Abstract
Purpose
We conducted a cohort study to clarify this relationship between asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome (ACOS) and pulmonary embolism (PE).
Methods
From the National Health Insurance Research Database of Taiwan, we identified patients who had a diagnosis of asthma and a diagnosis of COPD (defined as ACOS) and concurrent treatment between January 1999 and December 2009 (ACOS cohort: n = 14,150; non-ACOS cohort: n = 55,876). Cox proportional hazards regression analysis was performed to determine the adjusted hazard ratios (aHRs) for PE of the ACOS cohort compared with the non-ACOS cohort.
Results
Comparing the ACOS cohort with the non-ACOS cohort, the aHR of PE was 2.08 (95% confidence intervals [CIs]: 1.56–2.76). The risk of PE was higher in ACOS cohort than non-ACOS cohort, regardless of age, sex, comorbidity, inhaled corticosteroids (ICSs) and oral steroids (OSs) used. For ages ranging from 20 to 65 years, the aHR of PE was 2.53 (95% CI: 1.44–4.44) in the ACOS cohort. ACOS patients using ICSs (aHR: 1.97, 95% CI: 1.29–3.01) or OSs (aHR: 1.97, 95% CI: 1.46–2.65), the risk of PE was higher than in the non-ACOS cohort. The risk of PE increased with the number of outpatient visits and hospitalizations necessitated, ranging from 2.32 (95% CI: 1.54–3.52) in patients having 3–9 visits to 4.20 (95% CI: 2.74–6.44) for those having >9 visits.
Conclusions
ACOS is associated with increased risk of PE, particularly patients with a high frequency of AE—even in young adults or people without comorbidities.
Introduction
Pulmonary embolism (PE) is a life-threatening disease [1], the incidence of which has been increasing according to a recent report [2]. High mortality is associated with a delayed diagnosis of acute PE [3]. Recent studies have indicated that the predisposing factors for PE include numerous underlying diseases such as obstructive airway disease (causing asthma [4] and chronic obstructive pulmonary disease [COPD] [5]), atherosclerosis [6] (causing hypertension, diabetes [7], and stroke), heart failure [8] and cancer [9].
The triad of abnormalities in hypercoagulability (blood composition), endothelial injury (vessel wall function), and stasis (blood flow) has been attributed to the shear stress involved in venous thrombosis or thromboembolism [10]. In addition, the airways represent a body compartment in which coagulation self-initiates [11]. hypoxeamia -induced vascular endothelial dysfunction [12] reduces the activation of endothelial nitric oxide in patients with obstructive airway disorders [13]. Therefore, the levels of endogenous thrombin potential (ETP), plasminogen activator inhibitor type 1, and von Willebrand factor (vWF) increase [14]; primary pulmonary vascular remodelling [12] and increased vasoconstrictor tone may contribute to in situ thrombosis [4,5]. This combination of factors in acute exacerbation (AE) in asthma and COPD primarily results in PE without deep venous thrombosis [15,16]; this supports the notion that asthma and COPD with hypoxeamia are critical factors of PE [17].
Asthma-COPD overlap syndrome (ACOS) is a disorder comprising both asthma and COPD. The infiltration of neutrophil and eosinophil [18] in the airway caused by ACOS [19] are similar to the pathophysiology change of the airway in severe asthma [20] and COPD [21,22]. According to Ghebre et al., neutrophil plays a key role in ACOS [22]. Moreover, the level of neutrophil cells correlates with the levels of ETP and vWF, which play key roles in severe asthma with PE [14]. No previous report has mentioned the association between PE and ACOS. We hypothesized that ACOS is associated with the risk of PE; accordingly, we conducted a cohort study to evaluate this association.
Methods
Data Source
The study population cohort was established using data from the Longitudinal Health Insurance Database 2000 (LHID 2000), a subset of the National Health Insurance Research Database (NHIRD) of Taiwan. The NHIRD is a database of all registry and claims data from the National Health Insurance (NHI) program, including data on patient demographic characteristics, diagnoses, and prescription claims for ambulatory and inpatient care. The NHI program is a mandatory single-payer national health insurance scheme that has provided comprehensive medical care coverage in Taiwan since 1995, covering more than 99% of all residents in Taiwan. The LHID 2000 data set consists of claims data collected from one million people randomly selected from the total population of insurants in the 1996–2011 period. The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) is used to record diseases in the database. According to NHIRD procedures, the original identification numbers for insurant files were replaced with anonymous numbers for privacy protection.
Ethics Statement
The NHIRD encrypts patient personal information to protect privacy and provides researchers with anonymous identification numbers associated with relevant claims information, including sex, date of birth, medical services received, and prescriptions. Therefore, patient consent is not required to access the NHIRD. This study was approved to fulfill the condition for exemption by the Institutional Review Board (IRB) of China Medical University (CMUH104-REC2-115). The IRB also specifically waived the consent requirement.
Data Availability Statement
All data and related metadata were deposited in an appropriate public repository. The data on the study population that were obtained from the NHIRD (http://nhird.nhri.org.tw/en/index.html) are maintained in the NHIRD (http://nhird.nhri.org.tw/). The NHRI is a nonprofit foundation established by the government. Only citizens of the Republic of China who fulfill the requirements of conducting research projects are eligible to apply for the NHIRD. The use of NHIRD is limited to research purposes only. Applicants must follow the Computer-Processed Personal Data Protection Law (http://www.winklerpartners.com/?p=987) and related regulations of National Health Insurance Administration and NHRI, and an agreement must be signed by the applicant and his/her supervisor upon application submission. All applications are reviewed for approval of data release.
