Abstract
Temperature is an important factor in research on the biological effects of extremely low-frequency electromagnetic field (ELF-EMF), but interactions between ELF-EMF and temperature remain unknown. The effects of ELF-EMF (50 Hz, 3 mT) on the lifespan, locomotion, heat shock response (HSR), and oxidative stress (OS) of Canton-Special (CS) and mutant w1118 flies were investigated at 25°C and 35°C (thermal stress). Results showed that thermal stress accelerated the death rates of CS and w1118 flies, shortened their lifespan, and influenced their locomotion rhythm and activity. The upregulated expression levels of heat shock protein (HSP) 22, HSP26, and HSP70 indicated that HSR was enhanced. Thermal stress-induced OS response increased malondialdehyde content, enhanced superoxide dismutase activity, and decreased reactive oxygen species level. The effects of thermal stress on the death rates, lifespan, locomotion, and HSP gene expression of flies, especially w1118 line, were also enhanced by ELF-EMF. In conclusion, thermal stress weakened the physiological function and promoted the HSR and OS of flies. ELF-EMF aggravated damages and enhanced thermal stress-induced HSP and OS response. Therefore, thermal stress and ELF-EMF elicited a synergistic effect.
Introduction
Extremely low-frequency electromagnetic field (ELF-EMF) poses potential health hazards and bio-effects [1]. The United Nations and the International Telecommunications Union defined the influence of EMF as a core indicator of smart sustainable urban evaluation last year [2]. Epidemiological surveys and experimental research have indicated that ELF-EMF exposure is possibly associated with diseases, such as malignancies and cardiovascular, reproductive, and neurological disorders [3–5]. ELF-EMF unlikely elicits negative biological effects [6, 7] and plays a positive role under certain conditions [8–10]. Nevertheless, the bio-effect of ELF-EMF is controversial, and its mechanism remains unclear.
The periodic movement and collision of molecules and ions are detected in alternating electromagnetic fields [11]. As such, possible ELF-EMF-induced thermal effects should be demonstrated. Although non-thermal effects induced by long-term ELF-EMF exposure have been examined [12], thermal effects should also be considered in research on the bio-effects of ELF-EMF. For instance, ELF-EMF affects heat shock protein (HSP) accumulation in cells [13, 14]. The transcript levels of numerous heat shock genes, such as HSP22, HSP26, and HSP70, can be altered after an individual is subjected to long-term ELF-EMF exposure [15]. Oxidative stress (OS) is another important aspect in studies concerning the bio-effects of ELF-EMF; ELF-EMF can affect the generation of reactive oxygen species (ROS) and the activity of anti-oxidative enzymes, such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase [16, 17]. Therefore, HSR and OS are potential pathways involved in ELF-EMF responses; in most organisms, these pathways are also activated in response to thermal stress [18, 19]. ELF-EMF and temperature elicit interacting effects; for example, ELF-EMF can reduce cell damage at low temperatures and enhance stress response at high temperatures [9, 20]. However, the coupling effects between ELF-EMF and temperature have been rarely demonstrated, and the mechanism remains unclear.
In this study, adult flies were used to examine the effects of ELF-EMF (50 Hz, 3 mT) on lifespan, locomotion, HSR, and OS at 25°C and 35°C (thermal stress). Our results indicated that ELF-EMF aggravated the thermal stress-induced damages and reduced the thermal resistance of flies, especially w1118 flies. Therefore, a possible coupling mechanism exists between ELF-EMF and thermal stress, and they elicit a synergistic effect.
Material and Methods
Maintenance and treatment of flies
Wild-type Canton-Special (CS) and mutant w1118 flies (Core Facility of Drosophila Resource and Technique of SIBCB, CAS) were used. The flies were maintained on a sugar-yeast standard medium in an incubator at 25°C, 60% relative humidity (RH), and 12 h/12 h light/dark cycles; the incubator lights were turned on and off at 6 a.m. and 6 p.m. [21]. One- to two-day-old male and female flies were tested separately in all of the experiments. The following four treatment groups were prepared: 25°C (normal group); 25°C + ELF (ELF-EMF group); 35°C (thermal stress group); and 35°C + ELF (thermal and ELF-EMF co-stress group). Two stress factors were stimulated and terminated synchronously in all of the experiments. The flies were anesthetized with carbon dioxide.
ELF-EMF and thermal stress system
The ELF-EMF generating system was improved on the basis of our previous study [15]. In this study, ELF-EMF was produced by two parallel Helmholtz coils (260 turns of copper wire; diameter = 40 cm). Alternating current was applied using a variable-frequency AC power supply. The coil was electrified at 50 Hz, 82.3 V, and 6.75 A, and a variable magnetic field of 3.0 mT was produced between the two coils. The coils were then placed in an artificial climate incubator, which was utilized to control temperature, humidity, and light cycle during the experiment. A hose was wound around the coils and then connected to a condensed circulating water bath, which rapidly removed the heat produced by the coils. A temperature probe was set in the experimental zone to monitor, modify, and strictly control its actual temperature. The control system was similar to the exposure system except Helmholtz coils (S1 Fig).
Lifespan and locomotion analysis
One- to two-day-old male and female flies were separated under low-temperature anesthesia and loaded into glass tubes (5 mm × 7 cm; 1:1 tube-fly ratio) containing 10% sucrose/2% agar food pellet at one end and a cotton pellet at the other end. The glass tubes were then placed in Drosophila activity monitoring 2 (DAM2) boards (TriKinetics, Inc., Waltham, MA, USA). After the flies acclimated to the new environmental conditions overnight at room temperature (25°C), the boards were subjected to the following treatment conditions: 25°C; 25°C + ELF; 35°C; and 35°C + ELF. The activities of an individual fly were determined by using infrared beams crossing the center of each tube. The cumulative counts of the flies’ activities were recorded every 5 min.
The lifespan of flies was analyzed on the basis of the duration spanning the onset of and the completion of the last activity. Kaplan-Meier survival analysis was conducted, and cumulative survival curves were plotted. Log-rank (Mantel-Cox) test was performed to determine whether the difference among groups was significant. The average lifespan, median lethal time (calculated 50% mortality age), and maximum lifespan (calculated 90% mortality age) of female and male flies were also examined.
