Abstract
Telbivudine, a thymidine nucleoside analog, is a common therapeutic option for chronic hepatitis B infection. While raised serum creatine kinase is common, myopathy associated with telbivudine is rare. Reports on its myopathological features are few and immunohistochemical analyses of inflammatory cell infiltrates have not been previously described. We describe the clinical, myopathological and immunohistochemical features of four patients who developed myopathy after telbivudine therapy for chronic hepatitis B infection. All four patients presented with progressive proximal muscle weakness, elevation of serum creatine kinase and myopathic changes on electromyography. Muscle biopsies showed myofiber degeneration/necrosis, regeneration, and fibers with cytoplasmic bodies and cytochrome c oxidase deficiency. There was minimal inflammation associated with strong sarcolemmal overexpression of class I major histocompatibility complex (MHC class I). Upon withdrawal of telbivudine, muscle weakness improved in all patients and eventually completely resolved in three. In our series, telbivudine-associated myopathy is characterized by necrotizing myopathy which improved on drug withdrawal. Although the occasional loss of cytochrome c oxidase is consistent with mitochondrial toxicity, the overexpression of MHC class I in all patients could suggest an underlying immune-mediated mechanism which may warrant further investigation.
Introduction
Chronic hepatitis B virus (HBV) infection affects more than 360 million people globally and is a leading cause of liver cirrhosis, hepatocellular carcinoma and liver failure [1]. Of the two primary classes of antiviral therapy for chronic HBV infection, oral nucleoside/nucleotide analogs (NAs) have been found to be more effective than interferon-alpha/pegylated interferon-alpha [2]. To suppress HBV replication, NAs act by actively competing with endogenous substrates in viral DNA elongation and once incorporated act as chain terminators of viral DNA synthesis [3, 4]. Long-term NAs use has some drawbacks including the development of drug-resistant viral mutations and extra-hepatic serious adverse effects such as myopathy, nephropathy, neuropathy and lactic acidosis [4–7]. Clevudine was discontinued due to its myotoxicity while other NAs, including telbivudine and lamivudine, have been associated with raised serum creatine kinase (CK) and on rare occasions, clinical myopathy and even fatal rhabdomyolysis [3, 5, 7–11]. Furthermore, telbivudine has also been found to be associated with peripheral neuropathy especially when combined with pegylated interferon [5].
Nevertheless, NAs, including telbivudine, remain an important therapy and is the first line therapeutic recommendation in the current Asian-Pacific clinical practice guidelines on hepatitis B management [12]. The actual prevalence of telbivudine-associated myopathy is unknown, and reports of clinical myositis/myopathy from therapeutic trials for telbivudine did not have confirmatory muscle histopathology [13, 14]. In fact, there have only been a few muscle histopathology reports on telbivudine-associated myopathy, and none have provided any immunohistochemical (IHC) analyses of inflammatory cells involved [7, 9–11, 13–15]. We report the clinical and myopathological findings of four patients who developed myopathy after long-term administration of telbivudine for chronic HBV infection.
Patients and Methods
Patient characteristics
Four patients receiving telbivudine therapy for chronic HBV infection were referred to the Neuromuscular Clinic, University of Malaya Medical Centre (UMMC), Kuala Lumpur when they developed proximal muscle weakness and raised serum CK. As part of the standard investigative workup of patients with suspected acquired myopathy, they underwent clinical assessment, baseline blood tests, screening for myositis autoantibodies, electromyography (EMG) and muscle biopsy. All patients gave written informed consent for muscle biopsy and the study was approved by the medical ethics committee of UMMC (MECID. No: 20146–293).
