Abstract
Purpose
Dry eye syndrome (DES) affects millions of people worldwide. Homeopathic remedies to treat a wide variety of ocular diseases have previously been documented in the literature, but little systematic work has been performed to validate the remedies’ efficacy using accepted laboratory models of disease. The purpose of this study was to evaluate the efficacy of human milk and nopal cactus (prickly pear), two widely used homeopathic remedies, as agents to reduce pathological markers of DES.
Methods
The previously described benzalkonium chloride (BAK) dry eye mouse model was used to study the efficacy of human milk and nopal cactus (prickly pear). BAK (0.2%) was applied to the mouse ocular surface twice daily to induce dry eye pathology. Fluorescein staining was used to verify that the animals had characteristic signs of DES. After induction of DES, the animals were treated with human milk (whole and fat-reduced), nopal, nopal extract derivatives, or cyclosporine four times daily for 7 days. Punctate staining and preservation of corneal epithelial thickness, measured histologically at the end of treatment, were used as indices of therapeutic efficacy.
Results
Treatment with BAK reduced the mean corneal epithelial thickness from 36.77±0.64 μm in the control mice to 21.29±3.2 μm. Reduction in corneal epithelial thickness was largely prevented by administration of whole milk (33.2±2.5 μm) or fat-reduced milk (36.1±1.58 μm), outcomes that were similar to treatment with cyclosporine (38.52±2.47 μm), a standard in current dry eye therapy. In contrast, crude or filtered nopal extracts were ineffective at preventing BAK-induced loss of corneal epithelial thickness (24.76±1.78 μm and 27.99±2.75 μm, respectively), as were solvents used in the extraction of nopal materials (26.53±1.46 μm for ethyl acetate, 21.59±5.87 μm for methanol). Epithelial damage, as reflected in the punctate scores, decreased over 4 days of treatment with whole and fat-reduced milk but continued to increase in eyes treated with nopal-derived materials.
Conclusions
Whole and fat-reduced human milk showed promising effects in the prevention of BAK-induced loss of corneal epithelial thickness and epithelial damage in this mouse model. Further studies are required to determine whether human milk may be safely used to treat dry eye in patients.
Introduction
Dry eye syndrome (DES) affects millions of people worldwide, with an estimated prevalence of 5–30% in the aging population [1]. DES can significantly impact visual acuity and daily activities. Symptoms vary in type and severity. Common symptoms include dryness, red eyes, general irritation, excessive tearing, and blurred vision. DES can also inflict undue economic burden from medical costs incurred from frequent visits to health care providers and use of various therapies.
The pathophysiology of DES is complex and multifactorial but has classically been described as a product of decreased tear production, increased evaporative loss of tear film, or both. Even with numerous tests available, there is currently no gold standard test for diagnosing dry eye. Current diagnostic studies include symptom questionnaires, tear break-up time, ocular surface fluorescein staining, the Schirmer test, and meibomian function and structure tests [2]. Despite advances in technology to measure dry eye syndrome, effective treatment remains a major challenge.
Traditional eye medicine (TEM) has been widely used to treat ocular disease. Large-scale use of TEM for the treatment of corneal disease has been documented in Africa [3]. Human breast milk was the most commonly used TEM to treat corneal ulcers seen at a tertiary eye center in South India [4,5]. Human milk contains multiple components that have previously been studied in the treatment of DES. These components include epithelial growth factor (EGF), vitamin A, lactoferrin, oligosaccharides, and omega-3 and omega-6 fatty acids [6-10]. Human milk also contains components that protect against infection, including lysozymes, immunoglobulin (Ig) A and IgG, cytokines such as interleukin (IL)-10, and β-defensin-2 [11-13].
