Abstract
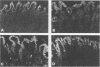
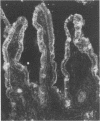
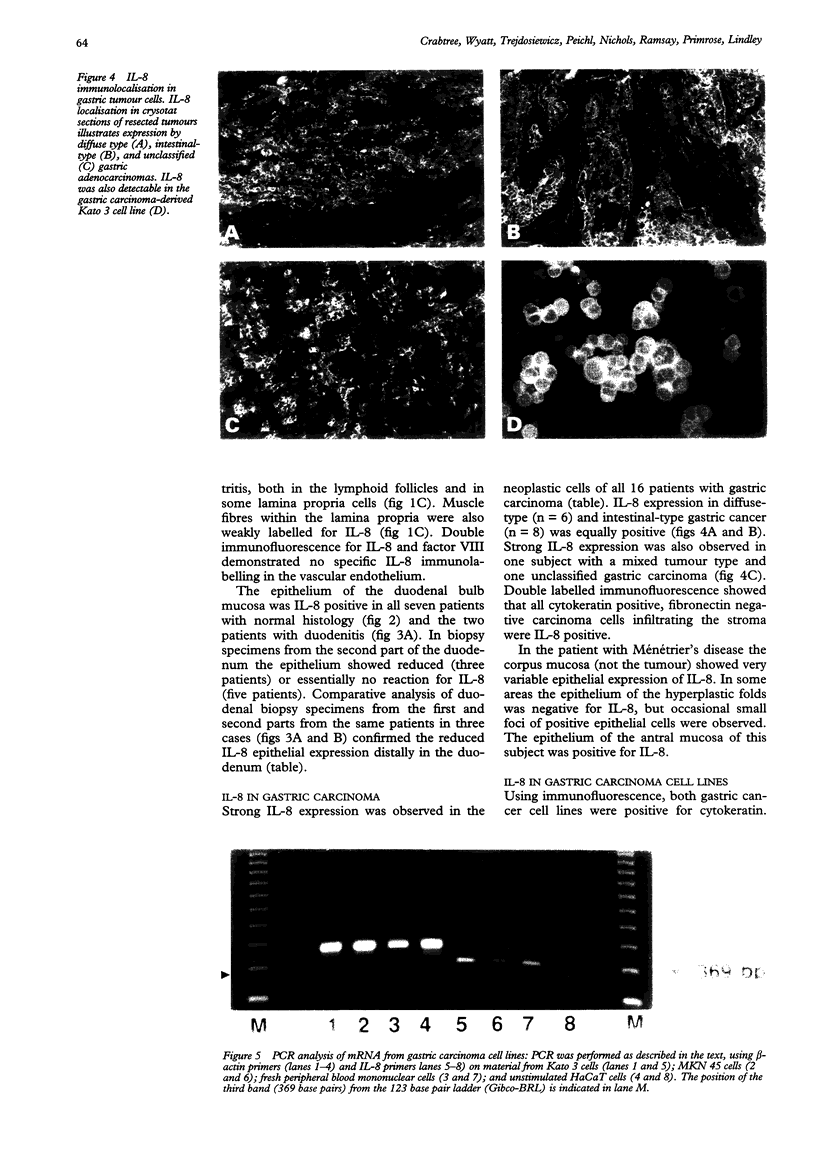
AIMS--To investigate the expression of interleukin-8 (IL-8) in Helicobacter pylori infected normal and neoplastic gastroduodenal mucosa, and in established gastric cancer cell lines. METHODS--Immunofluorescence techniques were used to localise IL-8 in cryosections of gastric (n = 25) and duodenal (n = 17) endoscopic biopsy specimens an in resected gastric tumour tissue samples from 16 patients. Two gastric cancer cell lines (Kato 3 and MKN 45) were examined for IL-8 protein expression by immunofluorescence and for the presence of IL-8 mRNA by reverse transcription followed by the polymerase chain reaction (RT-PCR). RESULTS--IL-8 was localised to the epithelium in histologically normal gastric mucosa, with particularly strong expression in the surface cells. IL-8 expression was also a feature of surface epithelium in the duodenal bulb, but was much reduced in the second part of the duodenum. In chronic H pylori-associated gastritis gastritis gastric epithelial IL-8 expression was increased and expression of IL-8 within the lamina propria was evident. By contrast, large areas of IL-8 negative epithelium were observed in the body mucosa of a subject with Ménétrier's disease. In gastric carcinoma the tumour cells were positive for IL-8. IL-8 was also detected by immunofluorescence in unstimulated Kato 3 and MKN 45 cells, and constitutive IL-8 gene expression in these cell lines was confirmed by detection of IL-8 mRNA by RT-PCR. CONCLUSIONS--Immunoreactive IL-8, a potent neutrophil chemotactic and activating factor, is present in the epithelium of both normal and inflamed gastric mucosa with increased expression in the latter. There is site dependent variation in epithelial IL-8 expression within the gastroduodenal mucosa. The expression of the pro-inflammatory cytokine IL-8 in gastric carcinoma cells may influence peritumoural cellular infiltrates.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abruzzo L. V., Thornton A. J., Liebert M., Grossman H. B., Evanoff H., Westwick J., Strieter R. M., Kunkel S. L. Cytokine-induced gene expression of interleukin-8 in human transitional cell carcinomas and renal cell carcinomas. Am J Pathol. 1992 Feb;140(2):365–373. [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M., Walz A., Kunkel S. L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989 Oct;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan F. M., Zachariae C. O., Chantry D., Larsen C. G., Turner M., Maini R. N., Matsushima K., Feldmann M. Detection of interleukin 8 biological activity in synovial fluids from patients with rheumatoid arthritis and production of interleukin 8 mRNA by isolated synovial cells. Eur J Immunol. 1990 Sep;20(9):2141–2144. doi: 10.1002/eji.1830200938. [DOI] [PubMed] [Google Scholar]
