Abstract
Cochlear synaptopathy (or hidden hearing loss), due to noise exposure or aging, has been demonstrated in animal models using histological techniques. However, diagnosis of the condition in individual humans is problematic because of (a) test reliability and (b) lack of a gold standard validation measure. Wave I of the transient-evoked auditory brainstem response is a noninvasive electrophysiological measure of auditory nerve function and has been validated in the animal models. However, in humans, Wave I amplitude shows high variability both between and within individuals. The frequency-following response, a sustained evoked potential reflecting synchronous neural activity in the rostral brainstem, is potentially more robust than auditory brainstem response Wave I. However, the frequency-following response is a measure of central activity and may be dependent on individual differences in central processing. Psychophysical measures are also affected by intersubject variability in central processing. Differential measures may help to reduce intersubject variability due to unrelated factors. A measure can be compared, within an individual, between conditions that are affected differently by cochlear synaptopathy. Validation of the metrics is also an issue. Comparisons with animal models, computational modeling, auditory nerve imaging, and human temporal bone histology are all potential options for validation, but there are technical and practical hurdles and difficulties in interpretation. Despite the obstacles, a diagnostic test for hidden hearing loss is a worthwhile goal, with important implications for clinical practice and health surveillance.
Keywords: noise-induced hearing loss, aging, cochlear nerve, auditory brainstem response, frequency-following response
Introduction
Hearing ability is usually assessed using pure-tone audiometry (Johnson, 1970), which measures the smallest detectable level of pure tones at a range of frequencies. The resulting audiogram is sensitive to dysfunction of the outer hair cells. However, it is becoming increasingly clear that the audiogram is much less sensitive to inner hair cell (IHC) loss (up to 80% IHC loss may occur without affecting audiometric thresholds; Lobarinas, Salvi, & Ding, 2013) and to some types of peripheral neural dysfunction. In particular, results from rodent models suggest that noise exposure and aging can cause permanent loss of synapses between the IHCs and auditory nerve fibers, without permanently affecting sensitivity to quiet sounds (Kujawa & Liberman, 2009, 2015; Sergeyenko, Lall, Liberman, & Kujawa, 2013). The disconnected nerve fibers subsequently degenerate. This disorder has been variously termed cochlear neuropathy, cochlear synaptopathy, and popularly hidden hearing loss (Schaette & McAlpine, 2011), because the loss is not thought to be detectable using pure-tone audiometry. The loss seems to affect selectively the low spontaneous rate (SR) fibers that have high thresholds and are thought to be responsible for coding sound intensity at moderate-to-high levels (Furman, Kujawa, & Liberman, 2013). This may explain why the loss does not affect sensitivity to quiet sounds.
The extent to which hidden hearing loss is a contributor to hearing difficulties experienced by humans is still unknown. There is evidence that listeners with a history of noise exposure but with normal audiograms have deficits in speech perception and temporal processing (Alvord, 1983; Kumar, Ameenudin, & Sangamanatha, 2012). Similarly, the aging process may affect speech perception in noise even when there are no significant increases in audiometric threshold (Dubno, Dirks, & Morgan, 1984; Rajan & Cainer, 2008), and this may be related to a deterioration in temporal processing (Ruggles, Bharadwaj, & Shinn-Cunningham, 2012; Snell, Mapes, Hickman, & Frisina, 2002). An open question concerns the extent to which these deficits are a consequence of cochlear synaptopathy or other types of dysfunction, for example, IHC dysfunction or central neural dysfunction.
A major obstacle to the academic investigation of hidden hearing loss, and to the eventual incorporation of the research findings into clinical practice, is the absence of a reliable and validated diagnostic test for the disorder. In the animal models, selective immunostaining and confocal microscopy can be used to determine directly the loss of synapses. However, such invasive procedures are not possible in humans, at least premortem. In this article, we will consider noninvasive measures of hidden hearing loss, their potential as a diagnostic test, and the challenges faced in developing them to this stage. Table 1 provides a summary of the techniques that will be discussed.
Table 1.
A Summary of Potential Diagnostic Techniques for Hidden Hearing Loss, and Their Advantages (“Pros”) and Disadvantages (“Cons”).
| Diagnostic technique | Hypothesized effect of synaptopathy | Pros | Cons |
|---|---|---|---|
| ABR | Reduction in Wave I amplitude at high levels | Relatively direct measure of auditory nerve function; objective | Highly variable in humans |
| FFR | Reduction in synchrony to amplitude modulation | Robust response; objective | Affected by variability in central processes |
| Behavioral | Increase in discrimination thresholds at high levels | Easy to measure | Affected by central processes; hypothesized effects are small |
Note. ABR: auditory brainstem response; FFR: frequency-following response.