Study Population
Employing a retrospective population-based cohort study design, we investigated the association between asthma–chronic obstructive pulmonary disease overlap syndrome (ACOS) and PE outcome. ACOS patients were defined as COPD (ICD-9-CM Codes: 491, 492, and 496) patients aged >20 years with concurrent [23] physician-diagnosed asthma (ICD-9-CM Code: 493) [24,25,26]. The index date represents the date of asthma diagnosis. We collected data for 14,150 ACOS patients who received treatment between 1999 and 2009 to establish the ACOS cohort. For each ACOS patient, four comparison subjects were matched for sex, age (5-year intervals), and index year; these subjects comprised patients not conforming to the diagnosis condition of ACOS (non-ACOS cohort; n = 55,876). Diagnosis of PE (ICD-9-CM: 415.1) during the follow-up period represented the outcome variables. Subjects with a history of PE at baseline or those aged<20 years were excluded.
Follow-up person-years were calculated for each subject from index date until December 31, 2011, through to the date of diagnosis of PE or withdrawal from the insurance system. Preexisting comorbidities for all subjects comprised atrial fibrillation (ICD-9-CM: 427.31), hypertension (ICD-9-CM: 401–405), diabetes (ICD-9-CM: 250), hyperlipidemia (ICD-9-CM: 272), stroke (ICD-9-CM: 430–438), heart failure (ICD-9-CM: 428), lower limb fracture (ICD-9-CM: 820–823), and cancer (ICD-9-CM: 140–208). Use of inhaled corticosteroids (ICSs) or oral steroids (OSs) [27] was measured until the study end date. The ICSs were included beclometasone (ATC code: R03BA01), budesonide (ATC code: R03BA02), fluticasone (ATC code: R03BA05), ciclesonide (ATC code: R03BA08), and formoterol and budesonide (ATC code: R03AK07). The betamethasone (ATC code: H02AB01); dexamethasone (ATC code: H02AB02); methylprednisolone (ATC code: H02AB04); paramethasone (ATC code: H02AB05); prednisolone (ATC code: H02AB06); triamcinolone (ATC code: H02AB08); hydrocortisone (ATC code: H02AB09); cortisone (ATC code: H02AB10) were categories of OSs. Drug users were defined as patients who used either drug for over 30 consecutive days [22–24].
Statistical Analyses
The distribution of categorical variables (e.g., sex, history of comorbidity, and drug used) were employed in analyzing the difference between the ACOS and non-ACOS cohorts through a chi-square test. The Student’s t test was employed to estimate the continuous age difference in both cohorts and to present the mean and standard deviation for the age variable. The incidence rates of PE (per 1,000 person-years) were calculated in the ACOS and non-ACOS cohorts for potential risk factors such as sex, age group (20–65 or ≥65 years old), comorbidity, and drug use. The incidence rate ratios (IRRs) and 95% CI of PE in the ACOS and non-ACOS cohorts were analyzed using a Poisson regression model. After adjustment for age, sex, comorbidity, ICSs used, and OSs used, the adjusted hazard ratios (aHRs) and 95% confidence intervals (95% CIs) of PE in the ACOS and non-ACOS cohorts were determined using Cox proportional hazard regression models. Additionally, the association between the number of outpatient visits and hospitalizations (per year) and that of PE events were estimated for the ACOS and non-ACOS cohorts by using a multivariable Cox proportional hazards model. The cumulative incidence curves for PE in both cohorts were plotted using the Kaplan–Meier method, and the curve difference was tested through a log-rank test. SAS Version 9.4 (SAS Institute, Cary, NC, USA) was employed to manage the data and for the statistical analysis. The significance level was set at p < 0.05 for two-sided testing.
Results
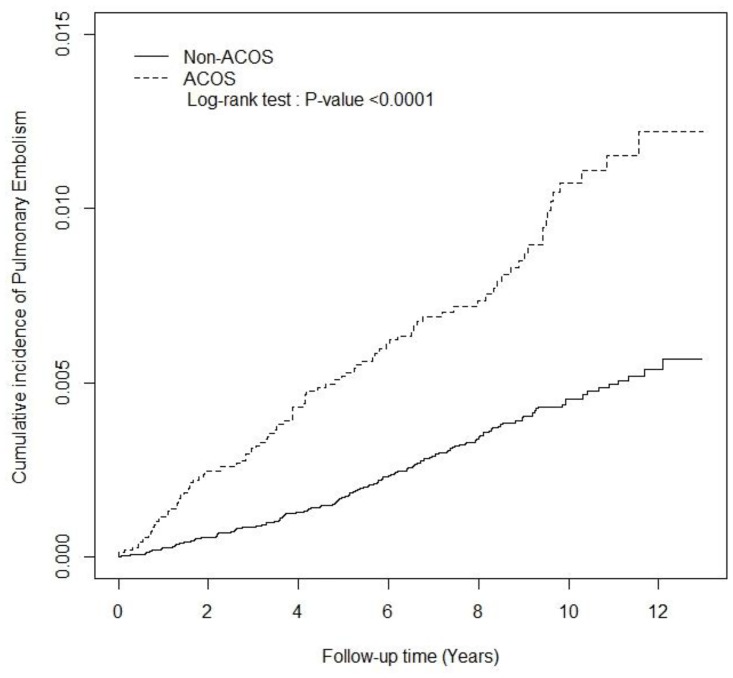
Table 1 shows the distribution of demographic risk factors among the ACOS and non-ACOS cohorts at baseline (Table 1). There were no significant differences based on the distributions of sex and age (mean of age: 65 years old) between the cohorts. The ACOS patients had a higher prevalence of preexisting comorbidities than did the non-ACOS patients (chi square test, p < 0.0001), excluding cancer. The percentages of ICSs (27.2%) and OSs (80.2%) users were higher in ACOS cohort than in the non-ACOS cohort. During the 13-year follow-up period, the ACOS cohort had a higher cumulative incidence of PE than did the non-ACOS cohort (log-rank test, p < 0.0001; Fig 1).
