Abstract
The welfare of animals in captivity is of considerable societal concern. A major source of stress, especially for wild animals, is the lack of control over their environment, which includes not being able to avoid contact with human beings. Paradoxically, some studies have shown that interactions with human beings may improve the welfare of wild animals in captivity. Here, we investigated the behavioural (behaviours indicative of cooperation or stress) and physiological (variations in salivary cortisol concentrations) effects of the increasingly used practice of training wild animals as a way to facilitate handling and/or as behavioural enrichment. We evaluated the effects of indoor training sessions with familiar caretakers on nine human-socialised individuals of a wild species, the wolf (Canis lupus), in comparison to nine individuals of its domesticated form, the dog (Canis lupus familiaris). All animals were raised and kept in intraspecific packs under identical conditions—in accordance with the social structure of the species—in order to control for socialisation with human beings and familiarity with training. We also collected saliva samples of trainers to measure GC and testosterone concentrations, to control for the effects of trainers’ stress levels on the responses of the animals. During the training sessions, separated from pack members, the animals stayed voluntarily close to the trainers and mostly adequately performed requested behaviours, indicating concentration to the task. Similarly to dogs, the salivary cortisol level of wolves–used as an index of stress—dropped during these sessions, pointing to a similar stress-reducing effect of the training interaction in both subspecies. The responses to the requested behaviours and the reduction in salivary cortisol level of wolves and dogs varied across trainers, which indicates that the relaxing effect of training has a social component. This points to another factor affecting the welfare of animals during the sessions, beside the rewarding effect of getting food and control over the situation by successfully completing a task. As all responses performed by the animals corresponded to cues already familiar to them, the reported effects were likely due to the above cited factors rather than to a learning process. Our results support previous findings suggesting that training is a potentially powerful tool for improving welfare in some wild social canids by creating structured and positive interactions between these animals and their human caretakers.
Introduction
Animals in captivity live in environments that may substantially differ from their natural habitat, in which they developed their behavioural repertoire [1]. In the wild, animals exert control over a significant proportion of their actions. For example, they decide when and how to get involved in particular activities. In contrast, captive animals face a schedule that is usually rigid and temporally regular, giving them few opportunities, for example, to find food and mates, and to exert control over situations to which they are exposed. This situation of reduced choice and controllability may create a context of under- or over-challenge, associated with frustration [2] and chronic stress [3], thereby affecting animal welfare.
The welfare of an animal may be evaluated via the efforts it makes to adapt to its environment [4–5]. Through physiological and behavioural coping strategies, individuals try to gain control over their interactions with the environment to maintain physical and mental homeostasis [6]. Environmental stimuli that lead to an imbalance of homeostasis are usually defined as “stressors” [7]. Behavioural responses to stressors include aggression, increased activity, and avoidance behaviours [6], while physiological response involves increased release of glucocorticoids (GC, e.g. cortisol [7–9]), and increases in heart and breathing rates [10]. Environmental stressors which surpass the coping capacity of the animals–for example, extreme temperatures, or social isolation in a social species—if prolonged or repeated [7] may result in chronic stress and, usually, in poor welfare [10].
The release of GC, which are the metabolic and stress coping hormones, occurs due to a modulation of the stress response system, regulated by the hypothalamic-pituitary-adrenal (HPA) axis [7–8]. Through its physiological and behavioural effects, the release of GC enables animals to cope with threatening or demanding situations [8–9]. However, frequently or chronically elevated GC may have detrimental consequences for learning and memory processes [11], result in the suppression of reproductive and immune systems [9, 12] and cause ill health [7].
