Abstract
Group A Streptococcus (GAS) causes diverse infections ranging from common pharyngitis to rare severe invasive infections. Invasive GAS isolates can have natural mutations in the virulence regulator CovRS, which result in enhanced expression of multiple virulence genes, suppressed the expression of the protease SpeB, and increased virulence. It is believed that CovRS mutations arise during human infections with GAS carrying wild-type CovRS and are not transmissible. CovRS mutants of invasive GAS of the emm1 genotype arise readily during experimental infection in mice. It is possible that invasive GAS arises from pharyngeal GAS through rare genetic mutations that confer the capacity of mutated GAS to acquire covRS mutations during infection. The objective of this study was to determine whether contemporary pharyngeal emm1 GAS isolates have a reduced propensity to acquire CovRS mutations in vivo compared with invasive emm1 GAS and whether emm3, emm12, and emm28 GAS acquire CovRS mutants in mouse infection. The propensity of invasive and pharyngeal emm1 and invasive emm3, emm12, and emm28 SpeBA+ isolates to acquire variants with the SpeBA- phenotype was determined during subcutaneous infection of mice. The majority of both invasive and pharyngeal emm1 SpeBA+ isolates and two of three emm12 isolates, but not emm3 and emm28 isolates, were found to acquire SpeBA- variants during skin infection in mice. All analyzed SpeBA- variants of emm1 and emm12 GAS from the mouse infection acquired covRS mutations and produced more platelet-activating factor acetylhydrolase SsE. Thus, contemporary invasive and pharyngeal emm1 GAS isolates and emm12 GAS have a similar capacity to acquire covRS mutations in vivo. The rarity of severe invasive infections caused by GAS does not appear to be attributable to a reduced ability of pharyngeal isolates to acquire CovRS mutations.
Introduction
Group A Streptococcus (GAS) is a major human pathogen that causes both relatively mild pharyngitis and superficial skin infections and potentially lethal, severe invasive infections [1]. Severe GAS infections were frequent and often fatal in the 19th century and reemerged in the 1980s [2]. The reemergence of severe invasive GAS infections in the 1980s is associated with the emergence of a virulent M1T1 clone of emm1 GAS [3–5] and virulent emm3 GAS [6]. The M1T1 clone of emm1 GAS is evolved by the acquisition of DNase Sda1- and superantigen SpeA-encoding prophages and the replacement of a 36-kb chromosomal region of pre-1980 M1 GAS with that of emm12 GAS that contains the NADase and streptolysin O genes [7–9]. Contemporary M3 GAS acquired a prophage that encodes the superantigen SpeK and phospholipase A2 SlaA [6]. Since 2000, M89 GAS without the genes for synthesis of the hyaluronic acid capsule has also emerged to cause severe invasive infections in the United Kingdom, as well as the United States, Finland, Iceland, and Portugal [10–12].
Invasive GAS isolates frequently exhibit a greater capacity to invade soft tissues and evade neutrophil responses in association with higher virulence in experimental animal infections in comparison to pharyngeal isolates. Natural acquisition of mutations of the two-component regulatory system CovRS (also known as CrsRS) leads to hypervirulence [13–19]. Invasive GAS isolates frequently carry CovRS mutations [20,21], which appear to arise during human infections with GAS carrying wild-type CovRS and are not transmissible [22]. CovRS negatively regulates many virulence factors, including most of those that are involved in the evasion of innate immunity [23–25]. As results of CovRS mutations, loss of production of the protease SpeB and enhanced production of the hyaluronic acid capsule and platelet-activating factor acetylhydrolase SsE contribute to the phenotype of hypervirulent invasive M1T1 and M3 GAS isolates [26–32].
The emergence of CovRS mutants of invasive M1T1 GAS has been readily demonstrated during experimental mouse infections [8,13,14,17,31–33]. Not all emm1 GAS strains are subject to selection of CovRS mutations during skin and soft tissue infections [8]. The DNase sda1 and the hasA and M protein (emm) genes are reported to be required for the selection of CovRS mutants in the M1T1 strain 5448 [31,32]. However, the role of Sda1 in the selection of CovRS mutants of M1T1 GAS has not been confirmed [33]. Among the host factors important for in vivo selection of invasive M1T1 GAS CovRS mutants, neutrophils are required for selection of covRS mutants in mouse infection, and M1T1 CovRS mutants exhibit greater resistance to neutrophils in vivo [17].
Approximately 15 million cases of streptococcal pharyngitis and 10,000 cases of severe invasive GAS disease occur annually in the United States. The basis for the rarity of severe invasive GAS infections compared with common strep throat is not well understood. It is well known that isolates that cause invasive disease are the same as those circulating in the upper respiratory tracts of the community [34], and contemporary pharyngeal emm1 isolates from Finland and emm1 invasive isolates from diverse geographic areas are closely related to the sequenced M1T1 GAS strain MGAS5005 [9]. However, there are single nucleotide polymorphisms (SNP) and indels among isolates of the M1T1 clone [9,14]. It is possible that invasive GAS arises from rare genetic mutations of pharyngeal GAS that confer the capacity of mutated GAS to acquire covRS mutations during infection. MGAS2221, a pharyngeal M1T1 isolate, has been shown to acquire covRS mutations during murine infection [14], inconsistent with the possibility. However, this strain was isolated from a patient with scarlet fever and may not represent a typical pharyngitis case. Thus, parallel comparison with multiple invasive and pharyngeal emm1 isolates in the capacity to acquire covRS mutations should be made to test whether pharyngeal and invasive emm1 isolates have differential capacity to acquire covRS mutations.
The most common emm genotypes associated with contemporary severe invasive infections in the United States are emm1, emm28, emm12, emm3, and emm11 in 1995–1999 [35], emm1, emm3, emm28, emm12, and emm89 in 2000–2004 [36], and emm1, emm12, emm28, emm89, and emm3 in 2005–2012 [37]. CovRS mutations have been frequently detected in clinical isolates of various emm genotypes [9,20,21,38]. However, emergence of CovRS mutants in experimental animal infections has been demonstrated only for emm1 GAS [8,13,14,17,31–33]. CovRS mutations of an emm3 strain are not detected in muscular infection in nonhuman primates; however, GAS isolates tested might be recovered from primates at day 1 after inoculation [39], which may be too soon to accumulate CovRS mutants. Thus, it is not known whether GAS isolates of the other most dominant invasive emm genotypes other than emm1 can readily acquire CovRS mutations in experimental animal infection.
This study was designed to determine whether pharyngeal emm1 GAS isolates have less propensity to acquire CovRS mutations in vivo than invasive emm1 GAS and whether emm3, emm12, and emm28 GAS can acquire CovRS mutants in mouse infection. The emm89 GAS was excluded for the second question because invasive emm89 GAS lacks the hyaluronic acid capsule [10,11] while the capsule has been shown to be critical for in vivo selection of emm1 CovRS mutants [32]. We first identified isolates that secrete the protease SpeB (SpeBA+ for the presence of SpeB activity in culture supernatant) among 176 invasive GAS isolates collected from patients with necrotizing fasciitis (NF) and/or streptococcal toxic shock syndrome (STSS) in 2010–2013 by the CDC Streptococcus Laboratory and 50 pharyngitis isolates collected in 2014 by the Harborview Medical Center Clinical Microbiology Laboratory at University of Washington School of Medicine. We then compared the capacity of SpeBA+ invasive and pharyngeal emm1 isolates to acquire SpeBA- variants during subcutaneous infection in mice. We also examined the capacity of SpeBA+ invasive emm3, emm12, and emm28 GAS isolates to acquire SpeBA- variants during mouse infection. We found that the majority of both invasive and pharyngeal emm1 SpeBA+ isolates and two of three emm12 isolates acquired SpeBA- variants during skin infection. Sixteen analyzed SpeBA- variants of emm1 and emm12 isolates all acquired covS mutations during infection in mice. Thus, we conclude that both invasive and pharyngeal contemporary emm1 GAS isolates and emm12 GAS have a similar capacity to acquire CovRS mutations in vivo.
