Abstract
Purpose
The aim of this study was to examine the association of preoperative thrombocytosis with the prognosis of patients with nonmetastatic renal cell carcinoma (RCC).
Materials and Methods
We conducted a retrospective analysis of 187 patients who underwent a radical nephrectomy for nonmetastatic RCC between July 1997 and June 2009. Thrombocytosis was defined as a platelet count≥400,000 µL, and patients were divided into 2 groups according to presence of preoperative thrombocytosis, and the cancer-specific survival rates and overall survival rates of the 2 groups after radical nephrectomy were compared.
Results
The mean age of the patients was 56.0±11.7 years and the mean follow-up period was 59.3±42.1 months; there were 20 patients with preoperative thrombocytosis. Thirty patients developed metastases and 9 patients died during the follow-up period. In Kaplan-Meier analysis using a univariate log-rank test, both cancer-specific survival rate (p=0.013) and overall survival rate (p=0.012) showed significant association with preoperative thrombocytosis. Controlling for pathological TNM stage, Fuhrman grade and tumor diameter, the Cox proportional hazards model for cancer-specific survival rates showed that preoperative thrombocytosis was an independent prognostic factor (p=0.025).
Conclusions
Preoperative thrombocytosis was associated with poorer prognosis in patients with nonmetastatic RCC. Thus, preoperative platelet count may be clinically useful for risk stratification of patients undergoing surgery for nonmetastatic RCC.
Keywords: Prognosis, Renal cell carcinoma, Thrombocytosis
INTRODUCTION
Renal cell carcinoma (RCC) is representative kidney cancer and has increased annually in the recent 2 decades [1]. Although surgery remains the standard curative treatment for RCC, approximately 30% of patients with RCC develop metastatic disease after surgery [2], and approximately one third of patients with RCC have metastasis at the time of diagnosis [3]. Several prognostic parameters have been evaluated and grade, histologic subtype, and stage remain important prognostic parameters in localized RCC [4,5,6]. In addition, some preoperative parameters, including platelet count, C-reactive protein (CRP), and erythrocyte sedimentation rate have been considered for their prognostic relevance [7,8]. Thrombocytosis (TC), particularly secondary type has been regarded as a poor prognostic factor in many malignant diseases including gastric, gynecologic, and lung cancer [9,10,11,12]. Recent studies have demonstrated an association of lower survival after operation with reactive TC for variable type of cancer, platelet count could have relation with the systemic inflammatory response, even though the exact relation between inflammation, platelet, and cancer outcomes is still not known. Reactive TC is caused in a background of hypercytokinemia related to tumor progression. [13]. The purpose of this study is to examine the association of preoperative TC with the prognosis of nonmetastatic RCC patients.
MATERIALS AND METHODS
This study was approved by the Ethics Committee of Yeungnam University School of Medicine and informed consents were waived because the operations were done between 1997 and 2009.
1. Patients
In this study the medical records of 187 patients who underwent radical nephrectomy for nonmetastatic RCC between July 1997 and June 2009 were reviewed retrospectively. All patients were examined by routine hematologic laboratories and radiologic imaging including chest X-ray and abdominal computed tomography. The definition of TC was platelet count≥400,000 µL, and patients were divided into 2 groups by presence of preoperative TC. Tumor stage was reassigned using the 2002 TNM classification of the American Joint Committee on Cancer (AJCC) [14]. Histologic and nuclear type was graded according to the Heidel-Fuhrman classification. Postoperatively, every 3 or 6 month, all patients were evaluated for the first 2 years and every 6 months for next 2 years, and annually thereafter. Preoperative variabels including sex, gender, platelet count, neutrophil to lymphocytes ratio (NLR), platelet to lymphocyte ratio (PLR), tumor size, tumor grade, histology and T stage were recorded. Moreover, distant metastasis during follow-up periods was also represented as a variable.
2. Statistical analysis
Fisher exact test and chi-square test were used for comparison of discrete variables between the 2 groups. Receiver operating characteristics (ROC) curves were generated to determine cutoff points for NLR and PLR. The overall survival (OS) and cancer-specific survival (CSS) were estimated using the Kaplan-Meier survival analysis and the log-rank test. Univariate and multivariable analysis was fulfilled using the Cox proportional hazard model and logistic regression for assessment of significant parameters associated with survival. Statistically significant difference was established at p<0.05.
