Abstract
The present review synthesizes lines of emerging evidence showing how several samples of children populations living in large cities around the world suffer to some degree neural, behavioral and cognitive changes associated with air pollution exposure. The breakdown of natural barriers warding against the entry of toxic particles, including the nasal, gut and lung epithelial barriers, as well as widespread breakdown of the blood-brain barrier facilitatethe passage of airborne pollutants into the body of young urban residents. Extensive neuroinflammation contributes to cell loss within the central nervous system, and likely is a crucial mechanism by which cognitive deficits may arise. Although subtle, neurocognitive effects of air pollution are substantial, apparent across all populations, and potentially clinically relevant as early evidence of evolving neurodegenerative changes. The diffuse nature of the neuroinflammation risk suggests an integrated neuroscientific approach incorporating current clinical, cognitive, neurophysiological, radiological and epidemiologic research. Neuropediatric air pollution research requires extensive multidisciplinary collaborations to accomplish the goal of protecting exposed children through multidimensional interventions having both broad impact and reach. While intervening by improving environmental quality at a global scale is imperative, we also need to devise efficient strategies on how the neurocognitive effects on local pediatric populations should be monitored.
Keywords: Air pollution, Child brain development, Children's health, Early prevention and intervention, Neurodegeneration, Neuroinflammation, Public health
Introduction
Clean air is critical for children′s health and well-being. Megacities around the world exceed the standards for air pollutants and many samples from children populations are showing an array of adverse short and long-term health outcomes, which include some of the most detrimental effects on brain development [1-3]. However, for the most part, current research and policy efforts link air pollution to respiratory and cardiovascular disease [4], and the effects on children’s central nervous system (CNS) are still not broadly recognized. As a result, wide reaching public health initiatives targeting pediatric populations are still considered premature or unwarranted. One of the goals of this review is to show that contrary to a hesitant approach, there is enough evidence supporting the perspective that air pollution brain effects on children should be one of the main public health targets linked with policies that are in the purview of the broader issue of global climate change.
In this paper, we briefly review current air pollutant standards, followed by experimental, clinical, epidemiologic and pathology studies associating air pollution exposures with children′s brain effects. This overview puts forward common denominators for the biological pathways linking air pollution to negative effects on the developing brain (Fig. 1). Then, we turn to outstanding challenges facing the development of dynamic and reciprocal intervention strategies aimed at children exposed to high levels of air pollution. Such challenges include the issues of how to establish links with the current mainstream concepts of cognition and neurodevelopment with the systemic biological and anatomical effects of air pollution, as well as the issues surrounding the formulation of strategies to study seemingly clinically healthy children exposed to air pollutants. Our goal is to provide sufficient evidence to justify the proposal of structured intervention strategies protecting exposed children from further damage due to air pollution. Our pediatric studies contrast age, gender, socio-economic status (SES), diets and physical activities matched children residents in extremes of low vs high air pollution (as defined below).
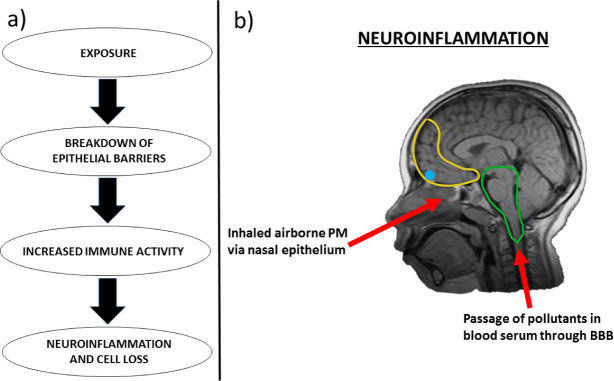
Figure 1.
A. Hypothesized progression by which air pollution may negatively impact the central nervous system, regarding the interaction of compromised epithelial barriers, normally protective against such pollutants, and a pro-inflammatory state of the body’s immune system. B. Sagittal view of magnetic resonance image of neuroinflammation in cortical structures in a male Mexico City resident aged 10 (Courtesy of Professor Lilian Calderón-Garcidueñas). Yellow = prefrontal cortex; Blue = olfactory bulb; Green = brainstem. Red arrows indicate plausible vectors by which airborne pollutants may enter the central nervous system and contribute to neuroinflammation/ cell loss. PM = particulate matter, BBB = blood-brain barrier.
Intervention strategies based on reciprocal interactions between multidisciplinary teams of researchers addressing the cognitive and developmental health effects of air pollution on children, and the public response to such initiatives, are of critical importance. Through further refining the context in which urban children and adolescents should become the primary target of multidisciplinary intervention strategies, the deleterious effects of exposure to air pollution may at the same time be more rigorously defined at a systemic developmental level, and lead to a more thorough understanding of the roles needed to protect exposed children.