Materials and Methods
Declaration of ethical approval
All animal experimental procedures were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health [40]. The protocols for mouse experiments were approved by the Institutional Animal Care and Use Committee at MSU (Permit numbers: 2011–57 and 2014–45).
GAS laboratory strains, clinical isolates, and M protein gene (emm) typing
M1T1 strain MGAS2221 [14] and its covS deletion mutant, MGAS2221 ΔcovS [16], have been previously described. A rocA deletion mutant of MGAS2221 (MGAS2221 ΔrocA) will be described elsewhere. One hundred seventy-six invasive GAS isolates from patients with NF and/or STSS were collected from 2010–2013, and their emm genotypes were determined by the Streptococcus Laboratory at the Centers for Disease Control and Prevention (CDC) (S1 Table). These CDC isolates were from 10 diverse geographic areas in the United States. Fifty GAS isolates (HMC isolates) were isolated from throat swabs obtained from patients with pharyngitis during the period of May-August 2014 by the Harborview Medical Center Clinical Microbiology Laboratory in Seattle, Washington (S2 Table). For these invasive isolates from CDC, we simply requested in 2013 the most recent isolates from patients with severe invasive infections of necrotizing fasciitis with or without streptococcal toxic shock syndrome (STSS) and STSS without necrotizing fasciitis. For the pharyngeal isolates, the Harborview Medical Center Clinical Microbiology Laboratory collected 50 pharyngitis isolates in 2014 without any selection criteria. The emm genotypes of the HMC pharyngeal isolates were determined by emm typing according to the CDC emm typing protocol [41] and by using the CDC emm database at http://www2a.cdc.gov/ncidod/biotech/strepblast.asp.
Bacterial growth
GAS bacteria were statically grown at 37°C in 5% CO2 in Todd–Hewitt broth supplemented with 0.2% yeast extract (THY). Tryptose agar with 5% sheep blood and THY agar were used as solid media.
PCR analysis of speA and sda1 in clinical M1 isolates
The presence of the DNase sda1 and exotoxin A (speA) genes in emm1 clinical isolates was determined by the presence of PCR products using primer pairs 5’-ATTATTCACTCTGCTCTGACC-3’/5’-CCCTCTTTACCATTTAT-3’ and 5’-CTTGGTTGTTAGGTAGACTTC-3’/5’-ATCTCGCAAGAGGTATTTGC-3’, respectively.
Selection of SpeBA- variants in mice
Analysis of in vivo selection of SpeBA- variants of clinical GAS isolates was performed as described previously [17]. More details regarding the experimental procedures and animal care are given below. Female, five-week old C57BL/6 mice used in the selection assay were bred at the Animal Resource Center at Montana State University (ARC) using breeding pairs of mice from the Jackson Laboratory (Bar Harbor, Maine). Adequate care of the animals used in experiments was provided by ARC in accordance with the standards incorporated in the Guide to the Care and Use of Laboratory Animals, 1996 edition (National Academy Press, Washington, D.C.). ARC, an 18,000-sq. ft facility, is fully accredited by the American Association for Accreditation of Laboratory Animal Care and is under the direction of a full-time, ACLAM-board-certified veterinarian. MSU complies with the NIH policy on animal welfare (letter of assurance filed), the Animal Welfare Act and all other applicable federal, state, and local laws. The facility is supplied with HEPA-filtered air and uses individually ventilated cages for mice.
GAS bacteria used in the selection assay were harvested from THY culture at the exponential growth phase and washed three times with pyrogen-free Dulbecco’s phosphate-buffered saline (DPBS) and resuspended in DPBS. Groups of 5 or 10 mice were subcutaneously inoculated for each strain with 0.2 ml of GAS suspension in DPBS with an OD600 of 0.9 after mice were anesthetized by isoflurane inhalation using an isoflurane vaporizer from VetEquip Inhalation Anesthesia Systems. Mice infected with GAS were monitored three times a day at about 8:00 am, 12:30 pm, and 16:30 pm. At day 4 after GAS inoculation, mice were euthanized by CO2 inhalation, which was done with a gradual fill method at a displacement rate of 30% CO2 of the chamber volume per minute, as recommended in The 2013 American Veterinary Medical Association Guidelines. SpeBA+ GAS isolates at the used dose caused ruffled fur but did not cause death at the endpoint of the experiment in this subcutaneous infection model.
Skin infection sites of the euthanized mice at day 4 after GAS inoculation were collected and homogenized in 1.0 ml DPBS using a Kontes pestle. The samples were then plated at 103-, 104-, and 105-fold dilutions to obtain well isolated colonies. Forty-eight colonies for each mouse of each test strain were randomly picked, inoculated in 200 μl THY in 96-well plates, and cultured overnight at 37°C in 5% CO2. Three μl of 10% β-mercaptoethanol were added into each well, and the cultures were centrifuged in the plate at 3,500 rpm for 10 min. The supernatant samples, 15 μl each, were loaded into wells on casein gel plates and incubated at 37°C for 3 h. The casein gel plate with 96 wells was prepared as follows: 125 ml of heat-solubilized agarose and casein aqueous solution containing 0.38 g Tris base, 1.1 g NaCl, 0.84 g casein, and 1.25 g agarose was poured into the cover plate of a cell culture plate (Greiner Bio-One International Cat.-No. 665 180), and the tube bottom of a 96-well PCR plate was inserted into the casein solution during gel formation. The formation of a cloudy ring around wells due to casein hydrolysis was the indication of the presence of the SpeB activity in culture supernatant (SpeBA+), and the lack of the cloudy ring indicated lack of the SpeB activity in the samples (SpeBA-). The number of SpeBA- colonies among 48 colonies from a mouse was counted to calculate the percentage of SpeBA- variants among GAS recovered from each mouse.
DNA sequencing
A DNA fragment containing the covRS genes was amplified from test strains using primers 5’-TCGCTAGAAGACTATTTGAC-3’ and 5’-TTCATGTCATCCATCATTGC-3’ and the Phusion high-fidelity PCR kit from New England BioLabs. DNA sequencing of the amplified PCR products was performed using the BigDye Terminator v3.1 Cycle Sequencing Kit and an Applied Biosystems 3130 genetic analyzer. Primers used for sequencing covRS were 5’-TCGCTAGAAGACTATTTGAC-3’, 5’-TTCATGTCATCCATCATTGC-3’, 5’-AACGGCTTCATCATATTTCC-3’, 5’-AAATCCACAAAACCGTTCAG-3’, 5’-TGATACACACGACCGATAG-3’, 5’-TTGATGACAGAAAGGGCAG-3’, 5’-TACGCGAACCATGTCTAAC-3’, and 5’-GTTGGGGTAAAGATGACAG-3’. DNA fragment containing the rgg gene was amplified and sequenced using primers 5’-GTAACAATAACCACATAGTAGGCG-3’ and 5’-TCGTCATTGCTTTTTATGATTTGTC-3’. DNA fragment in emm1 isolates containing the G/A polymorphism at base 989 of the nga gene was amplified using primers 5’-gaacagatgtgaaggttctgtg-3’ and 5’-gtctcttctagtgatacgatac-3’ and sequenced using primer 5’-GATTTGATGTTAGCTTTTGATGATG-3’. Sequence data were analyzed using the software Sequencer 5.1 from the Gene Codes Corporation.
Analyses for SsE production
Relative levels of SsE in culture supernatants of parent isolates and their SpeBA- variants were determined by measuring its platelet-activating factor acetylhydrolase activity using the 2-thio-PAF hydrolysis assay [42]. Briefly, 100 μl of the culture supernatant from the exponential growth phase (OD600 = ~0.4) was mixed with 100 μl of reactant solution containing 0.9 mM 2-thio-PAF and 1.3 mM 5,5'-dithiobis-(2-nitrobenzoic acid) at room temperature in wells of a 96-well plate. Absorbance change at 414 nm (ΔA414) as a measure of SsE-catalyzed 2-thio-PAF hydrolysis was recorded with time using a SPECTRAMax 384 Plus spectrophotometer (Molecular Devices).