Activity rhythm was evaluated by counting the number of activities every 30 min from the onset of stress. Activity pattern variations were analyzed on the basis of the number of activities in the first 2 h accounted for the proportion of the total number of activities within 24 h. Each experiment was performed in three replicates, with 96 males and 96 females allotted for each condition.
RT-PCR assay
The relative expression levels of HSP22, HSP26, and HSP70 were determined at 25°C, 25°C+ ELF, 35°C, and 35°C + ELF stress conditions. Ten surviving male and female flies were collected from each tube after 12 h of treatment. The samples were quick-frozen in liquid nitrogen for the analysis of HSP22, HSP26, and HSP70 expression levels. The experiment was repeated thrice. Total RNA was extracted using a TRIzol reagent (Invitrogen) and was reverse-transcribed using a PrimeScript RT Master Mix Perfect Real-Time kit (Takara). Quantitative real-time PCR was performed using a SYBR Premix Ex Taq II kit (Takara). All of the samples were tested in triplicate, and the CT values of the target genes were normalized to those of housekeeping gene RP49. Normalized data were considered to quantify the relative levels of the target genes by using 2-ΔΔCt method [22]. The primers used in this study are shown in S1 Table.
OS indicator analysis
OS indicators, including ROS, malondialdehyde (MDA), total antioxidant capacity (TAC), SOD, and CAT, were examined at 25°C, 25°C + ELF, 35°C, and 35°C + ELF. Twenty male and female flies were immediately homogenized in cold phosphate buffer saline (PBS, pH 7.4) after 12 h of treatment. The supernatant was centrifuged at 2,500 × g and used for subsequent assays.
ROS level was detected through DCF fluorescence [23]. The supernatant (5 μl) was loaded with 295 μl of DCFH2-DA with a final concentration of 10 μM and then incubated at 37°C for 40 min. Fluorescence intensity was monitored by using a multi-mode microplate reader (Spectrum M5) at 488 nm excitation and 525 nm emission. Results were expressed as fluorescent intensity per milligram of protein.
Corresponding assay kits (Jiancheng, Nanjing, China) were used to analyze MDA content, TAC, and SOD and CAT activities. The MDA content was detected using thiobarbituric acid method. TAC was measured on the basis of the ferric-reducing ability of plasma. SOD and CAT activities were determined using xanthine oxidation and molybdenum ammonia acid methods, respectively. The experiments were replicated thrice.
Statistical analysis
Statistical analysis was performed using SPSS 17 and Microsoft Excel. Kaplan-Meier survival analysis was conducted. A univariate general linear model was used for between-subject effects test. Post-hoc multiple comparisons (LSD) and one-way ANOVA were performed to analyze the significant differences among groups. Data were presented as mean ± standard error of the mean (SEM).
Results
ELF-EMF and thermal stress shortened the lifespan of flies
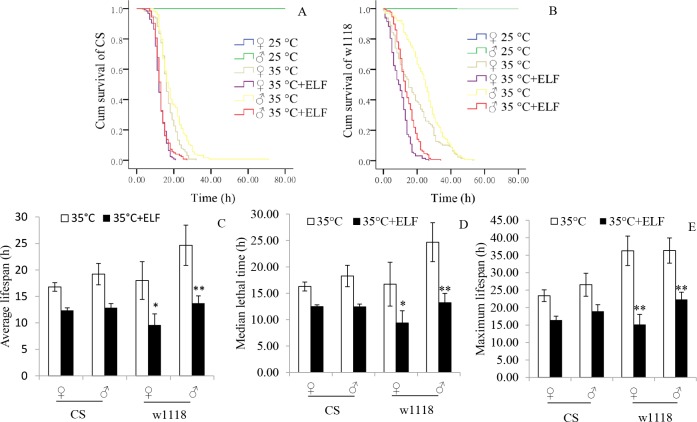
The lifespan of flies was analyzed on the basis of the monitoring results of DAM2. Survival analysis results show that the death rates of the male and female CS and w1118 flies at 25°C noticeably accelerated under thermal stress. At 35°C + ELF and 35°C, the death rate of CS (♀: X2 = 70.93, P < 0.001; ♂: X2 = 71.48, P < 0.001; Fig 1A) and w1118 (♀: X2 = 50.99, P < 0.001; ♂: X2 = 99.45, P < 0.001; Fig 1B) were further accelerated by ELF-EMF exposure. Because the flies could live several weeks at 25°C, much longer than that at 35°C, so only the lifespan data of flies at 35°C were provided. The average lifespan (♀: P = 0.013; ♂: P = 0.002; Fig 1C), median lethal time (♀: P = 0.038; ♂: P = 0.002; Fig 1D), and maximum lifespan (♀: P < 0.001; ♂: P = 0.002; Fig 1E) of w1118 were significantly shortened by ELF at 35°C (S2 Table). Similar phenomena were found in the CS line, but no significant influence was observed.
Fig 1. Survival functions and lifespan of flies.
(A) Survival functions of CS. (B) Survival functions of w1118. (C) Average lifespan of flies. (D) Median lethal time of flies. (E) Maximum lifespan of flies. Kaplan-Meier survival analysis was conducted, and log-rank (Mantel-Cox) test was performed to analyze significant differences among groups. Time durations from the start of stress exposure to the last recorded activity were considered to analyze their lifespan. Ages of 50% and 90% mortality were used as the evaluation indexes of median lethal time and maximum lifespan, respectively. Data are presented as mean ± SEM. Asterisk (*) indicates significant difference between the male and female flies of both lines exposed to 35°C and 35°C + ELF, respectively (*P < 0.05, **P < 0.01). Flies of three replicates were used for analysis. The numbers of CS and w1118 flies were as follows: 25°C group (♀: n = 96, ♂: n = 94); 35°C group (♀: n = 128, ♂: n = 128); and 35°C + ELF group (♀: n = 124, ♂: n = 124).
