SYNOPSIS
Rodent epigenetic models of early maternal care have demonstrated that natural variations in maternal behavior shape the development of stress reactivity and social behavior in offspring. Rodent models have also revealed the “hidden” regulatory functions of specific dimensions of maternal behavior. Here we present research that has extended rodent models of early care to the study of biobehavioral development in human infants. Research showing contemporaneous and predictive associations between quality of maternal caregiving behavior (MCB) and early biobehavioral development is reviewed. New evidence demonstrating the proximal effects of MCB in early infancy on infant stress reactivity is reported and highlights the value of examining early parenting at the specific behavioral level. Future research should extend this domain-specific approach to the study of infant contributions to the early care environment. Implications for intervention are discussed.
INTRODUCTION
Central to Bowlby’s (1969) attachment theory is the evolutionary advantage of proximity to a caregiver who fulfills infant needs for safety and comfort. Although it is now well-established that early, sensitive maternal care is associated with the eventual development of a secure attachment (De Wolff & van IJzendoorn, 1997), we know little about the mechanisms of influence that underlie this association in humans. Animal models continue to identify the mechanisms embedded in early parental influence, and they have done so by rigorously examining the neurological substrates and biobehavioral sequelae of very specific dimensions of parenting behavior. Here we describe our approach to the study of parenting that has been informed by rodent epigenetic models of early maternal care. We offer new directions for this approach that we believe will elucidate the mechanisms of influence involved in the association between maternal sensitivity and biobehavioral development.
RODENT MODELS OF MATERNAL CAREGIVING
Rodent models of maternal caregiving have focused on postnatal programming by examining maternal contributions to the development of stress reactivity and social behavior. Meaney and his colleagues have demonstrated that naturally occurring individual differences in the frequency of licking and grooming (LG) behavior are associated with neurological and behavioral differences that persist into adulthood. They have shown that rat pups that experienced low levels of LG display, as adults, a stress-reactive neuroendocrine profile (Liu et al., 1997) and a corresponding behavioral profile of elevated stress reactivity to novelty, including higher frequencies of startle responses, less open-field exploration, and elongated latencies to eat food presented in a novel environment (Caldji et al., 1998). Cross-fostering research has demonstrated that these effects are reversible, providing compelling experimental evidence for a causal link between early maternal care and stress reactivity (Francis, Diorio, Liu, & Meaney, 1999). New evidence is revealing that early maternal care changes gene expression in the rodent (Champagne, 2011).
Rodent work has also directed our attention to the function of specific dimensions of maternal behavior. Through a series of elegant studies, Hofer and his colleagues (2006) examined the mechanisms of influence of maternal care that support physiological regulation. They showed that the acute response to maternal separation seen in infantile offspring results from the loss of several individual components of maternal behavior, each of which plays a critical role in supporting homeostasis in pups. For instance, loss of warmth provided by the dam was associated specifically with brain changes that result in slowed activity level; loss of tactile stimulation provided by mother resulted in behavioral reactivity to novelty. This evidence demonstrates that embedded in early care are “hidden regulators”, which act in concert to support biobehavioral development. The behavioral specificity of Hofer’s approach elucidates how each facet of maternal behavior contributes individually, via different mechanisms, to biobehavioral development.
Extending the Rodent Model to Humans
Among the many virtues of these rodent models are the careful attention to context and examination of parenting at the specific behavioral level. In their meta-analysis, de Wolff and Van IJzendoorn (1997) showed that research examining maternal sensitivity at the specific behavioral level yielded stronger effect sizes with attachment than that which relied on global composites. Leerkes and her colleagues (Leerkes, Weaver, & O’Brien, 2012) have demonstrated differential origins and prediction for maternal sensitivity to infant distress vs. non-distress cues. Our approach has been to examine maternal behavior in the specific context of caregiving. Routine tasks such as changing, dressing, and feeding occur frequently in the first weeks of life, and these tasks likely lay the foundation for other forms of social interaction, insuring proximity between mother and infant from the moment of birth. Caregiving may offer more ecological challenge for mother and infant than free-play paradigms. Limits are necessarily placed on infants as they are fed and changed and the invasive stimulation involved in certain caregiving tasks may be stressful for infants. For instance, cortisol increases have been documented in healthy 3-month-olds following a tub bath (Albers, Riksen-Walraven, Sweep, & de Weerth, 2008). We have shown that infant negativity is more salient to mothers when it is manifest during routine caregiving tasks than during object-focused play (Hane et al., 2006). Hence, observation of mother-infant interaction during care-focused routines provides a snapshot of dyadic functioning in a commonplace, yet mutually challenging dyadic context.