Nopal (Opuntia ficus), a cactus endemic to Mexico, is also recognized as a homeopathic therapy for many diseases [5,14,15]. Nopal is a succulent plant containing many health-promoting constituents, including minerals, antimicrobials, antioxidants, and high levels of fibrous polysaccharides and lipids that may function to enhance water retention under arid conditions [14,16,17]. Extracts of plant tissue obtained from the cladodes and fruits of nopal have been shown to have beneficial effects in IL-1β-stimulated inflammatory models [18], but no work in models of ocular inflammation that involve cytokine stimulation such as in DES has been reported.
Although there are many anecdotal reports of human milk and nopal to treat ocular and inflammatory diseases, few laboratory studies have been reported to substantiate the efficacy of these therapies. We conducted the present study to assess the ability of human milk and nopal to normalize various markers of DES in a mouse model.
Methods
Benzalkonium chloride dry eye model
Eight-week-old female BALB/c mice weighing approximately 20 gm (Jackson Laboratory, Bar Harbor, ME) were used in this study. The mice were housed in a standard environment with alternating 14 h:10 h light-dark cycles. All experimental procedures were performed in adherence with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and with approval from the Institutional Animal Care and Use Committee. One eye of all mice was treated twice daily with topical administration of 5 μl of 0.2% BAK (Sigma, St. Louis, MO) to induce dry eye pathology. The contralateral eye was treated twice daily with topical administration of 5 μl PBS (150 mM NaCl, 5.6 mM Na2HPO4, 1 mM KH2PO4, pH 7.4) and used as the normal eye control group.
Experimental procedure
The left eyes of all mice received only PBS throughout the study serving as the normal eye control group. Right eyes were treated with BAK until dry eye pathology was established based on clinical evaluations. Once dry eye pathology was established, the mice were randomly assigned to the various therapeutic groups. Milk treatments were administered topically as a 5 μl drop four times daily and included either whole milk or fat-reduced milk. Nopal (crude and clarified aqueous extracts) and nopal materials extracted with ethyl acetate or methanol were dissolved in PBS at 1 mg/ml and delivered as a 5 μl drop four times daily. Cyclosporine (0.05%) was administered similarly as a positive therapeutic control. Animals continued to receive BAK to the experimental eye throughout the entire phase of treatment with test agents, but the latter were applied 1 h after BAK to avoid dilution or possible interactions between BAK and the therapeutic agent.
Clinical evaluation of DES
We monitored the mice for dry eye pathology using fluorescein staining on a slit-lamp. At the end of therapy, all mice were sacrificed and the ocular tissue was dissected and harvested for histological analysis of corneal epithelial thickness. Tear production was measured in treated and control eyes using the Zone Quick test but was considered unreliable as a metric for DES in this model.
Fluorescein staining
Eyes were stained with fluorescein (Ful-Glo, Akorn, Lake Forest, IL) to visualize damage to the corneal epithelium. For staining, 50 μl of saline was dropped on Ful-Glo strips, which were then placed on the palpebral conjunctiva. The animals were allowed to blink for 90 s and then examined with a cobalt blue filter under a slit-lamp microscope DS11 (Lombart Instruments, Norfolk, VA). The eyes were scored (punctate score) and assigned a value in the range of 1 to 5 based on the amount of fluorescein staining of the cornea. A score of 1 was given for fluorescein staining that covered 1/16 of the cornea surface area, 2 for 1/8, 3 for 1/4, 4 for ½, or 5 for more than 50% of the surface area of the cornea. Scoring was performed by observers masked to treatment.
H&E staining of tissue sections
All eyes were enucleated at the end of the study and fixed in 4% paraformaldehyde (Sigma) for 24 h and then placed into 10% normal buffered formalin (Sigma) for 4 h, followed by incubation in 70% ethanol (Sigma) for 24 h. Tissues were then processed and embedded in paraffin for sectioning and stained with hematoxylin and eosin (H&E; Sigma). The sections were imaged using a Nikon Eclipse 80i (Nikon, Tokyo, Japan) with a DSFII camera (Nikon), and the corneal epithelial thickness was measured using NIS-Element software (Nikon).