Table 1. Comparison of demographics and history of comorbidity between ACOS and non-ACOS cohorts.
| ACOS | |||||
|---|---|---|---|---|---|
| No (N = 55876) | Yes (N = 14150) | ||||
| Variable | n | % | n | % | p-value |
| Sex | 0.27 | ||||
| Female | 23621 | 42.3 | 5909 | 41.8 | |
| Male | 32255 | 57.7 | 8241 | 58.2 | |
| Age, year | 0.15 | ||||
| 20–45 | 5688 | 10.2 | 1422 | 10.0 | |
| 45–75 | 35513 | 63.6 | 8898 | 62.9 | |
| ≥75 | 14675 | 26.3 | 3830 | 27.1 | |
| Mean (SD)# | 64.9 | (14.2) | 65.2 | (14.3) | 0.01 |
| Comorbidity | |||||
| Atrial fibrillation | 1136 | 2.03 | 514 | 3.63 | <.0001 |
| Hypertension | 26831 | 48.0 | 8693 | 61.4 | <.0001 |
| Diabetes | 11201 | 20.0 | 3438 | 24.3 | <.0001 |
| Hyperlipidemia | 13008 | 23.3 | 4205 | 29.7 | <.0001 |
| Stroke | 10306 | 18.4 | 3750 | 26.5 | <.0001 |
| Heart failure | 2928 | 5.24 | 1705 | 12.0 | <.0001 |
| Fracture of lower limb | 2338 | 4.18 | 767 | 5.42 | <.0001 |
| Cancer | 1989 | 3.56 | 496 | 3.51 | 0.7548 |
| Medicine | |||||
| Inhaled corticosteroids (ICSs) | 1923 | 3.44 | 3855 | 27.2 | <.0001 |
| Oral steroids(OCs) | 24401 | 43.7 | 11349 | 80.2 | <.0001 |
ACOS, asthma–COPD overlap syndrome; Chi-square test;
# Student’s t-test
Fig 1. The cumulative incidence of pulmonary embolism in asthma–COPD overlap syndrome (ACOS) (dashed line) and non-ACOS cohorts (solid line).
Though the PE event is relatively rare in ACOS cohort, the overall incidence rate of PE was significantly higher in the ACOS cohort than in the non-ACOS cohort (1.02 vs. 0.42 per 1,000 person-years, respectively), and the IRR was 2.42 (95% CI: 2.29–2.55; Table 2). After adjustment for the potential risk factors, the aHR of PE was 2.08 (95% CI: 1.56–2.76) between the ACOS and non-ACOS cohorts (Table 2). The risk of PE was higher in the ACOS cohort than in the non-ACOS cohort, regardless of whether the patients were female or male. The incidence rate of PE was increased with age. Comparing the ≥65-year-old groups in each cohort, the ACOS cohort had a 1.87-fold risk of developing PE. In the comorbidity-stratified analysis and drug used-stratified analysis yielded similar results. For subjects with comorbidity, ACOS cohort had 2.00-fold the risk of PE than non-ACOS cohort (95% CI: 1.47–2.73; Table 2). The patients in the ACOS cohort who used drugs was presented 1.89-fold risk of PE than the comparison group (95% CI: 1.40–2.56; Table 2)
Table 2. Incidence rate and adjusted hazard ratio of pulmonary embolism between ACOS and non-ACOS cohorts stratified by sex, age, comorbidity (no/yes), and drug used (no/yes).
| ACOS | ||||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | Compared to non-ACOS cohort | ||||||
| Variables | Event | PY | Rate | Event | PY | Rate | IRR (95% CI) | Adjusted HR† (95% CI) |
| Overall | 168 | 399412 | 0.42 | 98 | 96387 | 1.02 | 2.42(2.29–2.55)*** | 2.08(1.56–2.76)*** |
| Sex | ||||||||
| Female | 84 | 174000 | 0.48 | 40 | 42226 | 0.95 | 1.96(1.80–2.14)*** | 1.63(1.06–2.50)* |
| Male | 84 | 225412 | 0.37 | 58 | 54160 | 1.07 | 2.87(2.68–3.08)*** | 2.52(1.73–3.69)*** |
| Age, year | ||||||||
| 20–65 | 35 | 192150 | 0.18 | 30 | 47308 | 0.63 | 3.48(3.20–3.78)*** | 2.53(1.44–4.44)*** |
| ≥65 | 133 | 207263 | 0.64 | 68 | 49078 | 1.39 | 2.16(2.01–2.32)*** | 1.87(1.34–2.61)*** |
| Comorbidity | ||||||||
| No | 38 | 174432 | 0.22 | 19 | 26933 | 0.71 | 3.24(2.95–3.56)*** | 2.97(1.54–5.71)*** |
| Yes | 130 | 224981 | 0.58 | 79 | 69453 | 1.14 | 1.97(1.84–2.10)*** | 2.00(1.47–2.73)*** |
| Drug used | ||||||||
| No | 73 | 224312 | 0.33 | 18 | 15564 | 1.16 | 3.55(3.22–3.92)*** | 3.21(1.89–5.43)*** |
| Yes | 95 | 175100 | 0.54 | 80 | 80822 | 0.99 | 1.82(1.70–1.96)*** | 1.89(1.40–2.56)*** |
ACOS, asthma–COPD overlap syndrome; Drug used, including subjects with inhaled corticosteroids (ICSs) or oral steroids(OSs); PY, person-year; Rate, incidence rate (per 1,000 person-years); IRR, incidence rate ratio; Adjusted HR†: multiple cox model analysis including age, sex, each comorbidity, inhaled corticosteroid (ICSs), and oral steroids(OSs);
*p<0.05,
***p<0.001
The stratified analysis in each comorbidity type was showed in Table 3. Compared with non-ACOS cohort, the IRR of PE was higher in the ACOS cohort in difference comorbidity, such as atrial fibrillation (IRR: 1.87, 95% CI: 1.38–2.52), hypertension (IRR: 1.86, 95% CI: 1.73–2.01), diabetes (IRR: 2.08, 95% CI: 1.85–2.33), hyperlipidemia (IRR: 1.63, 95% CI: 1.46–1.82), stroke (IRR: 1.65, 95% CI: 1.47–1.86), fracture of lower limb (IRR: 1.86, 95% CI: 1.44–2.39), and cancer (IRR: 3.43, 95% CI: 2.60–4.52). The aHR of pulmonary embolism was obviously higher in the ACOS cohort than the non-ACOS cohort regardless of hypertension (aHR: 1.80, 95% CI: 1.27–2.56), diabetes (aHR: 2.08, 95% CI: 1.21–3.59), cancer (aHR: 3.76, 95% CI: 1.17–12.1) existence or not.
