.
To address this, we attempted to increase the salience of chunk information by introducing a color cue in Phase 2. Color has been shown to be a high-salience stimulus dimension for pigeons (e.g., Garlick, Gant, Brakel, & Blaisdell, 2011), thus, each chunk was assigned a different color. This color information should highlight the similarities within each of the chunks, and signal the chunk boundaries. Figure 3 shows performance across trials from the last five sessions of Phase 2 training involving different colors for each of the chunks. A 2-way ANOVA found no main effect of Group, F(1,4)=2.21, p=.21, but did find a main effect of Position in the sequence, F(23,92)=4.38, p<.01, ηp2 =.52. The interaction, however, was not significant, F(23,92)=1.13, p=.32. Post-hoc Bonferroni analyses revealed significantly more errors on the first element than the second element of the 7th chunk for Group Run, p < .02. Finally, to test for rule-learning, a planned comparison found significantly fewer errors on the final element of the final chunk relative to the final element of the other chunks for the Violation group (p<.01) but not the Run group. Single-sample t-tests indicated that performance on all three elements was significantly better than chance (50%) for Violation birds, and better than chance on the second and third chunk elements for Run birds (ps<.05).
Figure 3.
Mean percentage of error rates for Experiment 1, Phase 2 where two circles were illuminated, and each chunk was signified by a different color. Performance across 5 sessions is shown after 30 sessions of training. Digits on the abscissa indicate the correct circles for successive trials of the pattern. The terminal elements (indicated by ‘x’) were the only trials in which the Run and Violation patterns differed. The ‘x’ represents Circle 2 for Run group and Circle 8 for the Violation group. The horizontal dotted line indicates chance performance (50%).
A mixed ANOVA collapsing across chunks with Group (Run versus Violation) as the between-group factor and chunk Element (1, 2, and 3) as the repeated measure was conducted on percent errors. There was no main effect of Group, F(1, 4)=2.21, p=.21, nor Group by Element interaction, F(2, 8)=1.06, p=.39, but there was a main effect of Element, F(2, 8)=52.76, p<.001, ηp2 = .86. Post-hoc Bonferroni analyses revealed significantly more errors to Elements 1 and 3 than to Element 2 for Group Run, ps<.05, showing a significant V-shaped pattern of errors with highest accuracy to the middle chunk element.
Again, this is strikingly different from the pattern of performance shown by rats, and also differs from the pattern shown by the pigeons in Phase 1 when color cues were not present. Rather than performance improving across the trials within a chunk, which would be characteristic of successful implementation of a sequence rule, errors were lowest on the middle trial of each chunk. Further, the Violation birds now performed better than the Run birds on the final trial of the final chunk, despite this trial being inconsistent with the within-chunk sequence for the Violation birds and not the Run birds.
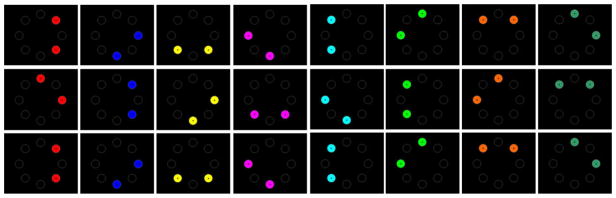
Closer inspection of the screen displays for all trial combinations of color and spatial location, however, reveals how such a result might have occurred (Figure 4).
Figure 4.
Illustration of all trial types presented to the pigeon in Experiment 1, Phase 2. Note that the first and third panels for each color are the same but require different responses, whereas the second panel for each color involves a unique configuration of color and position. See the digital version of the article for a color figure.
If we ignore sequence information, we can observe that it should be easier to memorize the correct response for the mid-chunk trials. In this situation, the unique configuration of color and position signifies that only one of the circles will be rewarded. The other circle will never be rewarded. On the other hand, in the first and third trials of a chunk, the same stimulus information is presented but the correct response differs between the first and third trial, creating an ambiguous situation on these trials. On half of the trials, one option is correct, and on the other half of the trials, the other option is correct. Hence, if sequence information is ignored, it would be expected that the first and third trials would be more difficult. Notably, performance on the first and third chunk elements was better than chance. Thus, birds do show evidence of learning the sequence rule or using the color of the circles on the prior trial as a cue for the correct choice on these trials. Nevertheless, performance was significantly worse than to the middle chunk element, reflecting behavioral control by color and position features.
Finally, in the case of the final chunk, the first and third trials were again identical in terms of color and position information, but for the Violation birds one option was always rewarded and the other option was never rewarded – in contrast to the Run birds where again each position was rewarded half of the time. This means that the Violation birds were also rewarded for this particular combination of color and position twice as often as for any other combination of color and position.
These suppositions describe the observed pigeon performance very accurately. They also suggest just how well pigeons are able to memorize many specific exemplars in visual displays (cf. Cook, Levison, Gillett, & Blaisdell, 2005; Pearce, 1989). We suspect that humans would have difficulty emulating the performance of the pigeons on this task. Since humans have a bias to look for rules or patterns, it may be argued that they would be less likely to recognize the statistical regularity that comes from treating each stimulus as an independent occurrence (cf. Pearce, 1989).
To assess whether our account is a satisfactory explanation of the pigeons’ performance, we randomized the order of presentation of the trials in Phase 3 of training. While it would be expected that randomizing the order of presentation would severely disrupt performance if it was based on learning the sequences or basing performance on both the current and the most recently experienced trials, it should not disrupt performance if performance was based only (or primarily) on color and positional information from the current trial.
Figure 5 shows performance across trials in the last five sessions of training on the randomized order in Phase 3. A 2-way ANOVA revealed a similar pattern of results to those observed in Phase 2. In particular, there was no main effect of Group, F(1,4)=1.41, p=.30, but there was a main effect of Position in the sequence, F(23,92)=2.76, p<.01, ηp2 =.41. The interaction was again not significant, F(23,92)=1.05, p=.40. A planned comparison found significantly fewer errors on the final element of the final chunk relative to the final element of the other chunks for the Violation group (p<.05) but not the Run group. Single-sample t-tests revealed that performance was better than chance only for the second chunk element in both groups (ps<.05).
Figure 5.
Mean percentage of error rates for Experiment 1, Phase 3 where two circles were illuminated, each chunk was signified by a different color, and order of presentation was randomized. Performance across 5 sessions is shown, with these 5 sessions using the randomized order following directly after the 5 sessions using a non-randomized order and shown in Figure 4. Digits on the abscissa indicate the correct circles for successive trials of the pattern. The terminal elements (indicated by ‘x’) were the only trials in which the Run and Violation patterns differed. The ‘x’ represents Circle 2 for Run group and Circle 8 for the Violation group. The horizontal dotted line indicates chance performance (50%).
The similar pattern of results between Phases 2 and 3 suggests that the pigeons were relying heavily on color and positional information alone to determine their responses. Nevertheless, performance on the first and third elements did not differ significantly to that predicted by chance alone. This is in contrast to Phase 2, where performance on these elements was above chance. This suggests that the pigeons had acquired some sequence information or learned to use inter-item associations even when performance was primarily controlled by the color and positional information. Thus, performance in Phase 2 was driven to a small but significant degree by the sequence rule defining a correct response to the first and third trials of each chunk.
To make the chunks more salient, the procedure was modified in Phase 4 of training so that reward was only given at the end of each chunk. This meant that the activation of the hopper served as a cue defining the chunk boundary, and also meant that the pigeons would not have attention to the screen interrupted by eating at the hopper following each trial within each chunk.
Figure 6 shows the performance of the birds on the last five sessions of training in Phase 4. A 2-way ANOVA revealed a main effect of Position in the sequence, F(23,92)=5.03, p<.01, ηp2 =.56, but no main effect of Group (Run versus Violation), F(1,4)<1.0. There was also no Group X Position interaction, F(23,92)=1.24, p=.23. A planned comparison failed to find a significant difference in performance on the final element of the final chunk compared to performance on the final element of the other chunks for either the Violation or Run groups. Thus, the Run birds still did not show better performance than the Violation birds on the violation trial, though the Bayes Factor of 2.22 does not provide strong support for similar performance as predicted by the Null hypothesis. Indeed, Violation birds showed marginally superior performance on the first element of the final chunk compared to the Run birds, p=.054. This suggests that the birds in the Violation condition were strongly influenced by circle 8 being reinforced every time the birds was given a choice between circles 8 and 2, whereas circle 2 was never correct on these trials. Single-sample t-tests revealed performance was better than chance (67%) on the second and third chunk elements for the Run birds and for all three chunk elements for the Violation birds (ps<.05). This suggests that the removal of the color cue eliminated the superior performance by the pigeons on the middle trial of each chunk.