The perspective guiding the present review indicates the need of a multidisciplinary approach; not only to address the issue’s complexity and challenges but also to make developmental, behavioral and clinical researchers and practitioners (in neuroscience and allied disciplines) aware of the wide spectrum of air pollution effects and the potential impact on their daily practice. Through the integration of various fields of research and expertise, informed and clear presentation of current air pollution research and dynamically structured intervention strategies, the negative impact of exposure to airborne pollutants on child development could be alleviated.
Air pollutants
Air quality is often defined by various indices reflecting the concentrations of six criteria air pollutants: particulate matter (PM), ozone (O3), carbon monoxide (CO), sulfur dioxide (SO2), nitrogen oxide (NO), and lead (Pb). These pollutants have been identified by the Environmental Protection Agency (EPA) to be hazardous to human health. The Air Quality Index (AQI) set standard values as a function of the characteristics and potential health/welfare impacts pollutants possess at varying concentrations. While the AQI values range from country to country, it is generally depicted as an open-ended scale ranging from 0 to 500+. Low values are typically color-coded green and represent minimal impact on health/welfare, whereas higher values are shown in orange-red and represent hazardous levels of pollution. Within the context of this review, exposure to PM (both fine and ultrafine), is particularly relevant. For instance, in the US alone, more than 103 million people are exposed to PM concentrations above the standards, while 123 million are exposed to ozone (a respiratory toxin). The two fractions of PM predominantly implicated in CNS effects are PM2.5 (particles with a diameter < 2.5 μm) and ultrafine PM (UFPM) (particles with a diameter < 100 nm). Outdoor PM2.5, mostly comes from tailpipe and brake emissions from mobile sources, residential fuel combustion, power plants, wildfires, oil refineries, and metal processing facilities. The primary contributors to UFPM are tailpipe emissions from mobile sources.
Indoor air pollutants, including tobacco smoke, emissions from cook stoves, mold, plasticizers, flame retardants, and pesticides also represent a major source of harmful substances. Indoor air quality in schools is a major issue, the presence of mold, poor air quality, close proximity to major highways, and contaminated playgrounds can result in serious health problems [5,6]. Moreover, there are major disparities in indoor air pollution exposures related to SES: the lower the SES, the higher indoor exposures [7].
Effects of air pollution on the developing brain
Animal models of air pollutants and brain development
There is a convergence of human, animal, and in vitro studies on the effects of air pollution on the brain. Animal models exposed to air pollutant components such as ozone, PM, diesel nanoparticles (NP), endotoxins, etc., have contributed to our understanding of the potential mechanisms acting upon the CNS. Depending on the pollutant component, doses, exposure protocol, age and gender, health status, etc., a wide, complex range of effects have been discovered including formation of free radicals and oxidative stress, dopaminergic neuronal damage, RNA, DNA damage, and identification of early hallmarks of Alzheimer’s and Parkinson’s diseases [8-13]. Notably, diesel exhaust particles (DEP), a major component of urban air pollution, have been linked in mice to neuroinflammation and the accumulation of Aß1-42, tau (linked to Alzheimer’s disease), along with α-synuclein, microglial activation and Parkinson’s disease-like pathology [8, 9]. Inhalation exposure to traffic-generated air pollutants promotes increased activity of powerful matrix metalloproteinases and degradation of tight junction proteins in the cerebral microvasculature resulting in altered brain-blood-barrier permeability and expression of neuroinflammatory markers [13].
Oxidative stress caused by low doses of ozone results in dysregulation of inflammatory responses, progressive neurodegeneration, altered brain repair in the hippocampus, and brain plasticity changes in the rat analogous to those seen in Alzheimer’s disease [14]. In rats, cigarette smoking a powerful source of oxidative stress and particles reduces the expression of pre-synaptic proteins, impairs axonal transport and produces neurodegenerative changes as those seen in Alzheimer’s disease [15]. In animal models, prenatal exposure to either one or a combination of criteria pollutants causes permanent changes in neurotransmitters and alters brain development, most commonly resulting in long-term deficits in functions associated with one or more memory systems [10,16-18].
The effects of air pollution on children’s brain
In spiteof thecomplexity of action and effects, the evidence shows that developmental detrimental effects in animals are analogous to the effects that are observed in children [19]. Children are among those most vulnerable to suffering adverse health effects due to exposure to high levels of air pollution. Due to their higher breathing rate to body size ratio, and less developed natural barriers in the lungs warding against inhaled particles children are subject to heightened sensitivity to airborne pollutants in the their environment [20]. The healthy development of natural barriers such as the blood-brain barrier, nasal, gut and lung epitheliums are of crucial importance for a child’s healthy developmental outcome. These barriers have been shown to be compromised in young urbanites exposed to air pollution [21], thus reducing the brain’s capacity to protect itself against potentially dangerous toxicants/ particles. Children also consume more air and water per unit of body size when compared to adults [22], and also spend a significant amount of time outdoors. This factor in particular elevates a child’s risk exposure to air pollution, especially when considering that not only are a child’s organs developing but also his/her sanitary behaviors respective to his/her environment. A child may not be aware of a potentially hazardous environment, and increased frequency of contact between the hands, face and mouth may elevate risk for ingesting/inhaling environmental toxicants.