The activity of the HPA axis can be non-invasively assessed from saliva samples [13]. Both biogenic amines and GC are released into the blood stream in the short-term–i.e., within seconds to minutes after a stressful event; GC have been preferable to study the effects of stress, among other factors, due to their longer permanence in the blood stream–from minutes up to days [12]. Salivary GC levels accurately reflect the biologically-active portion of total plasma GC, making them suitable for monitoring short-term physiological stress responses [14]. In dogs (Canis lupus familiaris), the estimated time for a change in adrenal activity be measured in the saliva is after 15–20 minutes of exposition to stressors, among which sometimes even handling [15, 16]. Regarding wolves (Canis lupus), there was so far no data in this estimated time. However, because they are closely related to dogs, we expect a very similar modulation of their HPA axis. A comprehensive understanding of the impact of stressors on animal welfare may be achieved by assessing behavioural (e.g., behaviours indicative of stress) and physiological (e.g., variations in cortisol concentrations, heart or breath rates) stress parameters in tandem.
Welfare and human-animal interactions
Intraspecific affiliative interactions have long been shown to produce calming effects on captive social animals [17, 18, 19, 20], having their corresponding neural circuitry already unveiled: a number of receptors in brain circuits related to reward or reinforcement were found linked to the formation of bonds in several species [21]. Even non-social species, such as tigers (Panthera tigris [22]), and less social ones, such as orangutans (Pongo pygmaeus [23]), have been shown to benefit from intraspecific social stimulation in captive settings. Positive human-animal interactions, understood as mutual and dynamic interactions between human beings and animals that have a relaxing effect on animals [24], have been recommended to improve the welfare of captive animals, domesticated or wild. It has been shown that interactions with human beings, in some situations, have the potential to reduce abnormal/stereotypic behavioural patterns and increase the duration of affiliative behaviours with group members [25–28], reduce GC concentrations [29–30], and support the expression of species-typical behaviours [31]. However, other studies have described potential negative effects of intense contact with human beings on the welfare of some mammals [25, 32–38]. Even the mere presence of a human being can be aversive for laboratory primates [39], and farm mammals in petting zoos, albeit domesticated, may show avoidance of contact with visitors [40].
Wild animals, which were not submitted to artificial selection for interacting with human beings, may be overwhelmed by close or frequent contact with them [41–42]. But wild mammals in captivity have also shown either improved welfare by enrichment through human contact [43–44], or increased stress responses [25, 33, 44, 45]. Hogan et al. [41], for example, conducted petting sessions with captive wombats (Lasiorhinus latifrons) that reacted to the sessions with avoidance behaviours and an increase in GC concentrations. Although the aversive responses of the animals have decreased as the sessions were repeated, their GC secretion remained high, suggesting that the quiet behaviour of wombats during petting sessions more likely reflected freezing consecutive to elevated stress than relaxation.
Whether interactions with human beings have a positive or a negative effect on animals likely depends on various factors. The level of sociality of the species is possibly one of the factors affecting the results of human-animal interactions, since most studies showing relaxing effects of such interactions have been developed with social species [24]. However, there are also studies reporting neutral or detrimental effects of such interactions on social species [25, 33, 40, 42]. Familiarity with the human partner may also support the relaxing effects of the interactions [46]. In addition, the frequency (i.e., how often they interact), the form and the quality of the interactions (i.e., how voluntary and rewarding the interaction is) an animal has with its caregivers, trainers or researchers also seem to matter [47–48]. However, research investigating the effect of these variables remains scarce, and mainly focused on farm animals [46, 49–51].
Previous work on interactions between human beings and captive wild mammals points at the relevance of the characteristics and experiences of the animals with human beings, for example, how often they have interacted with human beings, how rewarding these interactions were, if the animals were raised by their parents or by people, etc. These factors may contribute to differences in the temperament of the animals and, as a consequence, in their reactions to interactions with human beings [1, 47]. Early socialisation seems to play a crucial role, as demonstrated by a large-scale study with 219 individuals of four zoo-kept species in 46 zoos [47]. For example, wild-born black rhinos (Diceros bicornis michaeli and minor) and parent-reared maned wolves (Chrysocyon brachyurus) showed significantly less affinity to their keepers than their captive-born or hand-reared counterparts [47]. The same study reported a positive correlation between the experience of keepers and the fear of people exhibited by the animals, which these keepers were in charge of, which indicates that certain keeping styles or habits of experienced keepers might be fear-inducing. In order to avoid such detrimental effects on experimental animals, these are frequently hand-reared. In fact, hand-rearing is regarded as essential in some behavioural studies [52, 53, 54], when contact with human beings during testing is inevitable. Although the effects of hand-rearing have been evaluated only with social animals, such procedures are seemingly important for all wild species, as appropriate early socialisation with humans will reduce fearfulness, may promote cooperation from the part of the animals, and ensure safety for human partners [54, 47]. In summary, available data suggest that interactions with human beings can be beneficial, neutral or detrimental for captive wild mammals, depending on context, characteristics of the considered species, and specifics of the interactions (related to the personality of the human partner and the type of relationship developed with the partner).
