Abstract
The role of cerebral blood flow (CBF) on a maximal breath-hold (BH) in ultra-elite divers was examined. Divers (n = 7) performed one control BH, and one BH following oral administration of the non-selective cyclooxygenase inhibitor indomethacin (1.2 mg/kg). Arterial blood gases and CBF were measured prior to (baseline), and at BH termination. Compared to control, indomethacin reduced baseline CBF and cerebral delivery of oxygen (CDO2) by about 26% (p < 0.01). Indomethacin reduced maximal BH time from 339 ± 51 to 319 ± 57 seconds (p = 0.04). In both conditions, the CDO2 remained unchanged from baseline to the termination of apnea. At BH termination, arterial oxygen tension was higher following oral administration of indomethacin compared to control (4.05 ± 0.45 vs. 3.44 ± 0.32 kPa). The absolute increase in CBF from baseline to the termination of apnea was lower with indomethacin (p = 0.01). These findings indicate that the impact of CBF on maximal BH time is likely attributable to its influence on cerebral H+ washout, and therefore central chemoreceptive drive to breathe, rather than to CDO2.
Keywords: Apnea, Cerebral oxygen delivery, Hypercapnia, Hypoxia, Indomethacin
Introduction
The practice of extreme voluntary breath holding offers a unique opportunity to examine the physiologic responses to transient hypercapnic hypoxia. Breath holding of long durations (current world record is 11 min 35 s) in elite individuals produces blood gas partial pressures at the limits of human tolerance. The mediators underpinning maximal breath hold (BH) durations are undoubtedly integrative, involving not only severe changes in the partial pressures of arterial oxygen (PaO2) and carbon dioxide (PaCO2), but also lung and chemoreceptor afferents, and limitations in cerebral oxidative metabolism [1, 2]. We previously demonstrated the importance of increased cerebral blood flow (CBF) to maintain cerebral delivery of oxygen (CDO2) as arterial oxygen content (CaO2) decreases during apnea [3]. To mechanistically address this CBF compensation, the next logical step was to manipulate the CBF, and observe the consequence on maximal breath holding time. Efforts to describe the impact of CBF under acute hypoxic stress are fundamental for understanding pathology or circumstances of impaired CBF in hypoxia (e.g. with surgery, respiratory disease, and ischemic stroke).
Acute hypoxia increases CBF [4]. While the mechanisms involved are complex (including the interplay between vasoactive agents, deoxyhemoglobin and PaCO2 [5]), it is likely that the CBF is targeted to maintain CDO2 [6]. Indeed, in extreme breath holding (over 5 min), PaO2 is reduced to about 4 kPa, but CDO2 never falls below resting baseline levels [3]. The corollary effect of increased CBF is greater washout of CO2 from brain tissue [7, 8], and a consequent attenuation of central chemoreceptor stimulation, and ultimately the drive to breathe [9]. A reduced CBF may therefore reduce maximum BH duration not only by limiting CDO2, but also by augmenting brain tissue acidosis and the central drive to breathe.
The purpose of this study was to determine the impact of a reduced CBF (via the non-selective cyclooxygenase inhibitor indomethacin) on maximal BH time in ultra-elite apnea divers. The CBF, CDO2, PO2 and PaCO2 were quantified. Ultra-elite apnea divers were utilized to limit variability associated with BH motivation, and to examine the most severe levels of combined hypoxia and hypercapnia achievable in unanesthetized humans. It was hypothesized that a reduction in CBF with Indo would decrease maximal BH duration, consequent to a reduced CDO2, and increased cerebral acidosis; i.e., reduced CO2 washout.
Methods
Participants and experimental design
This study was approved by the University of British Columbia Clinical Research Ethics Board, and the University of Split School of Medicine Ethics Board, and conformed to the declaration of Helsinki. Following written informed consent, and consent to publish, seven ultra-elite apnea divers (one female and six male subjects, height (average ± standard deviation): 182 ± 9 cm, weight: 79 ± 13 kg, age: 30 ± 6 years) volunteered from the Croatian National Apnea Team. Caffeine, alcohol and strenuous exercise were avoided 24 h before experimental testing. All participants arrived to the laboratory in the morning after consuming a light breakfast (toast and orange juice). Subjects completed two maximal dry supine apneas on the same day. The first apnea acted as the control condition (Control). Immediately following the control apnea, 1.2 mg/kg body weight of oral indomethacin (Indo) was administered. Following a 90-min break to assure peak effect of Indo [10], the second, experimental, apnea was performed. Identical preparatory procedures (practice sub-maximal breath holds, mild hyperventilation) were performed before each maximal apnea [3].