When air pollutants enter the body, an innate immune response is generated. Such a response is observable by assessing blood and cerebrospinal fluid levels of small proteins, termed cytokines, such interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α). These cytokines are prominent inflammatory signaling mediators, promoting the swelling of tissue, the release of cytotoxic species by immune cells, and further pro-inflammatory signaling to additional cytokines. These actions contribute to widespread neuroinflammation in the brain, leading to the damage and diffuse loss of neural tissue in various areas of the brain. Affected structures include the prefrontal and frontal cortices and olfactory bulb (Fig. 1B), as well as midbrain structures such as the hippocampus [23]. These brain structures are critical to healthy cognitive function, and given the physiological changes observed in children as a result of chronic exposure to high levels of air pollution, present as tangible evidence for public health intervention.
The human brain contains receptors specific to Il-1 β, IL-6 and TNF-α.These proteins may also cross the blood-brain barrier, gaining ready access to developing regions of the brain. Once inside the brain these inflammatory molecules may signal to neurons and associated immune cells promoting inflammation. Exposure of elevated levels of air pollution results in high bodily concentrations of these inflammatory mediators [21], subsequently increasing their concentration in the CNS. White matter hyperintensities (WMH), or areas of demyelinated neurons resulting from reduced blood flow visible using magnetic resonance imaging (MRI), have been observed in children exposed to high levels of air pollution [24]. The presence of WMH has a negative impact on a neuron’s ability to synapse successfully, impairing its ability to communicate. WMH have been consistently associated with global cognitive deficits [25, 26]. In the context of children exposed to air pollution, WMH have been associated with inflammatory mechanisms, such as the presence TNF-α [24] in Mexico City urban adolescents. These findings suggest that exposure to air pollution may have deleterious effects on the myelination and thus function of neurons within the CNS, as well as a measureable increase in pro-inflammatory mediators contributing to neuroinflammation and cell loss. Taken together, these aspects of exposure to air pollution ultimately contribute to cognitive deficits, observable in clinically healthy children. Elevated levels of endothelin-1 (ET-1), a protein important for the constriction of blood vessels, has been associated with PM exposure [27]. Constriction of blood vessels will result in reduced blood flow to the affected area, negatively impacting the integrity of local cells/neurons (Harper et al., 1998). Disruptions in blood flow resulting from high ET-1 concentrations have been observed to negatively impact the circulatory architecture of the brain [27]. In children, these disruptions may contribute to a higher blood pressure, which has been associated with the development of respiratory disease [28], as well potentially damaging developing brain regions due to restricted blood flow and thus, less oxygen. Another finding in children exposed to high levels of air pollution is the presence of proteins characteristic of Alzheimer’s disease pathology. Two critical proteins in development of Alzheimier’s disease include hyperphosphorylated tau protein and the accumulation of Aβ1-42 plaques in and around neurons [29]. Neuropathology studies in children with accidental deaths and having been exposed to air pollution showed both hyperphosphorylation of tau protein and diffuse Aβ plaques in the frontal cortex of brains, compared to 0% in controls [30]. Of utmost importance for this review was the finding that children carrying the apolipoprotein E gene (APOE) allele ∊4 (well-known genetic risk factor for Alzheimer’s disease) displayed a greater number of these two marker proteins when compared to the more common APOE allele ∊3 carriers [30]. This finding suggests that genetic factors could make a significant portion of children exposed to air pollution more prone to developing Alzheimer’s disease later in life. The presence of such plaques also indicate an acceleration of Alzheimer’s disease pathology, and contribute to increased neuroinflammation and loss of communication between neurons. In Alzheimer’s disease patients, the presence and amount of both tau tangles and Aβ are a marker of disease progression, and the subsequent decline in cognitive function that is characteristic of the disease. The abnormal levels and diffuse presence of these protein complexes in clinically healthy children chronically exposed to airborne pollutants are disturbing at the very least, and may have a significant impact on cognitive outcome and neurodevelopment given their inherent and well-documented pathological nature.
Chronic damage to the lungs in response to prolonged inhaling of large amounts of PM is inevitable, and this is indeed the case with children [31]. This has been reported in Mexico City Metropolitan Area (MCMA) children, with boys being more affected than girls, likely due to longer daily outdoor activities and thus longer time of exposure [32]. Due to importance of physical activity in child’s development and the benefits of associated outdoor spaces [33], elevated levels of air pollution present a direct challenge to maintaining both cardiovascular and healthy neurodevelopment of children, by allowing them to play outside and engage in physical activities otherwise beneficial for their health and development [34].