Statistical analysis
The GraphPad Prism 7 software program (Graph-Pad Software, Inc.) was used for statistical analyses. The data of in vivo selection for SpeBA- variants in Figs 1 and 2 were analyzed using one way ANOVA Tukey’s multiple comparison test.
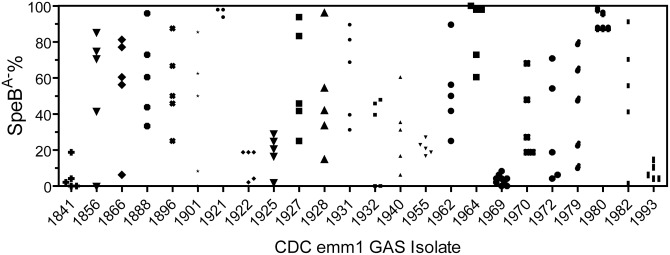
Fig 1. Selection of SpeBA- variants of SpeBA+ invasive emm1 isolates in subcutaneous infection of mice.
Five or 10 mice were subcutaneously inoculated with ~108 cfu of each of the indicated 24 emm1 invasive isolates. Four days later, GAS bacteria were recovered from skin infection sites, and 48 colonies from each mouse were tested for SpeB activity in overnight culture supernatant by the casein hydrolysis assay. Presented are percentages of variants without detectable SpeB activity among the 48 colonies.
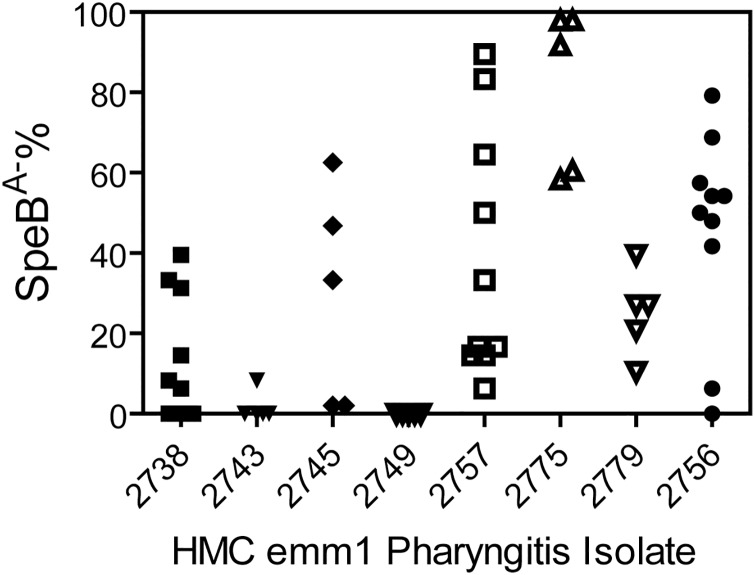
Fig 2. Selection of SpeBA- variants of SpeBA+ emm1 pharyngeal isolates in mice.
Shown are SpeBA−% among GAS bacteria recovered from skin infection sites of mice at day 4 after subcutaneous inoculation with ~108 cfu of each of the indicated 8 emm1 pharyngeal isolates. The SpeBA-% values were determined as described in Fig 1 legend.
Results
Clinical invasive and pharyngeal isolates
The objective of this study was to determine whether pharyngeal emm1 GAS isolates are able to acquire CovRS mutations as readily as invasive M1 isolates in vivo and whether emm3, emm12, and emm28 GAS can acquire CovRS mutants in mouse infection. To achieve this objective, we obtained 176 invasive GAS isolates from the CDC in the United States from 2010 through early 2013 (S1 Table). The 176 GAS samples included 102 isolates from patients with NF only, 60 ones from patients with STSS only, and 14 isolates with both NF and STSS. The Harborview Medical Center Clinical Microbiology Laboratory (Seattle, Washington) (HMC) collected 50 GAS isolates from patients with pharyngitis in May-August 2014 for this study (S2 Table).
The 5 most prevalent emm genotypes of these invasive isolates were emm1 (31 isolates), emm89 (21 isolates), emm3 (16 isolates), emm12 (15 isolates), and emm28 (11 isolates) (Table 1). These were the most common emm types in the US at that time [37]. The 4 most prevalent emm genotypes among the pharyngeal isolates were emm1 (9 isolates or 18%), emm12 (8 isolates or 16%), emm89 (5 isolates or 10%), emm28 (4 isolates or 8%) (Table 2). Each of the emm2, emm3, and emm87 genotypes was represented by 3 pharyngeal isolates (6%). A limitation of the HMC GAS collection is its small size and origin from a single location. Nevertheless, it is reasonable to conclude that the emm1 genotype appears to be prevalent among both invasive and pharyngeal GAS isolates and that the prevalent emm genotypes in severe invasive infections are similar to the prevalent emm genotypes of circulating pharyngeal GAS strains, which is discussed in more details in Discussion.
Table 1. Distribution of emm genotypes and %SpeBA- among the CDC Invasive GAS Isolates.
| emm genotype | No. of Isolates | No. of SpeBA- isolates | % SpeBA- isolates |
|---|---|---|---|
| 1 | 31 | 7 | 25.8 |
| 89 | 21 | 17 | 81.0 |
| 3 | 16 | 10 | 62.5 |
| 12 | 15 | 3 | 20.0 |
| 28 | 11 | 6 | 54.5 |
| 4 | 8 | 2 | 25.0 |
| 118 | 8 | 4 | 50.0 |
| 11 | 6 | 1 | 16.7 |
| 59 | 6 | 1 | 16.7 |
| 75 | 5 | 2 | 40.0 |
| 2 | 4 | 1 | 25.0 |
| 6 | 4 | 0 | 0.0 |
| 82 | 4 | 1 | 25.0 |
| 87 | 4 | 1 | 25.0 |
| 44 | 3 | 0 | 0.0 |
| 77 | 3 | 0 | 0.0 |
| 22 | 3 | 1 | 33.3 |
| 81 | 3 | 0 | 0.0 |
| 92 | 3 | 0 | 0.0 |
| 5 | 2 | 0 | 0.0 |
| 9 | 2 | 1 | 50.0 |
| 58 | 2 | 0 | 0.0 |
| 73 | 2 | 0 | 0.0 |
| 76 | 2 | 1 | 50.0 |
| 78 | 2 | 1 | 50.0 |
| 18 | 1 | 1 | 100 |
| 41 | 1 | 1 | 100 |
| 49 | 1 | 1 | 100 |
| 53 | 1 | 1 | 100 |
| 113 | 1 | 1 | 100 |
| 114 | 1 | 0 | 0.0 |
| total | 176 | 66 | 37.5 |
Table 2. Distribution of emm genotypes and %SpeBA- among the HMC pharyngeal isolates.