Arterial blood samples
A 20-gauge radial arterial catheter (Arrow, Markham, Ontario, Canada) was placed in the right radial artery. The catheter was attached to an in-line waste-less sampling system (Venous Arterial blood Management Protection System, VAMP, Edwards Lifesciences, Irvine, CA, USA). Blood samples (2 mL) were collected 30 sec prior to commencing the apnea, and immediately at apnea termination. Arterial PO2, PCO2, O2 saturation (SaO2%), and pH were measured immediately using a commercially available blood gas analyzer (ABL90 Flex, Radiometer Medical, Bronshoj, Denmark). Oxygen content was calculated from Equation 1:
Where; CaO2 is in mL/dL, Hb is in g/dL, PaO2in KPa, and SaO2in %
Cerebrovascular measures
Blood flow through the right internal carotid artery (QICA) was measured using duplex ultrasound, with concomitant measures of vessel diameter and velocity. Blood flow was quantified offline using edge detection software [11]. The latter stages of an extreme maximal apnea elicit strong inspiratory muscle contractions and contractions of the sternocleidomastoid muscles that, in some, prevented reliable recordings of QICA. As such, a transcranial Doppler probe (Spencer Technologies, Redmond, WA, USA) was fixed on the temporal window for continuous measures of middle cerebral (MCAv) and posterior cerebral artery (PCAv) blood velocity. End apnea QICA in all participants was therefore calculated by multiplying baseline QICA with the percent change in middle cerebral artery velocity from baseline to end apnea. ICA and MCA have previously been shown to follow the same pattern of change during maximal apnea. The CDO2 was measured as oxygen content (Eq. 1) multiplied by the QICA. Measures for QICA and PCAv were subsequently used to describe the responses in anterior and posterior CBF, respectively.
Statistical methods
All values are presented as mean ± standard deviation. Absolute values for blood gases, pH, CDO2, QICA and PCAv between Indo and Control were compared using a priori paired single-tailed T-tests. The change in QICA and PCAv from baseline to end apnea between Indo and Control was also compared using a paired single-tailed T-test. When significance was reached, effect size is shown using Cohen′s d (d). Significance was set at 95% certainty (a of 0.05).
Results
Maximal apnea time was significantly reduced with Indo (319 ± 57 s) compared to Control (339 ± 51 s), p = 0.04, d = 0.39 (Fig. 1).
Fig. 1.
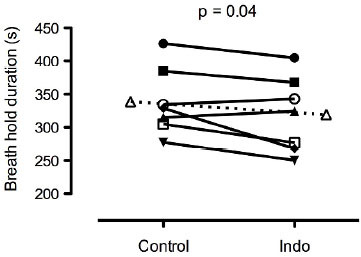
Maximal breath hold time during control and with 1.2 mg/kg of indomethacin. Dashed line indicates mean data.
Arterial blood gases
Absolute values for PO2, PaCO2, SO2 and pH during Indo and Control for baseline and end of apnea are presented in Table 1. There were no significant differences in any blood gas variable at baseline between Indo and Control. In contrast, the PO2 and SO2 were significantly higher at the end of apnea during the Indo compared to Control trial (p < 0.01, d = -1.67, and p< 0.01, d = -1.45, respectively). There was no significant difference in PaCO2 at the end of apnea between Indo compared to Control (p = 0.11).
Table 1.