Figure 6.
Mean percentage of error rates for Experiment 1, Phase 4 where three circles were illuminated and reward was only given at the end of each chunk. Performance across 5 sessions is shown after 50 sessions of training. Digits on the abscissa indicate the correct circles for successive trials of the pattern. The terminal elements (indicated by ‘x’) were the only trials in which the Run and Violation patterns differed. The ‘x’ represents Circle 2 for Run group and Circle 8 for the Violation group. The horizontal dotted line indicates chance performance (67%).
The relatively good performance of the violation birds on the violation trial may still reflect a positional cue. While the color cue had been removed, the location of the correct response for the violation trial was more rewarded relative to the other locations for the violation birds, and past experience with the frequency of reward for this location may still have biased the birds in the Violation group. To then completely eliminate this position bias across sessions, the start position for the sequence was randomized at the beginning of each session in Phase 5 of training, and we changed the procedure to display all 8 choice options simultaneously on each trial (Figure 1, right panel).
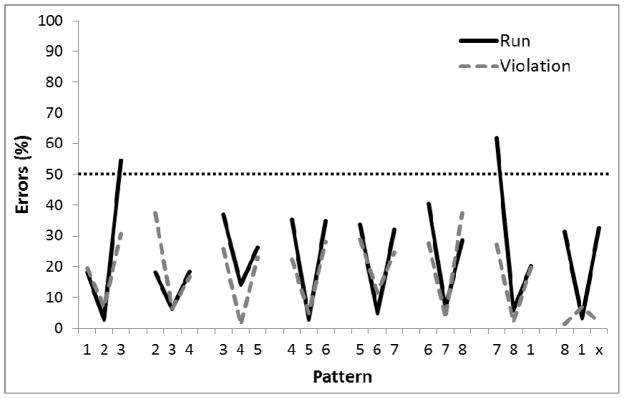
Figure 7 shows the performance of the birds on the last five sessions of training in Phase 5. A 2-way ANOVA revealed a main effect of Position in the sequence, F(23,92)=28.35, p<.01, ηp2 =.88, and a Group X Position interaction, F(23,92)=3.19, p<.01, ηp2 =.44, but no main effect of Group, F(1, 4)<1.0. A planned comparison found a significant difference on the third element of the final chunk relative to the third element on the other chunks for the Violation group but not the Run group (p<.05). Furthermore, there were significantly more errors on the final element of the final chunk for birds in the Violation group than in the Run group (p<.05). Single-sample t-tests revealed better-than-chance (87.5%) performance only for the second and third chunk elements for the Violation birds (ps<.01), but a tendency towards better than chance performance on the second (p=.063) and third (p=.056) chunk element for Run birds.
Figure 7.
Mean percentage of error rates for Experiment 1, Phase 5 where all 8 circles were illuminated, reward was only given at the end of each chunk, and start position was randomized at the beginning of each session. Performance across 5 sessions is shown after 60 sessions of training. Digits on the abscissa indicate the correct circles for successive trials of the pattern. The terminal elements (indicated by ‘x’) were the only trials in which the Run and Violation patterns differed. The ‘x’ represents Circle 2 for Run group and Circle 8 for the Violation group. The horizontal dotted line indicates chance performance (87.5%).
A mixed ANOVA collapsing across chunks with Group (Run versus Violation) as the between-group factor and chunk Element (1, 2, and 3) as the repeated measure was conducted on percent errors. There was no main effect of Group nor Group by Element interaction, Fs<1.0, but there was a main effect of Element, F(2, 8)=42.47, p<.001, ηp2 = .91. Post-hoc Bonferroni analyses revealed significantly more errors to Element 1 than Elements 2 and 3 for both groups, ps<.05. The finding of significantly higher error rates on the first element of each chunk indicates that the pigeons had difficulty finding the correct circle after eating from the hopper. An analysis of the incorrect responses on the first trial of each chunk revealed that the correct response was, however, the most frequent response (Figure 9). This suggests that the pigeons had difficulty remembering the correct location but did have some knowledge of it, rather than the pigeons making a systematic error such as choosing the location that was most recently rewarded or choosing a location that continued the sequence (i.e., if the previous chunk was 123, choosing 4 as the next response). Consistent with the overall low error rate, their responses to the second and third elements of the chunk were overwhelming to the correct location.
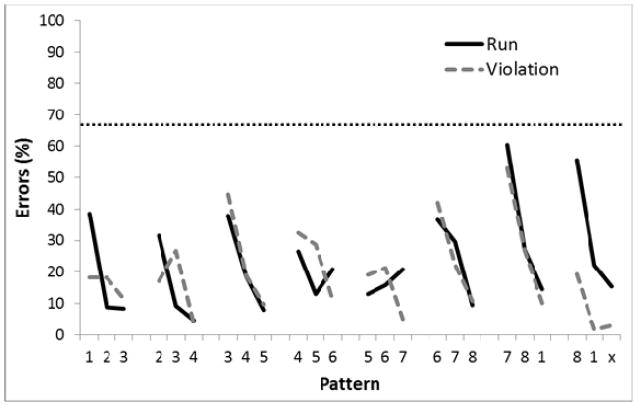
Figure 9.
Mean percentage of error rates for Experiment 2, Phase 1 where the circles were either presented closer together or further apart, grouped by Run vs Violation (top panel) and by Transformation (bottom panel). Digits on the abscissa indicate the correct circles for successive trials of the pattern. The terminal elements (indicated by ‘x’) were the only trials in which the Run and Violation patterns differed. The ‘x’ represents Circle 2 for Run group and Circle 8 for the Violation group. The horizontal dotted line indicates chance performance (87.5%).
The finding that performance did not differ between the second and third elements indicates that once the pigeons found the correct starting point for the chunk, they were able to perform equally well for the rest of the chunk sequence. Most importantly, we also finally found that the Violation birds performed significantly worse than the Run birds on the violation trial. This is consistent with what has been observed with rats (Fountain & Rowan, 1995a), and suggests that the pigeons were finally basing their behavior on a generalizable within-chunk rule. Applying this rule to the violation chunk results in the violation birds exhibiting substantially lower performance on this final trial in this chunk where a correct response violates this general rule. Consistent with this, an examination of their incorrect choices on the violation trial indicated that 82.75% of their choices were to the location that would be correct based on a run sequence.
Experiment 2
The results of Experiment 1, Phase 5 suggest that the pigeons had learned a general rule. It may then be asked exactly what had been learned? Experiment 2 explored this by manipulating the display and seeing whether the pigeon’s performance was still governed by the rules. The animals continued to be trained on the usual display, and were then presented with alternative probe sessions once every 5 days. During the probe sessions, once the first element had been identified, the next two elements were treated as correct to the pigeon in terms of the circle flashing, and then reward being delivered after the final element, irrespective of whether these circles followed the rule or not (i.e., nondifferential reinforcement). The only restriction was that a choice could not be the previously-correct choice as this would likely indicate perseveration by the pigeon rather than an actual choice. Rewarding any other choice meant that the animal’s responding later in the session was not influenced by a particular reward structure for the current display.
For Phase 1 of Experiment 2, eight circles were still displayed simultaneously, but the display was manipulated so that the circles were either closer together (contraction) or further apart (expansion). This meant that while the configuration of the circles was not changed, their absolute positions on the screen were. If performance was governed by some precise motor mechanism, such a manipulation may be expected to impede performance. On the other hand, if performance was governed by a more abstract representation of the locations with respect to the array, then changing the distance between them should not impair performance.
Another question that may be asked is what happens when the spatial configuration is changed? For instance, the number of circles could be halved so that there are only 4 circles in either a diamond or square pattern. Alternatively, the number of circles could be doubled, so that there are 16 circles total in the display. We explored these questions in Phase 2. If the birds have learned a general rule of moving clockwise around the display, these manipulations should also not affect performance.
Another way to test for generality of the within-chunk rule is to manipulate chunk size rather than number of elements in the array. During training, reward comes after choosing 3 circles in a clockwise direction. This raises the question; if the third circle is not rewarded, would the pigeons go on to choose additional circles that conform to the within-chunk rule until a reward is given? This was examined in Phase 3.
Subjects & Apparatus
The same subjects and apparatus from Experiment 1 were used in Experiment 2.