| emm genotype | No. of isolates | No. of SpeBA- isolates | % SpeBA- isolates |
|---|---|---|---|
| 1 | 9 | 1 | 11.1 |
| 12 | 8 | 1 | 12.5 |
| 89 | 5 | 0 | 0.0 |
| 28 | 4 | 0 | 0.0 |
| 2 | 3 | 0 | 0.0 |
| 3 | 3 | 1 | 33.3 |
| 87 | 3 | 0 | 0.0 |
| 9 | 2 | 1 | 50.0 |
| 4 | 1 | 0 | 0.0 |
| 6 | 1 | 1 | 100 |
| 22 | 1 | 1 | 100 |
| 48 | 1 | 0 | 0.0 |
| 58 | 1 | 0 | 0.0 |
| 59 | 1 | 0 | 0.0 |
| 75 | 1 | 0 | 0.0 |
| 77 | 1 | 0 | 0.0 |
| 81 | 1 | 0 | 0.0 |
| 82 | 1 | 0 | 0.0 |
| 85 | 1 | 0 | 0.0 |
| 111 | 1 | 0 | 0.0 |
| 238 | 1 | 0 | 0.0 |
| total | 50 | 6 | 12.0 |
Presence of sda1, speA, and nga 989G allele in all emm1 isolates
To determine whether all the emm1 isolates in both collections belonged to the M1T1 subclone, emm1 isolates were analyzed by PCR using sda1- and speA-specific primers, and all strains generated PCR products of expected sizes. Thus, all the emm1 isolates carried the sda1 and speA genes. It has been reported that isolates of the M1T1 clone have a replacement of a 36-kb, slo- and nga-containing chromosomal region of pre-1980 emm1 GAS with that of serotype emm12 GAS, which results in enhanced expression of SLO and NADase and has the 989G allele in the NADase gene nga [6–9]. We attempted to compare SLO levels in mid-exponential growth phase culture supernatants of SpeBA+ emm1 isolates, M1T1 strain MGAS2221 (positive control), and SF370 (a pre-1980 M1 strain and negative control) by Western blotting using SLO-specific antibodies. However, we could not consistently distinguish SF370 from MGAS2221 or SpeBA+ emm1 isolates on the basis of SLO levels. Thus, we determined the polymorphism at base 989 of the nga gene, and all the emm1 isolates had the 989G allele. Based on these results, all of the emm1 isolates in the two collections are most likely similar to the contemporary Finland pharyngeal emm1 isolates that are closely related to MGAS5005 [9], a representative strain of the currently pandemic M1T1 clone.
SpeB production in clinical invasive and pharyngeal isolates
Next, SpeB protease activity in the supernatants of overnight cultures of the clinical isolates was assayed by casein hydrolysis. Overall, 37.5% of the 176 invasive isolates were SpeBA-. SpeBA+ isolate samples did not contain SpeBA- bacteria as determined by checking SpeB activity of 5 or more independent colonies of each sample. Among the 5 most frequent emm genotypes of the invasive isolates, about 20% of emm1 and emm12 isolates, 60% of emm3 and emm28 isolates, and 80% of 21 invasive emm89 isolates were SpeBA- (Table 1). The SpeBA- percentage of the invasive emm1 isolates is slightly higher than reported for a larger invasive emm1 GAS collection [38]; however, the percentage of our invasive emm89 isolates that were SpeBA- is 4 times higher than observed in invasive emm89 isolates in the earlier study [38]. As our 5 emm89 pharyngeal isolates were all SpeBA+, the association of the SpeBA- phenotype with emm89 NF and/or STSS isolates appears to be genuine. The percentages of SpeBA- isolates among NF, STSS, and NF/STSS isolates were 39.2%, 33.3%, and 42.8%, respectively. Thus, isolates from these three different forms of severe invasive infection exhibit similar percentages of isolates with the SpeBA- phenotype.
Six of the 50 HMC pharyngeal isolates were SpeBA-, including 1 isolate each from the emm1, emm3, emm6, emm9, emm12, and emm22 genotypes (Table 2). Notably, all 5 emm89 isolates and 8 out of 9 emm1 isolates were SpeBA+. Thus, pharyngeal emm1 and emm89 isolates appear to have fewer percentages of isolates with the SpeBA- phenotype than NF and/or STSS isolates.
Selection of SpeBA- variants of invasive and pharyngeal emm1 SpeBA+ isolates
The screening of invasive and pharyngeal isolates by the SpeB activity assay identified 24 invasive and 8 pharyngeal emm1 SpeBA+ isolates (Table 3). These isolates allowed us to determine whether invasive and pharyngeal emm1 GAS isolates have a different tendency to acquire CovRS mutations in vivo. The study was mainly the comparison of pharyngeal emm1 isolates with severe invasive emm1 isolates in the capacity to acquire CovRS mutants in vivo, and 19 of the 31 severe invasive emm1 isolates were from patients with necrotizing fasciitis. Thus, both pharyngeal and subcutaneous infection models should be relevant for the purpose. We had preliminary data for in vivo selection of MGAS2221 covRS mutants in intranasal infection model. However, the intranasal model is more technically challenging because of the presence of non-GAS bacteria. Thus, we chose the model of subcutaneous infection of mice. We used the SpeB activity assay to identify SpeBA- variants among isolates that were recovered from skin infection sites in mice on day 4 after inoculation of each SpeBA+ emm1 GAS isolate. SpeBA- variants were detected at skin infection sites for all 24 invasive SpeBA+ emm1 isolates (Fig 1). Twenty-one of them had mean SpeBA-% values ranging from 12.5% to 96.5%, and 3 others had low mean SpeBA-% values ranging from 3.5% to 7.9% (Table 3). SpeBA- variants were detected at skin infection sites for 7 of the 8 SpeBA+ pharyngeal emm1 isolates (Fig 2). Six had mean SpeBA-% values from 13.3% to 81.2%, and one had 1.7% SpeBA- variants (Table 3). One pharyngitis isolate had no detectable SpeBA- variants. Thus, like contemporary invasive emm1 isolates, the majority of contemporary pharyngeal emm1 GAS isolates are able to give rise to SpeBA- variants in vivo.
Table 3. Average percentages of SpeBA- variants selected from invasive and pharyngeal emm1 SpeBA+ isolates and P values in statistical analysis.
| Strain number | Infection | Avg. SpeBA- variants (%)a | Group | Significant P valueb |
|---|---|---|---|---|
| 1921 | Invasive | 96.5 | iG1 vs iG2: <0.0001; iG1 vs iG4: ≤0.0372; iG1 vs pG2: <0.0001; iG1 vs pG4: ≤0.0216; iG2 vs pG1: ≤0.0003; iG3 vs iG2: <0.05 |
|
| 1964 | Invasive | 85.8 | iG1 | |
| 1980 | Invasive | 91.3 | ||
| 1841 | Invasive | 5.0 | ||
| 1969 | Invasive | 3.5 | iG2 | |
| 1993 | Invasive | 7.9 | ||
| 1856 | Invasive | 54.6 | ||
| 1866 | Invasive | 56.2 | ||
| 1888 | Invasive | 61.3 | ||
| 1896 | Invasive | 55.0 | ||
| 1901 | Invasive | 41.2 | ||
| 1927 | Invasive | 57.9 | iG3 | |
| 1928 | Invasive | 47.9 | ||
| 1931 | Invasive | 62.1 | ||
| 1962 | Invasive | 52.5 | ||
| 1970 | Invasive | 36.1 | ||
| 1979 | Invasive | 45.0 | ||
| 1982 | Invasive | 52.5 | ||
| 1922 | Invasive | 12.5 | ||
| 1932 | Invasive | 26.7 | ||
| 1925 | Invasive | 18.8 | iG4 | |
| 1940 | Invasive | 30.0 | ||
| 1955 | Invasive | 21.3 | ||
| 1972 | Invasive | 30.8 | ||
| 2775 | Pharyngeal | 81.2 | pG1 | pG1 vs pG2: <0.0001; pG1 vs pG3: ≤0.0119; pG1 vs pG4: ≤0.0059; pG2 vs pG3: ≤0.0405 |
| 2743 | Pharyngeal | 1.7 | pG2 | |
| 2749 | Pharyngeal | 0 | ||
| 2756 | Pharyngeal | 46.0 | pG3 | |
| 2757 | Pharyngeal | 39.0 | ||
| 2745 | Pharyngeal | 29.4 | ||
| 2738 | Pharyngeal | 13.3 | pG4 | |
| 2779 | Pharyngeal | 25.0 |
Significant difference among emm1 isolates in the capacity to acquire SpeBA- variants in murine subcutaneous infection
Isolates of both the invasive and pharyngeal emm1 collections had variable average SpeBA-% value in the in vivo selection for SpeBA- variants in mice. Statistical analysis of these data using one way ANOVA Tukey’s multiple comparison test could divide the isolates into four groups on the basis of significant difference in the SpeBA-% data for both invasive (iG1-iG4) and pharyngeal (pG1-pG4) isolates (Table 3). The iG1 and pG1 isolates had >80% of average SpeBA-% and were significantly different in SpeBA-% from iG2 and pG2 with average SpeBA-% of <10% (P <0.0001), and the average SpeBA-% values of the iG4 and pG4 isolates ranging from 10% to 30% were significantly different from those of the iG1 and pG1 isolates (P <0.05). Both iG3 and pG3 had average values of SpeBA-% ranging from 30% to 60% and were not significantly different from isolates in the iG, iG2, pG1, and pG2 groups. Thus, some isolates in both invasive and pharyngeal emm1 isolates have significantly higher capacity to acquire SpeBA- variants than others in the same categories.