Yields, Mn, Mw and PDI data obtained from SEC measurements of PCL and PLA samples
| PaC02(kPa) | PaC02(kPa) | SaO2(%) | pH | CDO2 | |
| Control | |||||
| BL | 14.16±1.89 | 4.73±0.83 | 98.7±0.8 | 7.47±0.05 | 4.44±9.4 |
| End | 3.44±0.32 | 49.7±6.8 | 7.35±0.04 | 44.9±8.0 | 4.44±9.4 |
| Indo | |||||
| BL | 13.96±2.0 | 4.60±0.73 | 98.5±0.9 | 74.6±0.05 | 32.8±9.3* |
| End | 4.05±0.45* | 7.00±0.83 | 59.3±7.5* | 7.34±0.03 | 38.4±11.0 |
* denotes a p < 0.05 between Indo and Control
Cerebrovascular measures
The absolute change from baseline to end apnea in QICA and PCAv for Indo and Control are presented in Figure 2. Baseline QICA and PCAv was decreased by 26 ± 16% (d = 1.14) and 22 ± 14% (d = 1.07), respectively, with Indo compared to Control (p < 0.01 for both). Likewise, the CDO2 at baseline was reduced by 26 ± 16% with Indo compared to Control (p < 0.01, d = 1.34). At the end of apnea, absolute QICA and PCAv were lower by 27 ± 16% (d = 1.46) and 24 ± 6% (d = 1.64), respectively, during Indo compared to Control (p < 0.01 for both). The CDO2 at the end of apnea was 14 ± 22% lower with Indo compared to Control (non-significant; p = 0.07, d = 0.73). Within condition, there was no significant change in CDO2 from baseline to end apnea, for Control and Indo (p > 0.05 for both).
Fig. 2.
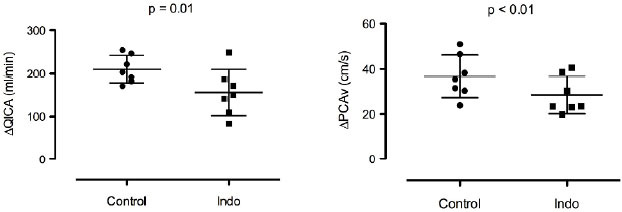
Absolute change in internal carotid artery flow (QICA; left panel) and posterior cerebral artery velocity (PCAv; right panel) from baseline to the end of the breath hold during Control and Indo.
The relative increase in QICA (Indo: 101 ± 29%, Control: 103 ± 30%) and PCAv (Indo: 109 ± 37%, Control: 114 ± 49%) from baseline to end apnea was similar with Indo compared to Control (p = 0.44 and p = 0.35, respectively). However, the absolute increase in QICA (Indo: 156 ± 54 ml.min-1, Control: 210 ± 32 ml.min-1) and PCAv (Indo: 28 ± 8 cm.sec-1, Control: 37 ± 9 cm.sec-1) was significantly lower with Indo compared to Control (p = 0.01, d = 1.32, and p < 0.01, d = 1.00, respectively), see Figure 2.
Accounting for differences in BH lengths, the relative change from baseline to end apnea per unit time in QICA (Indo: 19.6 ± 6.8 %min-1, Control: 18.3 ± 4.9 %min-1) and PCAv (Indo: 20.9 ± 7.1 %min-1, Control: 19.9 ± 7.9 %min-1) was similar with Indo compared to Control (p > 0.05 for both). In contrast, the absolute PCAv change from baseline to end apnea per unit time was lower in Indo (5.5 ± 2.0 cms-1min-1) compared to Control (6.6 ± 1.8 cms-1 min-1; p = 0.01, d = 0.58). The absolute QICA change from baseline to end apnea per unit time also trended to be lower with Indo (31.1 ± 15.2 mlmin-1min-1) compared with Control (37.7 ± 6.8 mlmin-1.min-1; p = 0.07).
Discussion
This study demonstrates that Indo attenuates CBF resulting in a moderate reduction of maximal BH tolerance. Given that CDO2 at baseline did not differ from CDO2 at end-apnea in both Indo and Control trials, the reduced maximal BH following CBF attenuation is likely not related to CDO2, and likely more related to the decreased cerebral CO2 washout, and therefore cerebral acidosis at the level of the central chemoreceptors leading to an augmented drive to breathe. Indeed, the tolerable level of hypoxemia (SO2) was about 9% higher during the Indo, compared to the Control apnea
What determines the apnea breakpoint?