Measures of Hidden Hearing Loss in Humans
The Auditory Brainstem Response
The click-evoked electrophysiological auditory brainstem response (ABR, see Figure 1) is a prime candidate for a measure of hidden hearing loss in humans. An advantage of the ABR is that many audiology clinics already have the necessary equipment and expertise to make the recordings. The ABR can be recorded in humans using electrodes placed on the scalp; typically, an electrode is attached to a mastoid and to another location such as the contralateral mastoid, forehead, or vertex. The differential response at the two electrodes determines the recorded ABR. Wave I of the ABR reflects auditory nerve function and, in the rodent models, has been shown to be sensitive to the effects of noise exposure (Kujawa & Liberman, 2009) and aging (Sergeyenko et al., 2013). In these models, the amplitude of Wave I is reduced at moderate-to-high levels but not at low levels, consistent with a selective loss of low-SR fibers. Furthermore, Wave I amplitude corresponds to the proportion of intact synapses (Kujawa & Liberman, 2009; Sergeyenko et al., 2013), which provides validation for the measure in rodents.
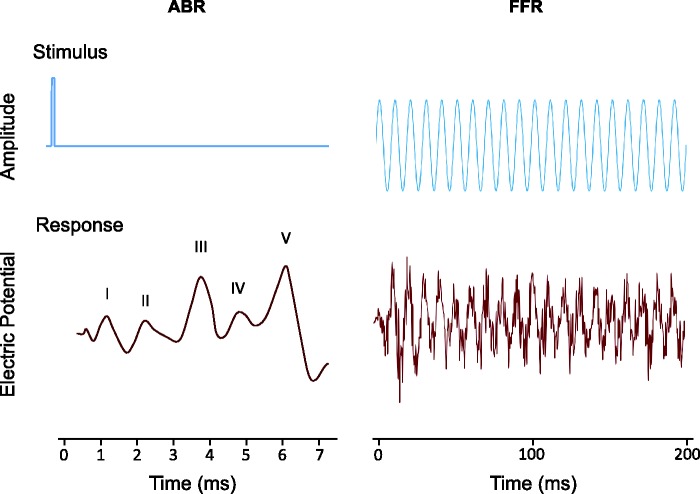
Figure 1.
An illustration of typical stimuli and recorded waveforms (response) for two electrophysiological measures of auditory neural coding: the auditory brainstem response (ABR) and the frequency-following response (FFR).
In humans, the evidence is less compelling, but both aging (Konrad-Martin et al., 2012) and, recently, noise exposure (Stamper & Johnson, 2015) have been shown to be associated with a reduction in ABR Wave I amplitude for high-level clicks in the absence of, or controlling for, an increase in audiometric threshold. In addition, Wave I amplitude for high-level clicks is reduced in listeners with tinnitus even when the audiogram is normal (Schaette & McAlpine, 2011): Schaette and McAlpine suggested that loss of auditory nerve fibers may induce tinnitus due to a compensatory increase in central neural gain. However, there are some problems associated with the use of ABR Wave I as a diagnostic test for hidden hearing loss. First, unlike the rodent models in which the ABR can be measured accurately using subcutaneous electrodes, in humans ABR Wave I has a relatively low amplitude and shows high variability both between and within individuals (Beattie, 1988; Lauter & Loomis, 1988). In addition to the variability due to cochlear synaptopathy itself, the variability in Wave I may be the result of a number of factors unrelated to cochlear synaptopathy. These factors include variation in sex and head size (Mitchell, Phillips, & Trune, 1989); individual variation in synchronization across cochlear place (Don, Ponton, Eggermont, & Masuda, 1994); variation in physiological noise or other sources of electrical noise, between individuals, or between sessions; and individual variation in preneural loss. The use of intracanal electrodes, including tympanic membrane electrodes, can not only increase the amplitude of Wave I but may also increase the variability (Stamper & Johnson, 2015). Hence, at present, while Wave I may be useful for demonstrating group differences in synaptopathy, between those noise exposed and those not for example, it is probably not useful for determining if an individual has hidden hearing loss.
Another issue is that the amplitude of Wave I in response to a broadband click is strongly influenced by activity in basal regions of the cochlea (Don & Eggermont, 1978). Even if the audiogram is normal over the clinical range, up to 4 kHz or 8 kHz, hair cell loss in higher characteristic frequency regions may affect the amplitude of the response. Hence, to identify synaptopathy, the results may have to be controlled for high-frequency audiometric thresholds, or, alternatively, the high-frequency region may be masked using high-pass noise during recording of the ABR to prevent the basal region contributing to the response (Don & Eggermont, 1978).