Statistical analysis
Statistical analysis was performed with GraphPad Prism (GraphPad Software, La Jolla, CA) using one-way ANOVA with the Tukey post-hoc test. Asterisks refer to P values according to groupings where *p<0.05, **p<0.01 and ***p<0.001.
Preparation of nopal extracts
Nopal leaves (prickly pear cactus) purchased fresh from a local supermarket or rehydrated from lyophilized leaves were dethorned, homogenized, and strained using a commercial juicer (Omega Model J8006, Harrisburg, PA). The juice was centrifuged for 15 min at 19,722 ×g in a microcentrifuge and passed through a 0.2 μm filter to remove large polysaccharides. Two chemical extractions were performed, the first with 100% ethyl acetate for lipid emulsion. After centrifugation at 13,226 ×g for 20 min at 4 °C, the resulting precipitant from this first extraction was then re-extracted, and the supernatants were mixed. In the second case, a portion of the nopal juice was combined with 30% methanol solution to isolate phenolic compounds and centrifuged at 13,226 ×g for 20 min at 4 °C. Extracts were lyophilized and resuspended in PBS at 1 mg/ml.
Preparation of human milk
Human milk was provided by a lactating donor and aliquoted without the addition of preservatives for storage at −20 °C. To produce fat-reduced milk, whole milk was thawed and then centrifuged for 20 min at 19,722 ×g. The aqueous layer, designated fat-reduced milk, was removed, stored at 4 °C, and used within 8 days.
Results
Fluorescein staining
Treatment with BAK induced DES-like pathology, as evidenced by increased fluorescein staining detected with slit-lamp microscopy (Figure 1, Figure 2). Increased fluorescein staining and epithelial thinning are characteristic features of human dry eye syndrome and thus were taken as quantifiable endpoints to measure the efficacy of DES therapies [2,19,20].
Figure 1.
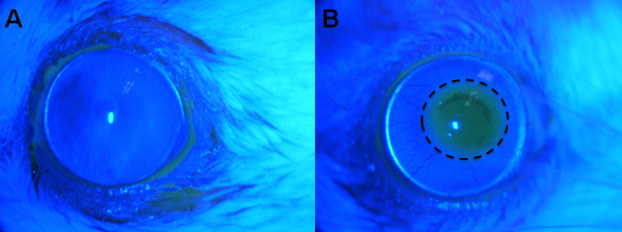
Fluorescein staining of the cornea. After staining with fluorescein, the corneal surface was imaged using a cobalt blue filter fitted to a slit-lamp microscope. Images were taken on day 4 of treatment. A: Normal eye control group without evidence of corneal surface damage. B: Dry eye group with fluorescein staining indicating corneal surface damage (dotted circle).
Figure 2.
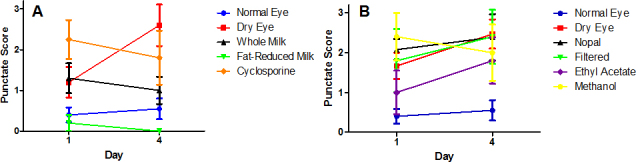
Punctate scores of dry eye therapy. Graphical representation of data reporting fluorescence staining punctate score for treatments spanning days 1–4. Controls include normal eye without benzalkonium chloride (BAK) treatment, dry eye receiving no therapy, and cyclosporine as a positive therapeutic control. A: Punctate scores following therapy with milk components. B:Punctate scores following therapy with nopal components.
As shown in Figure 2A, application of whole and fat-reduced human breast milk to BAK-treated eyes led to a decrease in fluorescein staining over a 4 day treatment period. Similar reductions in staining were observed when the mice received treatment with cyclosporine, a current first-line agent for human DES. In contrast, fluorescein staining increased over this time period in BAK-treated eyes receiving therapy with crude or filtered nopal extracts, or their solvent controls. Staining was essentially unchanged in the normal eye control (Figure 2B).
