Abstract
Background
Five-year survival rates among stage IIIA lung cancer patients range between 2% and 15%, and there is currently no consensus regarding optimal treatment approaches for these patients. The current investigation evaluated survival outcomes among stage IIIA lung cancer patients receiving 2 different treatment modalities, neoadjuvant chemotherapy followed by resection versus chemoradiation alone.
Material/Methods
This retrospective study is based on 127 patients attending the Lung Cancer Evaluation Center at Stony Brook Cancer Center between 2002 and 2014. Patients were treated either with neoadjuvant chemotherapy followed by resection or a regimen of chemoradiation alone. Kaplan-Meier curves were used to compare survival outcomes between groups and Cox proportional hazard models were used to evaluate treatment effects on survival, while adjusting for possible confounders.
Results
Approximately one-fourth (n=33) of patients received neoadjuvant chemotherapy followed by surgery, whereas 94 patients received definitive chemoradiation. Patients in the surgical group were found to be significantly younger than those receiving chemoradiation alone (60.1 vs. 67.9 years, respectively; p=0.001). Five-year survival among patients receiving preoperative chemotherapy followed by resection was significantly higher than that among patients receiving chemoradiation alone (63% vs. 19%, respectively; p<0.001), whereas the hazard ratio (HR) was 3–4 times greater in the latter group (HR=3.77, 95% confidence interval=1.87, 7.61).
Conclusions
Findings from this study indicate that preoperative chemotherapy followed by resection can improve survival outcomes for stage IIIA lung cancer patients compared with chemoradiation alone. The results reflect a select surgical group of patients; thus, the data highlight the need to develop new therapies that may result in more patients being viable surgical candidates.
MeSH Keywords: Antineoplastic Agents; Carcinoma, Non-Small-Cell Lung; Thoracic Surgery
Background
Lung cancer is the leading cause of cancer mortality in the United States for both men and women with nearly 158,000 deaths expected this year [1]. Stage IIIA lung cancer patients represent a heterogeneous group with a diversity of tumor characteristics and varying degrees of lymph node involvement ranging from microscopic ipsilateral mediastinal lymph node invasion to chest wall infringement and superior sulcus tumors. An estimated 15% of lung cancer patients present with stage IIIA non-small cell lung cancer (NSCLC) [2] and those with clinical stage IIIA N2 disease have an overall 5-year survival rate of 10–15%. Patients with bulky mediastinal involvement tend to have poorer outcomes with 5-year survival reported between 2% and 5% [2].
Current treatment modalities typically include surgery followed by adjuvant chemotherapy, neoadjuvant therapy followed by surgical resection, or definitive chemoradiation; however, to date a consensus has not been reached as to which approach is most efficacious [3–9]. The management of stage IIIA lung cancer patients remains controversial because of a lack of evidence-based treatment guidelines [10] mainly resulting from concerns related to treatment toxicities, surgical complications, and other factors [7]. We compared stage IIIa patients with proven N2 disease who were treated with neoadjuvant chemotherapy followed by resection with patients who received definitive chemoradiation.
Material and Methods
A retrospective investigation of patients attending the Lung Cancer Evaluation Center (LCEC) at the Stony Brook Cancer Center, Stony Brook, New York, was undertaken to evaluate survival outcomes of stage IIIA lung cancer patients. All biopsy-proven stage IIIA patients with cancers confined to the lung and mediastinum diagnosed with NSCLC between 2002 and 2014 who had obtainable survival status for 5 years postdiagnosis were included in this study. Data abstracted from the LCEC database included age, gender, tumor size, treatment modality (neoadjuvant chemotherapy plus resection or definitive chemoradiation), tumor type (adenocarcinoma, squamous cell, or other type), N2 status (positive vs. negative), lymph node involvement/staging, and survival time. Only patients with clinically staged IIIA disease were included. Upstaged patients after surgery were not included.