The mechanisms of BH breakpoint have been topic of several reviews (e.g. [2, 12, 13]). As described by Mithoefer in 1965 [14], the physiological apnea breakpoint, that is, the point of involuntary contraction of the inspiratory muscles, is undoubtedly governed from a combination of factors -e.g., phrenic, glossopharyngeal, and central chemoreceptor afferents [1, 2, 15]. This breakpoint derives from general discomfort and an increased drive to breathe, but is overcome in the ultra-elite where voluntary maximal BH is often extended until unconsciousness (a grounds for disqualification in regulated competitions). We have previously demonstrated that the motivated physiological breakpoint in the ultra-elite generally falls at a critical PO2 (of ~4 kPa) [1], and is likely governed in large part by consciousness. Nevertheless, given that CDO2 was maintained and PO2 was lower at the termination of the Control compared to Indo apnea, the disparate apnea breakpoint in the current study is likely reflective for an additional role of central chemoreception when CBF and its reactivity to elevations in PaCO2 is impaired.
Cerebral blood flow and CO2/H+ ‘washout’
By administering Indo, the central drive to breathe was likely increased via reductions in the CBF response to apnea, which reduces CO2/H+ washout from the medulla and in turn decreases pH [7, 8] and see Fig 7. in [9]. Theoretically, in a closed system independent of metabolism, flow equivalent to three times cerebral blood volume must pass through the cerebral vasculature for cerebral CO2 to reach 95% equilibrium between arterial and tissue compartments with a steady state step increase in CO2 [see discussion in [16]]. Therefore, while true equilibrium is never reached in vivo, CBF will dictate the magnitude of partial equilibration (i.e., tissue-arterial gradient). The average 24% reduction in posterior CBF (PCAv) with Indo compared to Control would have thus increased the time constant for CO2 tissue/arterial equilibrium in the brainstem by ~24%, meaning that the magnitude of partial equilibrium would be reduced. In turn, despite the ~0.27 kPa higher PaCO2 at the termination of the Control vs. Indo apnea (related to the longer apnea time), it is likely that the reduced CO2 washout with Indo would have more than offset the slight difference in PaCO2 so that cerebral tissue pH would have been comparable.
Limitations
Due to the extended half-life of indomethacin (˜4.5 h), we were unable to counter-balance the order of Indo vs. Control breath holds. Nevertheless, as confirmed in a previous counter balanced design [1], it is unlikely that an order effect would have influenced the present data. Moreover, maximal breath hold time is in fact increased with consecutive holds (the rationale for preparatory breath holds), therefore, had an order effect occurred, it would have confounded the data in the opposite direction of our primary finding; i.e., of a reduced maximal breath time hold following Indo. Each BH (Indo and Control) was performed following an identical preparatory phase (practice apneas), and ample recovery time (>90 min) was allocated between trials. We decided not to perform a second day placebo group to avoid multiple arterial catheterizations. Indomethacin reduces cerebrovascular reactivity to CO2 across the anterior and posterior circulation [9, 17] as well as cerebral O2 reactivity in the posterior circulation [9]. Although we were unable to measure blood flow in the vertebral artery, we observed an attenuated increase in absolute PCAv per unit time throughout the BH in the Control compared to the Indo condition. This finding supports the notion of reduced brainstem blood flow [9], and therefore CO2/H+ washout at the level of the central chemoreceptors.
Conclusion
We have previously demonstrated that a threshold PO2 of just below 4 kPa likely underscores the BH breakpoint in elite apnea divers [1]. The results from the present study extend these findings by suggesting that, when CBF and its reactivity is impaired, the reduced BH time is unrelated to cerebral O2 delivery per se. In turn, we suggest the moderately reduced BH time is consequent to a reduced cerebral CO2 / pH washout, and therefore increased central drive to breathe. Together, the results from this study highlight the multi-faceted nature of the BH breakpoint in ultra-elite apnea divers, and the influence of CBF in the extreme drive to breathe.
Acknowledgements
Conflict of interest statement: No authors declare any competing financial or conflict of interest in relation to the work herein. Drs. Ainslie, Dujić and Drvis were supported by a Croatian Science Foundation (IP-2014-09-1937). Mr. Bain was supported by a NSERC postgraduate award. ARB, PNA, CKW and ZD conceived the study idea; ARB drafted the manuscript; ARB, PNA, CKW, DBM, DM, PZM, ID, and ZD performed the experiments; ARB, PNA and RLH analyzed and interpreted the data. All authors edited and approved the final copy.