H&E staining of tissue sections
BAK treatment caused thinning of the corneal epithelium, easily measured in histological tissue sections representing the central region of the anterior eye surface (Figure 3). Quantification of treatment effects demonstrated that whole and fat-reduced human milk, similar to cyclosporine, essentially prevented BAK-induced corneal thinning in this model (p<0.001, Figure 4). Despite a trend toward improvement, administration of crude or filtered nopal extracts, as well as the ethyl acetate extract, to BAK-treated eyes failed to prevent statistically significant loss of corneal epithelium thickness. As with fluorescein staining, treatment with cyclosporine showed a significant protective effect (p<0.01). These results indicate that human milk is efficacious in the treatment of corneal surface damage in a dry eye mouse model. In contrast, nopal and its various extracts were ineffective at preserving the loss of corneal epithelial thickness in this dry eye model (Figure 5). The mean corneal epithelial thickness at the end of the study is displayed in Table 1 for all groups.
Figure 3.
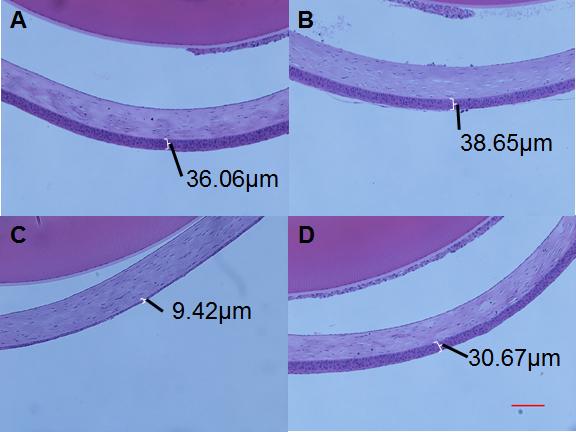
Corneal thickness after therapy. Whole eye tissue sections were stained with hematoxylin and eosin (H&E) to measure the corneal epithelial thickness. The epithelial layer is shown in white brackets with a representative thickness measurement. A: Normal eye. B: Cyclosporine. C: Dry eye, no therapy. D: Dry eye treated with whole milk, 10X magnification, red bar = 100 μm.
Figure 4.
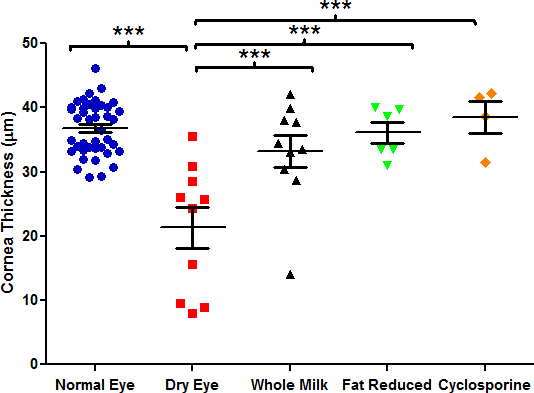
Corneal epithelial thickness after treatment with human milk therapies. Comparisons were analyzed with one-way ANOVA with Tukey’s post-hoc test. *p<0.05, **p<0.01, ***p<0.001; data are mean ± standard error of the mean (SEM).
Figure 5.
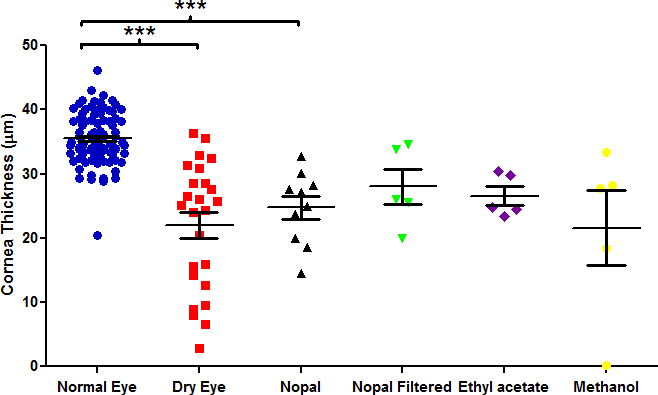
Corneal epithelial thickness after treatment with nopal and chemical extracts of nopal. Comparisons were analyzed with one-way ANOVA with Tukey’s post-hoc test. *p<0.05, **p<0.01, ***p<0.001. Data are mean ± standard error of the mean (SEM).