All patients initially underwent positron emission tomography/computed tomography (PET/CT) scan with endobronchial ultrasound bronchoscopy (adopted by Stony Brook Cancer Center as a standard practice in 2001) to assess the mediastinum. Patients who had bulky disease (lymph nodes 2.5 cm or contiguous disease extending from lung into mediastinum) were more likely to be treated with definitive chemoradiation, adjudicated by a multidisciplinary tumor board. During this time patients with proven N2 disease were not considered for up-front surgery with adjuvant therapy.
All other patients were deemed suitable for possible resection and received 2 to 3 cycles of neoadjuvant platinum-based chemotherapy (Taxol 200 mg/m2 and carboplatinum area under the curve [AUC]=6.0).
This group underwent repeat PET/CT and mediastinoscopy. If the mediastinoscopy was positive for residual N2 disease, the patients were treated with definitive chemoradiation, which included 4 cycles of concomitant platinum-based chemotherapy (50 mg/m2/week and carboplatinum AUC=2.0] and 58G over 30 sessions. Follow-up for those receiving resection included a CT scan at 1 month, CT scans every 6 months for 2 years, PET scan at 1 year, and CT scans annually during years 3 through 5. Negative mediastinoscopy was followed by surgery and 2 more cycles of chemotherapy (usually Taxol 200 mg/m2 and carboplatinum AUC=6.0 or cisplatin 75 mg/m2 and docetaxel 75 mg/m2). The up-front definitive chemoradiation group received 6 cycles of concomitant platinum-based chemotherapy (50 mg/m2/week and carboplatinum AUC=2.0) and 58G over 30 sessions Follow-up in this group included CT scans every 3 months and PET/CT at 1 year and 2 years, followed by a CT scan every 7 months thereafter, including at year 5. Follow-up for those receiving resection included a CT scan at 1 month, CT scans every 6 months for 2 years, PET scan at 1 year, and CT scans annually during years 3 through 5. Overall survival for both groups was compared at 1, 3, and 5 years, respectively, beginning at the date of diagnosis. This investigation was approved by Stony Brook University’s Institutional Review Board and Committee on Research Involving Human Subjects.
Statistical analyses
Descriptive statistics are presented as frequencies (percentages) for discrete variables and means (standard deviations) for continuous data. Differences between groups were evaluated using chi-square tests for categorical data, t-tests for continuous (independent) data, and paired t-tests for comparisons of baseline versus follow-up measurements. Kaplan-Meier curves and log-rank tests were used to display and evaluate the differences in survival outcomes between groups. Cox proportional hazard models were used to provide estimates of the treatment effects on survival while adjusting for age and gender. Hazard ratios (HRs) and 95% confidence intervals (CIs) are presented.
SPSS version 21 was used to conduct these analyses.
Results
This investigation included 127 patients with stage IIIA lung cancer confined to lung and mediastinum. Approximately one-fourth of cases (n=33) received neoadjuvant therapy followed by surgery, whereas 94 (74%) were treated with definitive chemoradiation. The demographic characteristics of these patients, stratified by treatment modality, are presented in Table 1. Although the gender distribution was similar in both groups, those receiving neoadjuvant chemotherapy with subsequent resection were significantly younger than those in the chemoradiation group (60.1 vs. 67.9 years, respectively; p=0.001). Among 32 patients with pathologically confirmed disease, one-half were diagnosed with adenocarcinoma and approximately one-third with squamous cell carcinoma. Bulky disease, as defined by lymph nodes ≥2.5 cm, was significantly more common in the definitive chemoradiation group than in the surgery group (p<0.0002).
Table 1.