ELF-EMF and thermal stress affected fly locomotion
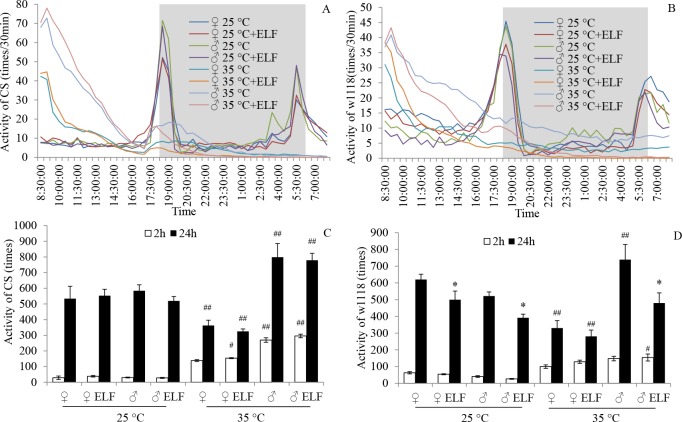
The locomotion rhythm of flies was analyzed on the basis of the number of activities, which were counted every 30 min from the onset of stress. At 25°C, CS and w1118 activities for each gender followed a circadian rhythm, and two activity peaks at dawn and dusk were recorded under our experimental condition. At 35°C, the number of activities of CS and w1118 for each gender increased sharply at the beginning, declined rapidly at the following time, and maintained a low level after about 12 h (Fig 2A and 2B). Compared with that at 25°C in both CS and w1118, the number of activities in the first 24 h significantly decreased in female flies but increased in male flies at 35°C (Fig 2C and 2D). The CS flies were mainly affected by temperature (F = 28.12; P < 0.001; S3 Table), and the w1118 flies were mainly influenced by ELF-EMF (F = 11.52; P = 0.002; S3 Table). ELF-EMF exposure significantly reduced w1118 activities at 25°C (♀: P = 0.045; ♂: P = 0.031) and 35°C (♂: P < 0.001; Fig 2D). The number of activities in the first 2 h accounted for more than 30% of the total number of activities within 24 h at 35°C. By contrast, this proportion was less than 10% at 25°C.
Fig 2. ELF-EMF and thermal stress effects on fly locomotion.
Activity frequencies of flies were counted every 30 min from the beginning of stress. (A) CS locomotion rhythm in the first 24 h. (B) w1118 locomotion rhythm in the first 24 h. White and gray backgrounds in the charts represent day and night, respectively. (C) CS activity frequencies in the first 2 and 24 h. (D) w1118 activity frequencies in the first 2 and 24 h. Data are presented as mean ± SEM. Hash (#) indicates significant difference between groups 25°C and 35°C when other factors are the same (# P < 0.05, ##P < 0.01). Asterisk (*) indicates significant difference between ELF-EMF and non-ELF-EMF exposure groups when other factors are the same (* P < 0.05).
ELF-EMF and thermal stress increased the transcript levels of HSP22, HSP26, and HSP70
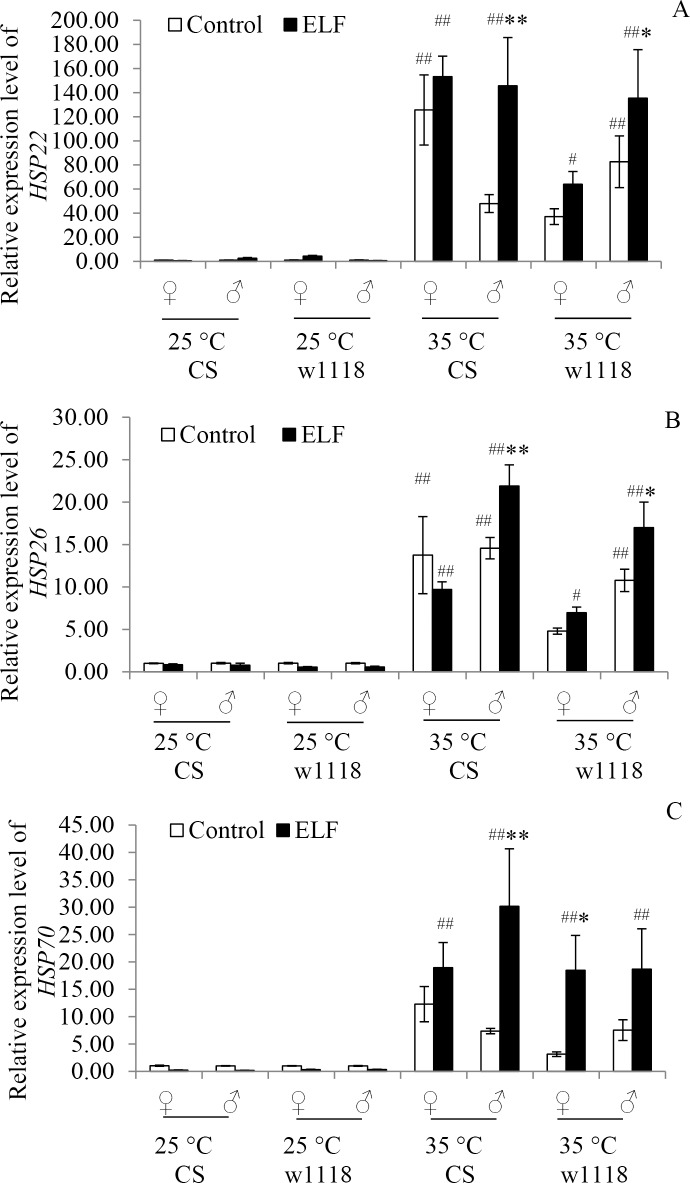
The transcript levels of HSP22, HSP26, and HSP70, which were sensitive to both ELF-EMF and thermal stress, were detected to evaluate the HSR of flies under each condition.
HSP22 transcript levels were mainly affected by the interaction of temperature and ELF (F = 6.58; P = 0.012; S4 Table). At 25°C, HSP22 transcript levels were not influenced by ELF-EMF in both CS and w1118 flies. However, they were significantly upregulated at 35°C (CS♀: P < 0.001; w1118♂: P = 0.002) and at 35°C + ELF (CS♀, CS♂, and w1118♂: P < 0.001; w1118♀: P = 0.044) conditions compared with their expression levels at 25°C. Moreover, HSP22 transcript levels at 35°C + ELF co-stress were significantly higher than those at 35°C in male flies of both CS (♀: P = 0.001) and w1118 (P = 0.046; Fig 3A).