- Clayton C. L., Wren B. W., Mullany P., Topping A., Tabaqchali S. Molecular cloning and expression of Campylobacter pylori species-specific antigens in Escherichia coli K-12. Infect Immun. 1989 Feb;57(2):623–629. doi: 10.1128/iai.57.2.623-629.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Peichl P., Wyatt J. I., Stachl U., Lindley I. J. Gastric interleukin-8 and IgA IL-8 autoantibodies in Helicobacter pylori infection. Scand J Immunol. 1993 Jan;37(1):65–70. doi: 10.1111/j.1365-3083.1993.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Crabtree J. E., Shallcross T. M., Heatley R. V., Wyatt J. I. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991 Dec;32(12):1473–1477. doi: 10.1136/gut.32.12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig P. M., Territo M. C., Karnes W. E., Walsh J. H. Helicobacter pylori secretes a chemotactic factor for monocytes and neutrophils. Gut. 1992 Aug;33(8):1020–1023. doi: 10.1136/gut.33.8.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denizot Y., Sobhani I., Rambaud J. C., Lewin M., Thomas Y., Benveniste J. Paf-acether synthesis by Helicobacter pylori. Gut. 1990 Nov;31(11):1242–1245. doi: 10.1136/gut.31.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. Y. Campylobacter pylori and peptic ulcer disease. Gastroenterology. 1989 Feb;96(2 Pt 2 Suppl):615–625. doi: 10.1016/s0016-5085(89)80057-5. [DOI] [PubMed] [Google Scholar]
- Graves D. T., Jiang Y. L., Williamson M. J., Valente A. J. Identification of monocyte chemotactic activity produced by malignant cells. Science. 1989 Sep 29;245(4925):1490–1493. doi: 10.1126/science.2781291. [DOI] [PubMed] [Google Scholar]
- LAUREN P. THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- Lindley I., Aschauer H., Seifert J. M., Lam C., Brunowsky W., Kownatzki E., Thelen M., Peveri P., Dewald B., von Tscharner V. Synthesis and expression in Escherichia coli of the gene encoding monocyte-derived neutrophil-activating factor: biological equivalence between natural and recombinant neutrophil-activating factor. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9199–9203. doi: 10.1073/pnas.85.23.9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Ceska M., Effenberger F., Kurlak L., Lindley I., Hawkey C. J. Enhanced synthesis of neutrophil-activating peptide-1/interleukin-8 in active ulcerative colitis. Clin Sci (Lond) 1992 Mar;82(3):273–275. doi: 10.1042/cs0820273. [DOI] [PubMed] [Google Scholar]
- Mai U. E., Perez-Perez G. I., Allen J. B., Wahl S. M., Blaser M. J., Smith P. D. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J Exp Med. 1992 Feb 1;175(2):517–525. doi: 10.1084/jem.175.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai U. E., Perez-Perez G. I., Wahl L. M., Wahl S. M., Blaser M. J., Smith P. D. Soluble surface proteins from Helicobacter pylori activate monocytes/macrophages by lipopolysaccharide-independent mechanism. J Clin Invest. 1991 Mar;87(3):894–900. doi: 10.1172/JCI115095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K., Oppenheim J. J. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL 1 and TNF. Cytokine. 1989 Nov;1(1):2–13. doi: 10.1016/1043-4666(89)91043-0. [DOI] [PubMed] [Google Scholar]
- Nielsen H., Andersen L. P. Chemotactic activity of Helicobacter pylori sonicate for human polymorphonuclear leucocytes and monocytes. Gut. 1992 Jun;33(6):738–742. doi: 10.1136/gut.33.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Peichl P., Ceska M., Broell H., Effenberger F., Lindley I. J. Human neutrophil activating peptide/interleukin 8 acts as an autoantigen in rheumatoid arthritis. Ann Rheum Dis. 1992 Jan;51(1):19–22. doi: 10.1136/ard.51.