Abstract
The emergence and dissemination of multidrug resistant bacterial pathogens necessitate research to find new antimicrobials against these organisms. We investigated antimicrobial production by eastern subterranean termites, Reticulitermes flavipes, against a panel of bacteria including three multidrug resistant (MDR) and four non-MDR human pathogens. We determined that the crude extract of naïve termites had a broad-spectrum activity against the non-MDR bacteria but it was ineffective against the three MDR pathogens Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus (MRSA), and Acinetobacter baumannii. Heat or trypsin treatment resulted in a complete loss of activity suggesting that antibacterial activity was proteinaceous in nature. The antimicrobial activity changed dramatically when the termites were fed with either heat-killed P. aeruginosa or MRSA. Heat-killed P. aeruginosa induced activity against P. aeruginosa and MRSA while maintaining or slightly increasing activity against non-MDR bacteria. Heat-killed MRSA induced activity specifically against MRSA, altered the activity against two other Gram-positive bacteria, and inhibited activity against three Gram-negative bacteria. Neither the naïve termites nor the termites challenged with heat-killed pathogens produced antibacterial activity against A. baumannii. Further investigation demonstrated that hemolymph, not the hindgut, was the primary source of antibiotic activity. This suggests that the termite produces these antibacterial activities and not the hindgut microbiota. Two-dimensional gel electrophoretic analyses of 493 hemolymph protein spots indicated that a total of 38 and 65 proteins were differentially expressed at least 2.5-fold upon being fed with P. aeruginosa and MRSA, respectively. Our results provide the first evidence of constitutive and inducible activities produced by R. flavipes against human bacterial pathogens.
Introduction
In recent years, insects have been recognized for having potent immune defenses that produce constitutive and inducible antimicrobial compounds to combat various pathogens [1]. Thus, they have been targeted as a potential source of antimicrobial compounds [2, 3]. Insects possess complex immune responses that act synergistically to provide protection against microbial infections [4]. When pathogens break through morphological barriers, insects evoke innate immune responses comprised of cellular and humoral reactions. Cellular reactions are hemocyte-mediated and include phagocytosis and encapsulation, while humoral reactions involve the production of antimicrobial proteins and activation of enzymatic cascades [5]. Over the last few decades, more than 150 insect antimicrobial peptides/proteins (AMPs) have been identified from naïve, microbe-challenged, or injured insects [6]. Reported insect AMPs include lysozymes, cecropins, attacins, defensins, and proline rich peptides [6, 7].
Recently, several constitutive antimicrobial proteins and peptides have been identified from three termite families: Termopsidae [8], Rhinotermitidae [9–12], and Termitidae [13–16]. The majority of these molecules have antifungal activities and only a few, including termicin, defensin-like peptides, spinigerin, and lysozymes, have weak antibacterial activities [13, 16]. Hussain et al. [17] reported induction of antibacterial activity from the whole body homogenates of Coptotermes formosanus Shiraki upon exposure with various bacteria, including a human pathogen Staphylococcus aureus. However, exposure to different bacteria did not stimulate activity against the inducing organisms except for Bacillus thuringiensis.
Subterranean termites (Blattodea: Isoptera: Rhinotermitidae), especially the Reticulitermes species, are widely distributed in the United States. These termites have developed disease resistance mechanisms that facilitated their survival and propagation as they nest and forage in soil [18]. Termite-produced AMPs, termicin (initially isolated from a fungus-growing termite) and tGNBPs (termite gram-negative binding proteins), have been described in the eastern subterranean termite R. flavipes and the dark southern subterranean termite R. virginicus [9, 10, 13]. GNBP2 has β-1, 3-glucanase activity in termites and contributes to external antifungal defense [13]. We previously reported the discovery of constitutive antibacterial activity from the cell-free extract (CFE) of R. flavipes against a common Gram-positive soil-borne entomopathogenic bacterium, B. subtilis [12]. In this study, we determined the presence, characteristics, and levels of constitutive and inducible antibacterial activities in R. flavipes against a panel of human bacterial pathogens including three common multidrug resistant nosocomial pathogens and five non-MDR pathogens.
Materials and Methods
Termite maintenance and induction of antimicrobial activity
R. flavipes were collected on the Auburn University campus as previously described [12, 19] and workers were maintained in Urban Entomology Laboratory at 25 ± 2°C for at least 20 days before being subjected to experiments. To examine the potentially inducible antibacterial activity, two heat-killed MDR pathogens, Gram-negative P. aeruginosa and Gram-positive MRSA, were selected to stimulate the immune response. Bacteria were grown in Lysogeny Broth (LB) [20] at 37°C with aeration overnight, subcultured, and grown in fresh LB to early-mid log-phase (OD600 = 0.3 ± 0.05). Twenty-four ml of heat-killed bacterial suspension was obtained as follows: cells from 48 ml of culture was harvested via centrifugation at 13,200 rpm for 5 min, washed twice with 48 ml of milli-Q (MQ) water, resuspended in 24 ml of MQ water (~6 x 108 cells/ml), and heat-killed at 100°C for 10 min. R. flavipes workers were surface sterilized with 70% ethanol to eliminate surface microbes immediately before being subjected to testing. To immunize the workers, a group of 3 g of surface-sterilized termites (8 groups per treatment) was introduced into Petri plates (15 cm × 2.5 cm) provisioned with sterile filter papers (12.5 cm in diameter, Whatman #1; 1 filter paper per Petri dish) moistened with 3 ml of heat-killed P. aeruginosa or MRSA suspension, respectively. Sterile filter papers were moisturized with the same amount of MQ water and were used as negative controls. The termites were allowed to feed in Petri plates for 24 hours and then harvested for analysis.
Preparation of whole body and size-fractionated CFE
Surface sterilized naïve and heat-killed pathogen challenged termite workers (24 g each) were suspended, separately, in 120 ml of 20 mM Tris-HCl, 20 mM NaCl (pH = 7.5) buffer and homogenized as previously described [12]. The crude CFE was quickly frozen in liquid nitrogen and lyophilized (Heto Lyolab 3000, Thermo Fisher Scientific, Pittsburgh, PA) at -57°C overnight before being dissolved in MQ water to achieve the final concentration of approximately 20 mg/ml protein concentration as determined by the Bradford assay (Bio-Rad, Hercules, CA) [21].
Additionally, a sample of each crude CFE (15 ml) was sequentially size fractionated with Microsep™ Advance Centrifugal Devices (Pall Corporation, Port Washington, NY) with the molecular weight cut-offs (MWCO) of 100K, 30K, 10K, and 3K. This separated the CFE into five fractions containing proteins with approximate molecular weight of >300 kDa, 90–180 kDa, 30–90 kDa, 10–20 kDa, and <10 kDa, respectively. The fractionated solutions were lyophilized and dissolved in MQ water to achieve the final protein concentration of approximately 20 mg/ml to match that of the crude extract. All samples were stored at -80°C until the antibacterial assays.
Protein denaturation
To denature proteins, 5 ml of the lyophilized crude extract of each treatment was heated to 100°C for five minutes as previously described [12]. Trypsin digestion was performed as follows. To 100 μl of lyophilized crude extract for each treatment, 5 μl of 200 mM dithiothreitol (DTT) in 100 mM NH4HCO3 was added and incubated for 30 min at room temperature. Then, 4 μl of the 1M iodoacetamide alkylating reagent was added to the sample, mixed, and incubated for 45 min at room temperature. Finally, 50 μl of trypsin (0.2 μg/μl in 100 mM NH4HCO3) was added to the sample and incubated overnight at 37°C.