A type of interspecific interaction that is generally suggested to promote improvements in welfare is Positive Reinforcement Training (PRT), a form of operant conditioning [55–57]. Positive Reinforcement Training is based on reinforcing specific behaviours by rewarding the individuals exhibiting this behaviour. Whereby, an intense and regular contact between trainers and animals may be established during interactions that are strongly controlled by the animal. This technique is being increasingly used as part of protocols for socialising wild mammals for the aims of comparative studies [58]. Although PRT has been shown efficient to reduce stress in some mammals (e.g., chimpanzees (Pan troglodytes [59]), gorillas (Gorilla gorilla [26]), baboons (Papio hamadryas [30]), dogs [29, 60], African wild dogs (Lycaon pictus [61]), other studies have reported limited or negative effects of such procedures (e.g., rhesus macaques—Macaca mulata [32, 42] and chimpanzees [33]). For most tested species there is a lack of evidence of the efficacy of PRT in improving welfare (e.g. [32, 62], and see [55] for a review). Possibly due to the considerable difficulties of standardising procedures and measuring the multifaceted welfare effects of PRT, studies on its effects in many species have not focused primarily on the effects of the procedure per se, but on indirect effects, i.e., on improvements in the specific trained behaviours. In turn, these improvements can promote a reduction in stress level through husbandry facilitation, avoiding the need for aversive procedures [27, 55, 63–69]. Further difficulty faced by these studies is the de-coupling of the effects of the learning process from the effects of the training interactions per se [55].
The study of the effects of training on wolves is important for at least two reasons. First, wolves are rather popular and accordingly, many more than the estimated 200000 wolves still living in the wild are kept in zoos or even privately [70–71, 72], despite their shy nature. Although wolves are highly social and cooperative with their pack mates [73, 74], as other untamed/non-socialised wild animals, they seem not well suited for captive life close to human beings [52–75]. During domestication, dogs have diverged from wolves in many ways, including in their behaviour and ecology [76, 77]. For example, even the social organisation of wolf packs and feral domestic dogs differ: wolves are more cooperative in hunting, offspring raising, and in defending a territory [78]. Second, wolves have been trained and used in captive settings as experimental subjects in comparative studies, primarily in comparison to dogs, in order to study the evolution of social behaviour and cognition [79–82]. Currently it is unknown whether intensive socialisation with human beings–through the reduction in fear and increase in cooperation this procedure is claimed to promote [53]—may allow wolves to benefit from the relaxing and stress-reducing effects of training in a way similar to that of human-socialised dogs. Alternatively, wolves, known for their shyness, might not benefit from training, and show an increase in GC concentrations and a higher frequency of behaviours non-related to training–i.e., behaviours different from the ones being requested by the trainers.
In this study we aimed at investigating the behavioural (behaviours indicative of cooperation or stress) and physiological (variations in cortisol concentrations) effects of PRT on human-socialised wolves, a wild species. Uniquely, we compared the effects of PRT on wolves to the effects on dogs raised and kept under identical conditions. As the positive effects of PRT have been repeatedly demonstrated in dogs—with no reported sex- or age-related differences [83–85], comparing the responses of dogs and wolves to the same procedures would give us an idea of the magnitude of this effect on wolves. Beyond the implication for welfare, a stress-reducing effect of training on wolves would support the validity of the current practice of studying the evolution of cognition by comparing the social behaviour of equally raised and kept wolves and dogs in interactions with human beings.