Patient demographics of N=127 stage IIIA lung cancer patients by treatment group.
| Characteristic | Neoadjuvant therapy + surgery (n=33) | Chemoradiation alone (n=94) | P-value |
|---|---|---|---|
| Age, years (mean ±sd) | 60.1±11.1 | 67.9±10.7 | 0.001 |
| Gender | 0.84 | ||
| Male, n (%) | 18 (54.5%) | 54 (57.4%) | |
| Female, n (%) | 15 (45.5%) | 40 (42.6%) | |
| Histology* | N/A | ||
| Adenocarcinoma, n (%) | 16 (50%) | ||
| Squamous cell, n (%) | 11 (34.4%) | ||
| Adenosquamous, n (%) | 2 (6.3%) | ||
| Other, n (%) | 3 (9.4%) | ||
| Lymph Node ≥2.5 cm | 6 (18.2%) | 68 (72.3%) | <0.0002 |
Histology findings among n=32 lung cancer cases with a confirmed pathological diagnosis.
Tumor characteristics, prior to and following preoperative chemotherapy, are presented in Table 2. Among those receiving neoadjuvant chemotherapy, tumor size was reduced in 48% of patients and remained stable in 39% of those treated, prior to surgery. The average tumor size was approximately 3.7 (±1.8) cm at diagnosis, and the neoadjuvant therapy yielded a 25% reduction. Standardized uptake value (SUV) tumor and lymph node measurements decreased by more than 50% as a result of the neoadjuvant therapy regimen. SUV tumor and lymph node responses yielded complete or partial reductions in more than 70% of patients, with stable values maintained in approximately one-fifth of those treated. Disease progression was noted in 5% to 15% of patients in this group.
Table 2.
Tumor characteristics for patients receiving neoadjuvant therapy prior to surgery.
| Characteristic | p-value* | |
|---|---|---|
| Tumor size, cm | <0.01 | |
| Initial (mean ±sd) | (3.7±1.8) | |
| Follow-up (mea n±sd) | (2.7±1.3) | |
| % change | 25% | |
| SUV tumor | <0.01 | |
| Initial (mean ±sd) | (11.6±4.4) | |
| Follow-up (mean ±sd) | (4.7±2.5) | |
| % change | 56% | |
| SUV lymph nodes | <0.01 | |
| Initial (mean ±sd) | (6.1±3.5) | |
| Follow-up (mean ±sd) | (2.5±2.2) | |
| % change | 51% |
Based on paired t-test.
Table 3 presents the 1-, 3- and 5-year survival outcomes for study patients stratified by treatment group. Both short-term (1-year) and longer term (5-year) survival rates were significantly higher among patients receiving neoadjuvant chemotherapy followed by resection compared with those treated with chemoradiation alone. Survival rates at 1 year postsurgery in the neoadjuvant group were 94% compared with 63% in the chemoradiation group (p=0.001). The 5-year Kaplan-Meier survival curves are presented in Figure 1 and indicate that rates were significantly more favorable among those treated with neoadjuvant therapy followed by surgery, with 5-year survival rates found to be 3 times higher among those resected compared with patients who did not undergo surgery (63% vs. 19%, respectively; p<0.001).
Table 3.
1-, 3-, and 5-year survival rates for patients with stage IIIA lung cancer stratified by treatment modality.
| Survival time | Neoadjuvant therapy + surgery (n=33) | Chemoradiation alone (n=94) | P-value |
|---|---|---|---|
| 1-year | 94% | 63% | 0.001 |
| 3-years | 69% | 27% | <0.001 |
| 5-years | 63% | 19% | <0.001 |
Figure 1.