CovS mutations and enhanced SsE expression in emm1 SpeBA- variants selected in mouse infection
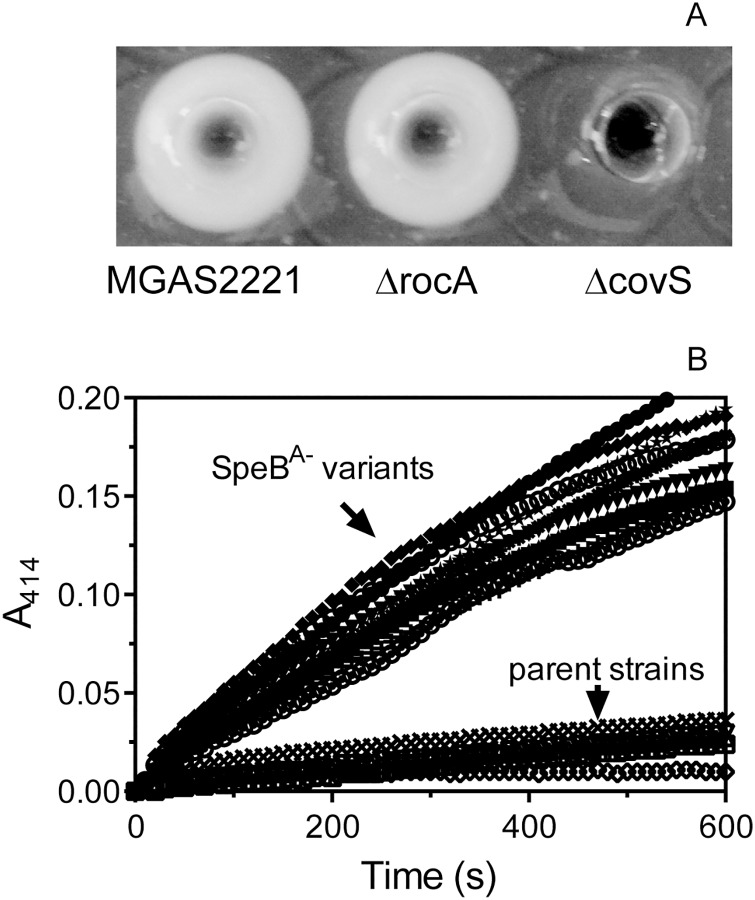
Although we have reported that the SpeBA- phenotype is a reliable indicator for CovRS mutations of MGAS2221 in subcutaneous infection of mice [17], CovRS mutations may not be the only cause of the SpeBA- phenotype. RocA has been shown to function through CovR, and rocA deletion enhances expression of CovRS-regulated virulence genes [43]. Thus, we determined whether rocA mutations are a cause of the SpeBA- phenotype by comparing the SpeB activity in culture supernatant of M1T1 strain MGAS2221 and its ΔrocA and ΔcovS mutants. MGAS2221 ΔcovS was SpeBA- but both MGAS2221 ΔrocA and MGAS2221 were SpeBA+ (Fig 3A). Thus, rocA mutations are not a cause of the SpeBA- phenotype. Another possible common cause of the SpeBA- variants is mutations in the rgg gene [38,39,44]. We sequenced the covRS and rgg genes in 5 emm1 invasive SpeBA+ isolates and 2 SpeBA- variants of each of them selected in skin infection. The 5 clinical isolates all had same covRS and rgg sequences with MGAS2221, and all 10 SpeBA- variants had missense mutations or indels of covS but have wild-type rgg (Table 4). Being consistent with the previous findings that natural CovRS mutations enhance SsE expression [16,29,33], all the SpeBA- variants had higher levels of SsE PAF acetylhydrolase activity than their parent strains in culture supernatant at the exponential growth phase (Fig 3B). Thus, SpeBA- variants of emm1 GAS selected in subcutaneous infection in mice are caused primarily by CovRS mutations and have enhanced virulence gene expression.
Fig 3. Evidence for CovRS mutations as the basis for the SpeBA- phenotype of variants selected in subcutaneous infection of mice.
(A) MGAS2221 and its rocA deletion mutant, but not its covS deletion mutant, had SpeB protease activity in overnight culture supernatant as determined by the casein hydrolysis assay. (B) Levels of SsE platelet-activating factor acetylhydrolase activity in culture supernatant at the exponential growth phase of 10 SpeBA- variants and their 5 emm1 SpeBA+ parent strains listed in Table 4. The SsE activity was measured using the colorimetric assay as described in the Methods.
Table 4. covS mutations of SpeBA- variants of emm1 and emm12 GAS in subcutaneous mouse infection.
| Straina | Parent strain | emm type | covS mutationb | Mutated CovS | rgg mutationb |
|---|---|---|---|---|---|
| 2253 | 1841 | 1 | C685T | Arg229Cys | No |
| 2254 | 1841 | 1 | A insertion at 1048 | Truncated | No |
| 2134 | 1856 | 1 | Δ1341A | Truncated | No |
| 2135 | 1856 | 1 | C59T | Ser20Phe | No |
| 2144 | 1866 | 1 | Δ83T | Truncated | No |
| 2145 | 1866 | 1 | Δ83T | truncated | No |
| 2154 | 1888 | 1 | Δ1215GAAAA | truncated | No |
| 2155 | 1888 | 1 | Δ1215GAAAA | truncated | No |
| 2258 | 1896 | 1 | C737T | Ser246Leu | No |
| 2259 | 1896 | 1 | Δ83T | Truncated | No |
| 3158 | 1845 | 12 | G1279T | Gly427Cys | No |
| 3159 | 1845 | 12 | Δ83T | truncated | No |
| 3160 | 1845 | 12 | A980T | Asp327Val | No |
| 3161 | 1845 | 12 | T1391C | Ile464Thr | No |
| 3162 | 1867 | 12 | Δ83T | truncated | No |
| 3164 | 1867 | 12 | C104T | Thr35Ile | No |
aTwo SpeBA- variants from the same mouse derived from each indicated parent strain except that 4 variants derived from strain 1845 were from two mice.
bMutations refer to genetic changes in comparison with the wt covS and rgg sequences in the parent strains.