Clinically healthy children from MCMA selected by stringent criteria including the absence of known risk factors for cognitive or neurological deficits, exhibited structural, neurophysiological and cognitive detrimental effects compared to low pollution exposed children matched for SES, gender, age and mother’s IQ [1, 35]. The cognitive deficits in MCMA children were associated with MRI volumetric alterations in their right parietal and bilateral temporal areas [24]. Dynamic changes of inflammatory mediators influence children’s CNS structural and volumetric MRI responses and cognitive correlates resulting from environmental pollution exposures. MCMA children performed more poorly across a variety of cognitive tests, compared to control children [1, 24, 35]. This is indicative of high air pollution contributing to a negative environment for healthy brain development and cognitive improvement.
Recent epidemiologic studies in cities across the world provide convergent confirmatory evidence of the link between exposure to similar air pollution components, and neurobehavioral outcomes similar to those observed in MCMA children [36]. In particular, vehicular emissions which are one important source of particulate pollution, have been associated with higher prevalence of frontal executive function deficits of preschool (2 to 5 year olds) and school aged children (6 to 14 year olds) in India [37], Boston [38], Cincinnati [39], New York City [40, 41], China [42], Barcelona [43] and Japan [44].
The reviewed evidence strongly suggests that air pollution exposed children experience a chronic intense state of environmental stress and exhibit an early brain imbalance in genes involved in inflammation, immune responses to their environment, cell integrity and neural communication [23, 24, 30]. Neuroinflammation, changes in endothelial barriers, alterations in blood vessel arrangement and the widespread blood-brain barrier breakdown contribute to cognitive impairment and pathogenesis and pathophysiology of neurodegenerative states [45, 46]. By its very nature, a mechanistic pathway (Fig. 1A) involving inflammation, the breakdown of the epithelial and the blood-brain barriers, and restricted blood flow has the potential to negatively impact cortical structures and the subsequent child’s development of brain regions central to behavior and cognitive performance. This collection of findings lies at the core of the pathology in exposed children, and structural changes are significant in areas of the prefrontal cortex, but have also been observed throughout the brain, including the brainstem (Fig. 1B)[35]. The blood-brain barrier in children has been observed to be more permeable during development than later in life [21], increasing the likelihood of ingested and inhaled pollutants entering the brain, causing inflammation of tissue and further damaging vulnerable brain regions. Taken together, the collected evidence gives weight to the importance of structured intervention strategies for children chronically exposed to air pollution. Children are amongst those individuals possessing the highest level of risk exposure, have underdeveloped natural defense mechanisms and consistently display a disturbing variety of physiological alterations associated with environmental imbalance with the potential to negatively affect neural and thus behavioral development.
From data to action: future directions
Air pollution effects on the developing brain may vary along a continuum from minor, subtle subclinical deficits in cognitive functioning to significant cognitive deficits that are identified readily by parents and/or teachers. The detrimental effects may also worsen with the age of the child, thus selected neurocognitive tools/screening ought to be useful for longitudinal studies, across educational backgrounds and expecting overlaps in the functional areas and tests affected. Complex cognitive responses that may be affected include: attention and short-term memory, information processing speed and executive function, verbal abstraction, and visuospatial and motor skills. Further refining the cognitive processes, as well as brain structures involved in/affected by exposure to air pollution would serve to broaden the current understanding of environment-mediated neurodevelopmental deficits. The inclusion of longitudinal and follow-ups to clinical studies is also of crucial importance.
Regarding the role of a mechanistic pathway (Fig. 1), one gap in our knowledge concerns the understanding of multiple concurrent underlying systemic mechanisms by which several observed developmental health effects and disease processes arise as a result of exposure to air pollution. In this context, particularly relevant is current evidence that air pollutants have an influence at the epigenetic levels [47]. The most recent and comprehensive reviews show that the majority of the findings to date reveal that polycyclic aromatic hydrocarbon (PAH) and PM2.5 may have modest effects on methylation levels of certain CpG (cytosine being 5’ to the guanine base) dinucleotide sites within candidate genes linked with cancer, cardiovascular and respiratory diseases [48, 49]. However, the data are too scarce and the methods too heterogeneous to afford conclusions with regard to effects directly related to the CNS and neuroinflammation. The latter aspects, in particular, would include investigation into complex brain-immune interactions resulting from exposure to air pollution, as well as genetic/epigenetic studies looking at changes in the expression of inflammatory genes. For instance, a very recent cross-sectional study [50] has indicated that exposure to short-term single (i.e., sulfate, black carbon) or combined air pollution components is associated with epigenetic alterations of mitogen-activated protein kinase (MAPK) pathway genes. Such epigenetic alterations are still not well understood since their effects on the biological pathways are too complex to be easily predicted on the basis of the current conceptual models. Future studies need to be designed so that this limitation can be overcome.
Related to the domain of epigenetics (see [51]), an increasing amount of studies suggests links between maternal, prenatal and early air pollution exposure, neuroinflammation and autism spectrum disorder (ASD) (for example, [52-54]). Findings have some degree of consistency, with associations seen with different components, including hazardous air toxics, ozone, particulate, and traffic-related pollution. However, very few studies are immune from residual confounds linked with multiple exposure, socioeconomic status and residential area. Among the exceptions are, notably, two large-scale US studies [55, 56] which found a specific association with air pollution exposure during the 3rd, but not the 1st, trimester, when both trimesters were modeled simultaneously and many key confounds were accounted for.