Table 1. Mean corneal epithelial thickness for each dry eye treatment.
| Treatment | Corneal Epithelial Thickness (μm) | N |
|---|---|---|
| Normal eye |
36.77±0.64 |
91 |
| Whole Milk |
33.20±2.50 |
15 |
| Fat-Reduced Milk |
36.10±1.58 |
9 |
| Nopal (crude) |
24.76±1.78 |
10 |
| Nopal (filtered) |
27.99±2.75 |
5 |
| Ethyl Acetate |
26.53±1.46 |
5 |
| Methanol |
21.59±5.87 |
5 |
| Dry eye (BAK) |
21.29±3.17 |
30 |
| Cyclosporine | 38.52±2.47 | 14 |
Statistics by one-way ANOVA with Tukey post-hoc test. Data are mean ± SEM.
Discussion
Given the high prevalence of DES worldwide and its significant burden on quality of life, there is considerable interest in the discovery of safe and effective agents to prevent symptoms associated with this condition. To evaluate potential new therapeutics, we used a mouse model of DES induced by repeated application of BAK, an agent once commonly used as a preservative in ophthalmic solutions. Application of BAK to the eye causes adverse effects, including decreased tear production and tear film instability, superficial punctate keratitis, and cytotoxicity to the corneal epithelium, conditions associated with DES in humans [20-22]. Thus, we used the BAK-induced murine dry eye model to evaluate the potential efficacy of natural products deemed to be of therapeutic value against DES.
Results from histological staining of the cornea provide a meaningful measure of dry eye induction and response to treatment. Corneal epithelial thickness was significantly decreased in the dry eye group compared to the normal eye control group. Treatment with cyclosporine, an established therapeutic agent, resulted in corneal epithelial thickness that was significantly thicker from the dry eye group. These results indicate the dry eye model successfully induces epithelial damage and responds to established dry eye therapy.
In this study, we observed that human whole milk and fat-reduced human milk were able to preserve corneal epithelial thickness in the dry eye mouse model. In addition, preservation of corneal epithelial thickness by human whole milk and fat-reduced milk was comparable to cyclosporine. We believe this is the first study to demonstrate that human milk can preserve corneal epithelial thickness in a dry eye model and that preservation of corneal epithelial thickness in these groups was comparable to topical cyclosporine. Although we did not observe reductions in tear volume measurement following the BAK treatment, we routinely observed thinning of the corneal epithelium on H&E staining in the dry eye group, which is consistent with the Lin et al. study [20]. Therefore, we used corneal epithelial thickness as a key metric to measure the efficacy of various therapeutic agents in this study.
The use of human milk as a therapeutic for DES has social and economic implications. With a resurgence in homeopathy, human milk provides an alternative to Western medicine practice [23,24]. Economically, human milk provides a relatively inexpensive and accessible medication. This may be especially valuable in rural communities or to patients with limited health care access. More importantly, it should not be forgotten that DES remains a difficult disease to treat, and new treatments are needed to provide longer and more complete relief of symptoms. Therefore, patients who have been unsuccessfully treated may favor TEM such as human milk.