Inspired by the rodent models and intrigued by the potential relevance of caregiving routines for humans, we explored the relation between maternal behavior in the context of caregiving activities and biobehavioral markers of infant stress reactivity (Hane & Fox, 2006). We examined the effects of early care within the context of a large, longitudinal study of low-risk mothers and their 9-month-old infants. Mothers and infants were observed in the home and rated for quality of maternal behavior for sensitive, non-intrusive interventions during the course of routine care tasks, including snack preparation, feeding, application of lotion, and dressing. A quality of maternal caregiving behavior (MCB) variable was derived from ratings of maternal sensitivity and intrusiveness. This variable was normally distributed, representing ordinary variability in maternal behavior during the course of routine care-focused tasks. Relative to infants who received high quality MCB, those who experienced low quality MCB displayed a biobehavioral profile marked by more fearfulness during the presentation of novel stimuli, less positive joint attention to a shared object with an experimenter, and more negative affect during these caregiving activities. Low MCB infants also showed a pattern of resting relative right frontal EEG asymmetry, which previous research has shown to be associated with higher basal and stress-induced salivary cortisol concentrations in 6-month-olds (Buss et al., 2003). The effects of MCB were significant when controlling for negative reactivity to novelty at age 4 months, suggesting that MCB’s contribution to this stress reactive biobehavioral profile was not driven by an earlier withdrawal motivational bias in the infant.
Phenotypic Plasticity and Defensive Responding
These findings offered novel evidence suggesting that the quality of MCB yields contemporaneous phenotypic changes to the systems involved in regulation of stress —effects that closely parallel the rodent epigenetic models. We have speculated that the mounting of a stress response in the context of maternal care may influence the organism’s propensity to manifest similar phenotypic changes in the future, a phenomenon documented by evolutionary biologists and referred to as phenotypic plasticity (Hane & Fox, 2007). Such phenotypic plasticity is evident in lower life forms. An interesting illustration comes from a study of the mollusks. Intertidal snails show morphological changes to the density of their shells on experimental exposure to predatory crabs. Trussel (1996) sampled specimens from their natural habitat, collecting specimens from tide pools where exposure to predatory crabs was high and those from tide pools where threat of predation was low. When exposed experimentally to a predatory crab in the laboratory, the snails sampled from tide pools with high risk of predation exhibited significantly greater shell density growth than snails sampled from tide pools in which the risk for predation was low. In essence, ecological adversity primed these organisms for change, increasing their malleability, insuring that a defensive response would be mounted more readily in the future.
Parent and her colleagues (2005) have suggested that a general profile of defensive reactivity develops in low LG rodents because behavioral defensiveness and the associated release of stress – related hormones are adaptive, allowing for detection of threat and the mobilization of metabolic resources under suboptimal early care conditions. Much like the intertidal snail, this mounting of a defensive response serves to prime the system to mount such a response more readily in the future. Such defensive responding is adaptive for organisms that remain in harsh ecologies. For instance, offspring of low LG dams exhibit enhanced memory formation under conditions of high, and not low, stress (Champagne et al., 2008). However, defensive reactivity costs the organism across the lifespan —for the intertidal snail, “protective” thickened shells result in attenuated growth of the organism itself (Trussell 1996).