Fig 3. Relative transcript levels of HSP22, HSP26, and HSP70.
Transcript levels of (A) HSP22, (B) HSP26, and (C) HSP70 were detected 12 h after exposure to 25°C, 25°C + ELF, 35°C, and 35°C + ELF conditions. Data are presented as mean ± SEM. Hash (#) indicates significant difference between 25°C and 35°C groups when other factors are the same (# P < 0.05, ##P < 0.01). Asterisk (*) indicates significant difference between ELF-EMF and non-ELF-EMF exposure groups when other factors are the same (* P < 0.05, ** P < 0.01).
HSP26 transcript levels were mainly affected by the interaction of temperature, gender, and ELF (F = 4.57; P = 0.035; S4 Table). At 25°C, HSP26 transcript levels were not influenced by ELF-EMF in both CS and w1118 flies. By contrast, they were significantly upregulated at 35°C (CS♀, CS♂, and w1118♂: P < 0.001) and 35°C + ELF (CS♀: P = 0.001; CS♂: P < 0.001; w1118♀: P = 0.013; w1118♂: P < 0.001) conditions compared with their expression levels at 25°C. Moreover, HSP26 transcript levels at 35°C + ELF co-stress were significantly higher than those at 35°C in the male flies of both CS (P = 0.007) and w1118 (P = 0.016; Fig 3B).
HSP70 transcript levels were mainly affected by the interaction of temperature and ELF (F = 11.39; P = 0.001; S4 Table). At 25°C, HSP70 transcript levels were not influenced by ELF-EMF in both CS and w1118 flies. Conversely, they were significantly upregulated at 35°C and 35°C + ELF (CS♀: P = 0.002; CS♂: P < 0.001; w1118♀: P = 0.003; w1118♂: P = 0.003) conditions compared with their expression levels at 25°C. Moreover, HSP70 transcript levels at 35°C + ELF co-stress were significantly higher than those at 35°C in male CS flies (P = 0.001) and female w1118 flies (P = 0.015; Fig 3C).
ELF-EMF and thermal stress enhanced OS
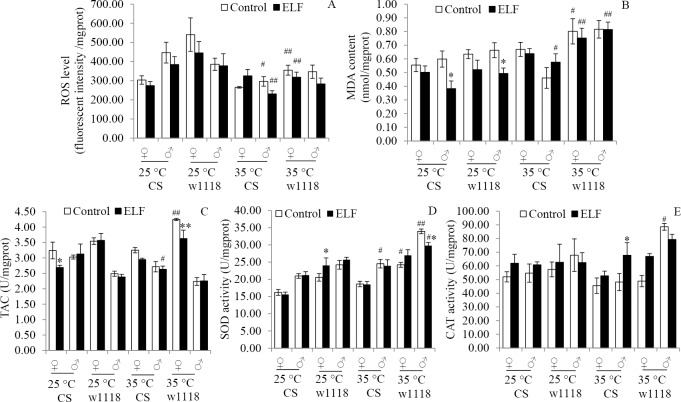
OS responses were investigated to determine the underlying factors of ELF-EMF-induced effects. OS indicators, including ROS, MDA, TAC, SOD, and CAT, were detected under four conditions.
ROS level was mainly influenced by temperature (F = 19.97; P < 0.001; S5 Table). Compared with those observed in the flies exposed to thermal stress at 25°C, ROS generations remarkably reduced at 35°C (CS♂: P = 0.011; w1118♀: P = 0.002) and 35°C + ELF (CS♂: P = 0.009; w1118♀: P = 0.032) (Fig 4A). At 25°C or 35°C, ELF-EMF did not significantly influence CS and w1118 flies (Fig 4A).
Fig 4. ELF-EMF and thermal stress effects on oxidative stress indicators.
(A) ROS. (B) MDA. (C) TAC. (D) SOD. (E) CAT. OS indicators were detected after 12 h of treatments. Data are presented as mean ± SEM. Hash (#) indicates significant difference between 25°C and 35°C group when other factors are the same (# P < 0.05, ##P < 0.01). Asterisk (*) indicates significant difference between ELF-EMF and non-ELF-EMF exposure groups when other factors are the same (* P < 0.05, ** P < 0.01).
MDA contents were mainly influenced by the interaction of temperature and ELF-EMF (F = 6.15; P = 0.014; S5 Table). At 25°C, the MDA contents were significantly reduced by ELF-EMF in male CS (P = 0.01) and w1118 (P = 0.047) flies. Compared with that at 25°C, the MDA content increased at 35°C (w1118♀: P = 0.046) and 35°C + ELF (CS♂: P = 0.021; w1118♀: P = 0.007; w1118♂: P < 0.001) (Fig 4B).
TACs were mainly influenced by ELF-EMF (F = 5.12; P = 0.024; S5 Table). They were significantly reduced by ELF-EMF in female CS flies (P = 0.022) at 25°C and in w1118 (P = 0.01) at 35°C. Compared with TAC at 25°C, TAC increased at 35°C (w1118♀: P = 0.003) and decreased at 35°C + ELF (CS♂: P = 0.038) (Fig 4C).
SOD activities were mainly influenced by temperature (F = 48.57, P < 0.001). They were significantly increased by ELF-EMF in female w1118 flies (P = 0.034) at 25°C and decreased in male w1118 flies (P = 0.012) at 35°C. Compared with that at 25°C, SOD increased at 35°C (CS♂: P = 0.03; w1118♀: P = 0.026; w1118♂: P < 0.001) and 35°C + ELF (CS♂: P = 0.013) (Fig 4D).
Temperature or ELF-EMF slightly influenced CAT activities (S5 Table). ELF-EMF significantly increased the CAT activities of the male flies at 35°C (P = 0.04). Compared with that at 25°C, CAT increased at 35°C (w1118 ♂: P = 0.046) (Fig 4E).
Discussion
ELF-EMF may share similar stress-response pathways with thermal stress. As such, thermal effect should be considered in research on the bio-effects of ELF-EMF. However, coupling effects between ELF-EMF and thermal stress have been rarely investigated, and underlying mechanisms remain poorly understood. Therefore, the coupling mechanisms of ELF-EMF and thermal stress on CS and w1118 flies were investigated in this study.