Furthermore, the generalizability of the available findings represents a further challenge given the variability of the diagnostic criteria across studies. For example, a study based on data from four large European population-based birth/child cohorts [57] squarely contradicts the US studies, by finding that prenatal exposure to NO2 and PM was not associated with autistic traits in children from 4 to 10 years of age. Analogously to the broader epigenetic effects discussed earlier, the available evidence requires complex models to decode the critical component(s) and causal pathway(s). The future generation of studies will need to address not only methodological discrepancies but also theoretical challenges. As a step towards this objectives, Weisskopf, Kioumourtzoglou, and Roberts [58] have illustrated the usefulness of directed acyclic graphs analysis to test models that differentiate the role of time-specific windows of susceptibility, with significant impact, from time-invariant confounds that have no effects. The latter is a good example of the type of work that will be necessary to advance knowledge about the nuanced, multi-level relationships between air pollution exposure, neuroinflammation mechanisms and neurodevelopmental processes, which although progressively gaining more clarity at the moment are at the level of sparse and isolated associations in the literature.
While our knowledge-base and understanding continue to grow, intervention strategies, such as the neuropsychological screening described above, being targeted at geographically defined populations should seek to not only gain an accurate assessment of current health and risk factors present, but also facilitate communication between health professionals, affected children/parents/ families, teachers and researchers in a reciprocal fashion. Further research inspired by the effectiveness of such programs, the usefulness of the information garnered by them and the logistical limitations of such tools should be prioritized. These research efforts would allow for the interplay between developmental researchers and the environment they are investigating, leading to context-dependent research investigating context-specific factors affecting populations at risk of high exposure to air pollution. Further refining definitions of high vs. low pollution, and establishing reliable proxies for air pollution exposure may render these applications more effective and clarify research regarding concentrations of specific pollutants.
The widespread nature of inflammation within the brain and the neuronal changes observed in exposed children suggests not to rely on a single study or measure but rather to employ a weight of evidence approach incorporating current clinical, neurophysiological, radiological and epidemiological research regarding exposure to single or mixtures of pollutants. Changes in immune system activity play a key role in the identification of children with structural changes and cognitive responses to their lifelong pollutant exposures [35, 36] and since inflammation of the brain/vascular damage/loss of neurons go hand in hand [23, 24], definition of biomarkers associated with immunological and cellular changes in the CNS establishing an observable relationship between brain development and behavioral/psychological outcomes in children are urgently needed. Identification of such biomarkers would allow for screening tools to non-invasively and accurately assess the presence of damage resulting from their exposure to air pollution, and provide health practitioners, educational professionals and families with empirical feedback regarding the need for intervention or therapy. Such a practice has its roots in preventative medical approaches [59], and would aid in further directing some of the applied developmental science research specializing on social justice [60] towards the issues of children’s outcomes and health behaviors in polluted environments.
Acknowledgment
We are indebted to Lilian Calderón-Garcidueñas for her most generous help with this paper.
References
- Calderón-Garcidueñas L, Mora-Tiscareño A, Ontiveros E, Gomez-Garza G, Barragán-Mejía G, Broadway J. et al. Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain Cogn. 2008;68:117–127. doi: 10.1016/j.bandc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Guxens M, Sunyer J. A review of epidemiological studies on neuropsychological effects of air pollution. Swiss Med. Wkly. 2012;141:w13322. doi: 10.57187/smw.2012.13322. [DOI] [PubMed] [Google Scholar]
- Suades-González E, Gascon M, Guxens M, Sunyer J. Air pollution and neuropsychological development: a review of the latest evidence. Endocrinology. 2015;156:3473–3482. doi: 10.1210/en.2015-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV. et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Everett-Jones S, Smith AM, Wheeler LS, McManus T. School policies and practices that improve indoor air quality. J. Sch. Health. 2010;80:280–286. doi: 10.1111/j.1746-1561.2010.00502.x. [DOI] [PubMed] [Google Scholar]