We have conjectured that phenotypic plasticity applies to early human maternal care, such that consistent exposure to insensitive care early in development leads to phenotypic changes that alter the infant’s response in the moment; and likely costs the infant across development as the physiological by-products of stress reactivity accumulate across time (Hane & Fox, 2007). We also suspected that receipt of insensitive care in the early relational context may prime the system for defensive responding in relational contexts in particular. For the infant of an insensitive caregiver, the mounting of a defensive response is tied to the social experience of being cared for by another. Like the rodent, the infant’s release of stress hormones while in the care of mother may support mobilization of metabolic resources necessary to respond to, or cope with, that experience. However, functioning in other social settings may be characterized by such defensiveness. This too is supported by animal models. Juvenile male offspring that received low LG as pups engaged in more play fighting in multiple play partners housing than high LG males (Parent & Meaney, 2008).
The Effects of Early Maternal Care in Early Childhood
In a longitudinal follow-up to Hane and Fox (2006), we examined whether the effects of the quality of MCB in infancy persist across time and influence social behavior in early childhood (Hane, Henderson, Reeb-Sutherland, & Fox, 2010). We found that, relative to children who experienced high quality MCB as infants (high MCB children), those who received low quality MCB (low MCB children) showed increased stress reactivity on measures that parallel those used in our earlier report, including inhibited social behavior with adults and right frontal EEG asymmetry. Low MCB children also manifested more aggression during play with a novel, same-sexed peer, and mothers reported that low MCB children tended to show more internalizing problems and more proneness to anger in social situations than high MCB children. Like Hane and Fox (2006), these effects were not influenced by earlier negative reactivity to novelty.
Our findings extend the research showing that early care environments influence the developing stress response system (see Gunnar, 2006 for a review) by illustrating that normative, non-extreme variation in caregiver behavior impacts biobehavioral development. The findings are consistent with the animal model, perhaps revealing a parallel between the species —with maternal behavior in the context of routine care serving a regulatory function on the developing stress response systems of both. However, it is critical to note that the dimensions of maternal behavior and their associated mechanisms of influence likely vary across species in accordance with the contextually determined priorities of early motherhood within each (see Hane et al., 2010). And like the animal models, the adaptive value of MCB (be it high or low quality) will depend on the nature of the ecology in which the child develops (see Beery & Francis, 2011).
MCB in Early Infancy
In the rodent epigenetic models, LG is examined shortly after birth, in the first week of life. In of our previous studies, MCB was examined at infant age 9 months. By 9 months, the dyad has established a rich interactional history. The quality of mother-infant interactions changes across the first year, and the snapshot obtained at 9 months may not resemble earlier transactions between mother and her infant. Also, our previous reports did not examine if experience of low MCB induces a defensive (Parent et al., 2005) stress response in infants.
In an effort to address these limitations, we are currently examining MCB during a home observation of mothers and their 4- to 8-week-old infants. We are observing mothers as they undress, bathe, and re-dress their infants (Albers et al., 2008). Commonplace and necessary although bathing is, the experience of being bathed is challenging for infants who must regulate their body temperature and cope with tactile stimulation associated with being washed. Albers and her colleagues (2008) showed that bathing was associated with a significant cortisol (CORT) response in 3-month-olds 25 min following removal from bath water.
We have applied the bathing methodology of Albers and her colleagues to our study of MCB in mothers and their very young infants. Thus far we have conducted preliminary analyses on a subsample of 27 mothers and their 4- to 8-week-old infants (16 males, M age = 7.4 weeks, SD = 2.2) in the home. To-date, 31 dyads have been observed in the home. For four infants, the observation was terminated early so the infant could feed prior to post-test CORT collection, resulting in missing CORT data. This sample consists of middle-class, predominately European American women and their infants recruited from rural New England. We rated quality of MCB during bathing based on an adaptation of Ainsworth’s original measure (Ainsworth, 1978). The dimensions of maternal behavior are listed and described in Table 1.
TABLE 1.