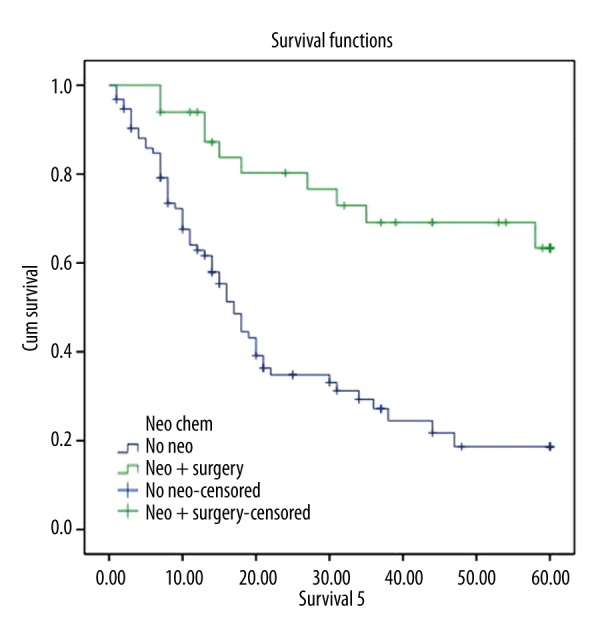
Five-year Kaplan-Meier survival curve for stage IIIA lung cancer patients receiving either neoadjuvant therapy followed by resection or definitive chemoradiation.
The effect of treatment modality on survival, while adjusting for age and gender, was estimated using HRs resulting from Cox Proportional Hazard Models. The HR was 3–4 times higher in the definitive chemoradiation group compared with the neoadjuvant chemotherapy plus resection group (HR=3.77, 95% CI=1.87, 7.61). Although age at diagnosis was not found to be significant in the Cox models, males tended to have poorer outcomes than females (HR=1.64, 95% CI=1.00, 2.68).
Discussion
This investigation yielded statistically significant improvements in survival for patients receiving neoadjuvant chemotherapy and surgery compared with those receiving definitive chemoradiation. Although there is continued debate surrounding optimal treatment options for stage IIIA lung cancer patients, neoadjuvant chemotherapy was shown to reduce tumor size by 25% and SUV (for tumor and lymph nodes) by more than 50% prior to surgery in this study. Inductive chemotherapy combined with resection resulted in significantly better outcomes at 5 years compared with a regimen of definitive chemoradiation alone (5-year survival rates: 63% vs. 19%, respectively). The debate thus is no longer if neoadjuvant chemotherapy works but rather why does it fail or why is it not used more often.
Treatment modalities
Confining the current discussion to stage IIIA lung cancer patients with N2 disease, the National Cancer Institute guidelines suggest that if complete resection of the tumor and lymph nodes is possible, such patients may benefit from surgery followed by postoperative chemotherapy, with the debate over the benefit of complete mediastinal lymph node dissection versus lymph node sampling ongoing despite a current stalemate [11]. A second treatment option includes the use of neoadjuvant chemotherapy prior to surgery. Proposed arguments for the benefits of adding preoperative chemotherapy to the treatment plan include a possible reduction in tumor size that may facilitate surgical resection, possible contribution to early eradication of developing metastases, and the fact that chemotherapy is often better tolerated preoperatively than postoperatively. A third strategy for treating advanced (resectable) lung cancer is to add a radiation regimen to the neoadjuvant chemotherapy protocol. It has been hypothesized that the addition may lead to intensified treatment and increased likelihood of downstaging the tumor. In the absence of conclusive evidenced-based trials, the controversy as to which treatment approach or combination of approaches is ongoing.
The Cochran Collaboration Group provided a systematic review and meta-analysis of 7 randomized controlled trials and reported an absolute benefit of 6% among patients receiving neoadjuvant chemotherapy followed by resection versus surgery alone at all stages (I through III) of resectable lung cancer [12,13]. Additionally, Gilligan et al. found a 12% relative survival benefit with preoperative chemotherapy, which was equivalent to an absolute improvement in survival of 5% at 5 years [13]. Although these investigations provide strong support for the use of neoadjuvant chemotherapy followed by resection, both investigations included patients with stage I through III lung cancer, and only a small representation of cases (7%) were diagnosed with stage III disease. Thus, it remains unclear whether the noted benefits are as compelling in patients with more advanced disease.