Termite hemolymph collection and hindgut extraction
Hemolymph was immediately drawn (~0.05–0.1 μl/individual) from surface sterilized termites by inserting a sterile insect needle into the dorsal intersegmental membrane of cold-immobilized insects. Any sample contaminated with the gut or fat was discarded. The extracted hemolymph (~200–400 μl) was transferred to a microcentrifuge tube containing 1 ml of 20 mM Tris-HCl, 20 mM NaCl (pH = 7.5) buffer and kept on ice. Hindguts for the same individuals were separated and rinsed in 5 ml of 20 mM Tris-HCl, 20 mM NaCl (pH = 7.5) buffer before being homogenized in 1ml of buffer on ice. The hemolymph and gut extracts were centrifuged at 12,000 rpm at 4°C for 5 min to acquire the cell-free samples. The protein concentration of each sample was measured and was adjusted to the final concentration of 25 mg/ml by either dilution or concentration following lyophilization.
Antibacterial assay
Antibacterial activity of each extract against a panel of selected bacteria (Table 1) was determined using a modified inhibition zone assay as previously described [12]. For every assay, each bacterium was freshly grown from a frozen stock. Briefly, a colony from a freshly streaked plate was inoculated into LB medium, grown overnight at 37°C with shaking at ~220 rpm, subcultured the next day into fresh LB medium and grown at 37°C with shaking at ~220 rpm to early log-phase of growth (OD600 = 0.3 ± 0.05), and diluted to ~2.5 × 107 CFU/ml. The antibacterial activities of crude extracts were examined by placing eight sterilized filter paper disks (5 × 5 mm) uniformly on the bacterial lawn in each plate. The paper disks were treated with one of the following eight samples, respectively: 20 μl of three crude extracts (naïve, P. aeruginosa-challenged, and MRSA-challenged) at a concentration of approximately 20 mg/ml (= 400 μg/disk), 20 μl of three heat-treated crude extracts (naïve, P. aeruginosa-challenged, and MRSA-challenged), 1 μl of ampicillin (= 25 μg/disk) as the positive control, and 20 μl of 80 mM Tris-HCl, 80 mM NaCl buffer as the negative control. The antibacterial activities of size-fractionated extracts, hemolymph, and gut extract were determined using the same assay. All plates were incubated at 37°C for 24 h to allow bacterial growth and the zones of clearing were measured. Every assay was repeated three times, each with 3 replicates, to acquire an N of 9 for each treatment.
Table 1. List of the tested bacteria.
| Bacterium | Gram Stain | Multi-Drug Resistant | Source or Reference |
|---|---|---|---|
| Staphylococcus aureus | + | No | ATCC 12600 via Robert Miller |
| Methicillin-resistant Staphylococcus aureus (MRSA) | + | Yes | James Barbaree |
| Streptococcus pyogenes | + | No | ATCC 19615 via Robert Miller |
| Pseudomonas aeruginosa (PAO1) | - | Yes | [22] |
| Escherichia coli O157:H7 (CDC B1409-C1) | - | No | ATCC 43889 |
| E. coli K-12 (MG 1655) | - | No | ATCC 700926 |
| Salmonella enterica serovar Typhimurium (LT2) | - | No | [23] via Jorge Escalante-Semerena |
| Acinetobacter baumannii (AYE) | - | Yes | ATCC BAA-1710 [24] |
Statistical analysis
The diameters (D; mm) of inhibition zones were compared using the ANOVA and Tukey’s method (PROC GLM; α = 0.05; SAS 9.2) to determine all possible pairwise differences among treatments. The ANOVA (PROC GLM; α = 0.05; SAS 9.2) was used to determine the difference in activities of the same treatment on each tested bacterium.
Gel electrophoretic analysis of proteins
Protein profiles of the MWCO of 30K and 100K fractionated samples of termite CFE were analyzed by polyacrylamide gel electrophoreses (PAGE) including both non-denaturing Native PAGE and denaturing SDS-PAGE. Approximately 20 μg of protein samples were loaded per lane. The protein ladders for Native PAGE (14–500 kDa) and SDS-PAGE (10–250 kDa) were purchased from Sigma-Aldrich (St Louis, MI) and Life Technologies (Green Island, NY), respectively. In addition, non-size fractionated hemolymph samples were analyzed on SDS-PAGE. The proteins on PAGE were stained with Coomassie brilliant blue R-250 (Bio-Rad, Hercules, CA) for visualization.
Two-dimensional gel electrophoretic analysis of the proteins in hemolymph was performed by the Kendrick Labs, Inc. (Madison, WI). Approximately 200 μg of total protein per sample was used to separate the proteins between pI of 3 to 10 for the first dimension and molecular weight of 14–220 kDa for the second dimension. Duplicate gels were run for each sample, stained with Sypro®Ruby (Bio-Rad), and scanned on a Typhoon FLA 9000 scanner (GE Healthcare, Piscataway, NJ). A total of 493 spots were analyzed using Progenesis SameSpots software (version 4.5, 2011, TotalLab, UK) and Progenesis PG240 software (version 2006, TotalLab, UK). The quantity of each spot was calculated as spot percentages (individual spot density divided by total density of all measured spots). The quantity differences between MRSA-challenged versus naïve and P. aeruginosa-challenged versus naïve were analyzed using two-sample t-test.
Results
Broad-spectrum constitutive antibacterial activity of R. flavipes
In order to understand the biological range of antibacterial activity of R. flavipes CFE, we tested a panel of non-MDR and MDR human bacterial pathogens for their susceptibility. Table 1 shows the panel of bacteria used in this study which includes both gram-positive and gram-negative bacteria. The CFE prepared from the whole body of naïve termite displayed significant inhibitory activity against the five non-MDR bacteria (Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli O157:H7, Salmonella enterica serovar Typhimurium, and E. coli K-12) but it was inactive against the three MDR pathogens (methicillin resistant S. aureus or MRSA, Pseudomonas aeruginosa, and Acinetobacter baumannii) (Table 2). Of the five susceptible bacteria, the strongest inhibitory effect was against S. aureus, followed by E. coli O157:H7 and S. Typhimurium. The weakest activity was against S. pyogenes. As expected, ampicillin inhibited the growth of all but the three MDR pathogens. We observed no obvious correlation between Gram-staining and the effectiveness of the termite CFE on growth inhibition. The antibacterial activity of termite CFE disappeared completely when it was heat-denatured (Table 2) or treated with trypsin (data not shown). These data suggest that the antibacterial activity of R. flavipes is likely to be proteinaceous in nature.
Table 2. Antibacterial activity of CFE of naïve termites.
| Bacteria | Termite crude extract (400 μg) | Ampicillin (25 μg) | Heat-treated crude extract | Control* | ||
|---|---|---|---|---|---|---|
| Non-infectious | E. coli | 9.41 ± 0.37d | 8.49 ± 0.33e | 0 | 0 | |
| Infectious | Non-MDR | S. aureus | 16.16 ± 0.73a | 27.99 ± 0.4a | 0 | 0 |
| S. pyogenes | 9.14 ± 0.30d | 16.41 ± 0.36b | 0 | 0 | ||
| E. coli O157:H7 | 14.51 ± 0.57b | 11.28 ± 0.24d | 0 | 0 | ||
| S. Typhimurium | 10.42 ± 0.50c | 12.39 ± 0.28c | 0 | 0 | ||
| MDR | MRSA | 0 | 0 | 0 | 0 | |
| P. aeruginosa | 0 | 0 | 0 | 0 | ||
| A. baumannii | 0 | 0 | 0 | 0 | ||
Different letters within a column indicate significant difference in inhibition zone diameters at significance level of α = 0.05 (ANOVA, F4, 40(CrudeExtract) = 1854.68, P < 0.0001; F6, 56(Ampicillin) = 2960.78, P < 0.0001). The experiment was performed three independent times with triplicate samples for a total N of 9 for each treatment. The diameter of zone of inhibition was measured following 24 h incubation at 37°C.