Phase 1: Generalization tests with contracted or expanded arrays
The procedure was the same as in Phase 5 of Experiment 1, except that the display was changed so that the circles were closer together for a total of two probe sessions, and were expanded further apart for a total of two probe sessions. In the closer sessions, the circles were located 27 mm apart and the bottom of the bottom circle was 38 mm from the bottom of the screen. In the expanded condition, the circles were located 56 mm apart and the bottom of the bottom circle was 2 mm from the bottom of the screen. The correction procedure was discontinued during this experiment. Training continued for 25 sessions. Following the 25th session of this phase, pigeons received one test session with only the contracted array. Following this, subjects received four more training sessions, followed by a second test session with the contracted array. Following this, subjects received four more training sessions, followed by one test with only the expanded array, followed by four more training sessions, and finally one more test session with the expanded array. Data analysis was performed on performance pooled across both test sessions with contracted and expanded arrays, respectively.
Phase 2: Generalization tests with 4 or 16 circles in the array
The procedure was the same as in Phase 5 of Experiment 1, except that the number of circles displayed was changed. In the 4-circle condition, half of the circles were taken away, leaving either a diamond or a square configuration. In the 16-circle condition, circles were added between each of the circles so that each circle was only 21 mm apart from its neighbors. The animals were tested on the diamond configuration for two probe sessions, the square configuration for two probe sessions, and the 16-circle configuration for four probe sessions. The correction procedure was discontinued during this experiment. Training continued for 25 sessions. Following the 25th session of this phase, pigeons received one test session per week, with four training sessions intervening between each test session. The order of test sessions was two 4-circle arrays with the diamond configuration followed by two 4-circle arrays with the square configuration, followed by four with the 16-circle configuration. Data analysis was performed on performance pooled across all four 4-circle probe sessions and 16-circle probe sessions, respectively.
Phase 3: Generalization to chunk lengths of 4 or 5 elements
The procedure was the same as in Phase 5 of Experiment 1, except that 4-element and 5-element chunks were used. Hence, in the 4-element procedure, reward would be provided at the end of the following chunks:
1234 2345 3456 4567 5678 6781 7812 81x3
In the 5-element procedure, reward would be provided at the end of 5-element chunks:
12345 23456 34567 45678 56781 67812 78123 81x34
Prior to probe sessions, subjects received training on the basic procedure for 30 sessions following the last Phase 2 probe session. Four probe sessions were given once per week using the 4-element chunks, and four probe sessions were given once per week using the 5-element chunks, with four sessions intervening between each probe session. Data were pooled across each type of probe session. The correction procedure was discontinued during this experiment.
Results & Discussion
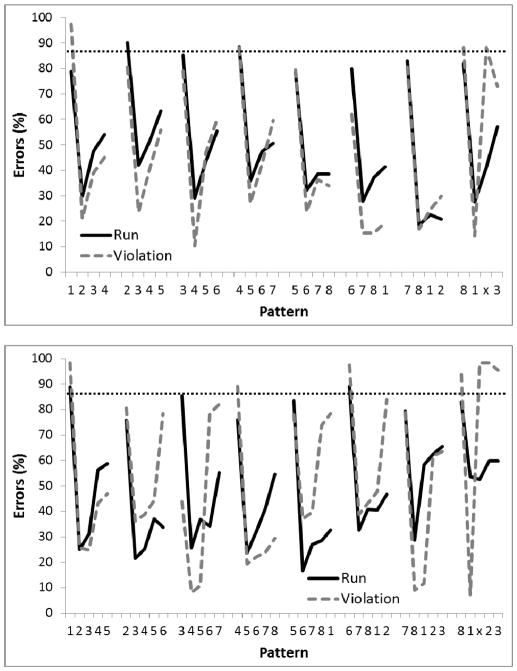
Figure 9 shows the performance of the birds when the display was either closer (two sessions; top panel) or expanded (two sessions, bottom panel). A 2-way ANOVA revealed a main effect for the Transformation of the display (expanded versus contracted), F(1, 4)=7.73, p<.05, ηp2 =.66, 95% CIs = (0, .83), a main effect for Position in the sequence, F(23,92) =16.56, p<.01, ηp2 =.81, and a small but significant Transformation X Position interaction, F(23,92)<1.76, p<.05, ηp2 =.31. Unlike Phase 5 of Experiment 1, however, there was no Group X Position interaction, F(23,92)=1.35, p=.16. A planned comparison indicated that there was a significant difference on the third element of the final chunk relative to the third element on the other chunks for the Violation group (p<.05) but not the Run group. Single-sample t-tests revealed that performance on the second and third chunk elements was significantly better than chance (87.5%) for both Groups (ps<.05).
Thus, performance did generalize readily to transformations involving expanded and contracted arrays that preserved intra-array relations between circles. Moreover, these transformations also did not disrupt the use of the within-chunk response rule, as evidence by the high percentage of errors on the violation trial for Violation birds. The results of Phase 1 suggest that the birds were insensitive to changes in the display when it was expanded or contracted, but the relative spatial configuration remained the same.
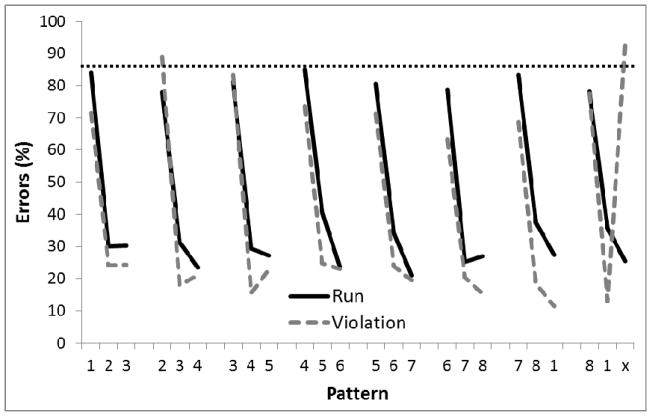
Figure 10 (top panel) shows the performance of the birds when the display contained only four circles (four sessions) in Phase 2. As can be seen, this manipulation resulted in much higher error rates than manipulating the distance between the circles but keeping the spatial configuration the same. It suggests that the birds had learned a rule that does not generalize as well to configurations with different numbers of circles compared to training. A 2-way ANOVA found a main effect of Position in the sequence, F(11,44)=5.60, p<.01, ηp2 =.58, but no effect of Group or Group X Position interaction, Fs<1.0. A planned comparison indicated that there was no a significant difference on the third element of the final chunk relative to the third element on the other chunks for the Violation group or the Run group. Single-sample t-tests revealed that performance on the first and second chunk elements was better than chance (75%) for both Groups (ps<.05).
Figure 10.
Mean percentage of error rates for Experiment 2, Phase 2 where the number of circles presented were reduced to 4, either in a diamond or square pattern (top panel) or increased to 16 (bottom panel). Digits on the abscissa indicate the correct circles for successive trials of the pattern. The terminal elements (indicated by ‘x’) were the only trials in which the Run and Violation patterns differed. The ‘x’ represents Circle 2 for Run group and Circle 4 (top panel) or Circle 16 (bottom panel) for the Violation group. The horizontal dotted line indicates chance performance (75% in the top panel, 93.75% in the bottom panel).
Figure 10 (bottom panel) shows the performance of the birds when the displayed contained 16 circles (four sessions) in Phase 2. One bird did not complete the entire sequence in any session, and thus its data were removed from analysis. For the remaining 5 birds, a 2-way ANOVA found a main effect for Position in the sequence, F(47,141)=2.56, p<.01, ηp2 =.46, but no effect of Group, F<1.0, and only a marginal Group X Position interaction, F(47,141)=1.43, p=.06, ηp2 =.32. A planned comparison found no difference in errors to the third element of the final chunk relative to the third element on the other chunks for either the Violation group or the Run group. Single-sample t-tests revealed that performance was only better than chance (93.75%) on the second chunk element for the Run birds (p<.05), though performance approached significance on the third chunk element for the Run birds as well (p=.062).
The results from Phase 2 suggest that there is a limit as to how generalizable the rule is that the pigeons have learned. Halving or doubling the number of elements in the circular array resulted in a partial disruption in application of the within-chunk +1 rule. This failure is interesting because generalizing beyond training history is often touted as a hallmark of abstract rule learning. While there are many cases that animals such as the pigeon do learn abstract rules that generalize beyond training history (e.g., Blaisdell & Cook, 2005), our results reveal that this is not universal.