Human milk contains components previously investigated and proven to be effective in other dry eye studies: EGF, vitamin A, lactoferrin, oligosaccharides, and omega-3 and omega-6 fatty acids [6-10]. EGF has multiple proposed therapeutic effects, including promoting epithelial proliferation, inhibiting apoptosis, and increasing goblet cell number to maintain the mucous component of tear film [6]. Deficiency in vitamin A has been linked to squamous metaplasia in the corneal epithelium [25,26]. Lactoferrin is an anti-inflammatory component of tear film. Dysfunction of the lacrimal functional unit may lead to decreased secretion of lactoferrin. Decreased lactoferrin, in turn, can lead to inflammation [8,27]. Dietary consumption of omega-3 fatty acids is thought to have anti-inflammatory actions on the meibomian glands, which could decrease evaporative losses of tear film. Recently, it was demonstrated that oligosaccharides present in human milk protect tear film stability [9].
Tsubota et al. previously demonstrated that topical autologous serum could supply EGF and vitamin A to the ocular surface, even after being preserved for 1 month in a refrigerator or 3 months in a freezer [7]. Human milk could potentially affect dry eye by providing EGF, vitamin A, lactoferrin, oligosaccharides, and omega-3 fatty acids to the ocular surface. Although not an autologous agent, human milk may have less antigenicity than other natural products such as nopal, thus increasing human milk’s chances of successfully delivering these components. Human milk also contains lysozymes, IgA, IgG, interleukin 10, and β-defensin-2 that may allow it to be preserved for extended periods of time free of contamination. Future studies will be needed to evaluate which specific components provided the beneficial effect or their mechanisms of action.
Topical human milk treatment demonstrated the ability to preserve corneal epithelial thickness in the BAK-induced dry eye mouse model and holds the potential to be an effective treatment for DES in humans. In addition, human milk may provide a viable alternative for DES treatment for patients more inclined to TEM or those with limited health care access. Further studies are indicated to determine whether human milk can be safely used to treat dry eye in humans, whether the benefit conferred in this study is applicable to more easily procured animal milk, and whether processing and long-term storage, such as in pasteurization, affect efficacy. Moreover, studies should be directed at elucidating the active components of human milk and their mechanisms of action, potentially expanding future treatment options for DES.
In Mexico, nopal (Opuntia ficus) is commonly consumed for treatment of a variety of ailments, including diabetes [28]. Although nopal appears to be safe to consume [29], little work has been reported on the use of the mucilaginous contents of nopal cladodes as a topical agent against tissue desiccation. Because unstable tear film is a common aspect of DES, it was thought that the polysaccharide and pectin content of nopal might increase tear viscosity and increase the stability and resilience of the tear film across the corneal surface [5,30]. Using either crude nopal juice or lipids and phenolic compounds enriched by extraction of nopal homogenates with ethyl acetate or methanol, respectively, no efficacy was observed with the use of nopal constituents against markers of DES in our mouse model. Thus, the homeopathic benefits of nopal do not extend to relief of ocular surface abnormalities associated with DES.
There is tremendous interest in development of effective treatment strategies for DES. A recent attempt to perform a meta-analysis of 49 clinical trials conducted during 1988–2010 was unsuccessful due to heterogeneity among the study endpoints [31]. Because the best treatments for DES are still undetermined, many patients now consider alternative therapies such as natural products to relieve the symptoms of DES. Results from the current study suggest that human milk may provide therapeutic benefits as evidenced by protection against corneal surface damage in a mouse model of DES and thus lay the groundwork for future studies in additional animal models that have features associated with DES in humans.
Acknowledgments
This work was supported in part by NIH grants R01EY021455 and R01EY005856 and by a Department of Ophthalmology unrestricted grant from Research to Prevent Blindness, New York, NY.