Muscle biopsy analysis
Biopsies were obtained from clinically-weak biceps brachii muscles from all four patients. The biopsies were snap frozen in isopentane cooled by liquid nitrogen, sectioned at 8 μM and stained with haematoxylin and eosin (H&E), modified Gomori trichrome, and a standard set of histochemical stains that included myofibrillar ATPase, nicotinamide adenine dinucleotide tetrazolium reductase (NADH-TR), acid phosphatase, cytochrome-c oxidase (COX), succinate dehydrogenase (SDH) and combined COX/SDH as previously described [16]. Standard IHC stains were used to identify CD4, CD8, CD20, CD68 cells and class I major histocompatibility complex (MHC class I) antigens (Dako, Glostrup, Denmark) as previously reported [17].
Autoantibody profiling
Serum samples were analyzed for myositis-specific antibodies (MSA) and myositis-associated antibodies (MAA) using the commercial assay Myositis Profile 3 EUROLINE according to the manufacturer’s instruction (Euroimmun; Lübeck, Germany). Assays were interpreted using the EUROLineScan software as negative, borderline or positive (Euroimmun; Lübeck, Germany).
Statistical analysis
Statistical analyses were performed using SPSS (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.).
Results
The clinical features of our patients: two men, and two women, are summarized in Table 1. All four patients (age range: 60 to 73 years; mean 67.3 years) developed progressive proximal muscle weakness after 12 to 24 months (mean 17.3 months) on telbivudine therapy for chronic HBV infection. Muscle weakness was present for 2 to 17 months (mean 9 months) before they were diagnosed to have telbivudine-associated myopathy. Using the National Cancer Institute (NCI) of the National Institute of Health (NIH) Common Terminology Criteria for Adverse Events (CTCAE) version 4.03, the severity of generalized muscle weakness was grade 2 in three patients and grade 3 in one (Patient #2) [18]. This meant that they had symptomatic weakness, evident of examination, and limited their activities of daily living (ADL), and was disabling in Patient #2. Serum CK was elevated with the mean of 984.5 IU/L (range: 426 to 1798 IU/L). Two patients on simvastatin and one on atorvastatin had their statin treatment stopped when they developed muscle weakness at 3, 8 and 6 months, respectively, before telbivudine was discontinued. However, this did not result in improvement in muscle strength or reduction in serum CK levels. None had a family history of neuromuscular disease. All patients were shown to have myopathic EMGs but normal nerve conduction studies. The MSA and MAA were negative in three of the four patients in whom it was done.
Table 1. The clinical data of patients with telbivudine-associated myopathy.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Age at presentation (years) | 68 | 68 | 60 | 73 |
| Sex | Male | Female | Female | Male |
| Ethnicity | Chinese | Malay | Chinese | Chinese |
| Duration with HBV (months) | 23 | 26 | 240 | 240 |
| HBV DNA, IU/mL | 1120000 | 809200 | 66930 | 340000 |
| ALT (IU/L, normal 30–65) | 107 | 46 | 61 | 39 |
| AST (IU/L, normal 15–37) | 108 | 95 | 46 | 56 |
| Daily telbivudine dose (mg) | 600 | 600 | 600 | 600 |
| Duration of telbivudine therapy before symptom onset (months) | 20 | 12 | 13 | 24 |
| Interval between symptom onset and diagnosis (months) | 3 | 14 | 17 | 2 |
| Proximal limb weakness | Present | Present | Present | Present |
| EMG findings | Myopathic | Myopathic | Myopathic | Myopathic |
| Peak serum CK (IU/L, normal 39–308 (men) and 26–192 (women)) | 1798 | 968 | 426 | 746 |
| MSA/MAA | ND | Absent | Absent | Absent |
| mRS score (at presentation) | 3 | 4 | 3 | 3 |
| CTCAE* (adverse events), grade | 2 | 3 | 2 | 2 |
| Statin therapy | None | Simvastatin | Atorvastatin | Simvastatin |
| Medication after diagnosis of telbivudine-associated myopathy | Entecavir | Entecavir | Tenofovir | Entecavir |
| Recovery time, months | 6 | 10 | 8 | 4 |
| mRS score (outcome) | 0 | 4 | 0 | 0 |
| Neurological outcome | Normal | Poor | Normal | Normal |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; CTCAE, common terminology criteria for adverse events; EMG, electromyography; HBV, hepatitis B virus; MAA, myositis-associated antibody; MSA, myositis-specific antibody; mRS, modified Rankin Scale; ND, not done.