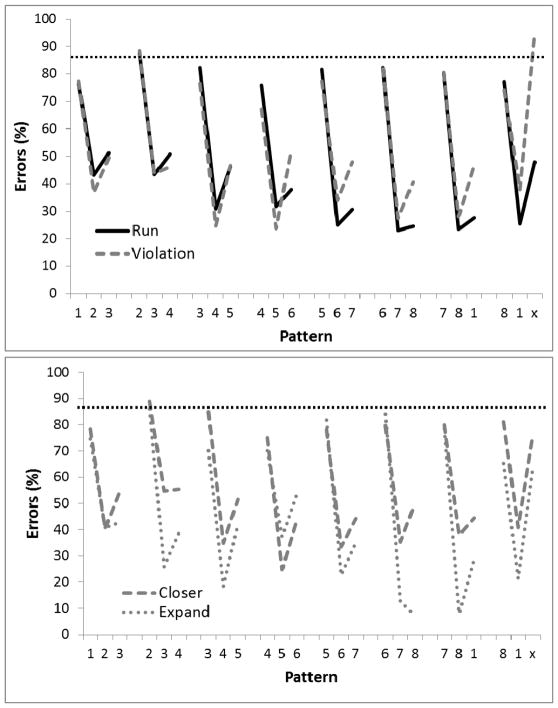
Figure 11 shows the birds’ performance on 4-element (4 sessions, top panel) and 5-element (4 sessions, bottom panel) chunks, respectively, during Phase 3. Unlike when the number of circles in the display were changed (Phase 2), performance was relatively unaffected by requiring the birds to peck to more circles before reward was delivered. For the 4-element chunk tests, a 2-way ANOVA found a significant main effect for Position in the sequence, F(31,124)=11.30, p<.01, ηp2 =.74, but no effect of Group or Group X Position interaction, Fs<1.0. A planned comparison indicated that there was a significant difference on the third element of the final chunk relative to the third element on the other chunks for the Violation group (p<.01) but not the Run group, replicating the effect of the violation manipulation found in Phase 5 of Experiment 1 and Phase 1 of this experiment. Single-sample t-tests found performance to be better than chance (87.5%) at chunk locations 2 and 3 for Run birds, and at chunk locations 2–4 for Violation birds.
Figure 11.
Mean percentage of error rates for Experiment 2, Phase 3 where reward was given after 4-element chunks (top panel) or 5-element chunks (bottom panel) following the +1 rule. Digits on the abscissa indicate the correct circles for successive trials of the pattern. The terminal elements (indicated by ‘x’) were the only trials in which the Run and Violation patterns differed. The ‘x’ represents Circle 2 for Run group and Circle 8 for the Violation group. The horizontal dotted line indicates chance performance (87.5%).
For the 5-element chunk tests, a 2-way ANOVA found a main effect of Position in the sequence, F(39,156)=4.64, p<.01, ηp2 =.54, but no effect of Group, F<1.0, or Group X Position interaction, F(39,156)=1.39, p=.08, ηp2 =.26. A planned comparison indicated that there was a significant difference on the third element of the final chunk relative to the third element on the other chunks for the Violation group (p<.01) and for the Run group (p<.05). Nevertheless, Violation birds made significantly more errors to the third element of the final (violation) chunk than did the Run birds, p<.02. Thus, the Violation birds continued to demonstrate the violation error even with the longer chunks were used during testing. Single-sample t-tests found performance to be better than chance (87.5%) at any chunk locations 2, 3, and 4 for the Run birds, and at chunk locations 2 and 3 for the Violation birds.
Overall, the results suggest that the rule they had learned is closer to pecking +1 until reward, rather than pecking specific configurations of circles such as 1-2-3. Nevertheless, pigeons in the Violation condition showed poorer performance on just about all chunks during the 5-chunk condition, especially for chunk elements 4 and 5. This suggests that the different rule in place on the violation trial resulted in a disruption in the application of the +1 rule within the remaining chunks.
Experiment 3
The previous experiments indicate that pigeons can correctly follow a Run sequence once they have identified the first element in the chunk, and that performance on a violation element is impaired. Because our task utilized a circular array of elements, a simple +1 rule cannot be implemented in our task using a simple motor pattern between elements within a chunk. The direction of movement in absolute space constantly changes as a function of the position of the element within the array. Pigeons were able to progress correctly through each chunk even when the starting element was randomized between chunks. Thus, pigeons appeared to have learned to use the circular array as an orienting structure or framework within which to base response choices.
Fountain & Rowan (1995a) found that rats were not only able to learn rules in Run sequences, but also when correct choices followed a Trill sequence. It is possible that pigeons were able to learn the Run sequence since it involves a general rule of proceed clockwise or in the same direction, but would have more difficulty when the sequence involves a change of direction, as do rats and humans (Fountain et al., 2007). Indeed, like humans, rats learned run sequences more easily than trill sequences, as reflected by rate of acquisition and types of errors (Fountain et al., 2007). This was examined by training different birds on the Trill sequence using a similar procedure to that described in Phase 5 of Experiment 1.
Subjects
Six new experimentally-naïve adult White Carneaux pigeons (Columba livia) participated in the experiment. Pigeons were housed and maintained as in Experiment 1.
Apparatus
The same apparatus was used as in Experiments 1–8.
Procedure
The pigeons were first autoshaped to peck to a white circle that appeared in the center of the screen. A single peck to the circle resulted in the circle disappearing and the hopper rising for 3 seconds before lowering again. This was followed by a 60-second intertrial interval (ITI) before the next circle was displayed. Once the pigeon was consistently responding to the circle, the entire array of eight circles was presented. Now, pecking to an illuminated circle led to the circle disappearing to a white outline, with the hopper again rising for 3 seconds. Pecking to a non-illuminated circle had no effect. Once the eighth illuminated circle was pecked, all eight circles would again be illuminated. There was no ITI between choices and each session lasted 60 minutes.
Phase 1: 8-choice SMC procedure with random start locations
After each pigeon had been rewarded for pecking to 960 total circles in the eight circle arrangement, they were allocated to a Trill or Violation group. The 3 pigeons in the Trill group were rewarded for choosing circles that corresponded to the consistent Trill sequence:
121 232 343 454 565 676 787 818
The 3 pigeons in the Violation group were rewarded for choosing the same circles except the final circle differed (indicated by the underline):
121 232 343 454 565 676 787 812
All 8-circles were illuminated simultaneously, and the start location in the sequence was randomly varied across sessions. The same randomly-determined start location for each session was given to all the birds. This prevented pigeons from learning to predict the violation element based on its absolute spatial location on the display. Pecking to incorrect circles had no effect on the display, while pecking to a correct circle would result in it darkening for half a second. Once the final circle in a chunk was pecked, all 8 circles would darken and the hopper was raised for 3 seconds. Following the hopper lowering again, there was an ITI of 3 seconds before the 8 circles were again illuminated for the next chunk. A correction procedure was implemented during all sessions of this experiment. Correction procedure trials were not included in data analysis. Data analysis was performed on data pooled across the last 5 sessions of training.
Phase 2: Chunk order randomized
Training on this procedure began immediately following the last session of Phase 1. Training was the same as in Phase 1 (including the correction procedure), except that whereas previously the next chunk would increment the start location by one relative to the final element of the previous chunk:
121 232 343 454 565 676 787 818
Now the start location at the beginning of each chunk could be any of the eight circle locations. Hence, for example:
232 565 121 121 787 454 232 676
To investigate the effect of training on performance with randomized chunks, data were analyzed for the first 5 sessions after initial exposure to the randomized chunk locations, and again for the last 5 of the 55 sessions of Phase 2 training.