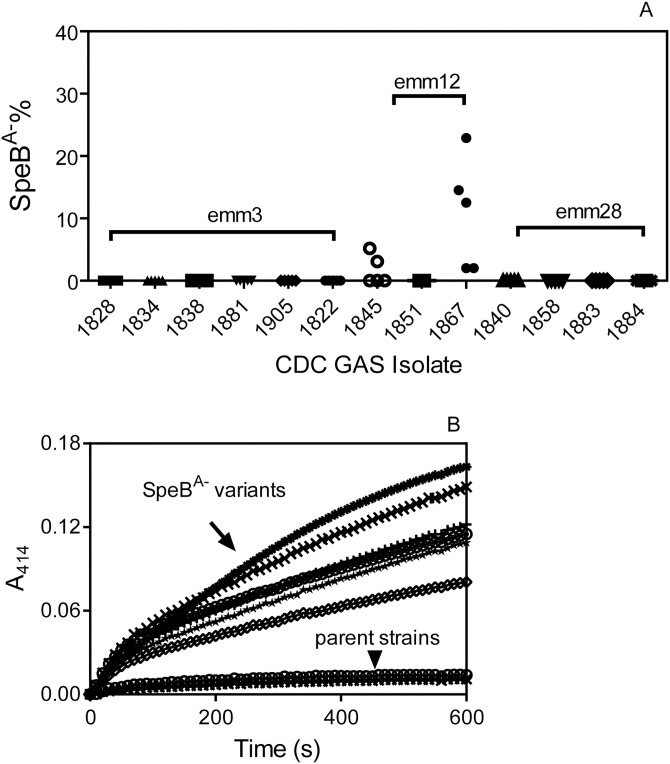
Selection of CovRS mutants of emm12 GAS in subcutaneous mouse infection
To determine whether emm3, emm12, and emm28 GAS has the capacity to acquire SpeBA- mutations in mouse infection, we tested 6 emm3, 3 emm12, and 4 emm28 SpeBA+ invasive isolates for selection of SpeBA- variants during cutaneous infection in mice. No SpeBA- variants were detected at day 4 after infection with the emm3 and emm28 isolates (Fig 4A). However, 10.8% of recovered M12 isolate 1867 were SpeBA-, and isolates recovered from emm12 strain 1845 infection contained 1.7% SpeBA- variants (Fig 4A). Four SpeBA- variants of GAS1845 and 2 SpeBA- variants of GAS1867 all had covS mutation or deletion and lacked rgg mutation compared with the parent strains (Table 4). Like the emm1 SpeBA- variants, all these emm12 SpeBA- variants had enhanced SsE PAF acetylhydrolase activity in their culture supernatant at the exponential growth phase (Fig 4B). Thus, arising of emm12 GAS CovRS mutants is demonstrated in subcutaneous infection of mouse.
Fig 4. Selection of CovRS mutants of emm12 GAS in subcutaneous mouse infection.
(A) The capacity of SpeBA+ emm3, emm12, and emm28 invasive isolates to give rise to SpeBA- variants in mice. Shown are SpeBA−% among GAS bacteria recovered from skin infection sites of mice at day 4 after subcutaneous inoculation with ~108 cfu of each of the indicated 6 emm3, 3 emm12, and 4 emm28 invasive isolates. (B) Levels of SsE platelet-activating factor acetylhydrolase activity in culture supernatant at the exponential growth phase of 6 SpeBA- variants and their 2 emm12 SpeBA+ parent strains listed in Table 4.
Discussion
The main objective of this study was to test the hypothesis that pharyngeal emm1 GAS isolates have less propensity to acquire CovRS mutations in vivo than invasive emm1 isolates, as a possible explanation for the rarity of severe invasive GAS infections caused by pandemic M1T1 GAS. Our experimental observations do not support this hypothesis, as contemporary emm1 isolates from pharyngitis patients and patients with NF and/or STSS exhibit similar capacity to acquire CovRS mutations following subcutaneous infection of mice. Thus, the explanation for the rarity of severe invasive GAS infections remains to be fully elucidated. We also showed that contemporary emm1 isolates from both pharyngitis and severe invasive infections can have significantly different propensity to acquire CovRS mutations. In addition, we found that emm12, but not emm3 and emm28, GAS isolates can acquire CovRS mutations in subcutaneous infection of mice. The findings provides information for the understanding of the arising of GAS mutants in a hostile environment.
Although contemporary emm1 isolates belong to the M1T1 clone, they have single nucleotide polymorphisms [9,14]. It is possible that some M1T1 GAS bacteria acquire rare genetic alterations that render them to selection for CovRS mutations, causing rare severe invasive infections. However, this possibility has been excluded by our finding that pharyngeal emm1 isolates have the capacity to acquire CovRS mutations during mouse infection at frequencies similar to those of invasive emm1 isolates. Nevertheless, our data are consistent with the consensus notion in the field that contemporary emm1 GAS strains cause severe invasive infections in only a small fraction of the population, suggesting that additional bacterial genetic determinants and/or host factors are involved.
As in a recent analysis of SpeB production by invasive GAS isolates, the majority (74.2%) of the CDC emm1 isolates were found to be SpeBA+ [38]. The prevalence of SpeBA- isolates is similar to that of SpeBA- variants identified on day 4 after murine infection with SpeBA+ emm1 isolates. Indeed, mixed GAS populations with or without CovRS mutations are observed in patients [45]. The likelihood to isolate a CovRS mutant depends on where and when isolates are recovered from the host [5]. Thus, the frequency of SpeBA- and SpeBA+ phenotypes among clinical invasive GAS isolates should be interpreted with caution. Mixed GAS populations with or without CovRS mutations are also consistent with the suggestion that CovRS mutations arise during human infection with GAS carrying wild-type CovRS and are not transmissible [22]. The inability of CovRS mutants to transmit suggests a "source-sink" dynamic in which GAS with wild-type CovRS has an advantage in transmission and pharyngeal infection while GAS with CovRS mutations contributes to rarer episodes of severe invasive disease in susceptible population.
The pharyngeal isolates in this study were from a single location whereas the CDC invasive isolates represent diverse geographic areas in the United States. Whether our comparison is relevant depends on whether the pharyngeal emm1 isolates in Seattle are similar to those in the other geographic areas in the United States. It is well established that contemporary emm1 GAS strains belonged to the invasive M1T1 subclone emerged in the 1980s and have essentially displaced antecedent emm1 strains across a broad geographic region [3,4,9]. Contemporary severe and non-severe invasive emm1 isolates in the United States are genetically related to the invasive M1T1 clone [9]. Contemporary pharyngeal emm1 isolates in the United States must be dominantly related to the invasive M1T1 clone because there are evidences for the origination of invasive GAS strains from pharyngitis strains [34,46]. This possibility is supported by the fact that contemporary pharyngeal and invasive emm1 strains in Canada and Finland were related to the invasive M1T1 clone [9]. All the pharyngeal and invasive emm1 isolates in this study carry the sda1 and speA genes and the nga 989G allele, consistent with the invasive M1T1 clone. These data and the previous findings support that emm1 pharyngeal GAS strains in the United States are dominantly related to the M1T1 subclone. Thus, the geographic limitation in our analyses unlikely invalidates our conclusion that contemporary pharyngeal and invasive emm1 isolates have a similar capacity to acquire CovRS mutations in vivo.
Invasive GAS isolates frequently have CovRS mutations resulting in a SpeBA-phenotype [20,21,38]. However, the selection of SpeBA- variants has been previously demonstrated in mouse infections only for M1T1 GAS [8,13,14,17,31–33]. Neutrophils are required for in vivo selection of M1T1 GAS SpeBA- variants [17]. The capsule and M protein are critical for the selection of SpeBA- variants of M1T1 GAS [32]. However, capsule and M protein are critical virulence factors for both contemporary and pre-1980 emm1 GAS strains. Reports are conflicting as to whether Sda1 is critical for the selection of SpeBA- variants of invasive M1T1 GAS [31,33]. Thus, the critical trigger for in vivo selection of M1T1 SpeBA- variants is presently unknown. The present study shows that selection of CovRS mutants of emm12 GAS isolates can also be demonstrated in mice. We also showed that some emm1 isolates are overpopulated by emerged CovRS mutants within a few days while others accumulate small percentages of CovRS mutants at the same time point in subcutaneous infection of mice. Further characterization of the selection of CovRS mutants of emm12 GAS and elucidation of the basis for the differential propensity of contemporary emm1 GAS to acquire CovRS mutants in mouse infection may provide additional clues regarding the trigger for in vivo selection of GAS CovRS mutations.
No SpeBA- variants were detected after cutaneous infection with SpeBA+ emm3 isolates. One possible reason is that emm3 isolates have a natural rocA mutation that enhances expression of CovRS-controlled virulence factors [43] and thus alleviates the selective pressure for CovRS mutants. However, this possibility is not consistent with the observation that invasive emm3 isolates are more likely to contain CovRS mutations than pharyngeal emm3 isolates [24]. Furthermore, even in a rocA null background, CovRS mutations enhance the expression of virulence genes and critically contribute to emm3 GAS virulence [21]. An alternative possibility is that the screening of M3 GAS by the SpeB activity assay failed to pick up certain CovRS mutations. For example, the CovSG457V point mutation of invasive M3 isolate MGAS315 enhances expression of virulence genes and critically contribute to its virulence; however, this point mutation does not cause a SpeBA- phenotype in MGAS315 [19]. It is also possible that human infections have a mechanism for the selection of emm3 GAS CovRS mutants that is not mimicked in the murine skin infection model.