Materials and Methods
Ethics Statement
All study animals were kept at the Wolf Science Center (www.wolfscience.at), located in Game Park Ernstbrunn in Austria (License No.: AT00012014). The CITES (www.cites.org) import permits for the animals used in this study are: 2008: Zoo Herberstein, Austria: AT08-B-0998, AT08-B-0996, AT08-B-0997; 2009: Triple D Farm, USA: AT09-E-0018; 2012: Minnesota Wildlife Connection, USA: 12AT330200INEGCJ93. The animals were housed in accordance with the Austrian Federal Act on the Protection of Animals (Animal Protection Act—TSchG, BGBl. I Nr. 118/2004). All animals tested in this study could voluntarily choose to enter the experimental enclosure and to participate in a training session. If the animals entered the experimental enclosure but were not motivated enough to participate–i.e., if they failed to respond to the cues during the first minute of the session—they were shifted back into their home enclosure. The methods used in this study were strictly non-invasive. Hence, in accordance with the Austrian Animal Experiments Act (BGBl. I Nr. 114/2012, Tierversuchsgesetz 2012 –TVG 2012) no ethical approval was required, but we obtained one from the University of São Paulo, Brazil (Committee of Ethics for Animal Research from the Institute of Psychology, University of São Paulo, Brazil, approval number 016.2009).
The individual pictured in Fig 1 (FR) has given written informed consent (as outlined in PLOS consent form) to publish her picture.
Fig 1. Picture of a training session showing the female wolf Shima performing the response to the cue down, within one metre from the trainer FR.
Subjects
We studied nine timber wolves and nine mixed breed dogs. The characteristics of the animals, such as sex and age are reported in Table 1. All the animals were captive-born and hand-reared with the participation of all trainers contributing to this project. This procedure allowed us to control for developmental experiences, human socialisation and familiarity with training. Hand-raised wolves and dogs had daily contact with human beings, 20 to 24 hours a day, 7 days a week, from their first week of age until the age of 16 to 20 weeks, which also included bottle feeding and, later, hand feeding. After this period of time, the animals were integrated into a pack with older conspecifics, and interactions with human beings were reduced to four to five days per week, approximately 30 min per day. From their third week of life, all animals consistently received PRT, becoming familiarised to cues such as “sit”, “down”, “stay”, “roll-over”. In all training sessions the responses of the animals were rewarded through a continuous reinforcement schedule, and behaviours were shaped with a secondary reinforcer (clicker). Besides that, each animal participated in several weekly food-rewarded behavioural tests. Each animal took voluntarily part in all training sessions; the animals were called by name and came to the door of the training room. No interactions of the animals with human beings involved domination, punishment, or any other negative interventions, and when items had to be taken away from the animals, they were generally exchanged for food.
Table 1. Characteristics of the studied animals.
| Subspecies | Name | Sex | Age at first testing (months) |
|---|---|---|---|
| Wolf | Aragorn | Male | 24 |
| Wolf | Shima | Female | 24 |
| Wolf | Kaspar | Male | 24 |
| Wolf | Tatonga | Female | 11 |
| Wolf | Nanuk | Male | 11 |
| Wolf | Geronimo | Male | 11 |
| Wolf | Yukon | Female | 11 |
| Wolf | Cherokee | Male | 11 |
| Wolf | Apache | Male | 11 |
| Dog | Meru | Male | 43 |
| Dog | Nuru | Male | 26 |
| Dog | Zuri | Female | 26 |
| Dog | Nia | Female | 25 |
| Dog | Layla | Female | 24 |
| Dog | Rafiki | Male | 12 |
| Dog | Alika | Female | 12 |
| Dog | Maisha | Male | 11 |
| Dog | Kilio | Male | 11 |
Both wolves and dogs lived in packs with conspecifics, in enclosures of 3500 and 900 m2 respectively, at the Wolf Science Center, Ernstbrunn, Austria. The animals had a diet of meat, milk products and dry food throughout the study period. As the two studied subspecies have different feeding requirements, each subspecies was fed following its own feeding rhythm: wolves had major meals of carcasses once to three times per week and dogs received smaller portions of dry food or meat every day. Water was permanently available for all animals.