Dimensions of Maternal Behavior Rated in the Context of Bathing and their Associations with Infant Stress Response
| Maternal Caregiving Behavior Dimension | Definition | Correlation with Change in Infant Cortisol |
|---|---|---|
| Maternal Acceptance- Rejection | Mother accepts the infant as an independent being, with interests and preferences that differ from her own. | −.23 |
| Sensitivity- Insensitivity | Mother bases her behavior on infant signals and promptly responds. | −.47* |
| Cooperation- Intrusiveness | Mother does not unnecessarily interfere with the infant’s agenda; she cooperates with the infant’s wishes; she is not intrusive | −.46* |
| Quality of Vocal Contact | Mother engages infant with high quality vocalizations, frequent use of “motherese” | −.20 |
| Quality of Physical Contact | Mother handles the infant appropriately, accommodating infant posture and assuring physical comfort | −.40* |
Note. Codes adapted from the Technical manual for the Systems for Coding Infant Attachment and Reciprocal Maternal Behaviors (Ainsworth, 1976).
p < .05
We are assessing infant stress response via CORT, tethering it to the experience of bathing (Albers et al., 2008). We collected saliva from the infant at baseline, immediately on arrival to the home, and immediately prior to bathing; and 15 min following the removal of the infant from the bath. Saliva was collected using 2 salivettes and frozen at -20 degrees C until assayed. Assays were performed in duplicate using the Salimetrics, LLC High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit. Raw CORT concentrations (ug/dl) were normalized using a logarithmic transformation prior to analyses.
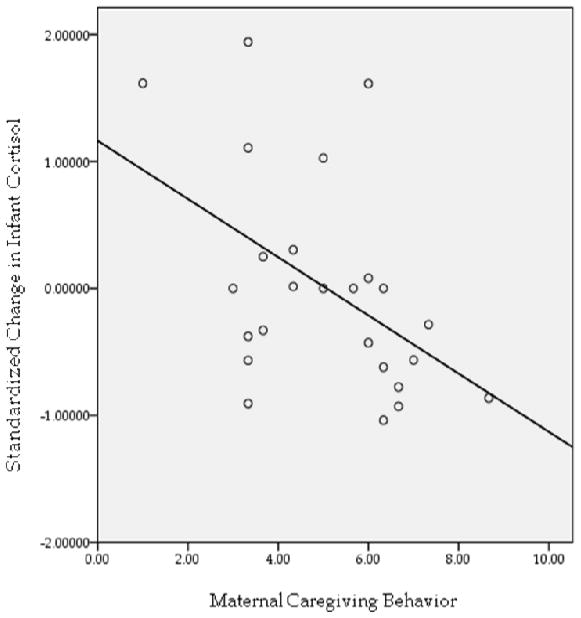
To examine if quality of MCB was associated with infant stress response, we computed zero-order correlations between each dimension of MCB and infant change in CORT, calculated as the standardized residual score obtained by regressing post-bath CORT values onto basal CORT values. As reported in Table 1, quality of maternal vocal contact and maternal acceptance of the infant were not associated with infant stress response, suggesting that these dimensions of maternal behavior were not of significant importance in supporting infant stress physiology during bathing. However, ratings for maternal sensitivity, cooperation, and quality of physical contact were associated with infant change in CORT, with less optimal maternal behavior predicting a larger neuroendocrine stress response in the infant. The pattern emerging in the data indicates that “optimal” sensitivity during bathing involves non-intrusive, physically accommodating, and infant-directed maternal handling. We derived a composite MCB variable by averaging the ratings on these 3 subscales (maternal sensitivity, cooperation, and quality of physical contact), and this MCB score accounts for approximately 20% of the variance in change in infant CORT, r(23)=−.45, p < .05 (Figure 1). These preliminary findings support the premise that the experience of low quality MCB is associated with the mounting of a stress response in the infant, very early in life. The variability in the magnitude of the effects for each dimension of MCB underscores the importance of examining maternal behavior at a specific behavioral level. We are continuing to enroll mothers in the project and track this sample across time to elucidate the underlying processes involved in early postnatal programming. To achieve this end, however, we must sharpen our focus, applying even more specificity to the construct of MCB itself and turning our focus to the role of the infant in these MCB effects.
Figure 1.
Quality of maternal caregiving behavior is negatively associated with increase in salivary cortisol across bathing.
ADVANTAGES OF DOMAIN SPECIFICITY
Domain-general approaches that view parenting through a wide-angled lens have hindered our ability to identify mechanisms of influence involved in socialization (Bornstein, 2006; Grusec & Davidov, 2010) . Nowhere are these short-comings more evident than in conceptualizations of maternal sensitivity. Ainsworth constructed a comprehensive battery for rating the quality of maternal behavior in both caregiving and play contexts, including scales tapping the concrete (e.g., frequency and quality of vocal behavior) and the abstract (e.g., acceptance-rejection of the infant). Such breadth is both a blessing and a curse, offering conceptual richness, but interpretive restrictions.