Results & Discussion
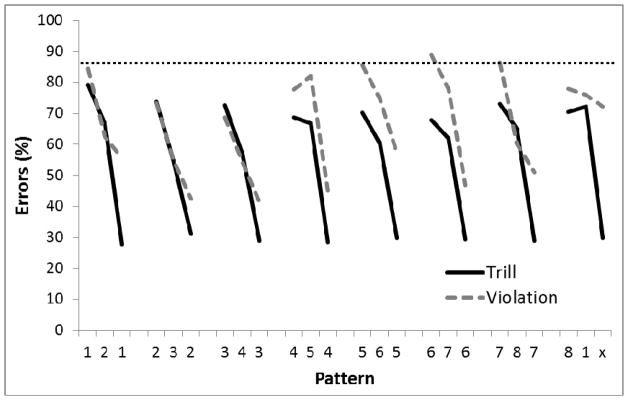
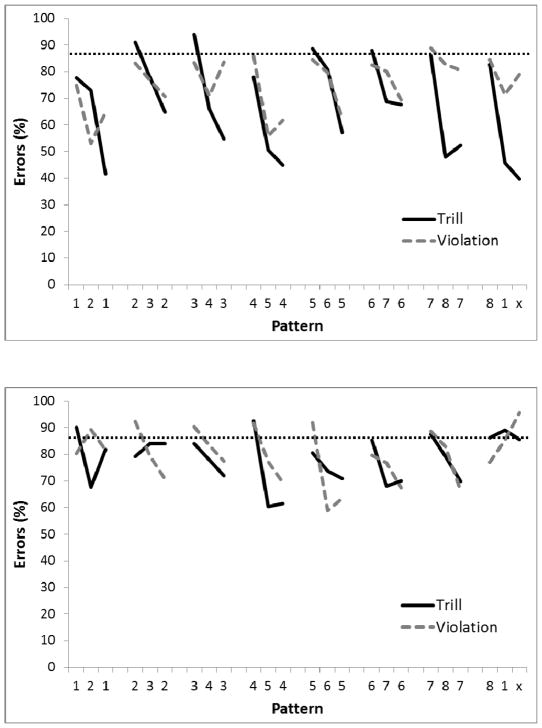
After 100 sessions, performance reached a stable asymptote for all birds. Figure 12 shows the performance on the last five sessions of training on the Trill sequence. A 2-way ANOVA conducted on percent errors with Group (Trial versus Violation) as a between-group factor and Position in sequence as a repeated measure found a main effect for Position in the sequence, F(23,92)=7.45, p<.01, ηp2 =.65, but no effect of Group, F(1, 4)=2.90, p=.16, nor a Group X Position interaction, F<1.0. A planned comparison indicated that there was a significant difference on the third element of the final chunk relative to the third element on the other chunks for the Violation group (p<.05) but not the Run group. Furthermore, errors on the third element of the final chunk were higher in the Violation than Run Group, p<.02. Single-sample t-tests found performance to be better than chance (87.5%) at chunk location 3 for the Run birds and at chunk locations 2 and 3 for the Violation birds (ps<.05). Performance on chunk location 2 for the Run birds approached significance (p=.059).
Figure 12.
Mean percentage of error rates for Experiment 3, Phase 1 using new birds and the Trill sequence. All 8 circles were illuminated, reward was only given at the end of each chunk, and start position was randomized at the beginning of each session. Performance across 5 sessions is shown after 100 sessions of training. Digits on the abscissa indicate the correct circles for successive trials of the pattern. The terminal elements (indicated by ‘x’) were the only trials in which the Run and Violation patterns differed. The ‘x’ represents Circle 8 for Run group and Circle 2 for the Violation group. The horizontal dotted line indicates chance performance (87.5%).
A mixed ANOVA collapsing across chunks with Group (Run versus Violation) as the between-group factor and chunk Element (1, 2, and 3) as the repeated measure was conducted on percent errors. There was no main effect of Group, F(1, 4)<2.90, p=.16. There was a main effect of Element, F(2, 8)=65.51, p<.001, ηp2 = .94, and a trend towards a Group by Element interaction, F(2, 8)=4.16, p=.058. Post-hoc Bonferroni analyses revealed significantly fewer errors to Element 3 than to Elements 1 and 2 for Group Run, and significantly fewer errors to Element 3 than to Element 1 for Group Violation, ps<.001.
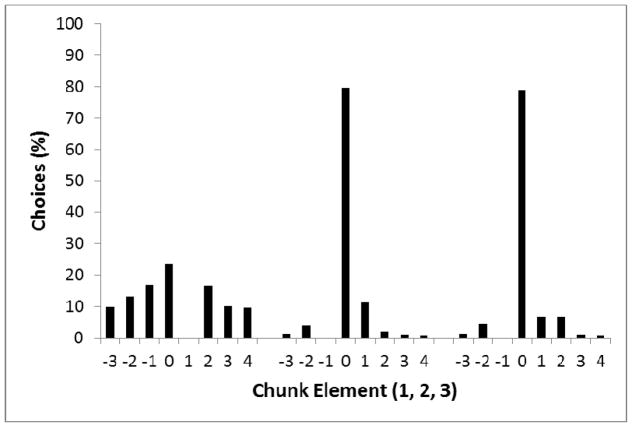
The results indicate that performance improved from the first element to the second element to the third element of the chunk, especially in Group Run. This differs from Phase 5 of Experiment 1, where there was no significant difference in performance on the second and third elements of the chunk. The high error rates on the first element of the chunk again indicate that the birds had difficulty remembering the correct location to return to after feeding from the hopper, but the top panel of Figure 13 illustrates that the correct location was still the most preferred. We also found evidence that pigeons had learned the within-chunk sequence rule, as indicated by the poor performance on the final element in the final chunk for the Violation condition but not in the Run condition.
Figure 13.
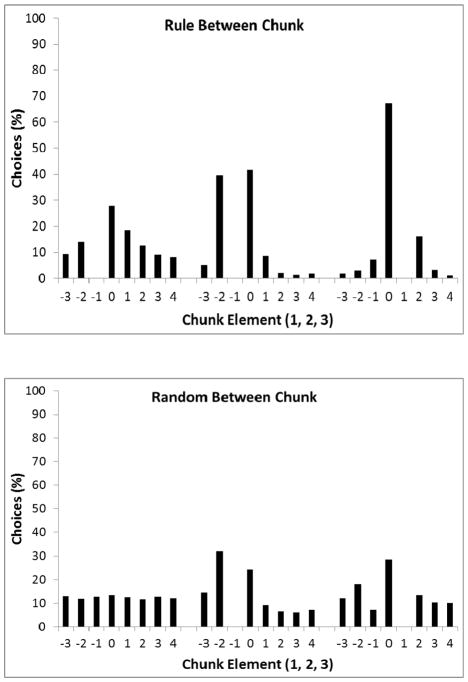
Top panel: Comparison of percentage of choices to each location based on position in the chunk for Experiment 3, Phase 1. Consistent with Figure 8, 0 indicates the correct location, 1 indicates the circle clockwise to the correct location, −1 indicates the circle anti-clockwise to the correct location, etc. Responses to the same location as the previously correct location were ignored. Bottom panel: Comparison of percentage of choices to each location based on position in the chunk for Experiment 3, Phase 3 when chunk order was randomized.
Given that the between-chunk rule did little to aid performance for the first element of the chunk, we wondered if taking away this structure would have any effect on performance within a chunk. To do this, we tested the immediate effect of randomization of the chunk start location at the beginning of each chunk in Phase 2.
Figure 14 (top panel) shows the performance on the first five trials of training with the start location for each chunk randomized. A 2-way ANOVA revealed a significant main effect for Position in the sequence, F(23,92)=7.45, p<.001, ηp2 =.65, but no effect of Group, F(1, 4)=2.90, p=.16, nor Group X Position interaction, F<1.0. Planned comparisons found no differences on the third element of the final chunk relative to the third element on the other chunks for either group. Nevertheless, error rates on the third element of the final chunk were higher in the Violation group than in the Run group, p<.02, suggesting that birds in the Violation group continued to misapply the abstracted trill rule common to the remaining chunks despite randomizing chunk order. Single-sample t-tests found performance to be better than chance (87.5%) at all three chunk locations for the Violation birds (ps<.05), but at none of the locations for the Run birds (smallest p=.08).
Figure 14.
Mean percentage of error rates for Experiment 3, after initial 5 (top panel) and last 5 (bottom panel) of 55 sessions of Phase 2 training where the start position for each chunk was randomized. Digits on the abscissa indicate the correct circles for successive trials of the pattern. The terminal elements (indicated by ‘x’) were the only trials in which the Run and Violation patterns differed. The ‘x’ represents Circle 8 for Run group and Circle 2 for the Violation group. The horizontal dotted line indicates chance performance (87.5%).
A mixed ANOVA collapsing across chunks with Group (Run versus Violation) as the between-group factor and chunk Element (1, 2, and 3) as the repeated measure was conducted on percent errors. There was no main effect of Group, F(1, 4)<1.79, p=.25. There was a main effect of Element, F(2, 8)=13.12, p<.01, ηp2 = .77, but no Group by Element interaction, F(2, 8)=2.63, p=.13. Post-hoc Bonferroni analyses revealed only a significant difference in errors between Elements 1 and 3 for Group Run, p<.02.