Procedures
Saliva collection
In the training sessions, we used samples of saliva of the animals for a physiological assessment of stress, through the measurement of cortisol concentration. Previously to the training experiment, all animals were trained for saliva sampling with the use of PRT. Saliva collection was performed by the introduction of a surgical hydrocellulose sponge (Sorbette, by Salivette ®) into the cheek pouch of the animal for as many times as necessary to collect enough saliva (two soaked sponges). During saliva collection, as well as during the training sessions, the animals were rewarded only with cheese, to control for the influence of proteins in the saliva samples [86]. Saliva collection was performed a few (2 to 10) minutes before and 15 minutes after the end of each training session. Immediately after saliva collection, the sponges were transferred into a plastic tube (Sarstedt ®) and stored at –20°C until analysis. As there was, so far, no estimation on the time of delay between exposition to stressors and detectable changes in salivary cortisol concentrations in wolves, we performed a validation procedure, which results pointed to the same time lag as the one recorded in dogs (i.e., 15–20 minutes, [S1 Text]). The procedure of attracting one animal and collecting its saliva took less than 10 minutes, around 4 minutes in most sessions, and thus the adrenal activity measured in the first sample could reliably be considered as not related to the handling procedure.
Saliva samples of the trainers were also collected before each training session, using the same material as the one used for the collection of saliva from dogs and wolves. The saliva samples of trainers were analysed to measure GC and testosterone concentrations, to control for the effect of the stress level of the trainer on the response of the animals [87].
Training sessions
Each studied animal took part in 15 different training sessions of five minutes each, for a total of 270 sessions. The training sessions were videotaped, and took place between May 2010 and March 2011. Each trainer (BB, FR, KK, RT, ZV) was equally involved in the training of every one of the animals, conducting three sessions with each studied animal. The sessions with each animal were run on average within four months. Each animal participated in the sessions in isolation from the pack, between 8:30 h and 17:00 h, in a training room (63.6 m2) located next to their home enclosure. The training room was empty, except for an elevated platform on one side, and did not allow visual contact with other animals or human beings. No animal was trained more than once a day. Each trainer, however, worked with four to nine animals on each training day, with the order of animals randomised across sessions.
Before training an animal, the trainer collected his/her own saliva, called the animal, led it into the training room and collected its first saliva sample. As soon as the saliva sample of the animals had been taken and stored, the trainer started giving cues (PRT, Fig 1) continuously, in a freely chosen order for five minutes. Although there was no pre-defined order for the cues in the sessions, the trainers used mostly the same proportions of each cue. The behaviours requested were sitting, laying down, turning around, walking around the trainer, giving the paw, allowing the placement of a muzzle, allowing the placement of a harness, rolling, standing, staying and looking into the trainer’s eyes. Using a continuous reinforcement schedule the animal was rewarded with pieces of cheese for presenting a correct response. If the animal failed to perform the requested response, the cue was given again after five seconds.
After the end of the session, the trainer and the animal interacted freely to keep the subject entertained for 15 more minutes until the second saliva sample was taken from the animal. Finally, the animal was sent back to the pack.
Data Analyses
All evaluated behavioural parameters–behaviour and orientation of the animals, requested cues and distance between the animal and the trainer—were coded through continuous sampling of the videos, using the program Solomon Coder (Beta version 11.03.28, 2006–2011 by András Péter).
Besides the responses to the cues, non-training behaviours (NTB) exhibited by the animals were also recorded. Non-training behaviours were all behaviours that did not contribute to the training process and took the animal, or its attention, away from the trainer. These behaviours were considered as indicators of lack of attention, boredom or of an increase in stress levels [88, 89], indicating that the animal was not focused on the activity.
The observed NTB were: 1—jumping on the trainer: the animal is either standing on its hind legs or jumping, with its four legs leaving the ground, and touches the trainer with its forelegs; 2 –leaving: the animal moves away from the trainer, abandoning the interaction, and 3 –exploring: the animal sniffs the ground or the walls.