- Kingsley SL, Eliot MN, Carlson L, Finn J, Macintosh DL, Suh HH. et al. Proximity of US schools to major roadways: a nationwide assessment. J. Expo. Sci. Environ. Epidemiol. 2014;24:253–259. doi: 10.1038/jes.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamkiewicz G, Zota AR, Fabian MP, Chahine T, Julien R, Spengler JD. et al. Moving environmental justice indoors: understanding structural influences on residential exposure patterns in low-income communities. Am. J. Public Health. 2011;101(Suppl. 1):S238–S245. doi: 10.2105/AJPH.2011.300119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque S, Taetzsch T, Lull ME, Kodavanti U, Stadler K, Wagner A. et al. Diesel exhaust activates and primes microglia: air pollution, neuroinflammation and regulation of dopaminergic neurotoxicity. Environ. Health Perspect. 2011;119:1149–1155. doi: 10.1289/ehp.1002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque S, Taetzsch T, Lull ME, Johnson JA, McGraw C, Block ML. The role of MAC1 in diesel exhaust particle-induced microglial activation and loss of dopaminergic neuron function. J. Neurochem. 2013;125:756–765. doi: 10.1111/jnc.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Xu X, Weil ZM, Chen G, Sun Q, Rajagopalan S. et al. Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol. Psychiatry. 2011;16:987–995. doi: 10.1038/mp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Zhu N, Guo Z, Li G K, Chen C, Sang N. et al. Particulate matter (PM10) exposure induces endothelial dysfunction and inflammation in rat brain. J. Hazard. Mater. 2012;213/214:28–37. doi: 10.1016/j.jhazmat.2012.01.034. [DOI] [PubMed] [Google Scholar]
- Brun E, Carrière M, Mabondzo A. In vitro evidence of dysregulation of blood-brain barrier function after acute and repeated/long term exposure to TiO2 nanoparticles. Biomaterials. 2012;33:886–896. doi: 10.1016/j.biomaterials.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Oppenheim HA, Lucero J, Guyot AC, Herbert LM, McDonald JD, Mabondzo A. et al. Exposure to vehicle emissions results in altered blood brain barrier permeability and expression of matrix metalloproteinases and tight junction proteins in mice, Part. Fibre Toxicol. 2013;10:62. doi: 10.1186/1743-8977-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-Arancibia S, Guevara-Guzmán R, López-Vidal Y, Rodríguez-Martínez E, Zanardo-Gomes M, Angoa-Pérez M. et al. Oxidative stress caused by ozone exposure induces loss of brain repair in the hippocampus of adult rats. Toxicol.Sci. 2010;113:187–197. doi: 10.1093/toxsci/kfp252. [DOI] [PubMed] [Google Scholar]
- Ho YS, Yang X, Yeung SC, Chiu K, Lau CF, Tsang AW. et al. Cigarette smoking accelerated brain aging and induced pre-Alzheimer-like neuropathology in rats. PLoS One. 2012;7:e36752. doi: 10.1371/journal.pone.0036752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Mizuo K, Shinkai Y, Oshio S, Takeda K. Prenatal exposure to titanium dioxide nanoparticles increases dopamine levels in the prefrontal cortex and neostratium of mice. J.Toxicol. Sci. 2010;35:749–756. doi: 10.2131/jts.35.749. [DOI] [PubMed] [Google Scholar]
- Umezawa M, Tainaka H, Kawashima N, Shimizu M, Takeda K. Effect of fetal exposure to titanium dioxide nanoparticle on brain development-brain region information. J.Toxicol. Sci. 2012;37:1247–1252. doi: 10.2131/jts.37.1247. [DOI] [PubMed] [Google Scholar]
- Schröder N, Silva-Figueiredo L, Martins de Lima MN. Role of brain iron accumulation in cognitive dysfunction: evidence from animal models and human studies. J. Alzheimers Dis. 2013;34:797–812. doi: 10.3233/JAD-121996. [DOI] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque P. Neurotoxicants are in the air: convergence of human, animal, and in vitro studies on the effects of air pollution on the brain. Bio. Med. Res. Int. 2014:736385. doi: 10.1155/2014/736385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanos JK. Children’s health and vulnerability in outdoor microclimates: a comprehensive review. Environ. Int. 2015;76:1–15. doi: 10.1016/j.envint.2014.11.016. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Vojdani A, Blaurock-Busch E, Busch Y, Friedle A, Franco-Lira M. et al. Air pollution and children: neural and tight junction antibodies and combustion metals, the role of barrier breakdown and brain immunity in neurodegeneration. J. Alzheimers Dis. 2015;43:1039–1058. doi: 10.3233/JAD-141365. [DOI] [PubMed] [Google Scholar]
- Sampson N. Environmental justice at school: understanding research, policy, and practice to improve our children’s health. J. Sch. Health. 2012;82:246–252. doi: 10.1111/j.1746-1561.2012.00694.x. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Franco-Lira M, Mora-Tiscareño A, Medina-Cortina H, Torres-Jardón R, Kavanaugh M. Early Alzheimer’s and Parkinson’s disease pathology in urban children: friend versus foe responses - it is time to face the evidence. Biomed. Res. Int. 2013:161687. doi: 10.1155/2013/161687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Mora-Tiscareño A, Styner M, Gómez-Garza G, Zhu H, Torres-Jardón R. et al. White matter hyperintensities, systemic inflammation, brain growth, and cognitive functions in children exposed to air pollution. J. Alzheimers Dis. 2012;31:183–191. doi: 10.3233/JAD-2012-120610. [DOI] [PubMed] [Google Scholar]