Table 3. Incidence rate and adjusted hazard ratio of pulmonary embolism between ACOS and non-ACOS cohorts stratified by each comorbidity types.
| ACOS | ||||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | Compared to non-ACOS cohort | ||||||
| Variables | Event | PY | Rate | Event | PY | Rate | IRR (95% CI) | Adjusted HR† (95% CI) |
| Atrial fibrillation | ||||||||
| No | 157 | 394087 | 0.40 | 89 | 94053 | 0.95 | 2.38(2.25–2.51)*** | 2.13(1.59–2.86)*** |
| Yes | 11 | 5325 | 2.07 | 9 | 2334 | 3.86 | 1.87(1.38–2.52)*** | 1.57(0.59–4.22) |
| Hypertension | ||||||||
| No | 63 | 222429 | 0.28 | 36 | 40282 | 0.89 | 3.16(2.92–3.41)*** | 2.68(1.67–4.30)*** |
| Yes | 105 | 176983 | 0.59 | 62 | 56104 | 1.11 | 1.86(1.73–2.01)*** | 1.80(1.27–2.56)** |
| Diabetes | ||||||||
| No | 128 | 327873 | 0.39 | 73 | 74866 | 0.98 | 2.50(2.35–2.66)*** | 2.04(1.46–2.84)*** |
| Yes | 40 | 71540 | 0.56 | 25 | 21521 | 1.16 | 2.08(1.85–2.33)*** | 2.08(1.21–3.59)** |
| Hyperlipidemia | ||||||||
| No | 123 | 311590 | 0.39 | 74 | 67643 | 1.09 | 2.77(2.60–2.95)*** | 2.37(1.70–3.31)*** |
| Yes | 45 | 87822 | 0.51 | 24 | 28743 | 0.83 | 1.63(1.46–1.82)*** | 1.47(0.86–2.52) |
| Stroke | ||||||||
| No | 120 | 338208 | 0.35 | 70 | 74789 | 0.94 | 2.64(2.48–2.80)*** | 2.25(1.61–3.16)*** |
| Yes | 48 | 61205 | 0.78 | 28 | 21598 | 1.30 | 1.65(1.47–1.86)*** | 1.62(0.96–2.71) |
| Heart failure | ||||||||
| No | 145 | 385130 | 0.38 | 81 | 87309 | 0.93 | 2.46(2.33–2.61)*** | 2.25(1.65–3.06)*** |
| Yes | 23 | 14282 | 1.61 | 17 | 9077 | 1.87 | 1.16(0.96–1.41) | 1.27(0.64–2.52) |
| Fracture of lower limb | ||||||||
| No | 159 | 386391 | 0.41 | 93 | 92487 | 1.01 | 2.44(2.31–2.58)*** | 2.08(1.55–2.78)*** |
| Yes | 9 | 13021 | 0.69 | 5 | 3900 | 1.28 | 1.86(1.44–2.39)*** | 1.95(0.58–6.60) |
| Cancer | ||||||||
| No | 161 | 389413 | 0.41 | 92 | 93884 | 0.98 | 2.37(2.24–2.51)*** | 2.00(1.49–2.68)*** |
| Yes | 7 | 9999 | 0.70 | 6 | 2502 | 2.40 | 3.43(2.60–4.52)*** | 3.76(1.17–12.1)* |
ACOS, asthma–COPD overlap syndrome; PY, person-year; Rate, incidence rate (per 1,000 person-years); IRR, incidence rate ratio; Adjusted HR†: multiple cox model analysis including age, sex, each comorbidity, inhaled corticosteroid (ICSs), and oral steroids(OSs);
*p<0.05,
**p<0.01,
***p<0.001
Table 4 presents the risk of PE in the ACOS patients using ICSs or OSs. Compared with the non-ACOS cohort, the risk of PE was presented 1.97-fold (95% CI: 1.29–3.01) in ACOS patients who received ICSs treatment. The aHR of PE decreased from 2.91 (95% CI: 1.82–4.63) to 1.97 (95% CI: 1.46–2.65) in the ACOS patients using OSs.
Table 4. Adjusted hazard ratio of pulmonary embolism found in the follow-up period associated with ACOS and prescriptions of ICSs and OSs.
| Variables | N | Event | Rate | IRR (95% CI) | Adjusted HR† (95% CI) |
|---|---|---|---|---|---|
| Non-ACOS cohort | 55876 | 168 | 0.42 | 1.00 | 1.00 |
| ACOS cohort | |||||
| Without Inhaled corticosteroids (ICSs) | 10295 | 71 | 1.05 | 2.50(2.36–2.66)*** | 2.22(1.66–2.98)*** |
| With Inhaled corticosteroids (ICSs) | 3855 | 27 | 0.93 | 2.21(2.03–2.42)*** | 1.97(1.29–3.01)** |
| Non-ACOS cohort | 55876 | 168 | 0.42 | 1.00 | 1.00 |
| ACOS cohort | |||||
| Without oral steroids(OSs) | 2801 | 20 | 1.11 | 2.64(2.39–2.92)*** | 2.91(1.82–4.63)*** |
| With oral steroids (OSs) | 11349 | 78 | 0.99 | 2.37(2.23–2.51)*** | 1.97(1.46–2.65)*** |
ACOS, asthma–COPD overlap syndrome; Rate, incidence rate (per 1,000 person-years); IRR, incidence rate ratio; Adjusted HR†: multiple cox model analysis including age, sex, each comorbidity, inhaled corticosteroid (ICSs), and oral steroid(OSs);
**p<0.01,
***p<0.001
Compared with the non-ACOS cohort, the risk of developing PE was more notably affected by the number of outpatient visits and hospitalizations necessitated in the ACOS cohort, ranging from 2.32 (95% CI: 1.54–3.52) for those having 3–9 visits to 4.20 (95% CI: 2.74–6.44) for those having >9 visits (p < 0.0001; Table 5).