The effects of temperature on flies have been widely explored. For example, the numbers of phenotypic modulations, metabolites, genes, and proteins associated with temperature damages, hardening, and tolerance have been identified [18, 24, 25]. Temperatures between 34°C and 37°C are often used as mild thermal stress conditions because acute HSR is likely induced at these temperature levels [18, 26, 27]. Likewise, our previous studies showed that the mortality of flies sharply increases, and the HSP70 transcript level is rapidly upregulated at >35°C under short-term stress (2 h) [28]. In our present study, the physiological function of flies declined under sustained thermal stress, as supported by the acceleration of death rates, the shortening of lifespan, and the effects of locomotion rhythm and activity of flies. Under thermal stress, the metabolism of flies is enhanced in the initial stage. With prolonged exposure to stress, heat damages accumulate and metabolic rate declines because of disrupted water-salt balance, damaged cell structure, and decreased enzyme activity [29–31]. Changes in locomotion rhythm confirmed these assumptions. The activities were sharply increased in the beginning stages of the experiment and then acutely decreased in succeeding hours. Moreover, the effects of thermal stress on the death rates, lifespan, and locomotion of flies, especially w1118 line, were enhanced by ELF-EMF. These findings indicated that ELF-EMF aggravated thermal stress-induced damages, although thermal effects were also dominant at 35°C + ELF. Previous studies demonstrated similar synergistic effects; for instance, ELF-EMF enhances cellular apoptosis induced by low doses of X-ray irradiation exposure [32], increases lipid peroxidation induced by lead in mouse [33], and increases the survival rate of flies and Escherichia coli at low temperature [9, 34]. These findings suggested that ELF-EMF can interact with various chemical or physical factors, including temperature.
HSR and OS usually share common stress response pathways under various stress conditions [35, 36], including thermal and ELF-EMF stress. They were used to estimate the differences in stress responses at 25°C, 25°C + ELF, 35°C, and 35°C + ELF. In this study, the transcript levels of HSP22, HSP26, and HSP70 were upregulated at 35°C possibly because the three HSP genes are heat-shock-induced proteins [37]. Similar variations of HSP70 have been found in flies exposed to mild thermal stress [38]. The transcript levels of the three HSP genes at 35°C + ELF were higher than those at 35°C. Hence, ELF promoted HSP gene expression under thermal stress. ELF-EMF can strongly enhance reporter gene expression under the control of HSP16 and HSP70 promoters in mild heat shock on Caenorhabditis elegans [20]. Moreover, several HSP genes, such as HSP16, HSP27, HSP70, and HSP90, respond to ELF-EMF [14, 20, 39]. HSP70 is closely related to self-protection mechanism [14, 40] and can be induced by ELF-EMF in flies, mice, and cells [41–43]. To the best of our knowledge, this study is the first to investigate HSP22 and HSP26 induced by ELF-EMF, although HSP22 and HSP26 have been described in other studies [15]. Similar to HSP27 and HSP16, HSP22 and HSP26 belong to the small HSP family, whose expression is affected by ELF-EMF [14]. HSP22 is mainly involved in aging, thermal, and oxidative stress pathways in flies and is necessary to adapt to stress [44, 45]. HSP26 mainly contributes to the stress response and senility of flies [46].
Thermal stress exacerbates OS by stimulating the accumulation of harmful metabolites and affecting antioxidant enzyme activities [47]. In our study, thermal stress and ELF-EMF elicited different effects on OS indicators. Thermal stress mainly influenced ROS level, MDA content, and SOD activity. ELF-EMF mainly affected MDA content and TAC. The MDA accumulation was the combined result of ELF-EMF and thermal stress. Under thermal stress, the MDA content of the flies was increased. Thus, oxidative damages were exacerbated under ELF-EMF and thermal co-stress conditions. Decreased ROS levels may be attributed to the stimulation of OS responses, such as SOD activity enhancement. ELF-EMF combined with lead exposure also causes a remarkable increase in MDA, GSH content, and TAC of mouse brain and liver [33]. Therefore, ELF-EMF and thermal stress affect OS. However, their pathways differ, and underlying mechanisms remain unknown.
Other possible common physiological processes, such as cell membrane permeability, protein synthesis, and cell proliferation, respond to thermal and ELF-EMF stress [48]. ELF-EMF-induced responses may influence the thermal stress-induced responses of flies and vice versa under co-stress condition. Different organisms, developmental stages, and organs or tissues exhibit various sensitivities to temperature and ELF-EMF [38, 49]. For example, each part of a fly can quickly respond to temperature variations. However, the magnetic sense of flies in terms of light-dependent magneto-reception is mediated by the Cry/MagR complex [50]. Cry is mainly expressed in specific subsets of a fly’s pacemaker neurons and is in the photoreceptor cells of its compound eyes [51]. Furthermore, the intense and irregular Brownian motion of molecules and ions is dominant in thermal stress [52]. By contrast, periodical and regular Lorentz force is dominant in ELF-EMF stress [53]. When combined, these forces may achieve equilibrium. Although this phenomenon is simply a hypothesis, a mutual influence between the two forces has been widely explored in interdisciplinary physics and chemistry research [54]. The effects of ELF-EMF and thermal co-stress may have resulted from the physical and chemical dynamic balance induced by stress and be associated with different sensitivities to each factor influencing organisms.