*Control: 80 mM Tris-HCl, 80 mM NaCl buffer.
MDR-induced alteration in antibacterial activities of R. flavipes
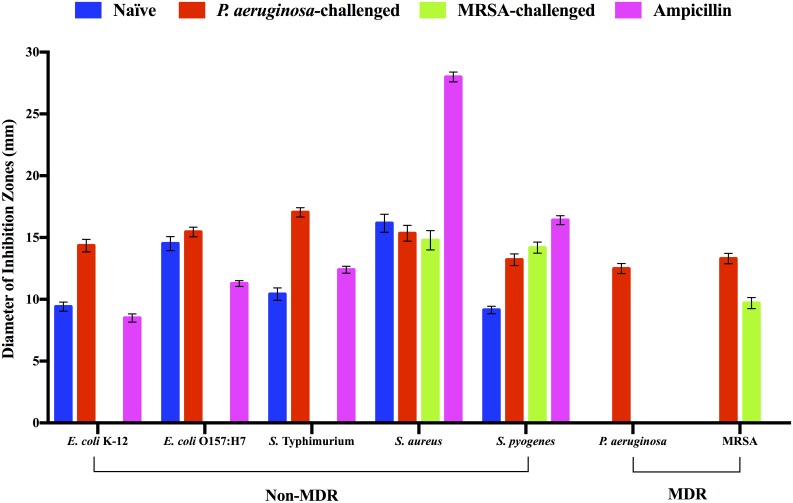
In addition to the constitutive antibacterial activity, we determined that R. flavipes possesses inducible antibacterial activities. Feeding termites with heat-killed P. aeruginosa or MRSA altered their antibacterial activities and stimulated specific anti-MDR activity as illustrated in Fig 1. In both cases, the termites produced new antibacterial activity that was effective against the inducer bacterium. Specifically, termite challenged by heat-killed P. aeruginosa produced activity against both P. aeruginosa and MRSA while maintaining or slightly increasing antibacterial activity against bacteria listed in Table 1. Termites challenged by heat-killed MRSA produced anti-MRSA activity while maintaining activity against two non-MDR Gram-positive pathogens. Interestingly, antibacterial activity against Gram-negative bacteria listed in Table 1 was completely abolished in MRSA-challenged termites (Fig 1). Neither P. aeruginosa nor MRSA induced antibacterial activity against A. baumannii in R. flavipes (data not shown).
Fig 1. Antibacterial activities of cell free extracts of R. flavipes.
Approximately 400 μg of CFE of the naïve, P. aeruginosa-challenged, and MRSA-challenged R. flavipes was applied respectively to each filter disk on a bacterial lawn. The zone of inhibition of growth was measured following incubation for 24 hours at 37°C. Ampicillin was used as the positive control at 25 μg per filter disk and 20 μl of buffer was used as the negative control. The data shown are a compilation of three independent experiments done in triplicate for a total N of 9 per sample (ANOVA, F27, 224 = 2635.47, P < 0.0001). Data for A. baumannii are not shown because the termite extracts were ineffective against the bacterium.
Size fractionation of antibacterial activities
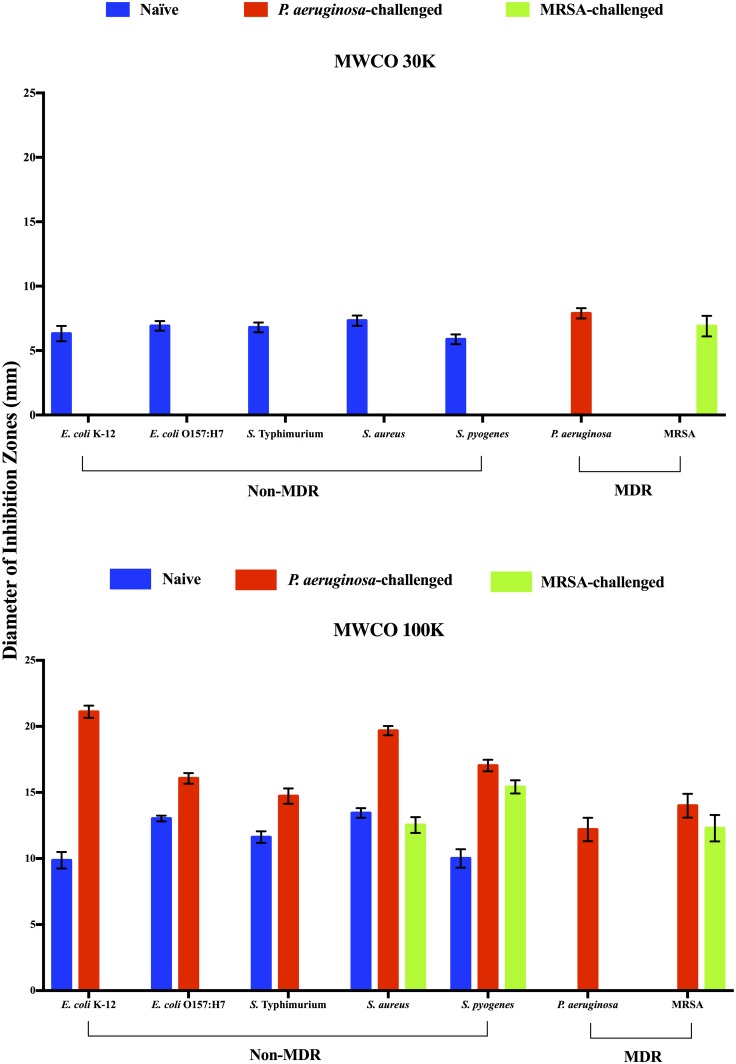
We previously demonstrated that multiple fractions of size-fractionated R. flavipes CFE had antibacterial activity against the soil-borne entomopathogenic B. subtilis [12]. This indicated that R. flavipes produced multiple proteins with anti-B. subtilis activity. In continuing our characterization, we tested size-fractionated CFE from the naïve, P. aeruginosa challenged, and MRSA challenged R. flavipes against the panel of bacteria listed in Table 1. We determined that all antibacterial activities of three groups of termites against this panel of bacteria were contained in fractions with proteins larger than 90 kDa in molecular weight (MWCO filters of 30K and 100K; S1 Fig). However, many of these large proteins appeared to be composed of smaller subunits because they migrated as proteins of 25–90 kDa on denaturing SDS-PAGE (S2 Fig). In all three groups of termites, the MWCO 100K fractions containing proteins of >300 kDa demonstrated greater activity against the susceptible bacteria than the MWCO of 30K fractions with 90–180 kDa proteins (Fig 2). These data support our previous finding that R. flavipes possesses multiple proteins with antibacterial activity.
Fig 2. Antibacterial activity of size-fractionated cell free extracts of R. flavipes.