Table 5. The adjusted hazard ratio of pulmonary embolism associated with number of outpatient visits and hospitalizations per year due to COPD or asthma exacerbation.
| Variables | N | Event | Rate | IRR (95% CI) | Adjusted HR† (95% CI) |
|---|---|---|---|---|---|
| Non-ACOS cohort | 55876 | 168 | 0.42 | 1.00 | 1.00 |
| ACOS cohort | |||||
| Number of outpatient visits and hospitalizations per year | |||||
| ≤ 3 | 7014 | 32 | 0.59 | 1.41(1.30–1.53)*** | 1.42(0.96–2.10) |
| 3–9 | 4052 | 32 | 1.17 | 2.79(2.57–3.02)*** | 2.32(1.54–3.52)*** |
| >9 | 3084 | 34 | 2.27 | 5.40(4.98–5.84)*** | 4.20(2.74–6.44)*** |
| p-value for trend | <.0001 | <.0001 |
ACOS, asthma–COPD overlap syndrome; Rate, incidence rate (per 1,000 person-years); IRR, incidence rate ratio; Adjusted HR†: multiple cox model analysis including age, sex, each comorbidity, inhaled corticosteroid (ICSs), and oral steroid(OSs);
***p<0.001
Discussion
The eosinophilic [18] and neutrophilic [22] inflammation [28] of the airway with pulmonary artery inflammation [12,13] might be the predisposing factors of PE [18] in previous study [15,16]. Meanwhile, the higher level of neutrophil cells with hemostatic markers (e.g., the ETP and vWF) of a prothrombotic state [14] have the effect on the hypercoagulation. Combined these factors, our study show that the ACOS have an impact on risk of the PE, especially in the patients receiving the high frequency of the healthcare utility [29]; even in the young adults or [30] people without comorbidities. In addition, complications such as hypertension [24], diabetes and cancer [31] were associated with the atherosclerosis in venous and arterial disease [32,33], which might play a role in the risk of PE [4,5] among the ACOS patients also.
These two phenotypes are found in the ACOS phenotypes such as Th2 high eosinophil predominant, Th2 low neutrophil predominant [19]. When the eosinophil predominant phenotype responds favorably to steroids [34], the level of hypoxeamia may be less [35], thus having the less risk of the hypercoagulation [36]. Similarly, in Wedzicha JA et al. report, the higher the blood eosinophil count, the greater the AE reduction response to ICSs. This finding might imply that the blood eosinophil count measured at a COPD with AE can direct the use of OSs at AE [28]. Moreover, according to Chen et al., high-dose ICSs use in patients with COPD improved their lung function status, decreased the frequency of exacerbations, and improved symptom relief, thus having the less risk of hypoxieamia [37]. These previous findings may explain why the ACOS cohort using steroids (aHR = 1.97 for ICSs and OSs) had a lower risk of PE than those not using steroids (aHR = 2.22 for ICSs; 2.91 for OSs) in the current study. Furthermore, Sneeboer et al. found that patients using OSs (odds ratio 0.5; p<0.01) or ICSs (odd ratio 0.5; p = 0.02) used these steroids >6 months prior to the PE event; this protective effect of PE in line with our findings [38].
Anti-inflammatory drugs, particularly ICSs [33], are the mainstay of treatment for asthma or AE of COPD cohort [33]. ACOS is also a systemic inflammation disease, for which ICSs are usually applied in treatment [39] The higher frequency of PE among ACOS patients receiving more medical services accords with this finding.
Steroids-induced hypercoagulability has reported for several years; however it remains controversial whether the use of steroids or the underlying disease itself contributes to the hypercoagulable state [15]. The present study highlights the role of PE in ACOS cohort; the patients who used steroids may have had a lower risk of PE than those who did not. Steroids use may reduce hypoxeamia [37,39], thus patients receiving steroids may have the less risk of hypercoagulation [28]. However, it may also induce the prothrombotic state, irrespective of the dose, the risk of PE may be higher in these patients with steroids use, especially during the first month of exposure [40]. Determining the appropriate steroids dosage and a means of shorting the duration of steroid use for the ACOS cohort with incidental PE, while avoiding PE, warrants randomized control study.
In the Kumbhare S et al. report [31], they compared the ACOS cohort with the pure COPD cohort and the pure asthma cohort. The ACOS cohort was more likely to have at least one co-morbidity, more hospitalization or emergency department visits, less exercise and more disability compared to the pure COPD cohort. Similarly, the comorbidities (e.g, the diabetes [24], hypertension, cancer [24]), acute respiratory event [24] (less exercise) and higher frequency of medical services (disability) are more associated with the ACOS cohort in our study. These factors were predisposing factors of the PE. In van Boven JF et al. study [41], they found that allergic rhinitis, anxiety, osteoporosis and gastroesophageal reflux disease are more related to the ACOS cohort than the pure COPD cohort, while ischemia heart disease (IHD) and chronic kidney disease were less associated. Regarding the impact on hospitalization risk in this previous study, the comorbidities such as lung cancer [9] and cor pulmonale [8] had relatively the strongest association with 1-year all-cause hospitalization risk in the ACOS cohort [41]. Meanwhile, these two factors [8,9] are associated with the PE also [42]. In their study did not include PE, and that would warrant further investigation, likewise for the differences between risk for PE in ACOS cohort and pure asthma cohort alone.