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl P., Ceska M., Effenberger F., Haberhauer G., Broell H., Lindley I. J. Presence of NAP-1/IL-8 in synovial fluids indicates a possible pathogenic role in rheumatoid arthritis. Scand J Immunol. 1991 Sep;34(3):333–339. doi: 10.1111/j.1365-3083.1991.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Seitz M., Dewald B., Gerber N., Baggiolini M. Enhanced production of neutrophil-activating peptide-1/interleukin-8 in rheumatoid arthritis. J Clin Invest. 1991 Feb;87(2):463–469. doi: 10.1172/JCI115018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi M., Sakakibara K., Fujii G. Establishment of cultured cell lines derived from a human gastric carcinoma. Jpn J Exp Med. 1978 Feb;48(1):61–68. [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Basha M. A., Chensue S. W., Lynch J. P., 3rd, Toews G. B., Westwick J., Strieter R. M. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest. 1990 Dec;86(6):1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Miyasaka H., Ota H., Yamakawa Y., Tagawa M., Kuramoto A., Mizuno S. Purification and partial primary sequence of a chemotactic protein for polymorphonuclear leukocytes derived from human lung giant cell carcinoma LU65C cells. J Exp Med. 1989 Jun 1;169(6):1895–1901. doi: 10.1084/jem.169.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejdosiewicz L. K., Southgate J., Hodges G. M., Goodman S. L. Micro-heterogenous expression of peanut agglutinin-binding sites in the extracellular matrix of cultured cells. Exp Cell Res. 1985 Jan;156(1):153–163. doi: 10.1016/0014-4827(85)90269-1. [DOI] [PubMed] [Google Scholar]
- Trejdosiewicz L. K., Southgate J., Kemshead J. T., Hodges G. M. Phenotypic analysis of cultured melanoma cells. Expression of cytokeratin-type intermediate filaments by the M5 human melanoma cell line. Exp Cell Res. 1986 Jun;164(2):388–398. doi: 10.1016/0014-4827(86)90037-6. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Arfors K. E., McKnight G. W. A monoclonal antibody against the CD18 leukocyte adhesion molecule prevents indomethacin-induced gastric damage in the rabbit. Gastroenterology. 1991 Apr;100(4):878–883. doi: 10.1016/0016-5085(91)90259-n. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Keenan C. M., Granger D. N. Gastric ulceration induced by nonsteroidal anti-inflammatory drugs is a neutrophil-dependent process. Am J Physiol. 1990 Sep;259(3 Pt 1):G462–G467. doi: 10.1152/ajpgi.1990.259.3.G462. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Yasumoto K., Okamoto S., Mukaida N., Murakami S., Mai M., Matsushima K. Tumor necrosis factor alpha and interferon gamma synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP-1 and NF-kB-like binding sites of the interleukin 8 gene. J Biol Chem. 1992 Nov 5;267(31):22506–22511. [PubMed] [Google Scholar]
- Yoshida M., Matsuzaki H., Sakata K., Takeya M., Kato K., Mizushima S., Kawakita M., Takatsuki K. Neutrophil chemotactic factors produced by a cell line from thyroid carcinoma. Cancer Res. 1992 Jan 15;52(2):464–469. [PubMed] [Google Scholar]
- Zachariae C. O., Thestrup-Pedersen K., Matsushima K. Expression and secretion of leukocyte chemotactic cytokines by normal human melanocytes and melanoma cells. J Invest Dermatol. 1991 Sep;97(3):593–599. doi: 10.1111/1523-1747.ep12481934. [DOI] [PubMed] [Google Scholar]