The Frequency-Following Response
In contrast to the click-evoked ABR, which largely reflects the onset response of auditory neurons, the frequency-following response (FFR) is a sustained auditory-evoked potential, thought to reflect neural activity in the brainstem synchronized (phase locked) to the waveform of the stimulus (Krishnan, 2006, see Figure 1). The FFR is particularly sensitive to amplitude modulation at modulation rates of a few hundred hertz, although it also reflects phase locking to temporal fine structure (the individual pressure fluctuations that carry the amplitude modulation) for frequencies up to about 1 kHz. Over recent years, the FFR has become popular as a measure of auditory temporal coding. The FFR can be recorded using similar electrode montages to the ABR, and for lower frequencies at least, is a more robust measure than ABR Wave I, with most participants showing a clear response above the noise floor. Importantly, FFR amplitude can be measured objectively using a discrete Fourier transform of the response at the component frequency, whereas ABR Wave I measurement sometimes requires a subjective intervention to analyze the waveform and determine the peak location.
There is evidence that the amplitude of the FFR to both stimulus envelope and temporal fine structure decreases with increasing age even when controlling for absolute threshold (Bones & Plack, 2015; Clinard & Tremblay, 2013; Marmel et al., 2013). The FFR is also predictive of behavioral performance on tasks that may be sensitive to synaptopathy for listeners with normal audiometric thresholds, such as frequency discrimination (Marmel et al., 2013) and modulation discrimination (Bharadwaj, Masud, Mehraei, Verhulst, & Shinn-Cunningham, 2015). There is also preliminary evidence that the FFR is reduced in noise-exposed ears for listeners with normal absolute thresholds (Plack, Barker, & Prendergast, 2014; see Figure 2). These results suggest that the FFR may be sensitive to synaptopathy.
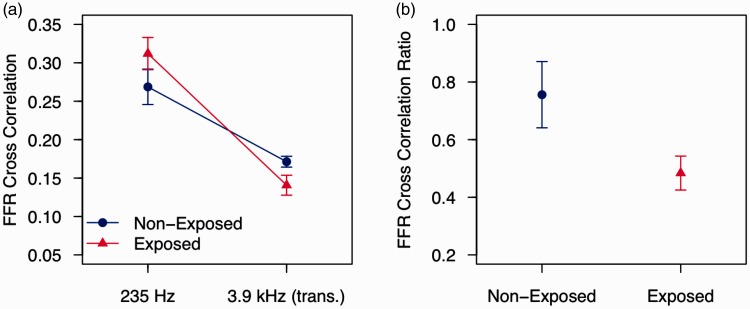
Figure 2.
Results from the conference presentation of Barker, Hopkins, Baker, and Plack (2014) reported by Plack et al. (2014). (a) FFR synchrony to a 235-Hz pure tone and to a 235-Hz tone transposed to 3.9 kHz (i.e., a 3.9-kHz pure-tone carrier amplitude modulated at 235 Hz) for groups of listeners with (red triangles) and without (blue circles) a history of recreational noise exposure. For each stimulus, the dependent variable was the coefficient of correlation between the FFR and a 235-Hz pure tone. (b) The ratios of the coefficients between the two carrier frequencies (3.9 kHz:235 Hz). Error bars are standard errors.
However, unlike ABR Wave I, the FFR is produced largely by generators in the brainstem, the largest component from the region of the inferior colliculus (Krishnan, 2006). Hence, differences in central auditory processing may well contribute to individual differences in FFR amplitude. For example, it is known that musicians and tone language speakers have stronger FFRs for certain types of stimuli (Krishnan, Xu, Gandour, & Cariani, 2005; Wong, Skoe, Russo, Dees, & Kraus, 2007), likely due to experience-related plasticity. Aging affects central neural function (Frisina & Walton, 2006), so an FFR deficit due to age could be a consequence of a combination of peripheral and central factors. Like the ABR, the FFR is also limited by between- and within-subject variability due to factors such as physiological noise, although the FFR may not be as sensitive as the ABR to sex differences (Hoormann, Falkenstein, Hohnsbein, & Blanke, 1992; Krizman, Skoe, & Kraus, 2012).