*CTCAE grading for generalized muscle weakness are as follows:—Grade 1: symptomatic, weakness perceived by patients but not evident on physical examination; Grade 2: symptomatic, weakness evident on physical examination and weakness limiting instrumental ADL; Grade 3: weakness limiting self-care ADL, disabling.
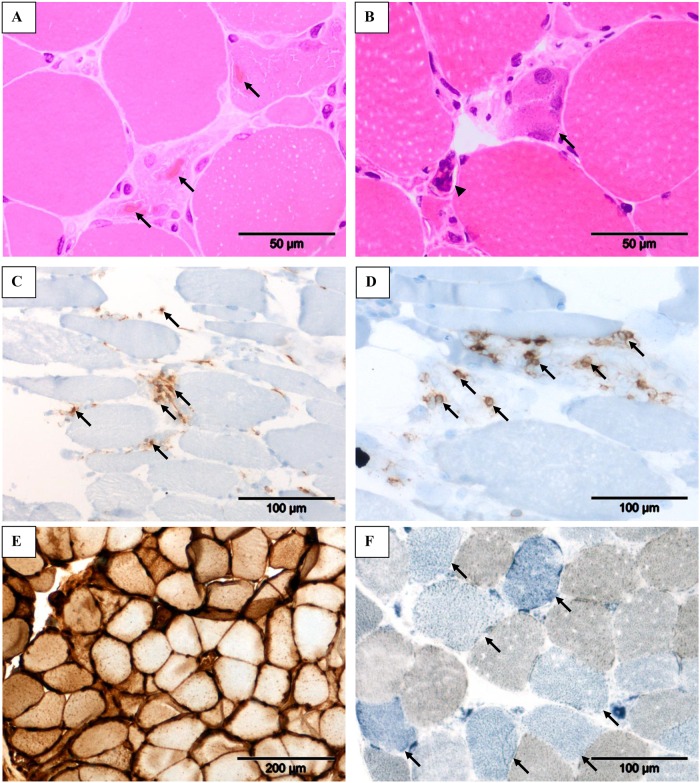
Muscle biopsy findings are shown in Table 2. The most prominent and consistent findings were fiber size variation, myophagocytosis, myofiber degeneration/necrosis and cytoplasmic bodies (Fig 1A), and fiber regeneration (Fig 1B). The inflammatory infiltrates consisted mainly of CD4+ (Fig 1C) and CD8+ T cells (Fig 1D). Additionally, all biopsies showed strong sarcolemmal overexpression of MHC class I antigens (Fig 1E) and occasional loss of COX activity (Fig 1F).
Table 2. The myopathological features of patients with telbivudine-associated myopathy.
| Patients | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Hypertrophic fibers | − | − | − | − |
| Small rounded atrophic/angular fibers | + | + | − | − |
| Degeneration/necrosis | + | + | + | + |
| Regeneration | + | + | + | + |
| Clumped nuclei | + | + | + | + |
| Cytoplasmic body | + | + | + | + |
| Acid phosphatase activity | + | +, Focal | − | + |
| Myofibrillar disarray | + | + | + | + |
| COX-negative fibers | + | + | + | + |
| Inflammatory cell infiltration† | ||||
| CD4 | ++ | ++ | + | ++ |
| CD8 | + | + | + | + |
| CD20 | − | − | − | − |
| CD68 | + | ++ | + | + |
| MHC Class I | Overexpressed | Overexpressed | Overexpressed | Overexpressed |
| Endomysial fibrosis | + | + | + | + |
Abbreviations: +, present; −, absence; CD, cluster of differentiation; COX, cytochrome c oxidase; MHC class I, class I major histocompatibility complex.