- Koppenborg RP, Nederkoorn PJ, Geerlings MI, van den Berg E. Presence and progression of white matter hyperintensities and cognition: a meta-analysis. Neurology. 2014;82:2127–2138. doi: 10.1212/WNL.0000000000000505. [DOI] [PubMed] [Google Scholar]
- Casado Naranjo I, Portilla Cuenca JC, Duque De San Juan G, García AF, Sevilla RR, Serrano Cabrera A. et al. Association of vascular factors and amnestic mild cognitive impairment: a comprehensive approach. J. Alzheimers Dis. 2015;44:695–704. doi: 10.3233/JAD-141770. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Vincent R, Mora-Tiscareño A, Franco-Lira M, Henríquez-Roldán C, Barragán-Mejía G. et al. Elevated plasma endothelin-1 and pulmonary arterial pressure in children exposed to air pollution, Environ. Health Perspect. 2007;115:1248–1253. doi: 10.1289/ehp.9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobyns EL, Eells PL, Griebel JL, Abman SH. Elevated plasma endothelin-1 and cytokine levels in children with severe acute respiratory distress syndrome. J. Pediatr. 1999;135:246–249. doi: 10.1016/s0022-3476(99)70029-6. [DOI] [PubMed] [Google Scholar]
- Braak H, DelTredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011;121:171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Kavanaugh M, Block M, D’Angiulli A, Delgado-Chávez R, Torres-Jardón R. et al. Neuroinflammation, Alzheimer’s disease-associated pathology and down regulation of the prion-related protein in air pollution exposed children and young adults. J. Alzhemers Dis. 2012;28:93–107. doi: 10.3233/JAD-2011-110722. [DOI] [PubMed] [Google Scholar]
- Lee BJ, Kim B, Lee K. Air pollution exposure and cardiovascular disease. Toxicol. Res. 2014;30:71–75. doi: 10.5487/TR.2014.30.2.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal-Calderón A, Acuña H, Villarreal-Calderón J, Garduño M, Henríquez-Roldán CF, Calderón-Garcidueñas L. et al. Assessment of physical education time and after-school outdoor time in elementary and middle school students in south Mexico City: the dilemma between physical fitness and the adverse health effects of outdoor pollutant exposure. Arch. Environ. Health. 2002;57:450–460. doi: 10.1080/00039890209601437. [DOI] [PubMed] [Google Scholar]
- Jerrett M, Almanza E, Davies M, Wolch J, Dunton G, Spruitj-Metz D. et al. Smart growth community design and physical activity in children. Am. J. Prev. Med. 2013;45:386–392. doi: 10.1016/j.amepre.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Ruangdej K, Suwanwaiphatthana W, Turner-Henson A. Outdoor air pollution and children’s health. Pediatr. Nurs. 2010;36:25–32. [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, D’Angiulli A, Kulesza RJ, Torres-Jardón R, Romero L, Keefe S. et al. Air pollution is associated with brainstem auditory nuclei pathology and delayed brainstem auditory evoked potentials. Int. J. Dev. Neurosci. 2011;29:365–75. doi: 10.1016/j.ijdevneu.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Engle R, Mora-Tiscareño A, Styner M, Gómez-Garza G, Zhu H. et al. Exposure to severe urban air pollution influences cognitive outcomes, brain volume and systemic inflammation in clinically healthy children. Brain Cogn. 2011;77:345–355. doi: 10.1016/j.bandc.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Siddique S, Banerjee M, Ray MR, Lahiri T. Attention-deficit hyperactivity disorder in children chronically exposed to high level of vehicular pollution. Eur. J. Pediatr. 2011;170:923–929. doi: 10.1007/s00431-010-1379-0. [DOI] [PubMed] [Google Scholar]
- Chiu YH, Bellinger DC, Coull BA, Anderson S, Barber R, Wright RO. et al. Associations between traffic-related black carbon exposure and attention in a prospective birth cohort of urban children. Environ. Health Perspect. 2013;121:859–864. doi: 10.1289/ehp.1205940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman NC, Ryan P, Lemasters G, Levin L, Bernstein D, Hershey GK. et al. Traffic-related air pollution exposure in the first year of life and behavioral scores at 7 years of age. Environ. Health Perspect. 2013;121:731–736. doi: 10.1289/ehp.1205555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Tang D, Wang S, Vishnevetsky J, Zhang B, Diaz D. et al. Prenatal polycyclic aromatic hydrocarbon (PAH) exposure and child behavior at age 6-7 years. Environ. Health Perspect. 2012;120:921–926. doi: 10.1289/ehp.1104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Chang HW, Tang D, Roen EL, Herbstman J, Margolis A. et al. Early-life exposure to polycyclic aromatic hydrocarbons and ADHD behavior problems. PloS One. 2014;9:e1 11670. doi: 10.1371/journal.pone.0111670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhang J, Zeng X, Zeng Y, Wang S, Chen S. Association of traffic-related air pollution with children’s neurobehavioral functions in Quanzhou, China. Environ. Health Perspect. 