Behavioral Measures
Behavioral measures, such as psychophysical thresholds, require a response from the listener. Hence, they may potentially depend on processing at all stages from the auditory periphery to the motor commands sent to the finger that presses the response key. As is the case for the FFR technique, there is the concern that performance may be influenced by central factors unrelated to synaptopathy. As well as purely auditory factors, these may include higher level functions such as memory and attention. However, behavioral techniques have been shown to provide reliable measures of some aspects of peripheral function, in particular frequency selectivity and cochlear compression (e.g., Oxenham & Plack, 1997).
Reduction in the numbers of low-SR fibers might be expected to affect discrimination tasks at high sound levels. However, as pointed out by Oxenham (2016), if considered in terms of signal detection theory, a 50% fiber loss (similar to that in the animal studies) would reduce the discrimination index, d’, by a factor of √2 only. This would result in a barely measurable increase in threshold for many psychophysical tasks. For example, based on the slopes of psychometric functions, a √2 decrease in d’ would result in an increase in threshold of about 1.5 dB in the case of signal detection in noise and from a baseline of 1% to about 1.4% in the case of frequency discrimination (Oxenham, 2016). Considering the between-subject variability in performance expected due to central factors, it is not clear from this analysis that these basic psychophysical measures have the necessary sensitivity to diagnose synaptopathy. However, it should be noted that this theoretical approach is based on assumptions about the way information from auditory nerve fibers is combined in the central auditory system and about the sources and nature of neural variability throughout the system (Oxenham, 2016). For example, the effect of fiber loss on performance could be increased if a central neural noise is added to information combined from different fibers. Importantly, there is also the assumption that the fiber loss is evenly distributed, which is not the case in cochlear synaptopathy which seems to affect mainly low-SR fibers. For example, if nearly all the synapses with low-SR fibers were lost in a given region of the cochlea, behavioral measures at high levels in the affected frequency region might well have the necessary sensitivity. It is also possible that performance on some psychophysical tasks is especially sensitive to synaptopathy because of the way the sensory information used in these tasks is coded. In particular, it has been suggested that temporal processing tasks, such as modulation detection, might be particularly affected (Bharadwaj, Verhulst, Shaheen, Liberman, & Shinn-Cunningham, 2014).
There are little available data directly relating synaptopathy to behavioral performance. Tinnitus patients with normal hearing, who exhibit a reduction in ABR Wave I amplitude consistent with synaptopathy (Schaette & McAlpine, 2011), have elevated intensity discrimination thresholds (Epp, Hots, Verhey, & Schaette, 2012). Noise exposure and aging have been related to deficits in temporal processing tasks and speech discrimination in noise (Alvord, 1983; Dubno et al., 1984; Füllgrabe, Moore, & Stone, 2015; Kumar et al., 2012; Ruggles et al., 2012).
Managing Variability
A common problem for measures of hidden hearing loss in humans is that of variability. Within-subject variability may be minimized for the electrophysiological techniques by using careful procedures. Physiological noise can be reduced by ensuring that participants are relaxed or asleep, and variability between participants can be reduced by ensuring that they are in the same state when measurements are taken. Any variability due to outer hair cell loss can be controlled by measurement of absolute threshold or otoacoustic emissions. In particular, measures such as these will be necessary to adjust for the effects of preneural loss if cochlear synaptopathy is to be detected in ears with audiometric thresholds outside the normal range. For psychophysical tests, practice and the use of a procedure that is easy to learn can ensure that performance is at asymptote (King, Hopkins, & Plack, 2013).
An approach for minimizing both within- and between-subject variability is to use a differential measure, in which two measures are compared for each individual: one measure that is assumed to be affected by synaptopathy and one that is not. Both measures should be affected equally by other sources of variability for this variability to be minimized, or even cancelled out. Such an approach may be effective for both electrophysiological and behavioral measures and help to reduce or eliminate confounds due to central factors for the FFR and for the behavioral measures. There are at least two possible options for differential measures of synaptopathy based on stimulus characteristics: comparisons across frequency and comparisons across level (see Figure 3).
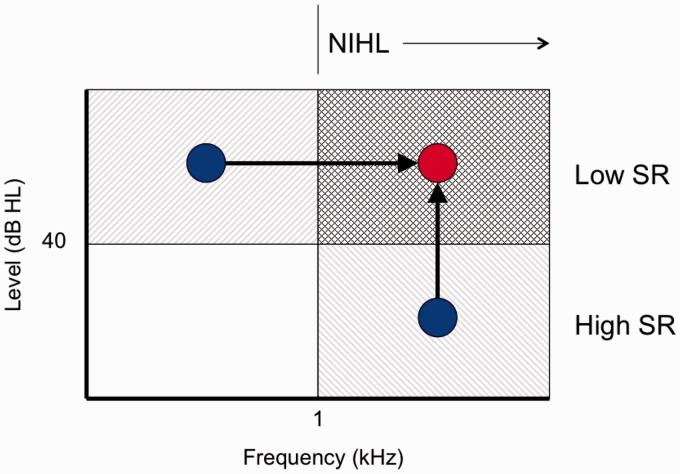
Figure 3.