The management in an individual patient with PE requires clinical assessment of risks and benefits and also depends on local availability of therapeutic interventions [43]. Incidental and isolated subsegmental PE should generally be managed in the same manner as symptomatic and non-subsegmental PE [43]. This hypothesis, however, should be assessed in prospective clinical trials [44]. By contrast, patients diagnosed with acute symptomatic PE, concomitant DVT was significantly associated with an increased risk of death within 30 days of PE diagnosis [40,45]. In addition, long-term Outcomes after PE (ELOPE) Study indicate that almost half of PE patients can be considered to have a "post-PE syndrome" characterized by exercise limitation at 1 year, which influences their quality of life (QOL) and degree of dyspnea [46]. The higher frequency of medical services [31] and the post-PE syndrome aggravated the disability [29]. Therefore, this study should alert the clinicians to be aware of the incident PE in the ACOS cohort, even in young adults or people without comorbidities.
Strengths
This is the first cohort study discussing the relationship between PE and ACOS based on the general population. PE-associated comorbidities and medicine (ICSs and OSs) were considered in this study. Multidisciplinary services for care of ACOS [24] and PE patients are well established in Taiwan [25]. Courses of ACOS and PE treatment can be followed up by public nurses and physicians. Furthermore, regular use of drugs such as ICSs can be monitored under these strict services. Meanwhile, our study cohort enrolled the patients aged >20 years <40 years [23] for avoid missing the young adults with ACOS.
Limitations
Several limitations were encountered in this study. The first limitation is the definition of ACOS in this paper and this is a very contentious area already, the ACOS cohort in this study is based on the COPD-subset. Second, even in ACOS, the incidence of PE is 1.02 in 1000 person years. Third, this study does not mention if the PE are incidental findings or not. The management of incidental PE is uncertain and patients with ACOS are at high risk of over-diagnosis of asymptomatic PE. Fourth, some of the important clinical variables/risk factors that have been associated with PE were not included such smoking, body mass index, pregnancy, use of oral anticonceptives (in women), anticoagulant drugs (aspirin, warfarin) and genetics. Fifth, we did not analyze the effect of bronchodilators or erythromycin on PE. Sixth, the ETP and vWF levels are unavailable in the NHIRD. Finally, clinical parameters related to asthma/COPD such as lung function parameters (e.g. forced expiratory volume in 1 second %, DLco %, 6MWT) are unavailable at NHIRD also. These confounding factors warrant investigation.
Conclusions
ACOS is associated with increased risk of PE, particularly patients with a high frequency of AE—even in young adults or people without comorbidities.
Supporting Information
(DOC)
Acknowledgments
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019), China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037), NRPB Stroke Clinical Trial Consortium (MOST 104-2325-B-039–005), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
Abbreviations
- aHR
adjusted hazard ratio
- CI
confidence interval
- NHIRD
National Health Insurance Research Database
- NHI
National Health Insurance
- NHRI
National Health Research Institutes
- LHID 2000
Longitudinal Health Insurance Database 2000
- ACOS
asthma–chronic obstructive overlap syndrome
- PE
pulmonary embolism
Data Availability
All data and related metadata were deposited in an appropriate public repository. The data on the study population that were obtained from the NHIRD (http://nhird.nhri.org.tw/en/index.html) are maintained in the NHIRD (http://nhird.nhri.org.tw/). The NHRI is a nonprofit foundation established by the government. Only citizens of the Republic of China who fulfill the requirements of conducting research projects are eligible to apply for the NHIRD. The use of NHIRD is limited to research purposes only. Applicants must follow the Computer-Processed Personal Data Protection Law (http://www.winklerpartners.com/?p=987) and related regulations of National Health Insurance Administration and NHRI, and an agreement must be signed by the applicant and his/her supervisor upon application submission. All applications are reviewed for approval of data release.
Funding Statement
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019), China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037), NRPB Stroke Clinical Trial Consortium (MOST 104-2325-B-039 -005), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
References
- 1.Piovella F, Iosub DI. Acute pulmonary embolism: risk assessment, risk stratification and treatment options. Clin Respir J. 2015. January 26 10.1111/crj.12264. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Dentali F, Ageno W, Pomero F, Fenoglio L, Squizzato A, Bonzini M. Time trends and case fatality rate of in-hospital treated pulmonary embolism during 11 years of observation in Northwestern Italy. Thromb Haemost 2016;115: 399–405. 10.1160/TH15-02-0172 [DOI] [PubMed] [Google Scholar]
- 3.Torres-Macho J, Mancebo-Plaza AB, Crespo-Giménez A, Sanz de Barros MR, Bibiano-Guillén C, Fallos-Martí R, et al. Clinical features of patients inappropriately undiagnosed of pulmonary embolism. Am J Emerg Med 2013;31: 1646–1650. 10.1016/j.ajem.2013.08.037 [DOI] [PubMed] [Google Scholar]
- 4.Chung WS, Lin CL, Ho FM, Li RY, Sung FC, Kao CH, et al. Asthma increases pulmonary thromboembolism risk: a nationwide population cohort study. Eur Respir J 2014;43: 801–807. 10.1183/09031936.00043313 [DOI] [PubMed] [Google Scholar]