2009;117:1612–1628. doi: 10.1289/ehp.0800023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forns J, Dadvand P, Foraster M, Alvarez-Pedrerol M, Rivas I, López-Vicente M. et al. Traffic-related air pollution, noise at school, and behavioral problems in Barcelona school children: a cross-sectional study, 2016. Environ. Health Perspect. doi: 10.1289/ehp.1409449. [Epub ahead of Print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorifuji T, Kashima S, HigaDiez M, Kado Y, Sanada S. Prenatal exposure to traffic-related air pollution and child behavioral development milestone delays in Japan. Epidemiology. 2016;27:57–65. doi: 10.1097/EDE.0000000000000361. [DOI] [PubMed] [Google Scholar]
- Paul D, Cowan AE, Ge S, Pachter JS. Novel 3D analysis of claudin-5 reveals significant endothelial heterogeneity among CNS microvessels. Microvasc. Res. 2013;86:1–10. doi: 10.1016/j.mvr.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roher AE, Debbins JP, Malek-Ahmadi M, Chen K, Pipe JG, Maze S. et al. Cerebral blood flow in Alzheimer’s disease, Vasc. Health Risk Manag. 2012;8:599–611. doi: 10.2147/VHRM.S34874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarelli A, Bollati V. Epigenetics and environmental chemicals. Curr. Opin. Pediatr. 2009;21:243–251. doi: 10.1097/mop.0b013e32832925cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton CV, Marutani AN. Air pollution and epigenetics: recent findings. pCurr. Environ. Health Rep. 2014;1:35–45. [Google Scholar]
- Ruiz-Hernandez A, Kuo CC, Rentero-Garrido P, Tang WY, Redon J, Ordovas JM. et al. Environmental chemicals and DNA methylation in adults: a systematic review of the epidemiologic evidence. Clin. Epigenetics. 2015;7:55. doi: 10.1186/s13148-015-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona JJ, Sofer T, Hutchinson J, Cantone L, Coull B, Maity A. et al. Short-term airborne particulate matter exposure alters the epigenetic landscape of human genes associated with the mitogen-activated protein kinase network: a cross-sectional study. Environ. Health. 2014;13:94. doi: 10.1186/1476-069X-13-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman S, Somogyi E, Coulon N, Kermarrec S, Cohen D, Bronsard G. et al. Genex Environment interactions in autism spectrum disorders: Role of epigenetic mechanisms. Front. Psychiatr. 2014;5 doi: 10.3389/fpsyt.2014.00053. http://dx.doi.org/10.3389/fpsyt.2014.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol D A, Genuis S J, Frye R E. Environmental toxicants and autism spectrum disorders: a systematic review. Trans. Psychiatry. 2014;4(2):e360. doi: 10.1038/tp.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Schmidt R J, Hertz-Picciotto I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int. J. Epi. 2014;43:443–464. doi: 10.1093/ije/dyt282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk H E, Kerin T, Lurmann F, Hertz-Picciotto I, McConnell R, Campbell D B. Autism spectrum disorder: interaction of air pollution with the MET receptor tyrosine kinase gene. Epidemiol. 2014;25:44–47. doi: 10.1097/EDE.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz R, Roberts AL, Lyall K, Hart JE, Just AC, Laden F. et al. Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: a nested case-control analysis within the Nurses’ Health Study II Cohort. Env. Health Perspect. 2015;123(3):264–270. doi: 10.1289/ehp.1408133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkbrenner AE, Windham GC, Serre ML, Akita Y, Wang X, Hoffman K, Thayer BP, Daniels JL. Particulate Matter Exposure, Prenatal and Postnatal Windows of Susceptibility, and Autism Spectrum Disorders. Epidemiol. 2015 Jan;26(1):30–42. doi: 10.1097/EDE.0000000000000173. [DOI] [PubMed] [Google Scholar]
- Guxens M, Ghassabian A, Gong T, Garcia-Esteban R, Porta D, Giorgis-Allemand L. et al. Air pollution exposure during pregnancy and childhood autistic traits in four European population-based cohort studies: The ESCAPE Project. Env. Health Perspect. 2015;124(1):133–140. doi: 10.1289/ehp.1408483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Kioumourtzoglou M-A, Roberts AL. Air pollution and autism spectrum disorders: causal or confounded? Curr. Environ. Health Rep. 2015;2:430–439. doi: 10.1007/s40572-015-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell M E, Boat T, Warner K. Preventing Mental, Emotional, and Behavioral Disorders Among Young People: Progress and Possibilities. The National Academies Press; Washington DC: 2009. [PubMed] [Google Scholar]
- Lerner R M, Fisher CB, Weinberg R A. Toward a Science for and of the People: Promoting Civil Society through the Application of Developmental science. Child Dev. 2000;71:11–20. doi: 10.1111/1467-8624.00113. [DOI] [PubMed] [Google Scholar]