Each video was watched three times. During the first viewing, the behaviour and orientation of the animal were registered. The second viewing aimed at coding the trainer’s cues. During the third viewing, the distance between the members of the dyad was recorded. The distance between the animal and the trainer was classified into three categories: a) less than one metre, b) between one and three metres, c) more than three metres.
In terms of behaviour, the following response variables were measured: a) proportion of time the animal spent oriented towards the trainer (the orientation of its head deviating less than 10° from the trainer’s face) [90]; b) proportion of time the animal spent within 1m of the trainer; c) mean latency to respond to the cue at first request; d) proportion of cues correctly responded at first request; e) number of cue repetition–if one cue had to be repeated because the animal did not respond to it at first request, only the responded cue was considered as correct; all cues that were not responded were coded as “not executed”; f) proportion of time spent exhibiting NTB.
As a physiological response variable, the variation between the salivary cortisol (SC) concentration of the animals before and after the sessions (measured as nanograms per ml of saliva–ng/ml) was used. The absolute values of SC of wolves and dogs, characteristic of each subspecies, were not compared; all comparisons were performed between the mean SC concentrations in the samples taken before and after the sessions. From the saliva samples of the trainers, we measured testosterone (picograms per ml of saliva–pg/ml) and SC (nanograms per ml of saliva–ng/ml) concentrations. All saliva samples were analysed using an enzyme immunoassay as described by Haubenhofer and Kirchengast [35], previously validated by Patzl [91].
As each animal was trained three times by each trainer (replication), a Linear Mixed Model was used [92], in which the animals themselves and the interactions between the trainer and the animal were considered as random effects, using the following covariates as fixed effects: 1) fasting, i.e., the number of days since the last full meal; 2) age of the animal (in months); 3) sex of the animal; 4) subspecies; 5) session period (morning or afternoon); 6) weather conditions–i.e., if the weather was (a) sunny, (b) cloudy, (c) rainy or (d) snowy; 7) SC concentration of trainer, and 8) testosterone concentration of trainer. The influence of the variable “fasting” was investigated only in wolves, as dogs were fed on a daily basis.
Behavioural and hormonal results are reported as mean ± standard error. Statistical analyses were performed with the software SPSS version 20. All videos were coded by the same observer (ASV), who was familiar with the animals and the coded behaviours. To confirm scoring consistency, 20% of videos were also coded by another observer (DS), and the records were correlated [93, 94]; Spearman’s rank correlations (rS) per video were all above 0.85.