Not surprisingly, the performance on the first element of the chunk for birds in Group Run was around 86% incorrect, which corresponds to chance performance when the location of the first element was unable to be predicted and was randomly allocated to one of eight locations. More surprisingly, performance on the other elements of the chunk also decreased, especially the final element of the chunk. This suggests that, even though performance on the first element of the chunk was poor when there was a between-chunk rule, this between-chunk rule still aided performance on subsequent elements within a chunk.
This raised the issue of whether the birds could be trained to perform better on the within-chunk rule with additional practice. After all, even though there may no longer be any between-chunk consistency, once the first element of the chunk is identified; correct responding is based on the simple alternation rule of +1, −1. This would suggest that pigeons do have the potential to master the trill rule if feeding from the hopper did not interfere with their memory for the start location.
Figure 14 (bottom panel) shows the performance from the last five sessions after training on random chunk order had continued for 55 sessions. A 2-way ANOVA found a main effect of Position in the sequence, F(23,92)=2.61, p<.01, ηp2 =.40, but no effect of Group nor Group X Position interaction, Fs<1.0. Planned comparisons failed to find differences on the third element of the final chunk relative to the third element on the other chunks for either group. Nor was there a difference between groups on performance on the final chunk of the final element (Bayes Factor=5.54), which contrasts with the results of Phases 1 and 2. Finally, single-sample t-tests failed to reveal better-than-chance performance at any chunk element for either group (ps>.10).
These results suggest that the birds performed worse at the task following additional training! This surprising result can be understood when their errors are examined for the second and third elements (see bottom panel of Figure 13). Following additional training, their performance has decreased on the second element because they more consistently apply the incorrect rule of −1 rather than the correct rule of +1. This likely reflects their training procedure. When the between-chunk element followed the rule, responding to the sequence 121 232 may be summarized as +1, +1, −1, +1, +1, −1. Hence, +1 are more likely to be a correct response. On the other hand, once the between-chunk rule was removed, the first element of a chunk was not necessarily +1. Correct responses were then equally distributed between +1 and −1, and the −1 response would then be rewarded with food from the hopper and more salient. Hence, the pigeons then learned the general rule of −1, which would impair their performance on the second element of the chunk. This suggests that the pigeons had difficulty creating separate memory representations for the second and third elements of the sequence, instead responding to a rule that applies across both of these elements.
General discussion
We found evidence for multiple processes that control behavior in a sequence learning task. Experiment 1 found evidence that sequence behavior in pigeons was controlled by low-level cues, such as through associative learning, rather than serial-pattern learning which involves learning the rules by which the elements in the sequence are structured. Experiment 1 used an adaptation of the 2-choice SMC task originally used by Fountain and Rowan (1995a) to study sequence learning in rats. Subsequent manipulations confirmed that low-level features, such as color, could cue correct choices, even when sequence order was randomized. Removal of color cues using a 3-choice SMC procedure still allowed for the use of spatial cues, such as the absolute position of choice items on the screen, to guide accurate choices. By moving to an 8-choice SMC procedure with absolute position cues removed by randomizing the start location across sessions, we finally found evidence for rule-learning in the pigeon. Especially strong evidence for rule learning comes from the high percentage of errors on the violation trial in Violation birds that learned a run sequence but with a violation element in the last position of the last chunk of the sequence which violated the +1 within-chunk rule from the other chunks of the sequence. This contrasts markedly with the performance by Run birds on the same item which conformed to the within-chunk +1 rule. Thus, pigeons finally showed evidence for a homologous process as has been demonstrated in people and rats (e.g., Fountain & Rowan, 1995b).
Following this, in Experiment 2 we tested how general was the application of this within-chunk rule. Pigeons were able to use the rule when the circles that made up the array were contracted or expanded, and when the chunk length was extended to four and five elements, but not when circles were removed or added to the array.
In Experiment 3, a replication of the procedure used in the final phase of Experiment 1 with new birds replicated the effect of sequence-rule learning for a trill sequence (cf. Fountain & Rowan, 1995a). Disrupting sequence information by randomizing chunk order (but maintaining within-chunk order), not only worsened performance on the first-chunk element, but also surprisingly on the second and third elements for which the within-chunk +1 rule was still viable. This suggests that pigeons had also encoded higher-order sequence information, with the removal of this information disrupting performance on all elements. Extended training on the randomized chunk orders for an additional 55 sessions resulted in the loss of control by the within-chunk rule despite its availability for use in guiding choices on second and third chunk elements.
Our results indicate that, like rats and humans, pigeons are able to abstract the higher-order structure of a repeating sequence. Nevertheless, unlike rats, pigeons displayed a high preference for using associative cues when those cues can lead to some success in performance. The preference of pigeons to rely on an absolute learning process is perhaps not surprising given previous evidence that pigeons have a great capacity to memorize discrete stimuli. For instance, Cook, Levison, Gillett, and Blaisdell (2005) examined the ability of pigeons to memorize a right or left response to pictorial stimuli. Two pigeons were trained for over 700 sessions each. Adjusted for guessing, the results indicated that the pigeons had access to approximately 830 memorized picture-response associations. These associations were also retained for months (cf. Vaughan & Greene, 1984). Possessing the capacity to store such a high number of associations is consistent with the pigeon’s performance in Experiment 1 (Phases 1–4) where low-level features could cue correct choices. This is particularly true in Phase 2, where the color and position cues could be used to predict with high certainty the rewarded location on any given trial. Interestingly, we suspect that pigeon performance on this task would even exceed that of humans. Humans would be more likely to observe the pattern from one trial to the next, and would not attend as closely to the unique color and position cues on each individual trial. Hence, we would predict that the observed performance of the pigeons in Phase 3 where the sequence was randomized would be greater than that of humans who were previously exposed to the sequences. Unfortunately, the extended training the pigeons were given makes a direct comparision with human performance difficult given the typically limited time that human participants are available (Silberberg & Kearns, 2009).
It may also be the case that the different performance shown by pigeons and rats reflects differences in the procedure or apparatus. Most of the rat experiments involved rats visiting locations on the surrounding walls. The richer set of spatial cues this situation provides may have facilitiated performance relative to the touchscreen. Indeed, when rats are trained on a 2-choice SMC task using a procedure where choices involve nose pokes to disks presented on a touchscreen, as in the pigeon procedure, higher error rates are found (Doyle et al., unpublished). Indeed, Doyle et al. report strikingly similar performance to our pigeons in rats trained on the touchscreen SMC procedure, providing strong support that differences between pigeon performance and rat performance found by Fountain and Rowan (1995b) are likely due to procedural differences rather than species differences. Differences in error rates shown in the octagonal versus touchscreen procedures may result from the increased memory load due to reinforcement being separated from the response in the touchscreen task, but not in the octagonal chamber, thus requiring touchscreen subjects to remember the location of the previous response to make the next correct choice. Supporting this interpretation, Colombo and Broadbent (2000) demonstrate that a spatial task presented in an operant chamber does not necessarily tap into the same neural systems (e.g., hippocampus) involved in a spatial task presented in an immersed environment that requires the animal to navigate space.
Despite the pigeon’s preference towards specific features of the stimulus display, there was evidence that the pigeons can learn sequential information when it is made more salient such as by reducing the reliability and salience of the specific low-level features. This was most clearly shown in Phase 5 of Experiment 1 and Phase 1 of Experiment 3, where the violation birds showed much higher error rates on the trial that violated the regular sequence. These results mirror those observed with rats (Fountain & Rowan, 1995a), and also mirror those observed with humans (Fountain & Rowan, 1995b).
Previous research has shown that serial pattern learning in the SMC task recruits multiple cognitive systems concurrently, including associative Stimulus-Response (S-R) learning, serial position learning involving timing or counting processes, and rule abstraction processes (Fountain & Benson Jr, 2006; Fountain et al., 2008; Fountain et al., 2012; Kundey & Fountain, 2010; Muller & Fountain, 2010; 2016). Learning to anticipate chunk-boundary elements has been shown to depend on both associative S-R learning and serial-position learning concurrently (Muller & Fountain, 2010; 2016; Stempowski, Carman, & Fountain, 1999). Learning to anticipate the violation element has been shown to depend on associative multiple-item learning involving cues from several preceding trials and ‘intra-box’ apparatus cues that signal the upcoming violation trial (Kundey & Fountain, 2010; Muller & Fountain, 2010; 2016). Learning to anticipate within-chunk elements, on the other hand, has been shown to depend on learning a motor program or abstract rules that are independent of external stimuli (Muller & Fountain, 2010).