Antibiotic activity was measured as diameter of inhibition zones caused by respective application of approximately 400 μg of size-fractionated CFE from the naïve, P. aeruginosa-challenged, and MRSA-challenged R. flavipes on a bacterial lawn. The data shown are a compilation of three independent experiments done in triplicate for a total N of 9 per sample (ANOVA, F27, 224 = 2635.47, P < 0.0001). (A) MWCO 30K (90–180 kDa) fraction. (B) MWCO 100K (>300 kDa) fraction. Data for fractions of <10 kDa, 10–20 kDa, 30–90 kDa are not shown because they did not demonstrate antibacterial activities.
The P. aeruginosa-induced antibacterial activities against the seven susceptible bacteria were all due to the protein fraction from the MWCO 100K filter except against the inducer bacterium. The anti-P. aeruginosa activity was present in both the MWCO 100K and 30K fractions, indicating the possibility of induction of multiple anti-P. aeruginosa proteins. In addition, the P. aeruginosa-challenged termites exhibited more effective antibacterial activity in the MWCO 100K protein fraction against those non-MDR bacteria than did the naïve termites. The increased activity in the MWCO 100K fraction was especially evident against E. coli K-12 and S. aureus, indicating that the induction of antibacterial activity by P. aeruginosa was independent of the Gram staining-based classification of bacteria. Interestingly, P. aeruginosa induced greater anti-MRSA activity in the MWCO 100K fraction than did MRSA.
Similar to P. aeruginosa induction, the MRSA-induced antibacterial activity was contained in the MWCO 100K fraction with the exception of anti-MRSA activity which was present in both the MWCO 30K and 100K fractions.
Origin of R. flavipes antibacterial activity
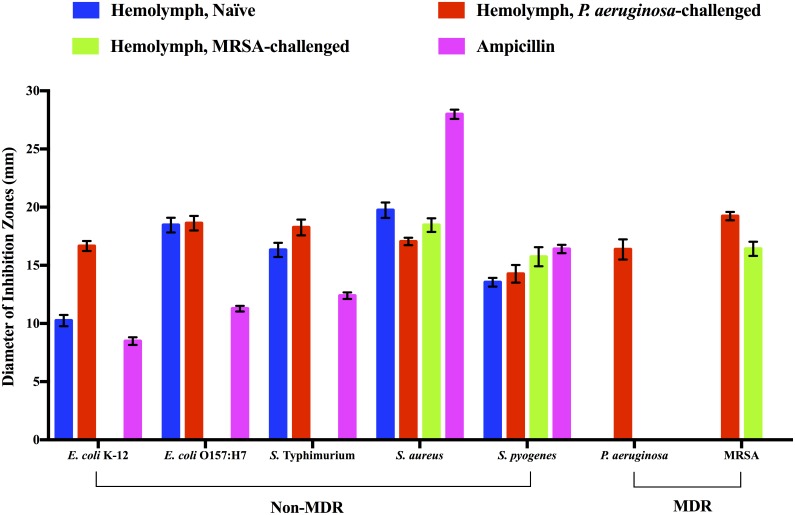
In order to determine whether the antibacterial activities were of termite origin or of the gut microbiota, we prepared extracts from the hindgut and compared the activity to the hemolymph. As illustrated in Fig 3, the antibacterial activity against our panel of bacteria were only observed in the hemolymph of the naïve, P. aeruginosa-challenged, or MRSA-challenged. Hindgut extracts from the same termites showed no perceived antibacterial activities (data not shown). The antibacterial activity profile of the hemolymph resembled that of the whole-body extract shown in Fig 1. Our data suggest that both constitutive and inducible antibacterial activities of R. flavipes are likely of termite origin and reside in hemolymph.
Fig 3. Antibacterial activity of R. flavipes hemolymph.
Approximately 400 μg of hemolymph extract from the naïve, P. aeruginosa-challenged, and MRSA-challenged R. flavipes was applied respectively on a bacterial lawn. The zone of inhibition was measured. Ampicillin was used as the positive control at 25 μg per filter disk. The data shown are a compilation of three independent experiments done in triplicate for a total N of 9 per sample (ANOVA, F41, 336 = 9762.44, P<0.0001).
Protein profiles of termite hemolymph
We analyzed the hemolymph protein profiles of naïve, P. aeruginosa-challenged, and MRSA-challenged termites via both one-dimensional and two-dimensional gel electrophoreses to identify differentially expressed proteins. On one-dimensional 8% Native PAGE, we observed very little difference between the naïve, MRSA-challenged, and P. aeruginosa-challenged termites for both 100K and 30K MWCO fractionated samples (S1 Fig). On denaturing 8% SDS-PAGE, we observed subtle differences between the three samples and between the 100K and 30K MWCO fractionated samples (S2 Fig). Specifically, there was a slight upregulation of ~50 kDa, ~110 kDa and ~150–200 kDa proteins, and a slight downregulation of ~35 kDa, ~55 kDa proteins in MRSA-challenged termites. For P. aeruginosa-challenged termites, we observed a slight upregulation of ~80 kDa, ~50 kDa, and ~35 kDa proteins and a slight downregulation of >250 kDa and ~60 kDa proteins. When the hemolymph samples were analyzed on 8% SDS-PAGE, we observed two abundant proteins between ~60–85 kDa that appeared to be conserved among the naïve, P. aeruginosa-challenged, and MRSA-challenged termites. We also observed some differences between three samples but especially for P. aeruginosa-challenged termites in which we saw disappearance of a prominent band of ~250 kDa and appearance of ~35 kDa, ~50 kDa, and ~65 kDa (S3 Fig).
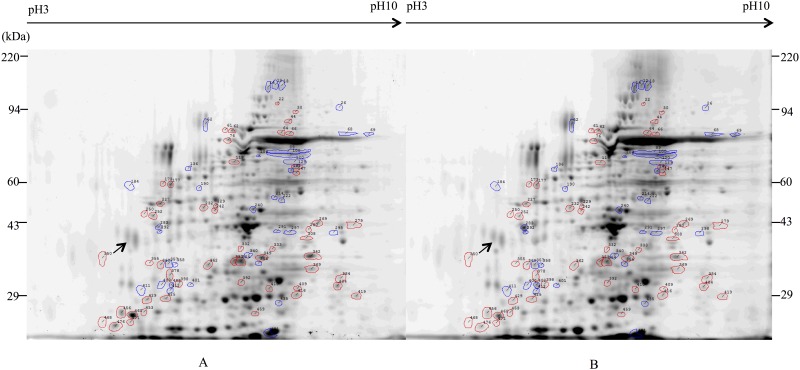
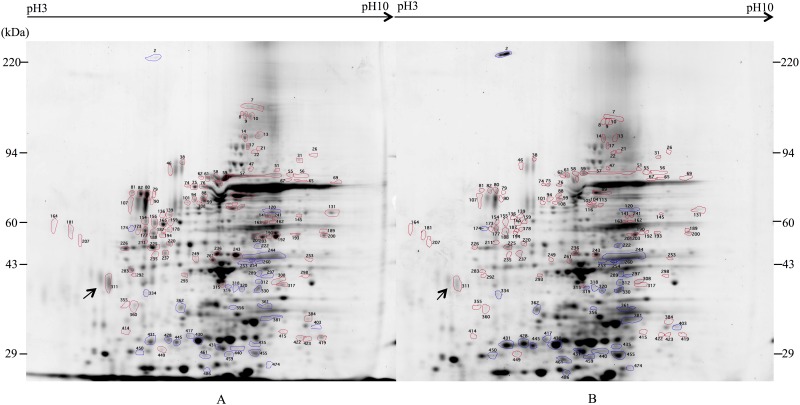
It was clear from our analyses that termite hemolymph was too complex to be accurately analyzed via one-dimensional PAGE. Thus, we performed two-dimensional electrophoretic analyses of the naïve, P. aeruginosa-challenged, and MRSA-challenged termite hemolymphs. Based on our data, the termite hemolymph contains approximately 493 proteins that are visible on two-dimensional gel stained with Sypro®Ruby. A comparison of the hemolymph proteins between the naïve (Fig 4A) and the P. aeruginosa-challenged termites (Fig 4B) indicated that 38 proteins were differentially expressed at least 2.5-fold (P<0.05). Of these, 18 proteins were upregulated and 20 proteins were downregulated (S1 Table). A comparison of the naïve termites (Fig 5A) and the MRSA-challenged termites (Fig 5B) showed that 65 proteins were differentially expressed at least 2.5-fold (P<0.05). Of these, 11 proteins were upregulated and 54 proteins were downregulated (S2 Table).