RESULTS
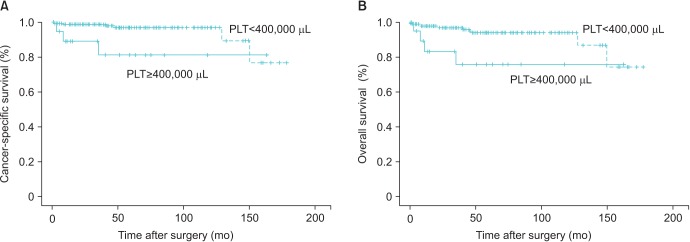
The clinicopathological data of 187 patients are described in Table 1. The mean follow-up period was 59.3±42.1 months and patients' mean age was 56.0±11.7 years. Mean tumor size was 5.2±2.9 cm. Twenty patients (10.7%) had T3 or T4 tumors, and 20 patients (10.7%) had preoperative TC. Thirty patients (16.0%) developed metastasis and 9 patients (4.8%) died during follow-up period. The cutoff value of NLR was 1.81 (area under the curve [AUC]=0.547; sensitivity, 0.5; specificity, 0.51) and PLR was 128.7 (AUC=0.703; sensitivity, 0.67; specificity, 0.51) in the ROC curve (Table 1). Preoperative TC showed significant correlation with T stage (p=0.028), and metastasis (p=0.001), NLR≥1.81 (p=0.003), PLR≥128.7 (p=0.001), but not with age, tumor histology subtype, gender, grade or tumor size (Table 2). In Kaplan-Meier analysis using univariate log-rank test, CSS rate (p=0.013) and OS rate (p=0.012) showed significant association with preoperative TC (Fig. 1).
Table 1. Clinical and pathological characteristics of 187 patients with nonmetastatic renal cell carcinoma.
| Characteristic | No. of patients (%) |
|---|---|
| Age (y) | |
| <60 | 112 (59.9) |
| ≥60 | 75 (40.1) |
| Sex | |
| Male | 136 (72.7) |
| Female | 51 (27.3) |
| Thrombocytosis | |
| No | 167 (89.3) |
| Yes | 20 (10.7) |
| Histology | |
| Clear cell | 158 (84.5) |
| Nonclear cell | 29 (15.5) |
| T stage | |
| T1 | 144 (77.0) |
| T2 | 23 (12.3) |
| T3 | 19 (10.2) |
| T4 | 1 (0.5) |
| Grade | |
| G1 | 29 (15.6) |
| G2 | 66 (35.3) |
| G3 | 69 (36.9) |
| G4 | 23 (12.2) |
| Tumor size (cm) | |
| <7 | 152 (81.3) |
| ≥7 | 35 (18.7) |
| Distant metastasis | |
| No | 157 (84.0) |
| Yes | 30 (16.0) |
| Neutrophil to lymphocyte ratio | |
| <1.81 | 86 (46) |
| ≥1.81 | 101 (54) |
| Platelet to lymphocyte ratio | |
| <128.7 | 85 (45) |
| ≥128.7 | 102 (55) |
Table 2. Comparison of perioperative parameters between with/without thrombocytosis group.
| Characteristic | Without thrombocytosis (n=167) | With thrombocytosis (n=20) | p-value |
|---|---|---|---|
| Age (y) | 0.992 | ||
| <60 | 100 (89.3) | 12 (10.7) | |
| ≥60 | 67 (89.3) | 8 (14.7) | |
| Sex | 0.069 | ||
| Male | 126 (92.6) | 10 (7.4) | |
| Female | 41 (80.4) | 10 (19.6) | |
| Histology | 0.215 | ||
| Clear cell | 143 (90.5) | 15 (9.5) | |
| Nonclear cell | 24 (82.8) | 5 (17.2) | |
| T stage | 0.028 | ||
| T1+T2 | 153 (91.6) | 14 (8.4) | |
| T3+T4 | 14 (70.0) | 6 (30.0) | |
| Grade | 0.122 | ||
| G1+G2 | 88 (92.7) | 7 (7.3) | |
| G3+G4 | 79 (85.7) | 13 (14.3) | |
| Tumor size (cm) | 0.446 | ||
| <7 | 137 (90.1) | 15 (9.9) | |
| ≥7 | 30 (85.7) | 5 (14.3) | |
| Distant metastasis | 0.001 | ||
| No | 147 (93.6) | 10 (6.4) | |
| Yes | 20 (66.7) | 10 (33.3) | |
| NLR | 0.003 | ||
| <1.81 | 83 (96.5) | 3 (3.5) | |
| ≥1.81 | 84 (83.2) | 17 (16.8) | |
| PLR | 0.001 | ||
| <128.7 | 84 (98.8) | 1 (1.2) | |
| ≥128.7 | 83 (81.4) | 19 (18.6) | |
| Cancer death | 0.001 | ||
| No | 161 (90.4) | 17 (9.6) | |
| Yes | 6 (67.0) | 3 (33.0) |
Values are presented as number (%).
NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio.
Fig. 1. Kaplan-Meier cancer–specific survival curves (A) and overall survival curves (B) based on preoperative platelet level. The cancer-specific and overall survival rate of patients with preoperative thrombocytosis (platelet count [PLT]≥400,000 µL) were significantly lower than that of patients without thrombocytosis (p=0.013, p=0.012, respectively).
TC, T stage, tumor size, and distant metastasis were significantly influencing parameters for CSS in both univariate and multivariable analysis. Controlling for pathological TNM stage, tumor size and Fuhrman grade, Cox proportional hazards model for CSS rates showed that preoperative TC was an independent prognostic factor (p=0.025) (Table 3). Cox proportional hazards model for OS rates showed that distant metastasis was an independent prognostic factor in multivariable analysis (p=0.001) (Table 4).
Table 3. Univariate and multivariable analysis of cancer-specific survival in 187 patients with renal cell carcinoma.
| Variable | Univariate | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age (y), <60 vs. ≥60 | 0.899 (0.740–1.062) | 0.786 | - | - |
| Sex, male vs. female | 1.793 (0.471–6.824) | 0.236 | - | - |
| Thrombocytosis, no vs. yes | 4.917 (1.223–19.769) | 0.013 | 10.830 (2.126–55.172) | 0.025 |
| Histology, conventional vs. nonconventional | 0.039 (0.01–122.731) | 0.188 | - | - |
| T stage, T1+T2 vs. T3+T4 | 11.490 (3.073–42.959) | <0.001 | 3.050 (0.517–17.990) | 0.218 |
| Grade, G1+G2 vs. G3+G4 | 2.194 (0.543–8.860) | 0.268 | - | - |
| Tumor size (cm), <7 vs. ≥7 | 6.402 (1.710–23.967) | 0.004 | 3.056 (0.548–17.047) | 0.028 |
| Distant metastasis, no vs. yes | 17.286 (3.569–83.715) | <0.001 | 12.215 (2.133–69.956) | 0.005 |
| NLR, <1.81 vs. ≥1.81 | 1.753 (0.437–7.039) | 0.435 | - | - |
| PLR, <128.7 vs. ≥128.7 | 2.822 (0.582–13.692) | 0.151 | - | - |
HR, hazard ratio; CI, confidence interval; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio.
Table 4. Univariate and Multivariable analysis of overall survival in 187 patients with renal cell carcinoma.
| Variable | Univariate | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age (y), <60 vs. ≥60 | 1.004 (0.956–1.054) | 0.875 | - | - |
| Sex, male vs. female | 1.800 (0.617–5.251) | 0.276 | - | - |
| Thrombocytosis, no vs. yes | 3.956 (1.237–12.656) | 0.012 | 2.428 (0.586–10.063) | 0.221 |
| Histology, conventional vs. nonconventional | 0.442 (0.058–3.384) | 0.419 | - | - |
| T stage, T1+T2 vs. T3+T4 | 5.149 (1.722–15.399) | 0.001 | 2.153 (0.508–9.116) | 0.298 |
| Grade, G1+G2 vs. G3+G4 | 1.457 (0.503–4.223) | 0.486 | - | - |
| Tumor size (cm), <7 vs. ≥7 | 3.839 (1.328–11.102) | 0.008 | 2.104 (0.598–7.410) | 0.247 |
| Distant metastasis, no vs. yes | 5.098 (1.779–14.607) | 0.001 | 6.522 (2.094–20.308) | 0.001 |
| NLR, <1.81 vs. ≥1.81 | 2.202 (0.689–7.035) | 0.172 | - | - |
| PLR, <128.7 vs. ≥128.7 | 3.076 (0.855–11.073) | 0.070 | - | - |
HR, hazard ratio; CI, confidence interval; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio.