The largest related phase III randomized clinical trial to date, the Intergroup 0139 Trial, included 396 stage IIIA NSCLC patients who received induction chemotherapy plus radiotherapy followed by either resection (n=202) or continued radiotherapy (n=194). The results indicated that surgery did not improve overall survival; however, those undergoing lobectomy (but not pneumonectomy) had improved outcomes compared with those receiving an additional radiotherapy regimen without resection [4]. The Intergroup 0139 Trial also found that although progression-free survival and less local recurrence were improved, this improvement came at a cost of increased treatment mortality of 8% versus 2%, respectively.
Additional investigations have been undertaken to evaluate the efficacy of adding radiotherapy to a neoadjuvant chemotherapy regimen prior to surgery. Again, the results have been inconsistent. A meta-analysis of 7 relatively small studies found that preoperative chemoradiation did not confer a survival benefit for stage IIIA lung cancer patients [3].However, the most recent study published to date by Darling et al. found that neoadjuvant chemoradiation and surgery led to better survival outcomes than definitive chemoradiation in a retrospective analysis of 215 resectable stage IIIA-N2 patients [6].
There remains a lack of consensus on the extent of surgical intervention, role of neoadjuvant therapies, and optimal chemotherapy and radiation regimens that would guide treatment in patients with advanced lung cancer [8]. Significant toxicities and high morbidity profiles have been reported among patients receiving chemoradiation [4,14], and surgical and postsurgical complications resulting from resection also represent serious concerns. Mortality rates resulting from lung resections have been reported to range from 1% to 6% among patients who did not receive preoperative therapy, with higher rates among those undergoing pneumonectomy compared with lobectomy [15–17].Corresponding rates among those receiving neoadjuvant therapy range from 2% to 12% [7,17–20]. Although it has been hypothesized that surgery may improve survival by removing microscopic disease [6], patients undergoing resection have been shown to experience higher treatment-related mortality due to respiratory issues and have been less able to complete postoperative chemotherapy protocols compared with patients who do not receive surgery [4]. Due to the toll that anatomic resection takes on the body, patients deemed suitable to receive surgical intervention are typically healthier and have a lower likelihood of operative complications [15–17,09,21].
In the present investigation, one-quarter of our patients underwent surgery after responding to neoadjuvant chemotherapy. It remains unclear whether the improved outcomes in this group were the result of their superior overall health status, positive response to the preoperative therapy, the surgery itself, or some combination of these and other factors.
Survival outcomes
According to the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) data, the overall 5-year survival rate for stage IIIA lung cancer patients is 14% [22]. Although the SEER estimates are based on large national statistics, data are limited with regard to survival outcomes stratified by specific treatment modalities. Available data from the Radiation Therapy Oncology Group phase III trial for stage IIIA NSCLC, which aimed to compare survival outcomes in patients receiving induction chemotherapy followed by either surgery (n=29) or radiotherapy (n=32) [23], found that the 1-, 3-, and 4-year survival rates for the surgery group were 70%, 33%, and 22%, respectively. The corresponding rates among those receiving radiotherapy (without resection) were 66%, 22%, and 22%, respectively. The findings were comparable between the surgical and nonsurgical treatment modalities, and the noted survival rates were consistent with those reported by SEER. In a second study, however, the 3-year overall survival rate for a selected group of resectable stage IIIA NSCLC patients receiving induction chemotherapy plus radiotherapy followed be surgery was significantly higher (51.7%) [7].
Inarguably, the healthiest patients were offered a surgical resection after neoadjuvant chemotherapy. However, in which patients does neoadjuvant therapy improve outcomes? When assessing the surgical risk, it is not just a question of immediate postoperative survival, but also of long-term survival. The criticism is that not enough patients were offered surgery. It is also clear that increasing the number of operations will lower the survival rate with present chemotherapy. Acceptable survival rates and determinations regarding an acceptable percentage of stage IIIA patients deemed eligible for surgery have not been established. Further, the role of bulky disease as a confounder for the choice of therapy, particularly involving surgery, is poorly understood.