Fig 4. Two-dimensional electrophoretic analysis of hemolymph proteins from naïve and P. aeruginosa-challenged R. flavipes.
Approximately 200 μg of hemolymph proteins were separated by two-dimensional gel electrophoresis and visualized with Sypro®Ruby. Blue circles indicate protein spots that are upregulated in P. aeruginosa challenged termite while red circles indicate protein spots that are downregulated. (A) Naïve termites. (B) P. aeruginosa-challenged termites.
Fig 5. Two-dimensional electrophoretic analysis of hemolymph proteins from naïve and MRSA-challenged R. flavipes.
Approximately 200 μg of hemolymph proteins were separated by two-dimensional gel electrophoresis and visualized with Sypro®Ruby. Blue circles indicate protein spots that are upregulated in MRSA challenged termite and red circles indicate protein spots that are downregulated. (A) Naïve termites. (B) MRSA-challenged termites.
In P. aeruginosa-challenged termites, the highest upregulated protein (approximately 11-fold increase) had MW of approximately 37 kDa. The 20 downregulated proteins displayed no discernable pattern in size. In MRSA-challenged termites, the majority of upregulated proteins (11 spots) had MW of approximately 18 to 58 kDa while downregulated proteins (53 spots) were all larger than 28 kDa. The alteration of hemolymph protein profile in response to bacterial challenge support our assertion that R. flavipes contains both constitutive and inducible antibacterial proteins.
We compared some of the differentially expressed hemolymph proteins to previously identified insect immune proteins based on their relative pI and MW. The results are shown in Tables 3 and 4 for P. aeruginosa-induced and MRSA-induced proteins, respectively. Among the P. aeruginosa-induced proteins, six proteins of approximately 30–55 kDa proteins had similar pI and MW to previously identified insect immune proteins. We did not find any insect immune proteins of ≥90 kDa in the literature that had similar pI and MW as the spots we identified (S1 Table). Among the MRSA-induced proteins, we found three proteins of approximately 29–60 kDa in MW that had similar pI and MW to previously identified insect immune proteins. Similar to P. aeruginosa-induced proteins, we did not find any insect immune proteins of ≥90 kDa in the literature that had similar pI and MW as the spots we identified in our MRSA-challenged hemolymph samples (S2 Table).
Table 3. Comparison of P. aeruginosa-induced termite hemolymph proteins to insect immune proteins.
| Spot | Fold change | pI | MW (Da) | Insect immune proteins | pI | MW (Da) | Reference |
|---|---|---|---|---|---|---|---|
| 491 | 3.7 | 7.1 | 17,661 | Antibacterial peptide (Bombyx mori) | 6.8 | 18,777 | [25] |
| 491 | 3.7 | 7.1 | 17,661 | Attacin-like immune protein | 7.0 | 17,588 | http://www.ncbi.nlm.nih.gov/protein/AHB11276.1 |
| 491 | 3.7 | 7.1 | 17,661 | Gloverin 4 (Bombyx mori) | 6.8 | 18,777 | [25] |
| 358 | 7.2 | 5.7 | 36,011 | Toll (Sitophilus oryzae) | 5.2 | 37,609 | [26] |
| 400 | 2.9 | 5.6 | 32,736 | Immune-related Hdd13 (Hyphantria cunea) | 5.7 | 29,691 | [27] |
| 402 | 3.1 | 5.7 | 32,692 | Antimicrobial protein 6 Tox precursor (Galleria mellonella) | 5.7 | 32,542 | [28] |
| 358 | 7.2 | 5.7 | 36,011 | Immune-related Hdd1 (Hyphantria cunea) | 5.2 | 35,611 | [27] |
| 283 | 3.2 | 5.6 | 42,081 | Termite GNBPs (Nasutitermes coniger) | 5.6 | 42,323 | [13] |
| 214 | 3.8 | 7.2 | 52,154 | β-1,3-glucan-binding protein/Gram negative bacteria-binding protein precursor (Hyphantria cunea) | 7.1 | 53,014 | [29] |
The pI and the MW of termite hemolymph proteins are based on the Kendrick Labs’ analysis of the two-dimensional gels. We used arbitrary cutoff values of ≤0.5 in pI and ≤5 kDa between our hemolymph proteins and previously reported insect proteins to determine potential relationship.
Table 4. Comparison of MRSA-induced termite hemolymph proteins to insect immune proteins.
| Spot | Fold change | pI | MW (Da) | Insect immune proteins | pI | MW (Da) | Reference |
|---|---|---|---|---|---|---|---|
| 486 | 7.7 | 6.6 | 18,674 | Antibacterial peptide (Bombyx mori) | 6.4 | 18,821 | [25] |
| 486 | 7.7 | 6.6 | 18,674 | Gloverin 2 (Bombyx mori) | 6.4 | 18,821 | [25] |
| 486 | 7.7 | 6.6 | 18,674 | Gloverin 4 (Bombyx mori) | 6.8 | 18,777 | [25] |
| 428 | 3 | 6.0 | 26,359 | Attacin-E (Hyalophora cecropia) | 6.1 | 25,438 | [30] |
| 450 | 2.5 | 5.7 | 27,591 | Possible antimicrobial peptide (Bombyx mori) | 6.3 | 27,469 | [31] |
| 440 | 3 | 7.1 | 29,320 | Phospholipase A2B precursor (Tribolium castaneum) | 7.6 | 29,425 | [32] |
| 440 | 3 | 7.1 | 29,320 | Spz1A, partial (Manduca sexta) | 7.6 | 29,195 | [33] |
| 440 | 3 | 7.1 | 29,320 | Scolexin A (Manduca sexta) | 7.0 | 30,373 | [34] |
| 254 | 6.3 | 7.2 | 44,637 | Putative hemolin (Hyphantria cunea) | 6.7 | 46,119 | [27] |
| 174 | 2.7 | 5.5 | 58,639 | Gram-negative bacteria binding protein 3 (Drosophila melanogaster) | 6.0 | 55,322 | [35] |
The pI and the MW of termite hemolymph proteins are based on the Kendrick Labs’ analysis of the two-dimensional gels. We used arbitrary cutoff values of ≤0.5 in pI and ≤5 kDa between our hemolymph proteins and previously reported insect proteins to determine potential relationship.