It is interesting that 17 of 21 invasive emm89 isolates (80%) are SpeBA- while all 5 pharyngeal emm89 isolates are SpeBA+. It has been reported that 20.7% of invasive emm89 isolates are SpeBA- [38], and these emm89 isolates belong to three major phylogenetic groups [11]. GAS in one of the tree groups lacks the hasABC capsule biosynthesis locus and has emerged as a frequent cause of severe invasive infections since 2000 [10–12]. The unusually high prevalence of the SpeBA- phenotype in CDC emm89 invasive isolates could be due to an enhanced selection pressure for acapsular emm89 GAS strains, leading to a propensity to cause necrotizing fasciitis and toxic shock syndrome.
Kazmi et al. first reported SpeBA+-to-SpeBA- phase shift of M1T1 GAS in mouse infection [47]. A covS mutant of M1T1 GAS arisen in mice confers the SpeBA- phenotype [14]. The regulator rgg also regulates speB [48]. The SpeBA- phenotype has been detected in many clinical isolates, and clinical SpeBA- isolates are associated dominantly with mutations in rgg and covS and less frequently with covR mutations [38]. Surprisingly, we only detected mutations of covS but not rrg in SpeBA- variants of emm1 and emm12 isolates in subcutaneous infection of mice in this study. Previous studies also reported that SpeBA- variants of emm1 GAS selected in mouse infection are associated with covRS mutations [8,13,14,15,17,31–33]. All these data resonate the lack of the selection of SpeBA- variants of emm3 GAS in subcutaneous infection of mice whereas covRS mutations are frequent in emm3 clinical isolates [21]. These findings suggest that different GAS strains may be subject to different stresses in different infections and that mutations of covRS and rgg confer different fitness advantage in serotype-dependent and infection-dependent ways.
Conclusions
We conclude that contemporary invasive and pharyngeal emm1 GAS isolates have a similar capacity to acquire CovRS mutations in vivo. We also conclude that emm2, but not emm3 and emm28, GAS can also readily acquire CovRS mutants in subcutaneous infection of mice.
Supporting Information
(DOCX)
(DOCX)
Acknowledgments
We thank Drs. Bernard Beall and Chris A. Van Beneden at National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention for providing invasive Group A Streptococcus isolates. We also acknowledge the CDC emm database at http://www2a.cdc.gov/ncidod/biotech/strepblast.asp for emm genotyping.
Data Availability
All relevant data are within the paper and supporting information.
Funding Statement
This work was supported in part by grants AI095704, AI097703, and GM110732 from the National Institutes of Health, Montana University System Research Initiative 51040-MUSRI2015-03, and the Montana State Agricultural Experimental Station.
References
- 1.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005; 5: 685–694. [DOI] [PubMed] [Google Scholar]
- 2.Katz AR, Morens DM. Severe streptococcal infections in historical perspective. Clin Infect Dis. 1992; 14: 298–307. [DOI] [PubMed] [Google Scholar]
- 3.Cleary PP, Kaplan EL, Handley JP, Wlazlo A, Kim MH, Hauser AR, et al. Clonal basis for resurgence of serious Streptococcus pyogenes disease in the 1980s. Lancet 1992; 339: 518–521. [DOI] [PubMed] [Google Scholar]
- 4.Musser JM, Kapur V, Szeto J, Pan X, Swanson DS, Martin DR. Genetic diversity and relationships among Streptococcus pyogenes strains expressing serotype M1 protein: recent intercontinental spread of a subclone causing episodes of invasive disease. Infect Immun. 1995; 63: 994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aziz RK, Kotb MZ. Rise and persistence of global M1T1 clone of Streptococcus pyogenes. Emerg Infect Dis. 2008; 14: 1511–1517. 10.3201/eid1410.071660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beres SB, Sylva GL, Barbian KD, Lei B, Hoff JS, Mammarella ND, et al. Genome sequence of a serotype M3 strain of group A Streptococcus: phage-encoded toxins, the high-virulence phenotype, and clone emergence. Proc Natl Acad Sci USA 2002; 99: 10078–10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sumby P, Porcella SF, Madrigal AG, Barbian KD, Virtaneva K, Ricklefs SM, et al. Evolutionary origin and emergence of a highly successful clone of serotype M1 group a Streptococcus involved multiple horizontal gene transfer events. J Infect Dis. 2005; 192: 771–782. [DOI] [PubMed] [Google Scholar]
- 8.Maamary PG, Ben Zakour NL, Cole JN, Hollands A, Aziz RK, Barnett TC, et al. Tracing the evolutionary history of the pandemic group A streptococcal M1T1 clone. FASEB J. 2012; 26: 4675–4684. 10.1096/fj.12-212142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasser W, Beres SB, Olsen RJ, Dean MA, Rice KA, Long SW, et al. Evolutionary pathway to increased virulence and epidemic group A Streptococcus disease derived from 3,615 genome sequences. Proc Natl Acad Sci USA. 2014; 111: E1768–1776. 10.1073/pnas.1403138111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner CE, Abbott J, Lamagni T, Holden MT, David S, Jones MD, et al. Emergence of a new highly successful acapsular Group A Streptococcus clade of genotype emm89 in the United Kingdom. MBio. 2015; 6: e00622 10.1128/mBio.00622-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu L, Olsen RJ, Nasser W, de la Riva Morales I, Musser JM. Trading capsule for increased cytotoxin production: Contribution to virulence of a newly emerged clade of emm89 Streptococcus pyogenes. MBio 2015; 6: e01378 10.1128/mBio.01378-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friães A, Machado MP, Pato C, Carriço J, Melo-Cristino J, Ramirez M. Emergence of the same successful clade among distinct populations of emm89 Streptococcus pyogenes in multiple geographic regions. MBio 2015; 6: e01780 10.1128/mBio.01780-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engleberg NC, Heath A, Miller A, Rivera C, DiRita VJ. Spontaneous mutations in the CsrRS two-component regulatory system of Streptococcus pyogenes result in enhanced virulence in a murine model of skin and soft tissue infection. J Infect Dis. 2001; 183: 1043–1054. [DOI] [PubMed] [Google Scholar]
- 14.Sumby P, Whitney AR, Graviss EA, DeLeo FR, Musser JM. Genome-wide analysis of Group A Streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog. 2006; 2: 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kansal RG, Datta V, Aziz RK, Abdeltawab NF, Rowe S, Kotb M. Dissection of the molecular basis for hypervirulence of an in vivo-selected phenotype of the widely disseminated M1T1 strain of group A Streptococcus bacteria. J Infect Dis. 2010; 201: 855–865. 10.1086/651019 [DOI] [PubMed] [Google Scholar]
- 16.Li J, Zhu H, Feng W, Liu M, Song Y, Zhang X, et al. Regulation of inhibition of neutrophil infiltration by the two-component regulatory system CovRS in subcutaneous murine infection with group A Streptococcus. Infect Immun. 2013; 81: 974–983. 10.1128/IAI.01218-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Liu G, Feng W, Zhou Y, Liu M, Wiley JA, et al. Neutrophils select hypervirulent CovRS mutants of M1T1 group A Streptococcus during subcutaneous infection of mice. Infect Immun. 2014; 82: 1579–1590. 10.1128/IAI.01458-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyoshi-Akiyama T, Ikebe T, Watanabe H, Uchiyama T, Kirikae T, Kawamura Y. Use of DNA arrays to identify a mutation in the negative regulator, csrR, responsible for the high virulence of a naturally occurring type M3 group A streptococcus clinical isolate. J Infect Dis. 2006; 193: 1677–1684. [DOI] [PubMed] [Google Scholar]