In the present investigation, comparing patients receiving neoadjuvant therapy followed by resection with those receiving chemoradiation alone, statistically significant differences in length of survival were noted. At 1 year, survival rates were 94% and 63% for the 2 groups, respectively, with 5-year rates found to be 63% and 19%, respectively. The survival outcomes found among LCEC patients in this study who received neoadjuvant chemotherapy plus surgery were 3–4 times higher than those in other reports [22,23]. Additionally, it is worth noting that in this investigation, 5-year survival among patients receiving chemoradiation alone (in the absence of surgery) was twice as high as national reported averages [22–24].
Conclusions
Our findings indicate that neoadjuvant chemotherapy followed by surgery results in improved 5-year survival of stage IIIA N2 lung cancer patients compared with definitive chemoradiation alone. These data support the use of preoperative chemotherapy followed by resection in patients for which surgery is an option and further highlights the need to develop new neoadjuvant therapies that would result in more patients being surgical candidates. Although this treatment approach is highly selective, with the healthiest and best-suited candidates being offered surgery, the findings add to a relatively limited evidence base regarding optimal treatment approaches for patients with stage IIIA lung cancer.
Footnotes
Source of support: Departmental sources
References
- 1.ACS. 2014 Cancer Survivorship Statistics – 10 Key Facts 2014. [cited 2015 May 26]. Available from: http://www.cancer.org/research/acsresearchupdates/more/2014-cancer-survivorship-statistics-10-key-facts.
- 2.Surveillance, epidemiology and end results: US National Institutes of Health. Bethesda, MD: National Cancer Institute; 2010. [Google Scholar]
- 3.Shah AA, Berry MF, Tzao C, et al. Induction chemoradiation is not superior to induction chemotherapy alone in stage IIIA lung cancer. Ann Thorac Surg. 2012;93(6):1807–12. doi: 10.1016/j.athoracsur.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: A phase III randomised controlled trial. Lancet. 2009;374(9687):379–86. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: Long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160) J Clin Oncol. 2007;25(3):313–18. doi: 10.1200/JCO.2006.08.2826. [DOI] [PubMed] [Google Scholar]
- 6.Darling GE, Li F, Patsios D, et al. Neoadjuvant chemoradiation and surgery improves survival outcomes compared with definitive chemoradiation in the treatment of stage IIIA N2 non-small-cell lung cancer. Eur J Cardiothorac Surg. 2015;48(5):684–90. doi: 10.1093/ejcts/ezu504. [DOI] [PubMed] [Google Scholar]
- 7.Uy KL, Darling G, Xu W, et al. Improved results of induction chemoradiation before surgical intervention for selected patients with stage IIIA-N2 non-small cell lung cancer. J Thorac Cardiovasc Surg. 2007;134(1):188–93. doi: 10.1016/j.jtcvs.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 8.Veeramachaneni NK, Feins RH, Stephenson BJ, et al. Management of stage IIIA non-small cell lung cancer by thoracic surgeons in North America. Ann Thorac Surg. 2012;94(3):922–26. doi: 10.1016/j.athoracsur.2012.04.087. discussion 926–28. [DOI] [PubMed] [Google Scholar]
- 9.Paul S, Mirza F, Port JL, et al. Survival of patients with clinical stage IIIA non-small cell lung cancer after induction therapy: Age, mediastinal downstaging, and extent of pulmonary resection as independent predictors. J Thorac Cardiovasc Surg. 2011;141(1):48–58. doi: 10.1016/j.jtcvs.2010.07.092. [DOI] [PubMed] [Google Scholar]