Our approach to MCB highlights the advantages of domain specificity. By examining the role of separate dimensions of maternal behavior during bathing, we are honing in on one mechanism through which MCB influences infant biobehavioral development. Like our earlier work, the findings of our work with young infants are reminiscent of the animal models, revealing that mothers support infant physiological regulation through ordinary variations in caregiving behavior. In the case of bathing, our early findings suggest that maintenance of homeostasis for the infant during the challenge of bathing is influenced by maternal behavior. A highly sensitive mother identifies the components of the bath that her infant finds aversive and attenuates their negative consequences. She prepares the bath in such a way that the water is warm and sufficiently covering the body. She removes clothing and applies soap gently, so the infant is not overly stimulated. She skillfully maneuvers the necessarily invasive washing of the face, particularly around the eyes. She prepares the clothing before application by readying the sleeves and opening the neck widely, to minimize pulling and tugging. This style of maternal caregiving may be “optimal” simply because it prevents infant discomfort, eliminating the need for the infant to mount a defensive response while being cared for. Mother is protecting the infant’s comfort in nuanced, subtle, and remarkably ordinary ways.
There is no single molecule through which parenting imparts its influence. Indeed, each mechanism of influence will be embedded within the context in which parenting was measured. Future MCB research should apply even more domain specificity, for example by examining if the stress-reducing, protective dimensions of MCB observed during bathing differ from the relevant dimensions involved in feeding and sleeping routines. We suspect the relevant dimensions of MCB will vary based on the nature of the task and the challenges involved for the infant in each context (e.g., regulating sucking and swallowing [feeding] or coping with fatigue and maternal separation [sleeping]).
In the most optimal of bathing interactions, the extremely sensitive mother moves beyond prevention of discomfort and induces pleasure. We have observed that, even with the young neonate and under the significant contextual challenge of bathing, some mothers manage to successfully achieve periods of mutual engagement. The experience of a tub bath rouses even the sleepiest of newborns and the alert state induced by the bath opens the floor for communication with the caregiver. Maternal behavior in this context may serve functions other than protection of physical comfort and its associated physiological regulatory support. High MCB mothers may also be engaging their infants with optimal levels of synchrony (Feldman, 2012). However, it is also possible that high MCB mothers do not necessarily engage in optimal parenting across all contexts (Grusec & Davidov, 2010).
The Baby in the Bath Water
Although we have shown that the effects of MCB are independent of earlier ratings of behavioral reactivity to novelty (Hane & Fox, 2006; Hane et al., 2010), we do not discount the role of the infant in MCB effects. In our work with 9-month-olds, infants who experienced low MCB manifested more negative affect during these caregiving tasks (Hane & Fox, 2006). Defensive responses to the experience of routine caregiving may have little to do with approach-withdrawal biases. After the first month, the experience of being dressed and washed is not novel. But infants differ in their response to these experiences, and some mothers will have to be more skillful in setting the stage for physical comfort than others. We contend that domain specificity is also critical for elucidating infant contributions to MCB effects, and the most relevant dimension of infant temperament may involve sensitivity to the discomforts associated with basic care tasks. Considerable attention has been paid to the construct of discomfort intolerance and its role in adult anxiety disorder (e.g., Schmidt, Richey, & Fitzpatrick, 2006). Our observations of neonates in the context of bathing have led us to surmise that reactivity to somatic discomfort —reactivity to the physical experiences of being cold, wet, and tactically stimulated —may be critically important to the study of early maternal care. Infants who are highly reactive to discomfort may evoke higher or lower quality MCB, and this likely depends on the nature of the sample (high- vs. low-risk). This type of reactivity may also interact with quality of MCB, such that highly reactive infants of low MCB mothers manifest the highest levels of defensive responding during the experience of insensitive care, and a profile of stress reactivity across development.