References
- 1.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- 2.Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:108–52. doi: 10.1016/s1542-0124(12)70083-6. [DOI] [PubMed] [Google Scholar]
- 3.Courtright P, Lewallen S, Kanjaloti S, Divala DJ. Traditional eye medicine use among patients with corneal disease in rural Malawi. Br J Ophthalmol. 1994;78:810–2. doi: 10.1136/bjo.78.11.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prajna NV, Pillai MR, Manimegalai TK, Srinivasan M. Use of Traditional Eye Medicines by corneal ulcer patients presenting to a hospital in South India. Indian J Ophthalmol. 1999;47:15–8. [PubMed] [Google Scholar]
- 5.Rodriguez-Fragoso L, Reyes-Esparza J, Burchiel SW, Herrera-Ruiz D, Torres E. Risks and benefits of commonly used herbal medicines in Mexico. Toxicol Appl Pharmacol. 2008;227:125–35. doi: 10.1016/j.taap.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao X, He H, Lin Z, Luo P, Zhou T, Zhou Y, Liu Z. Therapeutic effects of epidermal growth factor on benzalkonium chloride-induced dry eye in a mouse model. Invest Ophthalmol Vis Sci. 2012;53:191–7. doi: 10.1167/iovs.11-8553. [DOI] [PubMed] [Google Scholar]
- 7.Tsubota K, Goto E, Fujita H, Ono M, Inoue H, Saito I, Shimmura S. Treatment of dry eye by autologous serum application in Sjogren's syndrome. Br J Ophthalmol. 1999;83:390–5. doi: 10.1136/bjo.83.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pflugfelder SC. Antiinflammatory therapy for dry eye. Am J Ophthalmol. 2004;137:337–42. doi: 10.1016/j.ajo.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 9.Bucolo C, Musumeci M, Salomone S, Romano GL, Leggio GM, Gagliano C, Reibaldi M, Avitabile T, Uva MG, Musumeci S, Drago F. Effects of Topical Fucosyl-Lactose, a Milk Oligosaccharide, on Dry Eye Model: An Example of Nutraceutical Candidate. Front Pharmacol. 2015;6:280. doi: 10.3389/fphar.2015.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rashid S, Jin Y, Ecoiffier T, Barabino S, Schaumberg DA, Dana MR. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126:219–25. doi: 10.1001/archophthalmol.2007.61. [DOI] [PubMed] [Google Scholar]
- 11.Goldman AS, Smith CW. Host resistance factors in human milk. J Pediatr. 1973;82:1082–90. doi: 10.1016/s0022-3476(73)80453-6. [DOI] [PubMed] [Google Scholar]
- 12.Garofalo R, Chheda S, Mei F, Palkowetz KH, Rudloff HE, Schmalstieg FC, Rassin DK, Goldman AS. Interleukin-10 in human milk. Pediatr Res. 1995;37:444–9. doi: 10.1203/00006450-199504000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Baricelli J, Rocafull MA, Vazquez D, Bastidas B, Baez-Ramirez E, Thomas LE. beta-defensin-2 in breast milk displays a broad antimicrobial activity against pathogenic bacteria. J Pediatr (Rio J) 2015 doi: 10.1016/j.jped.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Espino-Diaz M, de Jesus Ornelas-Paz J, Martinez-Tellez MA, Santillan C, Barbosa-Canovas GV, Zamudio-Flores PB, Olivas GI. Development and characterization of edible films based on mucilage of Opuntia ficus-indica (L.). J Food Sci. 2010;75:E347–52. doi: 10.1111/j.1750-3841.2010.01661.x. [DOI] [PubMed] [Google Scholar]