A schematic illustration of the use of differential measures to provide an estimate of noise-induced cochlear synaptopathy. Synaptopathy is thought to affect low-SR fibers selectively, and therefore should be most evident at sound levels above about 40 dB HL, above the saturation level of the high-SR fibers. Furthermore, noise-induced hearing loss (NIHL) affects sensitivity in high frequency regions predominantly. Hence, cochlear synaptopathy due to noise exposure should mainly affect the response to stimuli with high levels and high frequencies (cross-hatched area). By comparing the response at low levels or low frequencies (blue circles) to the high-level or high-frequency response (red circle), it may be possible to provide an estimate of synaptopathy that is relatively unaffected by individual variations in overall response magnitude (in the case of the ABR, for example), or overall performance in the case of behavioral tasks.
Comparisons Across Frequency
One differential approach is to compare measures between a low-frequency region and a high-frequency region. It is generally reported that noise exposure causes most damage in higher frequency regions (around 4 kHz). If this is the case, then the low-frequency measure can be used as a within-subject comparison, although it should be noted that it is not yet confirmed that noise-induced synaptopathy is restricted to high-frequency regions. A preliminary study used this technique by comparing the FFR to a 235-Hz pure tone with that to a 235-Hz modulator imposed on a 3.9-kHz carrier (Plack et al., 2014, see Figure 2). The participants were audiogram matched. The noise-exposed group had no reduction in FFR amplitude to the low-frequency tone but showed a reduction in the amplitude of the FFR to the envelope of the high-frequency stimulus. Furthermore, the difference between the groups was greater when the ratio of high-frequency to low-frequency responses was used as the measure.
For the ABR, filtered or masked clicks can be used to probe different frequency regions, and hence allow a cross-frequency comparison. For behavioral measures, it is relatively simple to compare performance in different frequency regions using narrowband stimuli. Whenever narrowband stimuli are used, it may be advisable to include a broadband masking noise to ensure that high-SR fibers do not contribute to the response due to spread of excitation.
Comparisons Across Level
An alternative is to rely on the finding that synaptopathy appears to be selective to low-SR fibers, which have high thresholds and code intensity information at high levels, above the saturation level of the high-SR fibers. Hence, evoked-response amplitude and behavioral performance should be selectivity impaired at high levels. By comparing the measure across different levels, it may be possible to isolate the effects of synaptopathy from other sources of variability. In the study of Schaette and McAlpine (2011), it was observed that the reduction in ABR Wave I was greater for the 100 dB per SPL click than for the 90 dB per SPL click. Bharadwaj et al. (2015) have taken a similar approach for their FFR measures, by measuring the FFR to a modulator imposed on a high-level carrier. They reasoned that the FFR for a low modulation depth would be determined primarily by the response of low-SR fibers, whereas the FFR for a high modulation depth would depend in part on the response of high-SR fibers, since the dips in the modulation would fall within their level range. Bharadwaj et al. showed that the slope of the function relating FFR strength to modulation depth correlated more strongly with behavioral modulation detection performance than did FFR strength in isolation. Of course, differential measures based on across-level comparisons may be less sensitive to any loss that might affect both fiber groups. Some loss or dysfunction of high-SR fibers might occur due to aging, although the response of low-SR fibers seems to be particularly affected (Schmiedt, Mills, & Boettcher, 1996; Sergeyenko et al., 2013). There might also be IHC loss that could cause a reduction in ABR or FFR amplitude at low and high levels without affecting the audiogram. Hence, absolute measures might also be necessary to diagnose some conditions.
Comparisons Across Neural Generators
Schaette and McAlpine (2011) found that ABR Wave I was reduced at high levels in tinnitus patients compared with controls, whereas ABR Wave V amplitude (reflecting the response of auditory neurons in the rostral brainstem) was similar for the two groups. They attributed this pattern of results to the action of a central neural gain mechanism that effectively compensates for the reduced input from the auditory nerve by amplifying the response, so that by the stage in the pathway reflected by Wave V, there is no effect of synaptopathy on the overall response magnitude. For ABR measures of synaptopathy, therefore, another option is to assume that Wave V will be unaffected and to compare the amplitude ratio of Wave I to Wave V, which should be reduced in cases of synaptopathy. This should control to some extent for individual variation in overall ABR amplitude (due to head size, electrode placement, etc.). However, it has not yet been determined that central gain is an inevitable consequence of synaptopathy, and indeed aging is associated with a reduction in the amplitudes of both Wave I and Wave V (Konrad-Martin et al., 2012), in the latter case possibly due to central neural degeneration.