DISCUSSION
Prognostic factors for RCC have been investigated and studied. Factors can be classified according to clinical, molecular and anatomical, histopathological [15]. The European Association of Urology guidelines advise use of the TNM classification, Fuhrman nuclear grade, and histological subtype for treatment of RCC. These are valuable factors in assessing prognosis of RCC and treatment decision [16]. The pathological stage according to TNM classification is the most important prognostic factor in RCC, followed by Fuhrman grade [17,18]. Many recent studies have examined platelet counts and TC as a useful prognostic factor in predicting survival and recurrence of RCC [13,19,20]. TC has also been reported in various malignancies and has been investigated as a prognostic factor [10,11,13,21,22].
TC is classified according to 2 forms, primary and secondary. Primary TC is due to clonal TC and occurs in chronic myelodysplastic or myeloproliferative disorders. Secondary TC has been studied in many malignancies, including liver, gastrointestinal tract, lung, cervix, and others [23]. The exact mechanism of TC, which associated with RCC is not well known. Several theories have been suggested on how TC may be related to worse survival and the metastatic potential in malignancy [11,19,20,22]. One theory, TC in RCC patients may increase tumorigenesis by increasing platelet-derived growth factor, which is a mitogen for various cell types [24]. According to another theory, platelets may prohibit the immune system from clearing tumor cells from circulation [25]. In addition, platelets may provide for the adherence, penetration, and sequestration of malignant cells through the endothelial wall [11]. Many cytokines including interleukin-6 or interleukin-11, stem cell factor, granulocyte-macrophage colony-stimulating factor, and thrombopoietin are assumed to contribute to TC [23].
TC has been investigated as a valuable prognostic factor in predicting survival and recurrence in RCC patients. In their retrospective study of 1,422 patients who underwent radical or partial nephrectomy for RCC, either localized or metastatic, Wosnitzer et al. [26] reported that TC is a clinically significant predictor of OS and CSS. However, when excluding metastatic patients, TC was not an independent predictor of OS and CSS. A different result was reported by Brookman-May et al. [27] in their study of 3,139 patients who underwent surgery for RCC at four centers, preoperative TC was an independent predictor for decreased CSS in localized RCC patients and TC had no independent influence on CSS in metastatic disease. In a review of the records of 204 patients with RCC who underwent radical nephrectomy, O'Keefe et al. [20] reported that the overall and cancer specific death rate in 26 patients with TC was 50% and 42%. However, in the remaining the normal platelet counts 178 patients, the same variables were 15.2% and 7.3%. In their study the cancer-specific death rate was 5 times greater in patients with TC. They concluded that TC was a potent independent prognostic factor in patients with localized RCC. Cho et al. [28], who studied the association of TC and preoperative CRP elevation with prognosis of nonmetastatic RCC patients in Korea, found that CRP and TC were significant prognostic factors associated with recurrence-free survival in univariate analysis, whereas multivariate analysis showed that TC was not an independent prognostic factor but CRP was. In the current study, significantly lower OS and CSS was observed in the TC group and TC, T stage, tumor size, and distant metastasis were significantly influencing parameters for CSS in both univariate and multivariable analysis. Controlling for other parameters, preoperative TC was an independent prognostic factor in CSS. Similar to our study, Gogus et al. [29] reported that preoperative TC is a significant prognostic factor in localized RCC patients and TC was more frequent in advanced stage RCC patients, and patients with preoperative TC had worse survival compared to patients with normal platelet counts. We also examined the correlation among TC and NLR, PLR. NLR is recently, one of the most investigated inflammation prognostic markers of postoperative outcome. In fact, as it has relation with inflammatory response, the role in identifying patients with high-risk has been suggested in noncancer and cancer patients [13]. Different studies have emphasized the role of high for detection of poorer prognosis cancer patients, in terms of both overall and cancer disease-free survival and general comorbidities [30]. PLR was identified as a prognostic marker in advanced gastric cancer patients treated with chemotherapy, however mainly the studies are retrospective and pertain to only a few kinds of cancer [13]. In our study, preoperative TC showed significant correlation with both NLR≥1.81 (p=0.003), PLR≥1.28.7 (p=0.001), but NLR and PLR were not significant influencing factors for CSS in both univariate and multivariable analysis.