- 5.Chen WJ, Lin CC, Lin CY, Chang YJ, Sung FC, Kao CH, et al. Pulmonary embolism in chronic obstructive pulmonary disease: a population-based cohort study. COPD 2014; 11: 438–443. 10.3109/15412555.2013.813927 [DOI] [PubMed] [Google Scholar]
- 6.Cannegieter SC, Horváth-Puhó E, Schmidt M, Dekkers OM, Pedersen L, Vandenbroucke JP, et al. Risk of venous and arterial thrombotic events in patients diagnosed with superficial vein thrombosis: a nationwide cohort study. Blood 2015; 125: 229–235. 10.1182/blood-2014-06-577783 [DOI] [PubMed] [Google Scholar]
- 7.Chung WS, Lin CL, Kao CH. Diabetes increases the risk of deep-vein thrombosis and pulmonary embolism. A population-based cohort study. Thromb Haemost 2015;114: 812–818. 10.1160/TH14-10-0868 [DOI] [PubMed] [Google Scholar]
- 8.Motte S, Mélot C, Di Pierdomenico L, Martins D, Leclercq P, Pirson M. Predictors of costs from the hospital perspective of primary pulmonary embolism. Eur Respir J 2016;47: 203–211. 10.1183/13993003.00281-2015 [DOI] [PubMed] [Google Scholar]
- 9.O’Connell C. How I treat incidental pulmonary embolism. Blood 2015;125: 1877–1882. 10.1182/blood-2014-08-551879 [DOI] [PubMed] [Google Scholar]
- 10.Bagot CN, Arya R. Virchow and his triad: a question of attribution. Br J Haematol 2008;143: 180–190. 10.1111/j.1365-2141.2008.07323.x [DOI] [PubMed] [Google Scholar]
- 11.Levi M, Schultz MJ, Rijneveld AW, van der Poll T. Bronchoalveolar coagulation and fibrinolysis in endotoxemia and pneumonia. Crit Care Med 2003;31: S238–242. [DOI] [PubMed] [Google Scholar]
- 12.King PT. Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin Transl Med 2015;4: 68 10.1186/s40169-015-0068-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wanner A, Mendes ES. Airway endothelial dysfunction in asthma and chronic obstructive pulmonary disease: a challenge for future research. Am J Respir Crit Care Med 2010;182: 1344–1351. 10.1164/rccm.201001-0038PP [DOI] [PubMed] [Google Scholar]
- 14.Sneeboer MM, Majoor CJ, de Kievit A, Meijers JC, van der Poll T, Kamphuisen PW, et al. Prothrombotic state in patients with severe and prednisolone-dependent asthma. J Allergy Clin Immunol. 2016;137:1727–32. 10.1016/j.jaci.2015.10.038 [DOI] [PubMed] [Google Scholar]
- 15.Majoor CJ, Kamphuisen PW, Zwinderman AH, Ten Brinke A, Amelink M, Rijssenbeek-Nouwens L, et al. Risk of deep vein thrombosis and pulmonary embolism in asthma. Eur Respir J 2013;42: 655–661. 10.1183/09031936.00150312 [DOI] [PubMed] [Google Scholar]
- 16.Rizkallah J, Man SF, Sin DD. Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest 2009;135: 786–793. 10.1378/chest.08-1516 [DOI] [PubMed] [Google Scholar]
- 17.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation 2004;109: 159–165. [DOI] [PubMed] [Google Scholar]
- 18.Christenson SA, Steiling K, van den Berge M, Hijazi K, Hiemstra PS, Postma DS, et al. Asthma-COPD Overlap: Clinical Relevance of Genomic Signatures of Type 2 Inflammation in COPD. Am J Respir Crit Care Med. 2015;191:758–66. 10.1164/rccm.201408-1458OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnes PJ. Therapeutic approaches to asthma-chronic obstructive pulmonary disease overlap syndromes. J Allergy Clin Immunol 2015;136: 531–545. 10.1016/j.jaci.2015.05.052 [DOI] [PubMed] [Google Scholar]
- 20.Parulekar AD, Diamant Z, Hanania NA. Role of T2 inflammation biomarkers in severe asthma. Curr Opin Pulm Med 2016;22: 59–68. [DOI] [PubMed] [Google Scholar]
- 21.van den Berge M, ten Hacken NH, Cohen J, Douma WR, Postma DS. Small airway disease in asthma and COPD: clinical implications. Chest 2011;139: 412–423. 10.1378/chest.10-1210 [DOI] [PubMed] [Google Scholar]
- 22.Ghebre MA, Bafadhel M, Desai D, Cohen SE, Newbold P, Rapley L, et al. Biological clustering supports both "Dutch" and "British" hypotheses of asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol 2015;135: 63–72. 10.1016/j.jaci.2014.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Marco R, Pesce G, Marcon A, Accordini S, Antonicelli L, Bugiani M, et al. The coexistence of asthma and chronic obstructive pulmonary disease (COPD): prevalence and risk factors in young, middle-aged and elderly people from the general population. PLoS ONE 2013;8: e62985 10.1371/journal.pone.0062985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung WS, Lin CL, Kao CH. Comparison of acute respiratory events between asthma-COPD overlap syndrome and COPD patients: a population-based cohort study. Medicine (Baltimore) 2015;94: e755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang WC, Tsai YH, Wei YF, Kuo PH, Tao CW, Cheng SL, et al. Wheezing, a significant clinical phenotype of COPD: experience from the Taiwan Obstructive Lung Disease Study. Int J Chron Obstruct Pulmon Dis 2015;10: 2121–2126. 10.2147/COPD.S92062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braman SS. The chronic obstructive pulmonary disease-asthma overlap syndrome. Allergy and Asthma Proceedings 2015;36: 11–18. 10.2500/aap.2015.36.3802 [DOI] [PubMed] [Google Scholar]
- 27.Sneeboer MM H B, Majoor CJ, Bel EH, Kamphuisen PW. Oral and inhaled corticosteroid use and risk of recurrent pulmonary embolism. Thromb Res. 2016;46–50. [DOI] [PubMed] [Google Scholar]
- 28.Wedzicha JA. Eosinophils as Biomarkers of Chronic Obstructive Pulmonary Disease Exacerbation Risk. Maybe Just for Some? Am J Respir Crit Care Med 2016;193: 937–938. [DOI] [PubMed] [Google Scholar]
- 29.Alshabanat A, Zafari Z, Albanyan O, Dairi M, FitzGerald JM. Asthma and COPD Overlap Syndrome (ACOS): A Systematic Review and Meta Analysis. PLoS ONE 2015;10: e0136065 10.1371/journal.pone.0136065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Marco R, Marcon A, Rossi A, Antó JM, Cerveri I, Gislason T, et al. Asthma, COPD and overlap syndrome: a longitudinal study in young European adults. Eur Respir J 2015;46: 671–679. 10.1183/09031936.00008615 [DOI] [PubMed] [Google Scholar]