Discussion
The current study demonstrates that the eastern subterranean termite, R. flavipes, produces innate and inducible antibacterial activities that are effective against several human pathogens including two MDRs. The list of pathogens found to be susceptible to naïve R. flavipes’ extract includes bacteria that cause gastroenteritis (E. coli O157:H7 and S. Typhimurium), and common opportunistic and nosocomial pathogens (S. aureus and S. pyogenes). The presence of innate antibacterial proteins in R. flavipes parallels the results found in a fungus-growing termite Pseudacanthotermes spiniger and a pacific dampwood termite Zootermopsis angusticollis [8, 16]. Recent analysis of Z. nevadensis genome revealed that multiple effector immune response genes, including GNBPs, attacin, diptericin and termicin, are encoded in this termite [36]. It is likely that similar products may be found in the hemolymph of R. flavipes since there is a high degree of genetic conservation among termites. Interestingly, in contrast to other studies, including our own study demonstrating fractions of MWCO of 3K and 10K of naïve R. flavipes CFE inhibiting growth of the entomopathogenic B. subtilis [12], all of the activities against the infectious human pathogens we identified in this study were larger than ≥90 kDa contained within the MWCO of 30K and 100K fractions. However, based on our SDS-PAGE analyses, some of the larger proteins appear to be multisubunit complexes as they denatured into smaller proteins.
Constitutive defense mechanisms of insects usually rely on the response of hemocytes and several enzyme cascades such as phenoloxidase to defend against potential pathogens [1]. Although the exact identity or the molecular mechanisms of innate antibacterial activities of R. flavipes have yet to be characterized, we identified the hemolymph as the source of these activities. This suggests that the antibacterial activities seen in naïve termites are a part of R. flavipes’ constitutive immune system.
In addition to the constitutive antibacterial activities against several non-MDR human pathogens, we successfully demonstrated induction of specific activities using two MDR human pathogens as antagonists. Induction of antimicrobial activity in insects is not new. In 2012, Hussain et al. [17] described a low level induction of antibacterial activity in the Formosan subterranean termite, C. formosanus, when the termite was immersed in suspensions of an entomopathogenic fungus (M. anisopliae) or several bacteria. However, of the bacteria used in the study (S. aureus, B. thuringiensis, E. coli, and Ralstonia solanacearum), only B. thuringiensis, which produces anti-insect toxins, induced antimicrobial activity. Interestingly, our results suggest that both Gram-positive and Gram-negative bacteria can be strong inducers, and antibacterial responses can be observed 24 h after heat-killed bacteria challenge. This is supported by a recent study showing that specific combinations of immune genes in R. flavipes were expressed in responding to the exposure of various infective fungal spores [37]. Other studies have demonstrated specific response of the American cockroach, Periplaneta Americana [38], and the bumblebee, Bombus terrestris [39], to Gram-negative and Gram-positive bacteria. However, our study is the first one to demonstrate induction of specific activities in termite against MDR human pathogens.
The pattern of induced antibacterial activity based on the bacterium used as the antagonist suggests a complex phenomenon. P. aeruginosa-challenge induced anti-P. aeruginosa activity while maintaining or slightly increasing antibacterial activities across a broad spectrum of bacteria tested. In contrast, MRSA-challenge induced the activity against the antagonist while maintaining or slightly increasing activity only against two Gram-positive bacteria, S. aureus and S. pyogenes. Thus, these induction patterns appear to reduce the possibility of peptidoglycan or lipopolysaccharide serving as the major antagonist for R. flavipes against these bacteria. Both P. aeruginosa and MRSA possess peptidoglycan while only P. aeruginosa possesses lipopolysaccharide. Interestingly, heat-killed S. aureus failed to induce anti-MRSA activity (data not shown), thereby lending support that peptidoglycan is unlikely to be the inducer. Thus, MRSA likely invoked some immune response in R. flavipes that is specific to MRSA that is missing in S. aureus (i.e. staphyloccal cassette chromosome mec or SCCmec) [40, 41], while P. aeruginosa invoked a response that is effective against a multitude of bacteria.
Termites contain a complex microbiota in their alimentary tract that could have contributed to the observed inducible antibacterial activity [1, 42]. We suspected that the microbial symbionts from the hindgut, as well as termite immune proteins, could be the source of the antibacterial activity [43, 44] in our assays. However, our comparative analysis of the hemolymph versus the hindgut localized all of the observable antibacterial activity to the hemolymph, suggesting termite cells as the origin of antibacterial activity. Our finding agrees with a previous study that reported the inability of oral ingestion of fungal spores and bacteria to induce innate gut defenses in R. flavipes [45]. The authors of that study speculated a lack of inducible genes being present on the microarray or weak innate defense in this termite.
Several antimicrobials have been identified from termites including termicin, spinigerin, lysozymes, tGNBPs, and transferrin [8, 11, 14–16, 46]. However, given the small molecular weight of most these previously identified peptides/proteins ≤15 kDa), it is likely that we have discovered novel proteins or protein complexes, with antibacterial activity since most of our activity is limited to proteins of ≥90 kDa. This finding agrees with previous studies demonstrating various protein complexes functioning as antimicrobial effectors with large molecular weight (>150 kDa) in insects, plants, and microorganisms [47–51]. Because our hemolymph samples were CFE and the induced antibacterial proteins appeared to be involved in humoral reaction, it is possible that these proteins were induced by Relish [15]. Based on recent identification of differentially expressed proteins involved in stress response, immune signaling, biosynthesis and other functions in R. chinensis following an entomopathogenic fungal infection [52], mechanisms of insect immunity regulation appear to be complex.
Our two-dimensional gel analyses indicate that some of the upregulated proteins found in the induced termite hemolymph may be similar to previously identified insect immune proteins as listed in Tables 3 and 4. To compare our proteins to known insect immune proteins, we arbitrary designated a cutoff point of ≤0.5 in pI and ≤5000 Da in MW. However, given that mobility of a protein on IEF and SDS-PAGE can be affected by various factors including protein processing and modification, it is possible that our comparison is not comprehensive. We are currently in the process of identifying the differentially expressed proteins in our hemolymph samples to better understand the induction of the immune response in R. flavipes to bacterial challenge.
Supporting Information
Lanes 1–4: Protein ladders of α-lactalbumin from bovine milk, albumin from bovine serum, albumin from chicken egg white, and urease from jack bean, respectively. Lanes 5–7: MWCO of 100K fractions from P. aeruginosa-challenged, MRSA-challenged, and naïve termites, respectively. Lanes 8–10: MWCO of 30K fractions from P. aeruginosa-challenged, MRSA-challenged, and naïve termites, respectively.
(TIF)
Lane 1: Protein ladder (10–250 kDa); Lanes 2–4: MWCO of 100K fractions from P. aeruginosa-challenged, MRSA-challenged, and naïve termites, respectively. Lanes 5–7: MWCO of 30K fractions from P. aeruginosa-challenged, MRSA-challenged, and naïve termites, respectively.
(TIF)
Lane 1: Protein Ladder (10–250 kDa); Lanes 2–4: Hemolymph proteins from naïve, MRSA-challenged, and P. aeruginosa-challenged termites, respectively.
(TIF)
The pI and the MW of termite hemolymph proteins are based on the Kendrick Labs’ analysis of the two-dimensional gels.
(DOCX)
The pI and the MW of termite hemolymph proteins are based on the Kendrick Labs’ analysis of the two-dimensional gels.