Rule-based behavior may reflect an emergent consequence of underlying associative processes. Common features may be extracted from many ‘absolute cues’ (cf. Wallace & Fountain, 2002). These common features could then become associated with reward. The results from Phase 2 of Experiment 2 provide some support for this view. Performance established using an 8-element array failed to generalize to arrays containing either 4 or 16 elements. This generalization decrement suggests a limit on behavioral control by the common features extracted from the many ‘absolute cues’.
Since, for rats, the different pattern element types are learned using distinct cognitive mechanisms, it is not surprising to find that the same drug or toxic agent can result in differential facilitation of learning, impairment of learning, and no effect on learning for different element types in individual rats in the SMC task. Dissociations in learning and performance consistent with the foregoing behavioral and cognitive distinctions have been observed in rats following acute systemic treatment with MK-801, an N-methyl-D-aspartate receptor (NMDAr) antagonist, and with atropine, a muscarinic cholinergic antagonist (Chenoweth & Fountain, 2015; Fountain & Rowan, 2000; Fountain, Rowan, & Wollan, 2013). Similar dissociations have also been observed in adolescent nicotine effects on adult learning (Fountain et al., 2008; Pickens et al., 2013). Pickens et al. (2013) demonstrated sex-specific impairments of discrimination learning for chunk-boundary elements in male rats, but not female rats, and impairments of multiple-item discrimination learning for violation elements in female rats, but not male rats. Neither adult male nor female rats were impaired in rule-based learning for within-chunk elements after adolescent nicotine exposure. All the foregoing support the contention that serial pattern learning in rats depends on rats’ ability to use multiple concurrent cognitive processes in sequential learning tasks.
This raises the question of whether pigeons would show similar dissociations in the neural basis for different cognitive mechanisms of performance. Qadri and Cook (2015) have discussed how pigeon visual cognition can be similar to that of mammals in some cases (e.g., organizational principles of perceptual grouping), but divergent from that of mammals in other cases (e.g., perceptual completion). To what extent these similarities and differences in cognition arise from evolutionarily homologous and divergent neural systems remains a topic of interest. Given the extensive knowledge about the psychological and neural mechanisms of learning and memory processes in mammals, especially rats and primates, a fascinating avenue of comparative cognition research would be to understand how pigeon brains mediate the different cognitive strategies that we have shown to be involved in sequence learning.
The ability of pigeons to respond based on a sequential rule builds on work in other paradigms demonstrating rule-like behavior in pigeons, including control by a Same/Different relational concept (Blaisdell & Cook, 2005; Cook & Blaisdell, 2006; Daniel, Wright, & Katz, 2015; Wasserman, Hugart, & Kirkpatrick-Steger, 1995) and matching to sample (Bodily, Katz, & Wright, 2008).
The experiments suggest that the major difference between the pigeons’ performance in the SMC procedure in a touchscreen task and the rats’ performance in the SMC procedure in an octagonal-chamber task is not the overall ability to respond to sequential versus feature information, but a bias to engage one strategy over the other when both strategies are available. Rats (and humans) would seem to find sequential patterns highly salient when presented in 3D space, and will base their responding on these patterns if they are present. On the other hand, pigeons (and rats, Doyle et al., unpublished) tend to search more for feature-based cues or inter-item associations, at least when choices are presented on the 2D surface of a touchescreen. If these elements exist, they will use this information to govern their responding. Reducing the availability of this information, however, can bias responding to be based on sequential information. By establishing a touchscreen procedure for pigeons and potentially other species, we can investigate whether common or separate psychological and neural mechanisms support behavioral control by sequential information among different species.
Figure 8.
Mean percentage of choices to each location based on position in the chunk in Experiment 1, Phase 5. 0 indicates the correct location, 1 indicates the circle clockwise to the correct location, −1 indicates the circle anti-clockwise to the correct location, etc. Note that since pigeons tend to peck to a touchscreen in bursts, a response to the same location as the previously correct location was not considered to be an incorrect choice but rather a perseveration. Hence, these pecks were ignored in the analysis resulting in the locations having 0 responses.
Acknowledgments
Support for this research was provided by National Institutes of Health (NIH) Grant NS059076 (Aaron P. Blaisdell). This research was conducted following the relevant ethics guidelines for research with animals and was approved by UCLA’s institutional IACUC, and in compliance with the APA ethical standards in the treatment of animals.
References
- Aslin RN, Newport EL. Statistical learning from acquiring specific items to forming general rules. Current Directions in Psychological Science. 2012;21(3):170–176. doi: 10.1177/0963721412436806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaisdell AP, Cook RG. Two-item same-different concept learning in pigeons. Learning & Behavior. 2005;33:67–77. doi: 10.3758/bf03196051. [DOI] [PubMed] [Google Scholar]
- Bodily KD, Katz JS, Wright AA. Matching-to-sample abstract-concept learning by pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 2008;34:178–84. doi: 10.1037/0097-7403.34.1.178. [DOI] [PubMed] [Google Scholar]
- Brown MF, Sturz BR, Andriole K, Hardesty S, Place M. Precedence of spatial pattern learning revealed by immediate reversal performance. Behavioural Processes. 2010;85:252–264. doi: 10.1016/j.beproc.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Chenoweth AM, Fountain SB. Central muscarinic cholinergic involvement in serial pattern learning: Atropine impairs acquisition and retention in a serial multiple choice (SMC) task in rats. Neurobiology of Learning and Memory. 2015;123:18–27. doi: 10.1016/j.nlm.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Colombo M, Broadbent N. Is the avian hippocampus a functional homologue of the mammalian hippocampus? Neuroscience & Biobehavioral Reviews. 2000;24:465–484. doi: 10.1016/s0149-7634(00)00016-6. [DOI] [PubMed] [Google Scholar]
- Colombo M, Scarf D. Neurophysiological Studies of Learning and Memory in Pigeons. Comparative Cognition & Behavior Reviews. 2012;7:23–43. [Google Scholar]
- Cook RG, Blaisdell AP. Short-term item memory in successive same-different discriminations. Behavioural Processes. 2006;72:255–264. doi: 10.1016/j.beproc.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Cook RG, Katz JS, Blaisdell AP. Temporal properties of visual search in pigeon target localization. Journal of Experimental Psychology: Animal Behavior Processes. 2012;38:209–216. doi: 10.1037/a0026496. [DOI] [PubMed] [Google Scholar]
- Cook RG, Levison DG, Gillett S, Blaisdell AP. Capacity and limits of associative memory in pigeons. Psychonomic Bulletin & Review. 2005;12:350–358. doi: 10.3758/bf03196384. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Meyniel F, Wacongne C, Wang L, Pallier C. The neural representation of sequences: from transition probabilities to algebraic patterns and linguistic trees. Neuron. 2015;88 doi: 10.1016/j.neuron.2015.09.019. http://dx.doi.org/10.1016/j.neuron.2015.09.019. [DOI] [PubMed] [Google Scholar]
- Doyle KE, Renaud SM, Garlick D, Blaisdell AP, Fountain SB. Serial pattern acquisition in a touchscreen task for rats (in preparation) [Google Scholar]
- Doyon J, Gaudreau D, Laforce R, Castonguay M, Bedard PJ, Bedard F, Bouchard JP. Role of the striatum, cerebellum, and frontal Lobes in the learning of a visuomotor sequence. Brain and Cognition. 2997;34:218–245. doi: 10.1006/brcg.1997.0899. [DOI] [PubMed] [Google Scholar]
- Ferraro FR, Balota DA, Connor LT. Implicit memory and the formation of new associations in nondemented Parkinson’s disease individuals and individuals with senile dementia of the Alzheimer type: A serial reaction time (SRT) investigation. Brain and Cognition. 1993;21(2):163–180. doi: 10.1006/brcg.1993.1013. [DOI] [PubMed] [Google Scholar]