†The presence of these cells are graded as “−” means negative, “+” means grade I positive (very minimal to minimal), “++” means grade II positive (mild to moderate).
Fig 1. The myopathological features of our telbivudine-associated myopathy series.
Cytoplasmic bodies (A, arrows) within degenerate/necrotic fibers, fiber regeneration (B, arrow) and clumped nuclei (B, arrowhead), and inflammatory infiltrates consisting mainly of CD4+ T-cells (C, arrows) and CD8+ T-cells (D, arrows) as observed in Patient 4. Strong sarcolemmal overexpression of MHC class I in Patient 1 (E) and COX-negative fibers (F, arrows) in Patient 4. Stains: Hematoxylin and Eosin (A & C), immunohistochemistry with 3, 3’ diaminobenzidinetetrahydrochloride chromogen/hematoxylin (C–E) and combined COX/SDH histochemistry (F). Original magnification: x 40 objective (A & B); x 20 objective (C, D & F); x 10 objective (E).
Following the diagnosis of myopathy, telbivudine was discontinued and replaced with entecavir in three patients and with tenofovir in one (Table 1). Three patients recovered fully after a period between 4 to 7 months, while the other patient (Patient 2) showed only partial improvement of muscle strength and remained unable to stand and walk independently even at 10 months’ follow-up. She did, however, showed a reduction in CK levels to normal. In this patient, the diagnosis of myopathy had been delayed for 14 months and she would likely have had severe myofiber damage.
Discussion
The actual prevalence of telbivudine-associated myopathy is unknown but in large telbivudine trials, this has been reported to be low [13, 14]. In the phase III GLOBE trial, despite 12.9% of patients on telbivudine developing severe elevation of serum CK levels (defined as more than seven times the upper limits of normal), clinical myopathy which was defined by the authors as having muscle symptoms in addition to raised serum CK, was reported only in two (0.3%) patients [13]. In another series of 200 chronic hepatitis B patients from China treated with telbivudine, the 3-year cumulative incidence of elevated serum CK was high at 84.3%, occurring more commonly in men than women, patients aged less than 45 years and those who were HBeAg-negative [14]. However, only nine patients (5%) were reported to have clinical myopathy and no risk factors for myopathy were identified [14]. Subsequent follow-up studies have shown that on-treatment, elevated serum CK levels were often transient and were not predictive of the development of myopathy [19, 20]. In our patients, CK elevations were mild to moderate only, further confirming that CK levels did not correlate with the development of myopathy.
There have been only a few reports of telbivudine-associated myopathy with detailed muscle histopathology findings [7, 15]. In a patient treated with telbivudine for a duration of 6 months after being given other NAs, including lamivudine and adefovir, muscle biopsy showed the presence of necrotic/degenerating and regenerating fibers, and on electron microscopy (EM), no mitochondrial abnormalities were seen [15]. On the other hand, in a series of six chronic HBV patients from China treated with NAs (three with telbivudine, three with lamivudine), muscle pathology was reported as showing non-specific abnormalities, including variation in fiber sizes, presence of angulated and regenerating fibers, and type I and II fiber atrophy, with necrotizing myopathy seen in only one patient on telbivudine [7]. In addition, all patients had positive oil red O staining suggesting an accumulation of lipids in their muscle fibers [7]. In contrast, all our patients had necrotizing myopathy with prominent muscle fiber necrosis and myophagocytosis. These findings were also previously seen in clevudine-associated myopathy [21].