- 19.Stetzner ZW, Li D, Feng W, Liu M, Liu G, Wiley J, et al. Serotype M3 and M28 Group A Streptococci have distinct capacities to evade neutrophil and TNF-α responses and to invade soft tissues. PLoS One 2015; 10: e0129417 10.1371/journal.pone.0129417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikebe T, Ato M, Matsumura T, Hasegawa H, Sata T, Kobayashi K, et al. 2010. Highly frequent mutations in negative regulators of multiple virulence genes in group A streptococcal toxic shock syndrome isolates. PLoS Pathog. 6:e1000832 10.1371/journal.ppat.1000832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shea PR, Beres SB, Flores AR, Ewbank AL, Gonzalez-Lugo JH, Martagon-Rosado AJ, et al. Distinct signatures of diversifying selection revealed by genome analysis of respiratory tract and invasive bacterial populations. Proc Natl Acad Sci USA 2011; 108: 5039–5044. 10.1073/pnas.1016282108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole JN, Barnett TC, Nizet V, Walker MJ. Molecular insight into invasive group A streptococcal disease. Nat Rev Microbiol. 2011; 9: 724–736. 10.1038/nrmicro2648 [DOI] [PubMed] [Google Scholar]
- 23.Levin JC, Wessels MR. Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A Streptococcus. Mol Microbiol. 1998; 30: 209–219. [DOI] [PubMed] [Google Scholar]
- 24.Heath A, DiRita VJ, Barg NL, Engleberg NC. A two-component regulatory system, CsrR-CsrS, represses expression of three Streptococcus pyogenes virulence factors, hyaluronic acid capsule, streptolysin S, and pyrogenic exotoxin B. Infect Immun. 1999; 67: 5298–5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Federle MJ, McIver KS, Scott JR. A Response regulator that represses transcription of several virulence operons in the Group A Streptococcus. J Bacteriol. 1999; 181: 3649–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aziz RK, Pabst MJ, Jeng A, Kansal R, Low DE, Nizet V, et al. Invasive M1T1 group A Streptococcus undergoes a phase-shift in vivo to prevent proteolytic degradation of multiple virulence factors by SpeB. Mol Microbiol. 2004; 51: 123–134. [DOI] [PubMed] [Google Scholar]
- 27.Engleberg NC, Heath A, Vardaman K, DiRita VJ. Contribution of CsrR-regulated virulence factors to the progress and outcome of murine skin infections by Streptococcus pyogenes. Infect Immun. 2004; 72: 623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole JN, McArthur JD, McKay FC, Sanderson-Smith ML, Cork AJ, Ranson M, et al. Trigger for group A streptococcal M1T1 invasive disease. FASEB J. 2006; 20: 1745–1747. [DOI] [PubMed] [Google Scholar]
- 29.Zhu H, Liu M, Sumby P, Lei B. The secreted esterase of group a streptococcus is important for invasive skin infection and dissemination in mice. Infect Immun. 2009; 77: 5225–5232. 10.1128/IAI.00636-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu M, Zhu H, Li J, Garcia CC, Feng W, Kirpotina LN, et al. Group A Streptococcus secreted esterase hydrolyzes platelet-activating factor to impede neutrophil recruitment and facilitate innate immune evasion. PLoS Pathog. 2012; 8: e1002624 10.1371/journal.ppat.1002624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker MJ, Hollands A, Sanderson-Smith ML, Cole JN, Kirk JK, Henningham A, et al. DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat Med. 2007; 13: 981–985. [DOI] [PubMed] [Google Scholar]
- 32.Cole JN, Pence MA, von Köckritz-Blickwede M, Hollands A, Gallo RL, Walker MJ, et al. M protein and hyaluronic acid capsule are essential for in vivo selection of covRS mutations characteristic of invasive serotype M1T1 group A Streptococcus. MBio 2010; 1: e00191–10. 10.1128/mBio.00191-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu G, Feng W, Li D, Liu M, Nelson DC, Lei B. The Mga Regulon but Not Deoxyribonuclease Sda1 of Invasive M1T1 Group A Streptococcus Contributes to In Vivo Selection of CovRS Mutations and Resistance to Innate Immune Killing Mechanisms. Infect Immun. 2015; 83: 4293–4303. 10.1128/IAI.00857-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cockerill FR 3rd, MacDonald KL, Thompson RL, Roberson F, Kohner PC, Besser-Wiek J, et al. An outbreak of invasive group A streptococcal disease associated with high carriage rates of the invasive clone among school-aged children. JAMA. 1997; 277: 38–43. [PubMed] [Google Scholar]
- 35.O'Brien KL, Beall B, Barrett NL, Cieslak PR, Reingold A, Farley MM, et al. Epidemiology of invasive group a streptococcus disease in the United States, 1995–1999. Clin Infect Dis. 2002; 35:268–276. [DOI] [PubMed] [Google Scholar]
- 36.O'Loughlin RE, Roberson A, Cieslak PR, Lynfield R, Gershman K, Craig A, et al. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clin Infect Dis. 2007; 45: 853–862. [DOI] [PubMed] [Google Scholar]
- 37.Nelson GE, Pondo T, Toews KA, Farley MM, Lindegren ML, Lynfield R, et al. Epidemiology of Invasive Group A Streptococcal Infections in the United States, 2005–2012. Clin Infect Dis. 2016; 63: 478–486. 10.1093/cid/ciw248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsen RJ, Raghuram A, Cantu C, Hartman MH, Jimenez FE, Lee S, et al. The majority of 9,729 group A streptococcus strains causing disease secrete SpeB cysteine protease: pathogenesis implications. Infect Immun. 2015; 83: 4750–4758. 10.1128/IAI.00989-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carroll RK, Shelburne SA 3rd, Olsen RJ, Suber B, Sahasrabhojane P, Kumaraswami M, et al. Naturally occurring single amino acid replacements in a regulatory protein alter streptococcal gene expression and virulence in mice. J Clin Invest. 2011; 121: 1956–1968. 10.1172/JCI45169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Research Council. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC: 2011. [Google Scholar]
- 41.http://www.cdc.gov/streplab/M-ProteinGene-typing.html
- 42.Liu G, Liu M, Xie G, Lei B. Characterization of streptococcal platelet-activating factor acetylhydrolase variants that are involved in innate immune evasion. Infect Immun. 2013; 81: 3128–3138. 10.1128/IAI.00398-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller EW, Danger JL, Ramalinga AB, Horstmann N, Shelburne SA, Sumby P. Regulatory rewiring confers serotype-specific hyper-virulence in the human pathogen group A Streptococcus. Mol Microbiol. 2015; 98: 473–489. 10.1111/mmi.13136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kappeler KV, Anbalagan S, Dmitriev AV, McDowell EJ, Neely MN, Chaussee MS. A naturally occurring Rgg variant in serotype M3 Streptococcus pyogenes does not activate speB expression due to altered specificity of DNA binding. Infect Immun. 2009; 77: 5411–5417. 10.1128/IAI.00373-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flores AR, Sahasrabhojane P, Saldaña M, Galloway-Peña J, Olsen RJ, Musser JM, et al. Molecular characterization of an invasive phenotype of group A Streptococcus arising during human infection using whole genome sequencing of multiple isolates from the same patient. J Infect Dis. 2014; 209: 1520–1523. 10.1093/infdis/jit674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flores AR, Sahasrabhojane P, Saldaña M, Galloway-Peña J, Olsen RJ, Musser JM, et al. Molecular characterization of an invasive phenotype of group A Streptococcus arising during human infection using whole genome sequencing of multiple isolates from the same patient. J Infect Dis. 2014; 209: 1520–1523. 10.1093/infdis/jit674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kazmi SU, Kansal R, Aziz RK, Hooshdaran M, Norrby-Teglund A, Low DE, et al. Reciprocal, temporal expression of SpeA and SpeB by invasive M1T1 group a streptococcal isolates in vivo. Infect Immun. 2001; 69: 4988–4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chaussee MS, Ajdic D, Ferretti JJ. The rgg gene of Streptococcus pyogenes NZ131 positively influences extracellular SPE B production. Infect Immun. 1999; 67: 1715–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and supporting information.