- 15.Villa-Caballero L, Morello CM, Chynoweth ME, Prieto-Rosinol A, Polonsky WH, Palinkas LA, Edelman SV. Ethnic differences in complementary and alternative medicine use among patients with diabetes. Complement Ther Med. 2010;18:241–8. doi: 10.1016/j.ctim.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez E, Garcia S, Heredia N. Extracts of edible and medicinal plants damage membranes of Vibrio cholerae. Appl Environ Microbiol. 2010;76:6888–94. doi: 10.1128/AEM.03052-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medina-Torres L, Vernon-Carter EJ, Gallegos-Infante JA, Rocha-Guzman NE, Herrera-Valencia EE, Calderas F, Jimenez-Alvarado R. Study of the antioxidant properties of extracts obtained from nopal cactus (Opuntia ficus-indica) cladodes after convective drying. J Sci Food Agric. 2011;91:1001–5. doi: 10.1002/jsfa.4271. [DOI] [PubMed] [Google Scholar]
- 18.El-Mostafa K, El Kharrassi Y, Badreddine A, Andreoletti P, Vamecq J, El Kebbaj MS, Latruffe N, Lizard G, Nasser B, Cherkaoui-Malki M. Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules. 2014;19:14879–901. doi: 10.3390/molecules190914879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin H, Yiu SC. Dry eye disease: A review of diagnostic approaches and treatments. Saudi J Ophthalmol. 2014;28:173–81. doi: 10.1016/j.sjopt.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin Z, Liu X, Zhou T, Wang Y, Bai L, He H, Liu Z. A mouse dry eye model induced by topical administration of benzalkonium chloride. Mol Vis. 2011;17:257–64. [PMC free article] [PubMed] [Google Scholar]
- 21.Rosin LM, Bell NP. Preservative toxicity in glaucoma medication: clinical evaluation of benzalkonium chloride-free 0.5% timolol eye drops. Clin Ophthalmol. 2013;7:2131–5. doi: 10.2147/OPTH.S41358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim KA, Hyun LC, Jung SH, Yang SJ. The leaves of Diospyros kaki exert beneficial effects on a benzalkonium chloride-induced murine dry eye model. Mol Vis. 2016;22:284–93. [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher P. What is homeopathy? An introduction. Front Biosci. 2012;4:1669–82. doi: 10.2741/489. Elite Ed. [DOI] [PubMed] [Google Scholar]
- 24.Jonas WB, Kaptchuk TJ, Linde K. A critical overview of homeopathy. Ann Intern Med. 2003;138:393–9. doi: 10.7326/0003-4819-138-5-200303040-00009. [DOI] [PubMed] [Google Scholar]
- 25.Tseng SC, Farazdaghi M, Rider AA. Conjunctival transdifferentiation induced by systemic vitamin A deficiency in vascularized rabbit corneas. Invest Ophthalmol Vis Sci. 1987;28:1497–504. [PubMed] [Google Scholar]
- 26.el-Ghorab M, Capone A, Jr, Underwood BA, Hatchell D, Friend J, Thoft RA. Response of ocular surface epithelium to corneal wounding in retinol-deficient rabbits. Invest Ophthalmol Vis Sci. 1988;29:1671–6. [PubMed] [Google Scholar]
- 27.McCollum CJ, Foulks GN, Bodner B, Shepard J, Daniels K, Gross V, Kelly L, Cavanagh HD. Rapid assay of lactoferrin in keratoconjunctivitis sicca. Cornea. 1994;13:505–8. [PubMed] [Google Scholar]
- 28.Yeh GY, Eisenberg DM, Kaptchuk TJ, Phillips RS. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care. 2003;26:1277–94. doi: 10.2337/diacare.26.4.1277. [DOI] [PubMed] [Google Scholar]
- 29.De Smet PA. Herbal remedies. N Engl J Med. 2002;347:2046–56. doi: 10.1056/NEJMra020398. [DOI] [PubMed] [Google Scholar]
- 30.Guevara-Arauza JC, Ornelas Paz Jde J, Mendoza SR, Guerra RE, Paz Maldonado LM, Gonzalez DJ. Biofunctional activity of tortillas and bars enhanced with nopal. Preliminary assessment of functional effect after intake on the oxidative status in healthy volunteers. Chem Cent J. 2011;5:10. doi: 10.1186/1752-153X-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alves M, Fonseca EC, Alves MF, Malki LT, Arruda GV, Reinach PS, Rocha EM. Dry eye disease treatment: a systematic review of published trials and a critical appraisal of therapeutic strategies. Ocul Surf. 2013;11:181–92. doi: 10.1016/j.jtos.2013.02.002. [DOI] [PubMed] [Google Scholar]