- Fisher DH, Pazzani MJ, Langley P, editors. Concept formation: Knowledge and experience in unsupervised learning. Morgan Kaufmann; 2014. [Google Scholar]
- Fountain SB. The structure of sequential behavior. In: Wasserman EA, Zentall TR, editors. Comparative Cognition: Experimental Explorations of Animal Intelligence. Oxford: Oxford University Press; 2006. pp. 439–458. [Google Scholar]
- Fountain SB. Pattern structure and rule induction in sequential learning. Comparative Cognition & Behavior Reviews. 2008;3:66–85. [Google Scholar]
- Fountain SB, Benson DM., Jr Chunking, rule learning, and multiple item memory in rat interleaved serial pattern learning. Learning and Motivation. 2006;37:95–112. doi: 10.1016/j.lmot.2005.09.002. [DOI] [Google Scholar]
- Fountain SB, Doyle KE. Association and abstraction in sequential learning: “What is learned?” revisited. International Journal of Comparative Psychology. 2011;24(4):437–459. [Google Scholar]
- Fountain SB, Henne DR, Hulse SH. Phrasing cues and hierarchical organization in serial pattern learning by rats. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10(1):30–45. [Google Scholar]
- Fountain SB, Rowan JD. Sensitivity to violations of “run” and “trill” structures in rat serial-pattern learning. Journal of Experimental Psychology: Animal Behavior Processes. 1995a;21:78–81. [PubMed] [Google Scholar]
- Fountain SB, Rowan JD. Coding of hierarchical versus linear pattern structure in rats and humans. Journal of Experimental Psychology: Animal Behavior Processes. 1995b;21:187–202. doi: 10.1037//0097-7403.21.3.187. [DOI] [PubMed] [Google Scholar]
- Fountain SB, Rowan JD. Differential impairments of rat serial pattern learning and retention induced by MK-801, an NMDA receptor antagonist. Psychobiology. 2000;28(1):32–44. [Google Scholar]
- Fountain SB, Rowan JD, Carman HM. Encoding structural ambiguity in rat serial pattern: The role of phrasing. International Journal of Comparative Psychology. 2007;20:25–34. [Google Scholar]
- Fountain SB, Rowan JD, Wollan MO. Central cholinergic involvement in sequential behavior: Impairments of performance by atropine in a serial multiple choice task for rats. Neurobiology of learning and memory. 2013;106:118–126. doi: 10.1016/j.nlm.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain SB, Wallace DG, Rowan JD. The organization of sequential behavior. In: Fountain SB, Bunsey M, Danks JH, McBeath MK, editors. Animal Cognition and Sequential Behavior: Behavioral, Biological, and Computational Perspectives. Boston, MA: Kluwer Academic; 2002. pp. 115–150. [Google Scholar]
- Garlick D, Gant DJ, Brakel LAW, Blaisdell AP. Attributional and relational processing in pigeons. Frontiers in Comparative Psychology. 2011;2:14. doi: 10.3389/fpsyg.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrnstein RJ, Loveland DH, Cable C. Natural concepts in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1976;2:285–302. doi: 10.1037//0097-7403.2.4.285. [DOI] [PubMed] [Google Scholar]
- Kesner RP. Neural mediation of memory for time: Role of the hippocampus and prefrontal cortex. In: Fountain SB, Bunsey M, Danks JH, McBeath MK, editors. Animal cognition and sequential behavior: Behavioral, biological, and computational perspectives. Boston: Kluwer Academic; 2002. pp. 201–226. [Google Scholar]
- Knopman D, Nissen MJ. Procedural learning is impaired in Huntington’s disease: evidence from the serial reaction time task. Neuropsychologia. 1991;29(3):245–254. doi: 10.1016/0028-3932(91)90085-m. [DOI] [PubMed] [Google Scholar]
- Kundey SMA, Fountain SB. Irrelevant relations and the active search for pattern structure in rat serial pattern learning. Animal Cognition. 2011;14:359–368. doi: 10.1007/s10071-010-0370-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashley KS. The problem of serial order in behavior. In: Jeffress LA, editor. Cerebral mechanisms in behavior. New York: Wiley; 1951. pp. 112–146. [Google Scholar]
- McGonigle B, Chalmers M. The growth of cognitive structure in monkeys and men. In: Fountain SB, Bunsey M, Danks JH, McBeath MK, editors. Animal cognition and sequential behavior: Behavioral, biological, and computational perspectives. Boston; Kluwer Academic: 2002. pp. 269–314. [Google Scholar]
- Muller MD, Fountain SB. Concurrent cognitive processes in rat serial pattern learning: Item memory, serial position, and pattern structure. Learning and motivation. 2010;41(4):252–272. doi: 10.1016/j.lmot.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MD, Fountain SB. Concurrent cognitive processes in rat serial pattern learning: II. Discrimination learning, rule learning, chunk length, and multiple-item memories. Journal of the Experimental Analysis of Behavior. 2016;105:155–175. doi: 10.1002/jeab.186. [DOI] [PubMed] [Google Scholar]
- Murphy RA, Mondragón E, Murphy VA. Rule learning by rats. Science. 2008;319:1849–1851. doi: 10.1126/science.1151564. [DOI] [PubMed] [Google Scholar]
- Pearce JM. The acquisition of an artificial category by pigeons. The Quarterly Journal of Experimental Psychology Section B: Comparative and Physiological Psychology. 1989;41:381–406. [Google Scholar]
- Qadri MAJ, Cook RG. Experimental divergences in the visual cognition of birds and mammals. Comparative Cognition & Behavior Reviews. 2015;10:73–105. doi: 10.3819/ccbr.2015.100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Wedig MM, Wright CI, Martis B, McMullin KG, Shin LM, Cannistraro PA, Wilhelm S. Functional magnetic resonance imaging study of regional brain activation during implicit sequence learning in obsessive–compulsive disorder. Biological psychiatry. 2007;61(3):330–336. doi: 10.1016/j.biopsych.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Restle F. Serial patterns: The role of phrasing. Journal of Experimental Psychology. 1972;92(3):385. doi: 10.1037/h0033619. [DOI] [PubMed] [Google Scholar]
- Restle F, Brown ER. Serial pattern learning: Pretraining of runs and trills. Psychonomic Science. 1970;19:321–322. [Google Scholar]
- Sands SF, Wright AA. Primate memory: Retention of serial list items by a rhesus monkey. Science. 1980;209:938–940. doi: 10.1126/science.6773143. [DOI] [PubMed] [Google Scholar]
- Sands SE, Wright AA. Human and monkey pictorial memory scanning. Science. 1982;216:1333–1334. doi: 10.1126/science.7079768. [DOI] [PubMed] [Google Scholar]
- Siegert RJ, Taylor KD, Weatherall M, Abernethy DA. Is implicit sequence learning impaired in Parkinson’s disease? A meta-analysis. Neuropsychology. 2006;20(4):490–495. doi: 10.1037/0894-4105.20.4.490. http://dx.doi.org/10.1037/0894-4105.20.4.490. [DOI] [PubMed] [Google Scholar]
- Silberberg A, Kearns D. Memory for the order of briefly presented numerals in humans as a function of practice. Animal Cognition. 2009;12:405–407. doi: 10.1007/s10071-008-0206-8. [DOI] [PubMed] [Google Scholar]
- Simon HA. How big is a chunk. Science. 1974;183(4124):482–488. doi: 10.1126/science.183.4124.482. [DOI] [PubMed] [Google Scholar]
- Stempowski NK, Carman HM, Fountain SB. Temporal phrasing and overshadowing in rat serial-pattern learning. Learning and Motivation. 1999;30(1):74–100. [Google Scholar]
- Sun R, Giles CL. Sequence learning: Paradigms, algorithms, and applications. Springer-Verlag; Berlin Heidelberg: 2001a. [Google Scholar]
- Sun R, Giles CL. Sequence learning: from recognition and prediction to sequential decision making. IEEE Intelligent Systems. 2001b;16(4):67–70. [Google Scholar]
- Terrace HS, McGonigle B. Memory and representation of serial order by children, monkeys, and pigeons. Current Directions in Psychological Science. 1994:180–185. [Google Scholar]
- Vaughan W, Greene SL. Pigeon visual memory capacity. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10:256–271. [Google Scholar]
- Wallace DG, Fountain SB. What is learned in sequential learning? An associative model of reward magnitude serial-pattern learning. Journal of Experimental Psychology: Animal Behavior Processes. 2002;28:43–63. [PubMed] [Google Scholar]
- Wasserman EA, Kiedinger RE, Bhatt RS. Conceptual behavior in pigeons: Categories, subcategories, and pseudocategories. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:235–246. [Google Scholar]
- Wasserman EA, Hugart JA, Kirkpatrick-Steger K. Pigeons show same-different -different conceptualization after training with complex visual stimuli. Journal of Experimental Psychology: Animal Behavior Processes. 1995;21(3):248. doi: 10.1037//0097-7403.21.3.248. [DOI] [PubMed] [Google Scholar]
- Wright AA. Concept learning and learning strategies. Psychological Science. 1997;8:119–123. [Google Scholar]