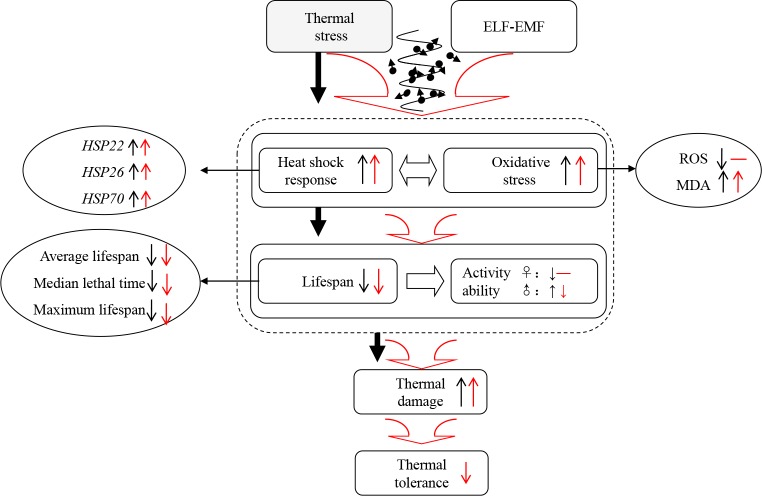
In conclusion, thermal stress weakened the physiological function and enhanced HSR and OS of CS and w1118 flies. ELF-EMF aggravated damages and enhanced thermal stress-induced HSP and OS response in flies, especially w1118 line (Fig 5). Therefore, thermal stress and ELF-EMF elicited a synergistic effect.
Fig 5. A concise summary of the study.
Black arrows indicate the observations in the groups exposed to 35°C compared with those subjected to 25°C thermal stress. Red arrows correspond to the results of the groups exposed to 35°C + ELF compared with those subjected to 35°C thermal stress. Upward arrows represent an increasing trend of the corresponding factor, whereas downward arrows show a decreasing trend of the corresponding factor.
Supporting Information
ELF-EMF was produced by two parallel Helmholtz coils (260 turns of copper wire with 40 cm diameter). The coils were then placed in an artificial climate incubator, which was utilized to control temperature, humidity, and light cycle during the experiment. A hose was wound around the coils and then connected to a condensed circulating water bath, which rapidly removed the heat produced by the coils. A temperature probe was set in the experimental zone to monitor, modify and strictly control its actual temperature.
(TIF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We are grateful to the Core Facility of Drosophila Resource and Technique of SIBCB, CAS for generously providing the Canton-S and w1118 flies.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by YZ201104, http://www.cas.cn/, Scientific Equipment Development Project of Chinese Academy of Sciences (CAS), Peng Cai; 3502Z20126012, http://www.xminfo.net.cn/, Xiamen Science and Technology Plans Project, Peng Cai; and 31270888, National Natural Science Foundation of China, Hui-Yong Lian. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wertheimer N, Leeper E. Electrical wiring configurations and childhood cancer. American journal of epidemiology. 1979;109(3):273–84. . [DOI] [PubMed] [Google Scholar]
- 2.Council EaS. The UNECE–ITU Smart Sustainable Cities Indicators. http://www.unece.org/fileadmin/DAM/hlm/projects/SMART_CITIES/ECE_HBP_2015_4.pdf.14/12/2015.
- 3.Zhao G, Lin X, Zhou M, Zhao J. Relationship between exposure to extremely low-frequency electromagnetic fields and breast cancer risk: a meta-analysis. European journal of gynaecological oncology. 2014;35(3):264–9. . [PubMed] [Google Scholar]
- 4.Panagopoulos DJ, Karabarbounis A, Lioliousis C. ELF alternating magnetic field decreases reproduction by DNA damage induction. Cell biochemistry and biophysics. 2013;67(2):703–16. 10.1007/s12013-013-9560-5 . [DOI] [PubMed] [Google Scholar]
- 5.Roosli M, Lortscher M, Egger M, Pfluger D, Schreier N, Lortscher E, et al. Mortality from neurodegenerative disease and exposure to extremely low-frequency magnetic fields: 31 years of observations on Swiss railway employees. Neuroepidemiology. 2007;28(4):197–206. 10.1159/000108111 . [DOI] [PubMed] [Google Scholar]
- 6.Guxens M, Slottje P, Kromhout H, Huss A, Ivar Martinsen J, Kauppinen T, et al. 0400 Occupational exposure to extremely low frequency magnetic fields or electric shocks and cancer incidence in four Nordic countries. Occupational and environmental medicine. 2014;71 Suppl 1:A50 10.1136/oemed-2014-102362.156 . [DOI] [Google Scholar]
- 7.Chen C, Ma X, Zhong M, Yu Z. Extremely low-frequency electromagnetic fields exposure and female breast cancer risk: a meta-analysis based on 24,338 cases and 60,628 controls. Breast cancer research and treatment. 2010;123(2):569–76. 10.1007/s10549-010-0782-6 . [DOI] [PubMed] [Google Scholar]
- 8.Podda MV, Leone L, Barbati SA, Mastrodonato A, Li Puma DD, Piacentini R, et al. Extremely low-frequency electromagnetic fields enhance the survival of newborn neurons in the mouse hippocampus. The European journal of neuroscience. 2014;39(6):893–903. 10.1111/ejn.12465 . [DOI] [PubMed] [Google Scholar]
- 9.Naito M, Hirai S, Mihara M, Terayama H, Hatayama N, Hayashi S, et al. Effect of a magnetic field on Drosophila under supercooled conditions. PloS one. 2012;7(12):e51902 10.1371/journal.pone.0051902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Z, Yu H, Sun Y, Yang C, Lian H, Cai P. The Energy Metabolism in Caenorhabditis elegans under The Extremely Low-Frequency Electromagnetic Field Exposure. Sci Rep. 2015;5:8471 10.1038/srep08471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawrence AF, Adey WR. Nonlinear wave mechanisms in interactions between excitable tissue and electromagnetic fields. Neurological research. 1982;4(1–2):115–53. . [DOI] [PubMed] [Google Scholar]
- 12.Frohlich H. What are non-thermal electric biological effects? Bioelectromagnetics. 1982;3(1):45–6. . [DOI] [PubMed] [Google Scholar]
- 13.Alfieri RR, Bonelli MA, Pedrazzi G, Desenzani S, Ghillani M, Fumarola C, et al. Increased levels of inducible HSP70 in cells exposed to electromagnetic fields. Radiat Res. 2006;165(1):95–104. . [DOI] [PubMed] [Google Scholar]
- 14.Bernardini C, Zannoni A, Turba ME, Bacci ML, Forni M, Mesirca P, et al. Effects of 50 Hz sinusoidal magnetic fields on Hsp27, Hsp70, Hsp90 expression in porcine aortic endothelial cells (PAEC). Bioelectromagnetics. 2007;28(3):231–7. 10.1002/bem.20299 . [DOI] [PubMed] [Google Scholar]