Translating the MCB Model
Another advantage of applying highly behaviorally or contextually specific assessments of parenting is the increased translational value in such work. We envision that an intervention program informed by our work might involve relatively simple, direct coaching to new mothers as they bathe, change, and dress their newborns. We have observed mothers make simple oversights that produce lower MCB ratings. Take, for instance, undressing the infant prior to preparing the bath. This is not what most might consider an egregious parenting error. It is not abusive or neglectful. Yet, we have observed that the impact on quality of MCB can be considerable: The freshly disrobed infant is held with one arm as mother fills the bath and gathers supplies. Across this task, mother’s arm becomes strained by the infant, and she shifts him awkwardly and frequently. The exposed infant is challenged by the chill of room temperature, and he squirms and cries. Mother’s arm is further taxed by the aroused infant; her holding becomes less accommodating. Placement in the bath is more abrupt as mother is eager to free her arm. The infant’s response to the water is more dramatic than it might have been, because his own body temperature has cooled, increasing the saliency of the water’s warmth.
Animal models have demonstrated that the effects of early suboptimal maternal care can be modified by subsequent placement in an enriched environment (Champagne & Meaney, 2007). Our observations of mothers in the home lead us to hypothesize that direct teaching to mothers as they perform caregiving rituals would make an important addition to a comprehensive home-based parenting intervention program. For instance, if an interventionist were present on an occasion such as that described above, one might envision that simple verbal directives to mother such as “prepare the bath first” or “reduce the duration of time your baby is unclothed,” might significantly increase quality of MCB and prevent infant discomfort. In short, an MCB-based intervention should be like the construct itself; focused on pragmatic, straightforward, and ordinary tactics.
FUTURE DIRECTIONS
We have outlined several new directions for extending research on maternal caregiving of human infants, arguing for further refinement in the assessment of MCB and the role of the infant in these MCB effects. As we extend our work examining MCB chronologically down to the neonatal period, we also continue to follow trajectories of low and high MCB children across development. It remains unclear if low MCB children will show a biobehavioral profile of risk across childhood, and if so, if that risk is specific to relational contexts. Behavioral specificity remains critical in terms of assessment of early care, infant contributions, and relevant outcomes across time. We believe that such behavioral specificity will serve to elucidate the “hidden regulators” (Hofer, 2006) embedded within the early care environments of the human infant, identifying the proximal and long-term mechanisms of influence underlying associations between child biobehavioral development and ordinary variations in early maternal care.
Acknowledgments
This research was supported by a grant from the National Institutes of Health (HD# 17899) to Nathan A. Fox. We thank Noah Sandstrom for consultation on salivary cortisol methodologies; Betty Zimmerberg for consultation on rodent models; and Lindsay Moore for behavioral coding. We extend our most sincere gratitude to Rosalie Girard for her support in recruiting and to the families who continue to participate in this research.
Contributor Information
Amie Ashley Hane, Email: ahane@williams.edu, Department of Psychology, Williams College, 18 Hoxsey Street, Williamstown, MA 01267.