Limitations of this study include the retrospective data assessment and no information was available concerning performance status, symptoms at the time of diagnosis, and further hematologic laboratory parameters such as CRP or hematocrit, and platelet count was assessed only before the operation. Furthermore, only the influence of TC on OS and CSS was evaluated, but not on recurrence-free survival, which can also be of interest.
CONCLUSIONS
Based on the results of the current study preoperative TC is a significant predictor for determining prognosis in nonmetastatic RCC patients and patients with preoperative TC had worse survival compared to patients with normal platelet counts. Thus, preoperative platelet count may be clinically useful for risk stratifying patients undergoing surgery for nonmetastatic RCC.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Lindblad P. Epidemiology of renal cell carcinoma. Scand J Surg. 2004;93:88–96. doi: 10.1177/145749690409300202. [DOI] [PubMed] [Google Scholar]
- 2.Dekernion JB, Ramming KP, Smith RB. The natural history of metastatic renal cell carcinoma: a computer analysis. J Urol. 1978;120:148–152. doi: 10.1016/s0022-5347(17)57082-2. [DOI] [PubMed] [Google Scholar]
- 3.Ljungberg B, Alamdari FI, Stenling R, Roos G. Prognostic significance of the Heidelberg classification of renal cell carcinoma. Eur Urol. 1999;36:565–569. doi: 10.1159/000020049. [DOI] [PubMed] [Google Scholar]
- 4.Van Brussel JP, Mickisch GH. Prognostic factors in renal cell and bladder cancer. BJU Int. 1999;83:902–908. doi: 10.1046/j.1464-410x.1999.00120.x. [DOI] [PubMed] [Google Scholar]
- 5.Ficarra V, Righetti R, Pilloni S, D'amico A, Maffei N, Novella G, et al. Prognostic factors in patients with renal cell carcinoma: retrospective analysis of 675 cases. Eur Urol. 2002;41:190–198. doi: 10.1016/s0302-2838(01)00027-6. [DOI] [PubMed] [Google Scholar]
- 6.Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27:612–624. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Karakiewicz PI, Suardi N, Capitanio U, Jeldres C, Ficarra V, Cindolo L, et al. A preoperative prognostic model for patients treated with nephrectomy for renal cell carcinoma. Eur Urol. 2009;55:287–295. doi: 10.1016/j.eururo.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 8.Saito K, Tatokoro M, Fujii Y, Iimura Y, Koga F, Kawakami S, et al. Impact of C-reactive protein kinetics on survival of patients with metastatic renal cell carcinoma. Eur Urol. 2009;55:1145–1153. doi: 10.1016/j.eururo.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Griesshammer M, Bangerter M, Sauer T, Wennauer R, Bergmann L, Heimpel H. Aetiology and clinical significance of thrombocytosis: analysis of 732 patients with an elevated platelet count. J Intern Med. 1999;245:295–300. doi: 10.1046/j.1365-2796.1999.00452.x. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen LM, Milman N. Prognostic significance of thrombocytosis in patients with primary lung cancer. Eur Respir J. 1996;9:1826–1830. doi: 10.1183/09031936.96.09091826. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez E, Lavine M, Dunton CJ, Gracely E, Parker J. Poor prognosis associated with thrombocytosis in patients with cervical cancer. Cancer. 1992;69:2975–2977. doi: 10.1002/1097-0142(19920615)69:12<2975::aid-cncr2820691218>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 12.Zeimet AG, Marth C, Muller-Holzner E, Daxenbichler G, Dapunt O. Significance of thrombocytosis in patients with epithelial ovarian cancer. Am J Obstet Gynecol. 1994;170:549–554. doi: 10.1016/s0002-9378(94)70225-x. [DOI] [PubMed] [Google Scholar]
- 13.Bugada D, Allegri M, Lavand'homme P, De Kock M, Fanelli G. Inflammation-based scores: a new method for patient-targeted strategies and improved perioperative outcome in cancer patients. Biomed Res Int. 2014;2014:142425. doi: 10.1155/2014/142425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, et al., editors. AJCC cancer staging manual. 6th ed. New York: Springer-Verlag; 2002. [Google Scholar]