(DOCX)
Acknowledgments
We thank the Auburn University Drs. Alan E. Wilson and Aaron M. Rashotte for access to their equipment, and Nannan Liu and Arthur Appel for advice. We also thank James Barbaree and Robert (Scott) Miller in the Department of Biological Sciences at Auburn University and Jorge Escalante-Semerena in the Department of Microbiology at the University of Georgia for MRSA, S. aureus, S. pyogenes, E. coli O157:H7, and S. Typhimurium. Finally, we thank Dr. Laura Silo-Suh of Mercer University School of Medicine for critical reading of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the National Institute of Food and Agriculture/United States Department of Agriculture Hatch program (ALA015-1-15005), unrestricted industry funds, the Auburn University Intramural Grant Program, the Auburn University Detection and Food Safety Peaks of Excellence program (USDA 2005-3439415674A) and NSF EPSCoR (NSF-EPS11-58862).
References
- 1.Haine ER, Moret Y, Siva-Jothy MT, Rolff J. Antimicrobial defense and persistent infection in insects. Science. 2008;322(5905):1257–9. 10.1126/science.1165265 . [DOI] [PubMed] [Google Scholar]
- 2.Dossey AT. Insects and their chemical weaponry: New potential for drug discovery. Nat Prod Rep. 2010;27(12):1737–57. WOS:000284317100001. 10.1039/c005319h [DOI] [PubMed] [Google Scholar]
- 3.Slocinska M, Marciniak P, Rosinski G. Insects antiviral and anticancer peptides: New leads for the future? Protein Peptide Lett. 2008;15(6):578–85. WOS:000259509400005. [DOI] [PubMed] [Google Scholar]
- 4.Tzou P, De Gregorio E, Lemaitre B. How Drosophila combats microbial infection: a model to study innate immunity and host-pathogen interactions. Current Opinion in Microbiology. 2002;5(1):102–10. WOS:000173791000016. [DOI] [PubMed] [Google Scholar]
- 5.Lavine MD, Strand MR. Insect hemocytes and their role in immunity. Insect Biochem Mol Biol. 2002;32(10):1295–309. . [DOI] [PubMed] [Google Scholar]
- 6.Yi HY, Chowdhury M, Huang YD, Yu XQ. Insect antimicrobial peptides and their applications. Appl Microbiol Biotechnol. 2014;98(13):5807–22. 10.1007/s00253-014-5792-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulet P, Hetru C, Dimarcq JL, Hoffmann D. Antimicrobial peptides in insects; structure and function. Dev Comp Immunol. 1999;23(4–5):329–44. WOS:000081403500007. [DOI] [PubMed] [Google Scholar]
- 8.Rosengaus RB, Cornelisse T, Guschanski K, Traniello JF. Inducible immune proteins in the dampwood termite Zootermopsis angusticollis. Naturwissenschaften. 2007;94(1):25–33. 10.1007/s00114-006-0151-9 . [DOI] [PubMed] [Google Scholar]
- 9.Bulmer MS, Lay F, Hamilton C. Adaptive evolution in subterranean termite antifungal peptides. Insect Mol Biol. 2010;19(5):669–74. 10.1111/j.1365-2583.2010.01023.x . [DOI] [PubMed] [Google Scholar]
- 10.Hamilton C, Bulmer MS. Molecular antifungal defenses in subterranean termites: RNA interference reveals in vivo roles of termicins and GNBPs against a naturally encountered pathogen. Dev Comp Immunol. 2012;36(2):372–7. 10.1016/j.dci.2011.07.008. WOS:000299190400015. [DOI] [PubMed] [Google Scholar]
- 11.Matsuura K, Tamura T, Kobayashi N, Yashiro T, Tatsumi S. The antibacterial protein lysozyme identified as the termite egg recognition pheromone. Plos One. 2007;2(8):e813 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng Y, Hu XP, Suh S-J. Multiple antibacterial activities of proteinaceous compounds in crude extracts from the Eastern Subterranean Termite, Reticulitermes flavipes Kollar (Blattodea: Isoptera: Rhinotermitidae). Adv Res. 2014;2(8):455–61. [Google Scholar]
- 13.Bulmer MS, Bachelet I, Raman R, Rosengaus RB, Sasisekharan R. Targeting an antimicrobial effector function in insect immunity as a pest control strategy. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(31):12652–7. 10.1073/pnas.0904063106. WOS:000268667600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bulmer MS, Crozier RH. Duplication and diversifying selection among termite antifungal peptides. Mol Biol Evol. 2004;21(12):2256–64. 10.1093/molbev/msh236 . [DOI] [PubMed] [Google Scholar]
- 15.Bulmer MS, Crozier RH. Variation in positive selection in termite GNBPs and Relish. Mol Biol Evol. 2006;23(2):317–26. 10.1093/molbev/msj037 . [DOI] [PubMed] [Google Scholar]
- 16.Lamberty M, Zachary D, Lanot R, Bordereau C, Robert A, Hoffmann JA, et al. Insect immunity: Constitutive expression of a cysteine-rich antifungal and a linear antibacterial peptide in a termite insect. The Journal Of Biological Chemistry. 2001;276(6):4085–92. . [DOI] [PubMed] [Google Scholar]
- 17.Hussain A, Wen SY. Induction of immune response among formosan subterranean termites, Coptotermes formosanus Shiraki (Rhinotermitidae: Isoptera). African Journal of Microbiol Res. 2012;6(5):995–1000. [Google Scholar]
- 18.Chovenc T, Efstathion CA, Elliott ML, Su NY. Extended disease resistance emerging from the faecal nest of a subterranean termite. Proc Royal Society B: Biology. 2013. 10.1098/rspb.2013.1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu XP, Appel AG. Seasonal variation of critical thermal limits and temperature tolerance in Formosan and eastern subterranean termites (Isoptera: Rhinotermitidae). Environmental entomology. 2004;33(2):197–205. IND43636883. [Google Scholar]
- 20.Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951;62(3):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–55. [DOI] [PubMed] [Google Scholar]
- 22.Holloway BW, Krishnapillai V, Morgan AF. Chromosomal genetics of Pseudomonas. Microbiol Rev. 1979;43:73–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lilleengen K. Typing Salmonella typhimurium by means of bacteriophage. Acta Pathol Microbiol Scan. 1948;Suppl 77:1–125. [Google Scholar]
- 24.Adams MD, Goglin K, Molyneaux N, Hujer KM, Lavender H, Jamison JJ, et al. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J Bacteriol. 2008;190(24):8053–64. 10.1128/JB.00834-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suetsugu Y, Futahashi R, Kanamori H, Kadono-Okuda K, Sasanuma S, Narukawa J, et al. Large Scale Full-Length cDNA Sequencing Reveals a Unique Genomic Landscape in a Lepidopteran Model Insect, Bombyx mori. G3-Genes Genom Genet. 2013;3(9):1481–92. 10.1534/g3.113.006239. WOS:000324244000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masson F, Vallier A, Vigneron A, Balmand S, Vincent-Monegat C, Zaidman-Remy A, et al. Systemic infection generates a local-like immune response of the bacteriome organ in insect symbiosis. J Innate Immun. 2015;7(3):290–301. 10.1159/000368928 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin SW, Park SS, Park DS, Kim MG, Kim SC, Brey PT, et al. Isolation and characterization of immune-related genes from the fall webworm, Hyphantria cunea, using PCR-based differential display and subtractive cloning. Insect Biochem Mol Biol. 1998;28(11):827–37. . [DOI] [PubMed] [Google Scholar]