- 10.Robinson LA, Ruckdeschel JC, Wagner H, Jr, Stevens CW American College of Chest Physicians. Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):243S–65S. doi: 10.1378/chest.07-1379. [DOI] [PubMed] [Google Scholar]
- 11.Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: Initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg. 2006;81(3):1013–19. doi: 10.1016/j.athoracsur.2005.06.066. discussion 1019–20. [DOI] [PubMed] [Google Scholar]
- 12.Burdett SS, Stewart LA, Rydzewska L. Chemotherapy and surgery versus surgery alone in non-small cell lung cancer. Cochrane Database Syst Rev. 2007;(3):CD006157. doi: 10.1002/14651858.CD006157.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilligan D, Nicolson M, Smith I, et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: Results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet. 2007;369(9577):1929–37. doi: 10.1016/S0140-6736(07)60714-4. [DOI] [PubMed] [Google Scholar]
- 14.Seder CW, Allen MS, Cassivi SD, et al. Stage IIIA non-small cell lung cancer: Morbidity and mortality of three distinct multimodality regimens. Ann Thorac Surg. 2013;95(5):1708–16. doi: 10.1016/j.athoracsur.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 15.Fang D, Zhang D, Huang G, et al. Results of surgical resection of patients with primary lung cancer: a retrospective analysis of 1,905 cases. Ann Thorac Surg. 2001;72(4):1155–59. doi: 10.1016/s0003-4975(01)02932-0. [DOI] [PubMed] [Google Scholar]
- 16.Ginsberg RJ, Hill LD, Eagan RT, et al. Modern thirty-day operative mortality for surgical resections in lung cancer. J Thorac Cardiovasc Surg. 1983;86(5):654–58. [PubMed] [Google Scholar]
- 17.Stamatis G, Djuric D, Eberhardt W, et al. Postoperative morbidity and mortality after induction chemoradiotherapy for locally advanced lung cancer: an analysis of 350 operated patients. Eur J Cardiothorac Surg. 2002;22(2):292–97. doi: 10.1016/s1010-7940(02)00266-x. [DOI] [PubMed] [Google Scholar]
- 18.Molnar TF, Baliko Z, Sarosi V, Horvath PO. Survival after surgery following chemotherapy for non-small-cell lung cancer. Asian Cardiovasc Thorac Ann. 2010;18(2):141–46. doi: 10.1177/0218492310361271. [DOI] [PubMed] [Google Scholar]
- 19.Martin J, Ginsberg RJ, Abolhoda A, et al. Morbidity and mortality after neoadjuvant therapy for lung cancer: the risks of right pneumonectomy. Ann Thorac Surg. 2001;72(4):1149–54. doi: 10.1016/s0003-4975(01)02995-2. [DOI] [PubMed] [Google Scholar]
- 20.Clamon GH, Parekh KR. Mortality related to neoadjuvant therapy and surgery for stage III non-small-cell lung cancer. Clin Lung Cancer. 2008;9(4):213–16. doi: 10.3816/CLC.2008.n.031. [DOI] [PubMed] [Google Scholar]
- 21.Doddoli C, Barlesi F, Trousse D, et al. One hundred consecutive pneumonectomies after induction therapy for non-small cell lung cancer: An uncertain balance between risks and benefits. J Thorac Cardiovasc Surg. 2005;130(2):416–25. doi: 10.1016/j.jtcvs.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Surveillance, epidemiology and end results: US National Institutes of health. Bethesda, MD: National Cancer Institute; 200. [Google Scholar]
- 23.Johnstone DW, Byhardt RW, Ettinger D, Scott CB. Phase III study comparing chemotherapy and radiotherapy with preoperative chemotherapy and surgical resection in patients with non-small-cell lung cancer with spread to mediastinal lymph nodes (N2); Final report of RTOG 89-01. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 2002;54(2):365–69. doi: 10.1016/s0360-3016(02)02943-7. [DOI] [PubMed] [Google Scholar]
- 24.Rowell NP, O’Rourke NP. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev. 2004;(4):CD002140. doi: 10.1002/14651858.CD002140.pub2. [DOI] [PubMed] [Google Scholar]