NA-associated myopathy is considered to be due to mitochondrial toxicity [7, 21, 22]. In pre-clinical studies, telbivudine was not shown to have any effect on human DNA polymerase γ, a key enzyme involved in the mitochondrial DNA replication, and in vitro toxicity studies showed no mitochondrial abnormalities in human hepatocytes, skeletal muscle and neuronal cells [23]. However, in the case series from China, there was evidence of mitochondrial dysfunction, including the presence of ragged red (or blue) and COX-deficient fibers, mitochondrial abnormalities on EM and depletion of mitochondrial DNA in muscle [7]. Findings of COX-deficient fibers in our patients would support the mitochondrial toxicity of telbivudine, but as they are of the older age group, these findings could also be attributed to aging [24].
Interestingly, we also found overexpression of MHC class I antigens on the muscle fibers associated with inflammatory infiltrates consisting of both CD4+ and CD8+ T cells. Overexpression of MHC class I and CD8+ T cells has also been reported in human immunodeficiency virus patients with zidovudine-induced myopathy [22]. These features could alternatively suggest an underlying immune-mediated mechanism for NA-associated myopathy, besides drug induced mitochondrial toxicity. However, myositis autoantibodies were absent in our patients, and three of four patients recovered fully after drug withdrawal without any immunotherapy. The patient (Patient 2) who did not have complete clinical recovery nevertheless had her serum CK reduced to normal levels after discontinuation of telbivudine. Her incomplete recovery of muscle strength could indicate a more severe myonecrosis and poorer muscle fiber regenerative capacity.
In conclusion, telbivudine-associated myopathy presents as a sub-acute or chronic necrotizing myopathy with some evidence of mitochondrial toxicity as its underlying mechanism. As the overexpression of MHC class I antigens and the presence of inflammatory cells were the prominent and consistent findings in our patients, the possibility of a secondary immune-mediated inflammation may need further investigation.
Acknowledgments
We thank the patients, the nurses, and the staff in the Neurology Laboratory and Anatomic Pathology Laboratory for their contributions to this work.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by the UM/MoHE/08 High Impact Research Grant H-20001-E00031, Ministry of Higher Education, Malaysia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. Journal of viral hepatitis. 2004;11(2):97–107. [DOI] [PubMed] [Google Scholar]
- 2.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology (Baltimore, Md). 2007;45(2):507–39. [DOI] [PubMed] [Google Scholar]
- 3.Fung J, Lai CL, Seto WK, Yuen MF. Nucleoside/nucleotide analogues in the treatment of chronic hepatitis B. The Journal of antimicrobial chemotherapy. 2011;66(12):2715–25. 10.1093/jac/dkr388 [DOI] [PubMed] [Google Scholar]
- 4.Fung J, Seto WK, Lai CL, Yuen MF. Extrahepatic effects of nucleoside and nucleotide analogues in chronic hepatitis B treatment. Journal of gastroenterology and hepatology. 2014;29(3):428–34. 10.1111/jgh.12499 [DOI] [PubMed] [Google Scholar]
- 5.Fleischer RD, Lok AS. Myopathy and neuropathy associated with nucleos(t)ide analog therapy for hepatitis B. Journal of hepatology. 2009;51(4):787–91. 10.1016/j.jhep.2009.06.011 [DOI] [PubMed] [Google Scholar]