- 28.Brown SE, Howard A, Kasprzak AB, Gordon KH, East PD. A peptidomics study reveals the impressive antimicrobial peptide arsenal of the wax moth Galleria mellonella. Insect Biochem Mol Biol. 2009;39(11):792–800. 10.1016/j.ibmb.2009.09.004 . [DOI] [PubMed] [Google Scholar]
- 29.Sun SC, Lindstrom I, Boman HG, Faye I, Schmidt O. Hemolin: an insect-immune protein belonging to the immunoglobulin superfamily. Science. 1990;250(4988):1729–32. . [DOI] [PubMed] [Google Scholar]
- 30.Sun SC, Lindstrom I, Lee JY, Faye I. Structure and expression of the attacin genes in Hyalophora cecropia. Eur J Biochem. 1991;196(1):247–54. . [DOI] [PubMed] [Google Scholar]
- 31.Taniai K, Lee JH, Lee IH. Bombyx mori cell line as a model of immune-system organs. Insect Molecular Biology. 2006;15(3):269–79. 10.1111/j.1365-2583.2006.00639.x. WOS:000237930000004. [DOI] [PubMed] [Google Scholar]
- 32.Shrestha S, Park Y, Stanley D, Kim Y. Genes encoding phospholipases A(2) mediate insect nodulation reactions to bacterial challenge. J Insect Physiol. 2010;56(3):324–32. 10.1016/j.jinsphys.2009.11.008. WOS:000275799700014. [DOI] [PubMed] [Google Scholar]
- 33.An C, Jiang H, Kanost MR. Proteolytic activation and function of the cytokine Spatzle in the innate immune response of a lepidopteran insect, Manduca sexta. The FEBS journal. 2010;277(1):148–62. 10.1111/j.1742-4658.2009.07465.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finnerty CM, Karplus PA, Granados RR. The insect immune protein scolexin is a novel serine proteinase homolog. Protein Sci. 1999;8(1):242–8. 10.1110/ps.8.1.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim YS, Ryu JH, Han SJ, Choi KH, Nam KB, Jang IH, et al. Gram-negative bacteria-binding protein, a pattern recognition receptor for lipopolysaccharide and beta-1,3-glucan that mediates the signaling for the induction of innate immune genes in Drosophila melanogaster cells. J Biol Chem. 2000;275(42):32721–7. 10.1074/jbc.M003934200 . [DOI] [PubMed] [Google Scholar]
- 36.Terrapon N, Li C, Robertson HM, Ji L, Meng XH, Booth W, et al. Molecular traces of alternative social organization in a termite genome. Nature communications. 2014;5 ARTN 3636 10.1038/ncomms4636. WOS:000337362200001. [DOI] [PubMed] [Google Scholar]
- 37.Gao Q. Social immunity and the expression of immune-related genes in the Eastern subterranean termite: University of Western Ontario; 2014. [Google Scholar]
- 38.Faulhaber LM, Karp RD. A diphasic immune response against bacteria in the American cockroach. Immunology. 1992;75(2):378–81. [PMC free article] [PubMed] [Google Scholar]
- 39.Sadd BM, Schmid-Hempel P. Insect immunity shows specificity in protection upon secondary pathogen exposure. Curr Biol. 2006;16(12):1206–10. 10.1016/j.cub.2006.04.047 . [DOI] [PubMed] [Google Scholar]
- 40.Ito T, Katayama Y, Asada K, Mori N, Tsutsumimoto K, Tiensasitorn C, et al. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45(5):1323–36. 10.1128/AAC.45.5.1323-1336.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katayama Y, Ito T, Hiramatsu K. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2000;44(6):1549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker BJ, Spragg CJ, Altincicek B, Gerardo NM. Symbiont-mediated protection against fungal pathogens in pea aphids: a role for pathogen specificity? Appl Environ Microbiol. 2013;79(7):2455–8. 10.1128/AEM.03193-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chouvenc T, Su NY, Robert A. Inhibition of Metarhizium anisopliae in the alimentary tract of the eastern subterranean termite Reticulitermes flavipes. J Invertebr Pathol. 2009;101(2):130–6. 10.1016/j.jip.2009.04.005. WOS:000267382800008. [DOI] [PubMed] [Google Scholar]
- 44.Rosengaus RB, Schultheis KF, Yalonetskaya A, Bulmer MS, DuComb WS, Benson RW, et al. Symbiont-derived beta-1,3-glucanases in a social insect: mutualism beyond nutrition. Frontiers in microbiology. 2014;5 ARTN 607 10.3389/fmicb.2014.00607. WOS:000345856400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sen R, Raychoudhury R, Cai Y, Sun Y, Lietze VU, Peterson BF, et al. Molecular signatures of nicotinoid-pathogen synergy in the termite gut. PloS One. 2015;10(4):e0123391 10.1371/journal.pone.0123391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson GJ, Crozier YC, Crozier RH. Isolation and characterization of a termite transferrin gene up-regulated on infection. Insect Mol Biol. 2003;12(1):1–7. . [DOI] [PubMed] [Google Scholar]
- 47.Anju KM, Archana MM, Mohandas C, Nambisan B. Purification and identification of an antibacterial protein from the symbiotic bacteria associated with novel entomopathogenic nematode, Rhabditis (Oscheius) sp. World J Microbiol Biotechnol. 2015;31(4):621–32. 10.1007/s11274-015-1816-3 . [DOI] [PubMed] [Google Scholar]
- 48.Beck G, Cardinale S, Wang L, Reiner M, Sugumaran M. Characterization of a defense complex consisting of interleukin 1 and phenol oxidase from the hemolymph of the tobacco hornworm, Manduca sexta. J Biol Chem. 1996;271(19):11035–8. . [DOI] [PubMed] [Google Scholar]
- 49.Benz J, Meinhart A. Antibacterial effector/immunity systems: it's just the tip of the iceberg. Curr Opin Microbiol. 2014;17:1–10. 10.1016/j.mib.2013.11.002 . [DOI] [PubMed] [Google Scholar]
- 50.James SG, Holmstrom C, Kjelleberg S. Purification and characterization of a novel antibacterial protein from the marine bacterium D2. Appl Environ Microbiol. 1996;62(8):2783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sitohy MZ, Osman AO, Mahgoub SA. Bioactive proteins against pathogenic and spoilage bacteria. Functional Foods in Health and Disease. 2014;4(10):451–62. [Google Scholar]
- 52.Liu L, Li G, Sun P, Lei C, Huang Q. Experimental verification and molecular basis of active immunization against fungal pathogens in termites. Scientific reports. 2015;5:15106 10.1038/srep15106 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lanes 1–4: Protein ladders of α-lactalbumin from bovine milk, albumin from bovine serum, albumin from chicken egg white, and urease from jack bean, respectively. Lanes 5–7: MWCO of 100K fractions from P. aeruginosa-challenged, MRSA-challenged, and naïve termites, respectively. Lanes 8–10: MWCO of 30K fractions from P. aeruginosa-challenged, MRSA-challenged, and naïve termites, respectively.
(TIF)
Lane 1: Protein ladder (10–250 kDa); Lanes 2–4: MWCO of 100K fractions from P. aeruginosa-challenged, MRSA-challenged, and naïve termites, respectively. Lanes 5–7: MWCO of 30K fractions from P. aeruginosa-challenged, MRSA-challenged, and naïve termites, respectively.
(TIF)
Lane 1: Protein Ladder (10–250 kDa); Lanes 2–4: Hemolymph proteins from naïve, MRSA-challenged, and P. aeruginosa-challenged termites, respectively.
(TIF)
The pI and the MW of termite hemolymph proteins are based on the Kendrick Labs’ analysis of the two-dimensional gels.
(DOCX)
The pI and the MW of termite hemolymph proteins are based on the Kendrick Labs’ analysis of the two-dimensional gels.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.