The Problem of Validation
In the rodent models, validation of electrophysiological or psychophysical measures is possible because synapses and nerve fibers can be counted postmortem using histological techniques. While human temporal bones are available to researchers and have been used to provide estimates of auditory nerve fiber loss due to aging (Makary, Shin, Kujawa, Liberman, & Merchant, 2011), it is not trivial to validate a test performed on a living human using a postmortem measure! The problem essentially is that we currently lack a gold-standard measure of synaptopathy that can be used with a living human to validate the diagnostic test. We are hence confronted by the serious problem of being unable to confirm that our diagnostic test is measuring what we want it to. There are, however, a number of potential approaches to validation that may be productive.
Validation With Animal Models
One approach to validation is to assume that between-species differences are insignificant with regard to the diagnosis of synaptopathy and to validate the measure using animal models. For the ABR, for example, there is good evidence from comparisons with synapse counts that Wave I is a reliable measure of synaptopathy in mice with normal sensitivity to quiet sounds (Kujawa & Liberman, 2009; Sergeyenko et al., 2013). The FFR could be validated in a similar way. It should also be possible to validate simple behavioral measures, such as psychophysical discrimination thresholds, in animals suited to behavioral tasks such as the chinchilla. These measures can then be compared with postmortem synapse counts taken shortly after threshold measurement.
Computational Modeling
There are now a number of computational models of the peripheral auditory system (e.g., Zilany, Bruce, Nelson, & Carney, 2009), based on animal and human data, that could be adapted to make predictions of the expected effects of synaptopathy on evoked potentials and behavioral performance. These results could help validate diagnostic tests based on these measures, to determine whether the pattern of results is consistent with the expected effects of synaptopathy. However, there are still too many uncertainties in these models to rely on them entirely, and these models of course cannot determine the actual synaptic loss for an individual. The utility of these models may lie in their use in conjunction with the animal data.
Auditory Nerve Imaging
Advanced imaging techniques, in particular magnetic resonance imaging (MRI), have the potential to provide a direct measure of nerve fiber loss. Although such a measure may not itself be cost effective or practical for routine use in the clinic as a diagnostic test, it could be used to validate a simpler clinical test. At present, it is not possible to image the auditory nerve noninvasively in humans with the resolution required to detect a proportional reduction in nerve fibers. However, it is conceivable that techniques such as high-field MRI, or diffusion MRI which can measure both axon and myelin degeneration (e.g., Song et al., 2003), may be refined to the point at which they can provide a direct estimate of the loss of fibers due to synaptopathy. It should be noted, however, that it may take months or years after the time of initial lesion for the neurons to atrophy to such an extent that the effects of synaptopathy are measurable using techniques such as these (Sergeyenko et al., 2013).
Human Temporal Bone Histology
Direct nerve fiber and synapse counts are certainly possible in humans postmortem using donated temporal bones. The problem then is how to use this information to validate a test, without having to repeatedly perform that test on the individual until they die to account for changes in performance over time. Terminally ill patients may be one option if consent can be obtained, although these individuals are predominantly elderly and may have a number of hearing-related complications, including hair cell loss. Another option is to test young participants in the military, or other occupations with higher than average mortality, who have agreed to donate their temporal bones.
Summary
The existence in humans of cochlear synaptopathy, or hidden hearing loss, would have major implications for audiological practice, health surveillance, and noise exposure regulations. Investigations of the disorder in humans are hampered by the lack of a reliable diagnostic test. The amplitude of Wave I of the ABR is the most direct noninvasive measure of auditory nerve function in humans but is limited by variability. The FFR and behavioral measures are less direct and are influenced by central factors, but may prove more reliable. Both variability and the influence of central factors may be reduced by the use of differential measures that compare performance across frequency or level, for example. There is also the problem of test validation. It may be necessary to rely on animal data relating comparable electrophysiological and behavioral measures with direct histological measures, although it is conceivable that technological innovations in neuroimaging may allow a direct estimate of auditory nerve fiber loss in humans. Such an estimate could be used to validate a more clinically useable test.