Lauren E. Philbrook, The Pennsylvania State University
References
- Ainsworth MDS. Technical manual for the Systems for Coding Infant Attachment and Reciprocal Maternal Behaviors. Princeton, NJ: Educational Testing Service; 1976. [Google Scholar]
- Albers EM, Riksen-Walraven M, Sweep FCGJ, de Weerth C. Maternal behavior predicts infant cortisol recovery from a mild everyday stressor. The Journal of Child Psychology and Psychiatry. 2008;49:97–103. doi: 10.1111/j.1469-7610.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- Beery AK, Francis DD. Adaptive significance of natural variations in maternal care in rats: A translational perspective. Neuroscience and Behavioral Reviews. 2011;35:1552–1561. doi: 10.1016/j.neubiorev.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH. Parenting science and practice. Handbook of child psychology. In: Renninger KA, Sigel IE, Damon W, Lerner RM, editors. Child psychology in practice. 6. Vol. 4. Hoboken, NJ US: John Wiley & Sons Inc; 2006. pp. 893–949. [Google Scholar]
- Bowlby J. Attachment and Loss: Vol. I. Attachment. NY: Basic Books; 1969. [Google Scholar]
- Buss KA, Malmstadt Schumacher JR, Dolski I, Kalin NH, Goldsmith H, Davidson RJ. Right frontal brain activity, cortisol, and withdrawal behavior in 6-month-old infants. Behavioral Neuroscience. 2003;117:11–20. doi: 10.1037//0735-7044.117.1.11. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proceedings of the National Academy of Sciences, USA. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA. Maternal imprints and the origins of variation. Hormones and Behavior. 2011;60:4–11. doi: 10.1016/j.yhbeh.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne DL, Bagot RC, can Hasselt F, Ramakers G, Meaney MJ, de Kloet R, Joels M, Krugers H. Maternal care and hippocampal plasticity: Evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. The Journal of Neuroscience. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wolff M, Van IJzendoorn MH. Sensitivity and attachment: A meta-analysis on parental antecedants of infant attachment. Child Development. 1997;68:571–591. [PubMed] [Google Scholar]
- Feldman R. Bio-behavioral Synchrony: A Model for integrating biological and microsocial behavioral processes in the Study of parenting. Parenting: Science and Practice. 2012;12:XX–XX. [Google Scholar]
- Francis DD, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress response in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Grusec J, Davidov M. Integrating different perspectives on socialization theory and research: A domain-specific approach. Child Development. 2010;81:687–709. doi: 10.1111/j.1467-8624.2010.01426.x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Social regulation of stress in early childhood. In: McCartney K, Phillips D, editors. Blackwell Handbook of Early Childhood Development. Malden: Blackwell Publishing; 2006. pp. 106–125. [Google Scholar]
- Hane AA, Fox NA. Ordinary variations in maternal caregiving of human infants influence stress reactivity. Psychological Science. 2006;17:550–556. doi: 10.1111/j.1467-9280.2006.01742.x. [DOI] [PubMed] [Google Scholar]
- Hane AA, Fox NA. A closer look at the transactional nature of early social development: The relations among early caregiving environments, temperament, and early social development and the case for phenotypic plasticity. In: Santoianni F, Sabatano C, editors. Brain Development in Learning Environments: Embodied and Perceptual Advancements. Newcastle, UK: Cambridge Scholars Publishing; 2007. pp. 1–15. [Google Scholar]
- Hane AA, Fox NA, Polak-Toste C, Ghera MM, Guner B. Contextual basis of maternal perceptions of infant temperament. Developmental Psychology. 2006;42:1077–1088. doi: 10.1037/0012-1649.42.6.1077. [DOI] [PubMed] [Google Scholar]
- Hane AA, Henderson HA, Fox NA, Reeb-Sutherland BC. Ordinary variations in human maternal caregiving in infancy and biobehavioral development in early childhood: A follow-up study. Developmental Psychobiology. 2010;52:558–567. doi: 10.1002/dev.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer MA. Psychobiological roots of early attachment. Current Directions in Psychological Science. 2006;15:84–88. [Google Scholar]
- Leerkes EM, Weaver J, O’Brien M. Differentiating maternal sensitivity to infant distress and non-distress. Parenting: Science and Practice. 2012;12:XX–XX. doi: 10.1080/15295192.2012.683353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Parent CI, Meaney MJ. The influence of natural variations in maternal care on play fighting in the rat. Developmental Psychobiology. 2008;50:767–776. doi: 10.1002/dev.20342. [DOI] [PubMed] [Google Scholar]
- Parent C, Zhang T, Caldji C, Bagot R, Champagne FA, Pruessner J, Meaney MJ. Maternal care and individual differences in defensive responses. Current Directions in Psychological Science. 2005;14:229–233. [Google Scholar]
- Schmidt NB, Richey JA, Fitzpatrick KK. Discmfort intolerance: Development of a construct and measure relevant to panic disorder. Journal of Anxiety Disorders. 2006;20:263–280. doi: 10.1016/j.janxdis.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Trussell GC. Phenotypic plasticity in an intertidal snail: The role of a common crab predator. Evolution. 1996;50:448–454. doi: 10.1111/j.1558-5646.1996.tb04507.x. [DOI] [PubMed] [Google Scholar]