- 15.Li SS, Zhang ZY, Yang CJ, Lian HY, Cai P. Gene expression and reproductive abilities of male Drosophila melanogaster subjected to ELF-EMF exposure. Mutation research. 2013;758(1–2):95–103. 10.1016/j.mrgentox.2013.10.004 . [DOI] [PubMed] [Google Scholar]
- 16.Buldak RJ, Polaniak R, Buldak L, Zwirska-Korczala K, Skonieczna M, Monsiol A, et al. Short-term exposure to 50 Hz ELF-EMF alters the cisplatin-induced oxidative response in AT478 murine squamous cell carcinoma cells. Bioelectromagnetics. 2012;33(8):641–51. 10.1002/bem.21732 . [DOI] [PubMed] [Google Scholar]
- 17.Reale M, Kamal MA, Patruno A, Costantini E, D'Angelo C, Pesce M, et al. Neuronal cellular responses to extremely low frequency electromagnetic field exposure: implications regarding oxidative stress and neurodegeneration. PloS one. 2014;9(8):e104973 10.1371/journal.pone.0104973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorensen JG, Loeschcke V, Kristensen TN. Cellular damage as induced by high temperature is dependent on rate of temperature change—investigating consequences of ramping rates on molecular and organismal phenotypes in Drosophila melanogaster. The Journal of experimental biology. 2013;216(Pt 5):809–14. 10.1242/jeb.076356 . [DOI] [PubMed] [Google Scholar]
- 19.Zhang S, Fu W, Li N, Zhang F, Liu TX. Antioxidant responses of Propylaea japonica (Coleoptera: Coccinellidae) exposed to high temperature stress. J Insect Physiol. 2015;73:47–52. 10.1016/j.jinsphys.2015.01.004 . [DOI] [PubMed] [Google Scholar]
- 20.Junkersdorf B, Bauer H, Gutzeit HO. Electromagnetic fields enhance the stress response at elevated temperatures in the nematode Caenorhabditis elegans. Bioelectromagnetics. 2000;21(2):100–6. . [DOI] [PubMed] [Google Scholar]
- 21.Sullivan W, Ashburner M, Hawley RS. Drosophila protocols Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 2000. xiv, 697 p. p. [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. 10.1006/meth.2001.1262 . [DOI] [PubMed] [Google Scholar]
- 23.Pandey A, Vimal D, Chandra S, Saini S, Narayan G, Kar Chowdhuri D. Long-term dietary exposure to low concentration of dichloroacetic acid promoted longevity and attenuated cellular and functional declines in aged Drosophila melanogaster. Age. 2014;36(3):9628 10.1007/s11357-014-9628-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams KD, Helin AB, Posluszny J, Roberts SP, Feder ME. Effect of heat shock, pretreatment and hsp70 copy number on wing development in Drosophila melanogaster. Mol Ecol. 2003;12(5):1165–77. . [DOI] [PubMed] [Google Scholar]
- 25.Sorensen JG, Nielsen MM, Kruhoffer M, Justesen J, Loeschcke V. Full genome gene expression analysis of the heat stress response in Drosophila melanogaster. Cell stress & chaperones. 2005;10(4):312–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hercus MJ, Loeschcke V, Rattan SI. Lifespan extension of Drosophila melanogaster through hormesis by repeated mild heat stress. Biogerontology. 2003;4(3):149–56. . [DOI] [PubMed] [Google Scholar]
- 27.Le Bourg E, Valenti P, Lucchetta P, Payre F. Effects of mild heat shocks at young age on aging and longevity in Drosophila melanogaster. Biogerontology. 2001;2(3):155–64. . [DOI] [PubMed] [Google Scholar]
- 28.Jing Zhang, Ziyan Zhang, Chuanjun Yang, Peng C. Co-effects of extremely low frequency electromagnetic field (ELF-EMF) and temperature on HSP22 and HSP26 expression in drosophila melanogaster. Mil Med Sci. 2014;38(5):321–6. [Google Scholar]
- 29.Walter MF, Petersen NS, Biessmann H. Heat shock causes the collapse of the intermediate filament cytoskeleton in Drosophila embryos. Dev Genet. 1990;11(4):270–9. 10.1002/dvg.1020110405 . [DOI] [PubMed] [Google Scholar]
- 30.Rolandi C, Iglesias MS, Schilman PE. Metabolism and water loss rate of the haematophagous insect Rhodnius prolixus: effect of starvation and temperature. The Journal of experimental biology. 2014;217(Pt 24):4414–22. 10.1242/jeb.109298 . [DOI] [PubMed] [Google Scholar]
- 31.Mutero A, Bride JM, Pralavorio M, Fournier D. Drosophila melanogaster acetylcholinesterase: identification and expression of two mutations responsible for cold- and heat-sensitive phenotypes. Mol Gen Genet. 1994;243(6):699–705. . [DOI] [PubMed] [Google Scholar]
- 32.Jian W, Wei Z, Zhiqiang C, Zheng F. X-ray-induced apoptosis of BEL-7402 cell line enhanced by extremely low frequency electromagnetic field in vitro. Bioelectromagnetics. 2009;30(2):163–5. 10.1002/bem.20461 . [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Weng E, Zhang Y, Hong R. [Effects of extremely low frequency electromagnetic field and its combination with lead on the antioxidant system in mouse]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2002;20(4):263–5. . [PubMed] [Google Scholar]
- 34.Morono Y, Terada T, Yamamoto Y, Xiao N, Hirose T, Sugeno M, et al. Intact preservation of environmental samples by freezing under an alternating magnetic field. Environ Microbiol Rep. 2015;7(2):243–51. 10.1111/1758-2229.12238 . [DOI] [PubMed] [Google Scholar]
- 35.Kim BM, Rhee JS, Jeong CB, Seo JS, Park GS, Lee YM, et al. Heavy metals induce oxidative stress and trigger oxidative stress-mediated heat shock protein (hsp) modulation in the intertidal copepod Tigriopus japonicus. Comparative biochemistry and physiology Toxicology & pharmacology: CBP. 2014;166:65–74. 10.1016/j.cbpc.2014.07.005 . [DOI] [PubMed] [Google Scholar]
- 36.Jammes Y, Steinberg JG, Delliaux S, Bregeon F. Chronic fatigue syndrome combines increased exercise-induced oxidative stress and reduced cytokine and Hsp responses. J Intern Med. 2009;266(2):196–206. 10.1111/j.1365-2796.2009.02079.x . [DOI] [PubMed] [Google Scholar]