- 31.Kumbhare S, Pleasants R, Ohar JA, Strange C. Characteristics and Prevalence of Asthma/Chronic Obstructive Pulmonary Disease Overlap in the United States. Ann Am Thorac Soc 2016;13: 803–810. 10.1513/AnnalsATS.201508-554OC [DOI] [PubMed] [Google Scholar]
- 32.Knoflach M, Kiechl S, Mayr A, Willeit J, Poewe W, Wick G. Allergic rhinitis, asthma, and atherosclerosis in the Bruneck and ARMY studies. Arch Intern Med 2005;165: 2521–2526. [DOI] [PubMed] [Google Scholar]
- 33.Fimognari FL, Scarlata S, Conte ME, Incalzi RA. Mechanisms of atherothrombosis in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2008;3: 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D'Urzo A, Donohue JF, Kardos P, Miravitlles M, Price D. A re-evaluation of the role of inhaled corticosteroids in the management of patients with chronic obstructive pulmonary disease. Expert Opin Pharmacother 2015;16: 1845–1860. 10.1517/14656566.2015.1067682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woods JA, Wheeler JS, Finch CK, Pinner NA. Corticosteroids in the treatment of acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2014;9: 421–430. 10.2147/COPD.S51012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips CL, McEwen BJ, Morel-Kopp MC, Yee BJ, Sullivan DR, Ward CM, et al. Effects of continuous positive airway pressure on coagulability in obstructive sleep apnoea: a randomised, placebo-controlled crossover study. Thorax 2012;67: 639–644. 10.1136/thoraxjnl-2011-200874 [DOI] [PubMed] [Google Scholar]
- 37.Cheng SL, Su KC, Wang HC, Perng DW, Yang PC. Chronic obstructive pulmonary disease treated with inhaled medium- or high-dose corticosteroids: a prospective and randomized study focusing on clinical efficacy and the risk of pneumonia. Drug Des Devel Ther 2014;8: 601–607. 10.2147/DDDT.S63100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sneeboer M, Hutten B, Majoor C, Kamphuisen P-W, Bel E. Oral and inhaled corticosteroid use has a time-dependent association with recurrent pulmonary embolism. European Respiratory Journal 2015;46: PA4567. [DOI] [PubMed] [Google Scholar]
- 39.Reddel HK. Treatment of overlapping asthma-chronic obstructive pulmonary disease: Can guidelines contribute in an evidence-free zone? J Allergy Clin Immunol 2015;136: 546–552. 10.1016/j.jaci.2015.06.043 [DOI] [PubMed] [Google Scholar]
- 40.Stuijver DJ, Majoor CJ, van Zaane B, Souverein PC, de Boer A, Dekkers OM, et al. Use of oral glucocorticoids and the risk of pulmonary embolism: a population-based case-control study. Chest 2013;143: 1337–1342. 10.1378/chest.12-1446 [DOI] [PubMed] [Google Scholar]
- 41.van Boven JF, Román-Rodríguez M, Palmer JF, Toledo-Pons N, Cosío BG, Soriano JB. Comorbidome, Pattern, and Impact of Asthma-COPD Overlap Syndrome in Real Life. Chest 2016;149: 1011–1020. 10.1016/j.chest.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 42.Meyer G, Vieillard-Baron A, Planquette B. Recent advances in the management of pulmonary embolism: focus on the critically ill patients. Ann Intensive Care 2016;6: 19 10.1186/s13613-016-0122-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Condliffe R, Elliot CA, Hughes RJ, Hurdman J, Maclean RM, Sabroe I, et al. Management dilemmas in acute pulmonary embolism. Thorax 2014;69: 174–180. 10.1136/thoraxjnl-2013-204667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoo HH, Queluz TH, El Dib R. Anticoagulant treatment for subsegmental pulmonary embolism. Cochrane Database Syst Rev 2016;(1):CD010222 10.1002/14651858.CD010222.pub3 [DOI] [PubMed] [Google Scholar]
- 45.Becattini C, Cohen AT, Agnelli G, Howard L, Castejón B, Trujillo-Santos J, et al. Risk Stratification of Patients With Acute Symptomatic Pulmonary Embolism Based on Presence or Absence of Lower Extremity DVT: Systematic Review and Meta-analysis. Chest 2016;149: 192–200. 10.1378/chest.15-0808 [DOI] [PubMed] [Google Scholar]
- 46.Kahn SR, Hirsch A, Beddaoui M, Akaberi A, Anderson D, Wells PS, et al. "Post-Pulmonary Embolism Syndrome" after a First Episode of PE: Results of the E.L.O.P.E. Study. Blood 2015;126: 650. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All data and related metadata were deposited in an appropriate public repository. The data on the study population that were obtained from the NHIRD (http://nhird.nhri.org.tw/en/index.html) are maintained in the NHIRD (http://nhird.nhri.org.tw/). The NHRI is a nonprofit foundation established by the government. Only citizens of the Republic of China who fulfill the requirements of conducting research projects are eligible to apply for the NHIRD. The use of NHIRD is limited to research purposes only. Applicants must follow the Computer-Processed Personal Data Protection Law (http://www.winklerpartners.com/?p=987) and related regulations of National Health Insurance Administration and NHRI, and an agreement must be signed by the applicant and his/her supervisor upon application submission. All applications are reviewed for approval of data release.
All data and related metadata were deposited in an appropriate public repository. The data on the study population that were obtained from the NHIRD (http://nhird.nhri.org.tw/en/index.html) are maintained in the NHIRD (http://nhird.nhri.org.tw/). The NHRI is a nonprofit foundation established by the government. Only citizens of the Republic of China who fulfill the requirements of conducting research projects are eligible to apply for the NHIRD. The use of NHIRD is limited to research purposes only. Applicants must follow the Computer-Processed Personal Data Protection Law (http://www.winklerpartners.com/?p=987) and related regulations of National Health Insurance Administration and NHRI, and an agreement must be signed by the applicant and his/her supervisor upon application submission. All applications are reviewed for approval of data release.