- 6.Jin JL, Hu P, Lu JH, Luo SS, Huang XY, Weng XH, et al. Lactic acidosis during telbivudine treatment for HBV: a case report and literature review. World journal of gastroenterology: WJG. 2013;19(33):5575–80. 10.3748/wjg.v19.i33.5575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu H, Wang Z, Zheng L, Zhang W, Lv H, Jin S, et al. Lamivudine/telbivudine-associated neuromyopathy: neurogenic damage, mitochondrial dysfunction and mitochondrial DNA depletion. Journal of clinical pathology. 2014;67(11):999–1005. 10.1136/jclinpath-2013-202069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baharin J, Sahari NS, Lim SM. Rhabdomyolysis due to Lamivudine administration in acute viral hepatitis B infection: a case report from Malaysia. Electronic physician. 2014;6(3):863–7. 10.14661/2014.863-867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finsterer J, Ay L. Myotoxicity of telbivudine in pre-existing muscle damage. Virology Journal. 2010;7:323 10.1186/1743-422X-7-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim EH, Park H, Lee KH, Ahn SH, Kim SM, Han KH. Two cases of telbivudine-induced myopathy in siblings with chronic hepatitis B. Clinical and molecular hepatology. 2013;19(1):82–6. 10.3350/cmh.2013.19.1.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SW, Jang JH, Kim BJ. Dysphagia could be the first presenting symptom of telbivudine-induced myopathy. Internal medicine journal. 2013;43(9):1048–9. 10.1111/imj.12237 [DOI] [PubMed] [Google Scholar]
- 12.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatology international. 2016;10(1):1–98. 10.1007/s12072-015-9675-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liaw YF, Gane E, Leung N, Zeuzem S, Wang Y, Lai CL, et al. 2-Year GLOBE trial results: telbivudine Is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136(2):486–95. 10.1053/j.gastro.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 14.Zou XJ, Jiang XQ, Tian DY. Clinical features and risk factors of creatine kinase elevations and myopathy associated with telbivudine. Journal of viral hepatitis. 2011;18(12):892–6. 10.1111/j.1365-2893.2010.01412.x [DOI] [PubMed] [Google Scholar]
- 15.Wang M, Da Y, Cai H, Lu Y, Wu L, Jia J. Telbivudine myopathy in a patient with chronic hepatitis B. International journal of clinical pharmacy. 2012;34(3):422–5. 10.1007/s11096-012-9633-3 [DOI] [PubMed] [Google Scholar]
- 16.Engel AG. The muscle biopsy In: Engel AG, Franzini-Armstrong C, editors. Myology: Basic and Clinical. New York: McGraw-Hill; 2004. p. 681–90. [Google Scholar]
- 17.Preuße C, Goebel HH, Held J, Wengert O, Scheibe F, Irlbacher K, et al. Immune-mediated necrotizing myopathy is characterized by a specific Th1-M1 polarized immune profile. The American journal of pathology. 2012;181(6):2161–71. 10.1016/j.ajpath.2012.08.033 [DOI] [PubMed] [Google Scholar]
- 18.Department of Health U.S. and Human Services, National Institute of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events v4.0 2009. Available: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 19.Jung M, Uddin A, Dong Y, Kraev A, Trylesinski A. Creatine kinase elevations during 4 years telbivudine anti-hepatitis B virus treatment are not predictive of muscle events (abstract). Hepatol Int. 2011;5(1):117. [Google Scholar]
- 20.Dong Y, Uddin A, Kraev A, Koehne C, Trylesinski A. Analyses of multiple telbivudine studies show creatine kinase elevations do not predict muscle events during chronichepatitis B treatment (abstract). Hepatol Int. 2012;6:120. [Google Scholar]
- 21.Seok JI, Lee DK, Lee CH, Park MS, Kim SY, Kim HS, et al. Long-term therapy with clevudine for chronic hepatitis B can be associated with myopathy characterized by depletion of mitochondrial DNA. Hepatology (Baltimore, Md). 2009;49(6):2080–6. [DOI] [PubMed] [Google Scholar]
- 22.Dalakas MC, Illa I, Pezeshkpour GH, Laukaitis JP, Cohen B, Griffin JL. Mitochondrial myopathy caused by long-term zidovudine therapy. The New England journal of medicine. 1990;322(16):1098–105. [DOI] [PubMed] [Google Scholar]
- 23.Bryant ML, Bridges EG, Placidi L, Faraj A, Loi AG, Pierra C, et al. Antiviral L-nucleosides specific for hepatitis B virus infection. Antimicrobial agents and chemotherapy. 2001;45(1):229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller-Hocker J. Cytochrome c oxidase deficient fibres in the limb muscle and diaphragm of man without muscular disease: an age-related alteration. Journal of the neurological sciences. 1990;100(1–2):14–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.