Acknowledgments
The content of this article was strongly influenced by discussions at the recent NIH/NIDCD Workshop on Noise-Induced Hearing Loss in Bethesda, MD. The authors are grateful to the workshop attendees and in particular to the organizers: Janet Cyr, Amy Donohue, Sharon Kujawa, Charlie Liberman, and Rick Davis. The authors also thank the Editor and two anonymous reviewers for very constructive comments that greatly improved the manuscript.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by grants from the Medical Research Council (MR/L003589/1) and from Action on Hearing Loss.
References
- Alvord L. S. (1983) Cochlear dysfunction in “normal-hearing” patients with history of noise exposure. Ear and Hearing 4: 247–250. [DOI] [PubMed] [Google Scholar]
- Barker, D., Hopkins, K., Baker, R., & Plack, C. J. (2014). Detecting the early effects of noise exposure. Paper presented at the Midwinter Meeting of Association for Research in Otolaryngology, San Diego.
- Beattie R. C. (1988) Interaction of click polarity, stimulus level, and repetition rate on the auditory brainstem response. Scandinavian Audiology 17: 99–109. [DOI] [PubMed] [Google Scholar]
- Bharadwaj H. M., Masud S., Mehraei G., Verhulst S., Shinn-Cunningham B. G. (2015) Individual differences reveal correlates of hidden hearing deficits. Journal of Neuroscience 35: 2161–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj H. M., Verhulst S., Shaheen L., Liberman M. C., Shinn-Cunningham B. G. (2014) Cochlear neuropathy and the coding of supra-threshold sound. Frontiers in Systems Neuroscience 8: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bones O., Plack C. J. (2015) Losing the music: Aging affects the perception and subcortical neural representation of musical harmony. Journal of Neuroscience 35: 4071–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinard C. G., Tremblay K. L. (2013) Aging degrades the neural encoding of simple and complex sounds in the human brainstem. Journal of the American Academy of Audiology 24: 590–599. quiz 643–594. [DOI] [PubMed] [Google Scholar]
- Don M., Eggermont J. J. (1978) Analysis of the click-evoked brainstem potentials in man using high-pass noise masking. Journal of the Acoustical Society of America 63: 1084–1092. [DOI] [PubMed] [Google Scholar]
- Don M., Ponton C. W., Eggermont J. J., Masuda A. (1994) Auditory brainstem response (ABR) peak amplitude variability reflects individual differences in cochlear response times. Journal of the Acoustical Society of America 96: 3476–3491. [DOI] [PubMed] [Google Scholar]
- Dubno J. R., Dirks D. D., Morgan D. E. (1984) Effects of age and mild hearing loss on speech recognition in noise. Journal of the Acoustical Society of America 76: 87–96. [DOI] [PubMed] [Google Scholar]
- Epp B., Hots J., Verhey J. L., Schaette R. (2012) Increased intensity discrimination thresholds in tinnitus subjects with a normal audiogram. Journal of the Acoustical Society of America 132: EL196–EL201. [DOI] [PubMed] [Google Scholar]
- Frisina R. D., Walton J. P. (2006) Age-related structural and functional changes in the cochlear nucleus. Hearing Research 216–217: 216–223. [DOI] [PubMed] [Google Scholar]
- Füllgrabe C., Moore B. C. J., Stone M. (2015) Age-group differences in speech identification despite matched audiometrically normal hearing: Contributions from auditory temporal processing and cognition. Frontiers in Aging Neuroscience 6: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman A. C., Kujawa S. G., Liberman M. C. (2013) Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. Journal of Neurophysiology 110: 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoormann J., Falkenstein M., Hohnsbein J., Blanke L. (1992) The human frequency-following response (FFR): Normal variability and relation to the click-evoked brainstem response. Hearing Research 59: 179–188. [DOI] [PubMed] [Google Scholar]
- Johnson E. W. (1970) Tuning forks to audiometers and back again. Laryngoscope 80: 49–68. [DOI] [PubMed] [Google Scholar]
- King A., Hopkins K., Plack C. J. (2013) Differences in short-term training for interaural phase difference discrimination between two different forced-choice paradigms. Journal of the Acoustical Society of America 134: 2635–2638. [DOI] [PubMed] [Google Scholar]
- Konrad-Martin D., Dille M. F., McMillan G., Griest S., McDermott D., Fausti S. A., Austin D. F. (2012) Age-related changes in the auditory brainstem response. Journal of the American Academy of Audiology 23: 18–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A. (2006) Frequency-following response. In: Burkhard R. F., Don M., Eggermont J. (eds) Auditory evoked potentials: Basic principles and clinical application, Philadelphia, PA: Lippincott Williams and Wilkins, pp. 313–333. [Google Scholar]