References
- [1].Bain A R, Dujić ž, Hoiland R L, Barak O F, Madden D, Drviš I. et al. Peripheral chemoreflex inhibition with low-dose dopamine; new insight into mechanisms of extreme apnea. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;309:R1162–R1171. doi: 10.1152/ajpregu.00271.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Parkes M J. The limits of breath holding. Sci. Am. 2012;306:74–79. doi: 10.1038/scientificamerican0412-74. [DOI] [PubMed] [Google Scholar]
- [3].Willie C K, Ainslie P N, Drviš I, MacLeod D B, Bain A R, Madden D. et al. Regulation of brain blood flow and oxygen delivery in elite breath-hold divers. J. Cereb. Blood Flow Metab. 2015;35:66–73. doi: 10.1038/jcbfm.2014.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Willie C K, MacLeod D B, Shaw A D, Smith K J, Tzeng Y C, Eves N D. et al. Regional brain blood flow in man during acute changes in arterial blood gases. J. Physiol. 2012;590:3261–3275. doi: 10.1113/jphysiol.2012.228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Willie C K, Tzeng Y C, Fisher J A, Ainslie P N. Integrative regulation of human brain blood flow. J. Physiol. 2014;592:841–859. doi: 10.1113/jphysiol.2013.268953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ainslie P N, Shaw A D, Smith K J, Willie C K, Ikeda K, Graham J. et al. Stability of cerebral metabolism and substrate availability in humans during hypoxia and hyperoxia. Clin. Sci. 2014;126:661–670. doi: 10.1042/CS20130343. [DOI] [PubMed] [Google Scholar]
- [7].Lee L Y, Milhorn H T Jr. Central ventilatory responses to O2 and CO2 at three levels of carotid chemoreceptor stimulation. Respir. Physiol. 1975;25:319–333. doi: 10.1016/0034-5687(75)90007-9. [DOI] [PubMed] [Google Scholar]
- [8].Neubauer J A, Santiago T V, Posner M A, Edelman N H. Ventral medullary pH and ventilatory responses to hyperperfusion and hypoxia. J. Appl. Physiol. 1985;58:1659–1668. doi: 10.1152/jappl.1985.58.5.1659. [DOI] [PubMed] [Google Scholar]
- [9].Hoiland R L, Ainslie P N, Wildfong K W, Smith K J, Bain A R, Willie C K. et al. Indomethacin-induced impairment of regional cerebrovascular reactivity: implications for respiratory control. J. Physiol. 2015;593:1291–1306. doi: 10.1113/jphysiol.2014.284521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xie A, Skatrud J B, Morgan B, Chenuel B, Khayat R, Reichmuth K. et al. Influence of cerebrovascular function on the hypercapnic ventilatory response in healthy humans. J. Physiol. 2006;577:319–329. doi: 10.1113/jphysiol.2006.110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Woodman R J, Playford D A, Watts G F, Cheetham C, Reed C, Taylor R R. et al. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J. Appl. Physiol. 2001;91:929–937. doi: 10.1152/jappl.2001.91.2.929. [DOI] [PubMed] [Google Scholar]
- [12].Parkes M J. Breath-holding and its breakpoint. Exp. Physiol. 2006;91:1–15. doi: 10.1113/expphysiol.2005.031625. [DOI] [PubMed] [Google Scholar]
- [13].Fowler W S. Breaking point of breath-holding. J. Appl. Physiol. 1954;6:539–545. doi: 10.1152/jappl.1954.6.9.539. [DOI] [PubMed] [Google Scholar]
- [14].Mithoefer J. Fenn W. American Physiological Society; New York USA: 1965. Breath holding; Handbook of Physiology; pp. 1011–1025. [Google Scholar]
- [15].Noble M I, Eisele J R, Frankel H L, Else W, Guz A. The role of the diaphragm in the sensation of holding the breath. Clin. Sci. 1971;41:275–283. doi: 10.1042/cs0410275. [DOI] [PubMed] [Google Scholar]
- [16].Mithoefer J, Kazemi H. Gas exchange during rebreathing. Ann. NY Acad. Sci. 1963;109:743–755. [Google Scholar]
- [17].Fan J L, Burgess K R, Thomas K N, Peebles K C, Lucas S J, Lucas R A. et al. Influence of indomethacin on the ventilatory and cerebrovascular responsiveness to hypoxia. Eur J. Appl. Physiol. 2011;111:601–610. doi: 10.1007/s00421-010-1679-0. [DOI] [PubMed] [Google Scholar]