- 37.Grover N, Sankhyan N, Bisht JP. A five year review of clinical profile in HSP. JNMA J Nepal Med Assoc. 2007;46(166):62–5. . [PubMed] [Google Scholar]
- 38.Krebs RA, Feder ME. Tissue-specific variation in Hsp70 expression and thermal damage in Drosophila melanogaster larvae. The Journal of experimental biology. 1997;200(Pt 14):2007–15. . [DOI] [PubMed] [Google Scholar]
- 39.Malagoli D, Lusvardi M, Gobba F, Ottaviani E. 50 Hz magnetic fields activate mussel immunocyte p38 MAP kinase and induce HSP70 and 90. Comparative biochemistry and physiology Toxicology & pharmacology: CBP. 2004;137(1):75–9. 10.1016/j.cca.2003.11.007 . [DOI] [PubMed] [Google Scholar]
- 40.Yang J, Tower J. Expression of hsp22 and hsp70 transgenes is partially predictive of drosophila survival under normal and stress conditions. The journals of gerontology Series A, Biological sciences and medical sciences. 2009;64(8):828–38. 10.1093/gerona/glp054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tipping DR, Chapman KE, Birley AJ, Anderson M. Observations on the effects of low frequency electromagnetic fields on cellular transcription in Drosophila larvae reared in field-free conditions. Bioelectromagnetics. 1999;20(2):129–31. . [PubMed] [Google Scholar]
- 42.Villarini M, Ambrosini MV, Moretti M, Dominici L, Taha E, Piobbico D, et al. Brain hsp70 expression and DNA damage in mice exposed to extremely low frequency magnetic fields: a dose-response study. International journal of radiation biology. 2013;89(7):562–70. 10.3109/09553002.2013.782449 . [DOI] [PubMed] [Google Scholar]
- 43.Heredia-Rojas JA, Rodriguez de la Fuente AO, Alcocer Gonzalez JM, Rodriguez-Flores LE, Rodriguez-Padilla C, Santoyo-Stephano MA, et al. Effect of 60 Hz magnetic fields on the activation of hsp70 promoter in cultured INER-37 and RMA E7 cells. In Vitro Cell Dev Biol Anim. 2010;46(9):758–63. 10.1007/s11626-010-9342-y . [DOI] [PubMed] [Google Scholar]
- 44.Morrow G, Samson M, Michaud S, Tanguay RM. Overexpression of the small mitochondrial Hsp22 extends Drosophila life span and increases resistance to oxidative stress. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2004;18(3):598–9. 10.1096/fj.03-0860fje . [DOI] [PubMed] [Google Scholar]
- 45.Tower J, Landis G, Gao R, Luan A, Lee J, Sun Y. Variegated expression of Hsp22 transgenic reporters indicates cell-specific patterns of aging in Drosophila oenocytes. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(3):253–9. 10.1093/gerona/glt078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liao PC, Lin HY, Yuh CH, Yu LK, Wang HD. The effect of neuronal expression of heat shock proteins 26 and 27 on lifespan, neurodegeneration, and apoptosis in Drosophila. Biochemical and biophysical research communications. 2008;376(4):637–41. 10.1016/j.bbrc.2008.08.161 . [DOI] [PubMed] [Google Scholar]
- 47.Chatelain EH, Pichaud N, Ballard JW, Tanguay RM, Morrow G, Blier PU. Functional conservatism among Drosophila simulans flies experiencing different thermal regimes and mitochondrial DNA introgression. Journal of experimental zoology Part B, Molecular and developmental evolution. 2011;316B(3):188–98. 10.1002/jez.b.21389 . [DOI] [PubMed] [Google Scholar]
- 48.Sharma S, Thakurdas P, Sinam B, Joshi D. Paradoxical masking effects of bright photophase and high temperature in Drosophila malerkotliana. Chronobiol Int. 2012;29(2):157–65. 10.3109/07420528.2011.644875 . [DOI] [PubMed] [Google Scholar]
- 49.Al-Akhras MA. Influence of 50 Hz magnetic field on sex hormones and body, uterine, and ovarian weights of adult female rats. Electromagnetic biology and medicine. 2008;27(2):155–63. 10.1080/15368370802072125 . [DOI] [PubMed] [Google Scholar]
- 50.Qin S, Yin H, Yang C, Dou Y, Liu Z, Zhang P, et al. A magnetic protein biocompass. Nat Mater. 2015. 10.1038/nmat4484 . [DOI] [PubMed] [Google Scholar]
- 51.Yoshii T, Todo T, Wulbeck C, Stanewsky R, Helfrich-Forster C. Cryptochrome is present in the compound eyes and a subset of Drosophila's clock neurons. J Comp Neurol. 2008;508(6):952–66. 10.1002/cne.21702 . [DOI] [PubMed] [Google Scholar]
- 52.Baker MK, Abrams CF. Dynamics of lipids, cholesterol, and transmembrane alpha-helices from microsecond molecular dynamics simulations. J Phys Chem B. 2014;118(47):13590–600. 10.1021/jp507027t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ward BK, Roberts DC, Della Santina CC, Carey JP, Zee DS. Vestibular stimulation by magnetic fields. Ann N Y Acad Sci. 2015. 10.1111/nyas.12702 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hofling F, Munk T, Frey E, Franosch T. Critical dynamics of ballistic and Brownian particles in a heterogeneous environment. J Chem Phys. 2008;128(16):164517 10.1063/1.2901170 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ELF-EMF was produced by two parallel Helmholtz coils (260 turns of copper wire with 40 cm diameter). The coils were then placed in an artificial climate incubator, which was utilized to control temperature, humidity, and light cycle during the experiment. A hose was wound around the coils and then connected to a condensed circulating water bath, which rapidly removed the heat produced by the coils. A temperature probe was set in the experimental zone to monitor, modify and strictly control its actual temperature.
(TIF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.