- 15.Medina Lopez RA, Conde Sánchez JM, Congregado Ruiz CB, Gonzalez Resina R, Mármol Navarro S, Torrubia Romero FJ. Prognostic factors in renal cell carcinoma. Actas Urol Esp. 2009;33:575–583. doi: 10.1016/s0210-4806(09)74192-0. [DOI] [PubMed] [Google Scholar]
- 16.Ljungberg B, Cowan NC, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010;58:398–406. doi: 10.1016/j.eururo.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 17.Kontak JA, Campbell SC. Prognostic factors in renal cell carcinoma. Urol Clin North Am. 2003;30:467–480. doi: 10.1016/s0094-0143(03)00020-x. [DOI] [PubMed] [Google Scholar]
- 18.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Inoue K, Kohashikawa K, Suzuki S, Shimada M, Yoshida H. Prognostic significance of thrombocytosis in renal cell carcinoma patients. Int J Urol. 2004;11:364–367. doi: 10.1111/j.1442-2042.2004.00808.x. [DOI] [PubMed] [Google Scholar]
- 20.O'Keefe SC, Marshall FF, Issa MM, Harmon MP, Petros JA. Thrombocytosis is associated with a significant increase in the cancer specific death rate after radical nephrectomy. J Urol. 2002;168(4 Pt 1):1378–1380. doi: 10.1016/S0022-5347(05)64453-9. [DOI] [PubMed] [Google Scholar]
- 21.McDougal WS, Garnick MB. Clinical signs and symptoms of renal cell carcinoma. In: Vogelzang NJ, Scardino PT, Shipley WU, Coffey DS, editors. Comprehensive textbook of genitourinary oncology. 2nd ed. Baltimore: Lippincott Williams & Wilkins; 2000. pp. 111–128. [Google Scholar]
- 22.Partin AW, Criley SR, Steiner MS, Hsieh K, Simons JW, Lumadue J, et al. Serum ferritin as a clinical marker for renal cell carcinoma: influence of tumor volume. Urology. 1995;45:211–217. doi: 10.1016/0090-4295(95)80007-7. [DOI] [PubMed] [Google Scholar]
- 23.Erdemir F, Kilciler M, Bedir S, Ozgok Y, Coban H, Erten K. Clinical significance of platelet count in patients with renal cell carcinoma. Urol Int. 2007;79:111–116. doi: 10.1159/000106322. [DOI] [PubMed] [Google Scholar]
- 24.Westermark B, Heldin CH. Platelet-derived growth factor: structure, function and implications in normal and malignant cell growth. Acta Oncol. 1993;32:101–105. doi: 10.3109/02841869309083897. [DOI] [PubMed] [Google Scholar]
- 25.Karpatkin S, Pearlstein E, Salk PL, Yogeeswaran G. Role of platelets in tumor cell metastases. Ann N Y Acad Sci. 1981;370:101–118. doi: 10.1111/j.1749-6632.1981.tb29726.x. [DOI] [PubMed] [Google Scholar]
- 26.Wosnitzer M, Polland A, Hai Q, Hruby G, McKiernan J. Role of preoperative platelet level in clinical and pathological outcomes after surgery for renal cortical malignancies. BJU Int. 2011;108:73–79. doi: 10.1111/j.1464-410X.2010.09889.x. [DOI] [PubMed] [Google Scholar]
- 27.Brookman-May S, May M, Ficarra V, Kainz MC, Kampel-Kettner K, Kohlschreiber S, et al. Does preoperative platelet count and thrombocytosis play a prognostic role in patients undergoing nephrectomy for renal cell carcinoma? Results of a comprehensive retrospective series. World J Urol. 2013;31:1309–1316. doi: 10.1007/s00345-012-0931-0. [DOI] [PubMed] [Google Scholar]
- 28.Cho DS, Kim SJ, Lee SH, Ahn HS, Kim YS, Kim SI. Prognostic significance of preoperative C-reactive protein elevation and thrombocytosis in patients with non-metastatic renal cell carcinoma. Korean J Urol. 2011;52:104–109. doi: 10.4111/kju.2011.52.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gogus C, Baltaci S, Filiz E, Elhan A, Bedük Y. Significance of thrombocytosis for determining prognosis in patients with localized renal cell carcinoma. Urology. 2004;63:447–450. doi: 10.1016/j.urology.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 30.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]