- Krishnan A., Xu Y., Gandour J., Cariani P. (2005) Encoding of pitch in the human brainstem is sensitive to language experience. Cognitive Brain Research 25: 161–168. [DOI] [PubMed] [Google Scholar]
- Krizman J., Skoe E., Kraus N. (2012) Sex differences in auditory subcortical function. Clinical Neurophysiology 123: 590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa S. G., Liberman M. C. (2009) Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. Journal of Neuroscience 29: 14077–14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa S. G., Liberman M. C. (2015) Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hearing Research 330: 191–199. doi:10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar U. A., Ameenudin S., Sangamanatha A. V. (2012) Temporal and speech processing skills in normal hearing individuals exposed to occupational noise. Noise and Health 14: 100–105. [DOI] [PubMed] [Google Scholar]
- Lauter J. L., Loomis R. L. (1988) Individual differences in auditory electric responses: Comparisons of between-subject and within-subject variability. II. Amplitude of brainstem Vertex-positive peaks. Scandinavian Audiology 17: 87–92. [DOI] [PubMed] [Google Scholar]
- Lobarinas E., Salvi R., Ding D. (2013) Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas. Hearing Research 302: 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makary C. A., Shin J., Kujawa S. G., Liberman M. C., Merchant S. N. (2011) Age-related primary cochlear neuronal degeneration in human temporal bones. Journal of the Association for Research in Otolaryngology 12: 711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmel F., Linley D., Carlyon R. P., Gockel H. E., Hopkins K., Plack C. J. (2013) Subcortical neural synchrony and absolute thresholds predict frequency discrimination independently. Journal of the Association for Research in Otolaryngology 14: 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C., Phillips D. S., Trune D. R. (1989) Variables affecting the auditory brainstem response: Audiogram, age, gender, and head size. Hearing Research 40: 75–86. [DOI] [PubMed] [Google Scholar]
- Oxenham A. J. (2016) Characterizing individual differences in frequency coding: Implications for hidden and not-so-hidden hearing loss. Trends in Hearing 20. [Google Scholar]
- Oxenham A. J., Plack C. J. (1997) A behavioral measure of basilar-membrane nonlinearity in listeners with normal and impaired hearing. Journal of the Acoustical Society of America 101: 3666–3675. [DOI] [PubMed] [Google Scholar]
- Plack C. J., Barker D., Prendergast G. (2014) Perceptual consequences of “hidden” hearing loss. Trends in Hearing 18: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan R., Cainer K. E. (2008) Ageing without hearing loss or cognitive impairment causes a decrease in speech intelligibility only in informational maskers. Neuroscience 154: 784–795. [DOI] [PubMed] [Google Scholar]
- Ruggles D., Bharadwaj H., Shinn-Cunningham B. G. (2012) Why middle-aged listeners have trouble hearing in everyday settings. Current Biology 22: 1417–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaette R., McAlpine D. (2011) Tinnitus with a normal audiogram: Physiological evidence for hidden hearing loss and computational model. Journal of Neuroscience 31: 13452–13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt R. A., Mills J. H., Boettcher F. A. (1996) Age-related loss of activity of auditory nerve fibers. Journal of Neurophysiology 76: 2799–2803. [DOI] [PubMed] [Google Scholar]
- Sergeyenko Y., Lall K., Liberman M. C., Kujawa S. G. (2013) Age-related cochlear synaptopathy: An early-onset contributor to auditory functional decline. Journal of Neuroscience 33: 13686–13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K. B., Mapes F. M., Hickman E. D., Frisina D. R. (2002) Word recognition in competing babble and the effects of age, temporal processing, and absolute sensitivity. Journal of the Acoustical Society of America 112: 720–727. [DOI] [PubMed] [Google Scholar]
- Song S.-K., Sun S.-W., Ju W.-K., Lin S.-J., Cross A. H., Neufeld A. H. (2003) Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage 10: 1714–1722. [DOI] [PubMed] [Google Scholar]
- Stamper G. C., Johnson T. A. (2015) Auditory function in normal-hearing, noise-exposed human ears. Ear and Hearing 36: 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. C. M., Skoe E., Russo N. M., Dees T., Kraus N. (2007) Musical experience shapes human brainstem encoding of linguistic pitch patterns. Nature Neuroscience 10: 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilany M. S., Bruce I. C., Nelson P. C., Carney L. H. (2009) A phenomenological model of the synapse between the inner hair cell and auditory nerve: Long-term adaptation with power-law dynamics. Journal of the Acoustical Society of America 126: 2390–2412. doi:10.1121/1.3238250. [DOI] [PMC free article] [PubMed] [Google Scholar]