Results
Variables related to stress response
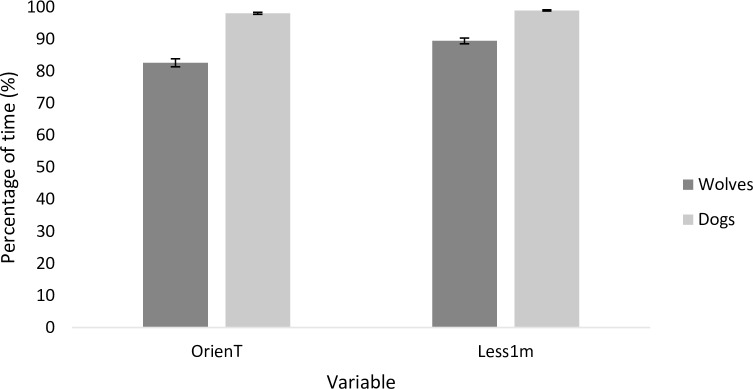
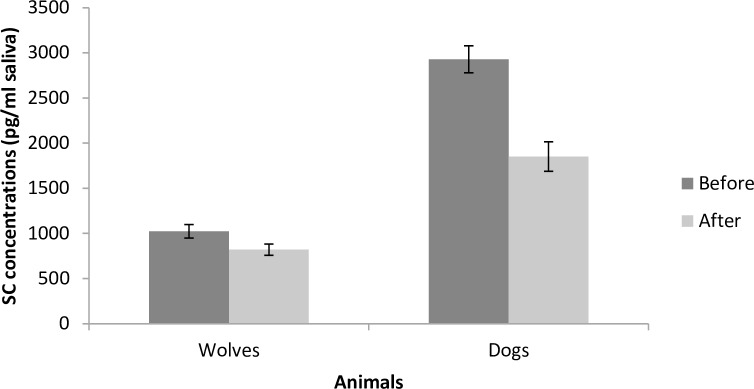
The animals showed no behaviour suggesting distress (i.e. panting, lip licking, pacing, tail tucked between legs etc. [88, 89]) during the sessions. Both wolves and dogs spent most of the time of the session voluntarily within one metre of the trainer and were attentive to him/her. Dogs spent significantly more of the training time within one metre of the trainer than wolves (99±0.2% versus 89.5±0.9%; t = 4.760, p < 0.0001, Fig 2). There was a tendency of decrease in the time the animals spent within one metre of the trainer as the trainer’s SC concentrations increased (t = 1.924, p < 0.056). In dogs and wolves together, 16% of variability in the time spent close to the trainer was explained by the identity of the trainer. No other measured variable was affected by the measured SC concentrations of the trainers. Dogs spent significantly more time with their face oriented towards the trainers than wolves (98.1±0.3% versus 82.7±1.2%; t = -7.870, p < 0.001, Fig 2), and 11% of orientation variability for dogs and wolves together was due to trainer identity. The longer the period the wolves had been fasting before the training session, the greater the proportion of time they spent oriented towards the trainer (t = 2.155, p < 0.033). A proportion of the variability in the effect of fasting on the wolves’ orientation (5.9%) was affected by trainer identity. Behaviours not related to training were infrequently observed (<10% of the time; i.e., on average less than 30 seconds per session). Wolves showed explorative behaviour towards the test room for a greater proportion of the session than dogs (7.2±0.6% versus 0.8±0.1%; t = 6.221, p < 0.001). Also, wolves spent more time temporarily quitting the training situation than dogs (1.6±0.2% versus 0.03±0.02%, t = 6.095, p < 0.001). Regarding jumping, there was no difference between wolves and dogs (wolves 0.4±0.06%, dogs 0.2±0.004%, t = 0.977, p = 0.341). After each training session, the SC concentration of animals was reduced compared to the measurement taken before the training session in both wolves and dogs (t = -2.864, p = 0.004, Fig 3; wolves before training–BT = 1023.03 ± 75.99 ng/ml; wolves after training–AT = 820.13 ± 64.03 ng/ml, a decrease of 19.83%; dogs BT = 2280.87 ± 153.2 ng/ml; dogs AT = 1851.99 ± 162.9 ng/ml, a decrease of 18.8%). The drop in GC concentrations was observed in most wolves (eight out of nine) and dogs (seven out of nine).
Fig 2. Mean percentage of time (±SE) spent by dogs and wolves within one metre of the trainer (Less1m) and with their face oriented towards the trainer (OrienT) during the training sessions.
Fig 3. Mean SC concentrations (±SE) in samples collected from wolves and dogs (1) before and (2) 15 minutes after the end of the training sessions.
Variables related to the performance of the animals during the training sessions
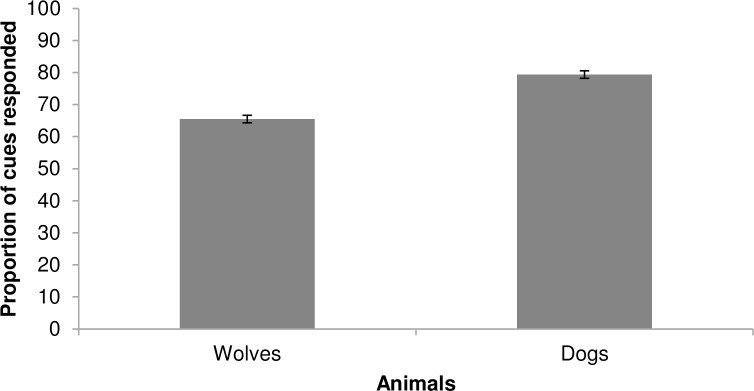
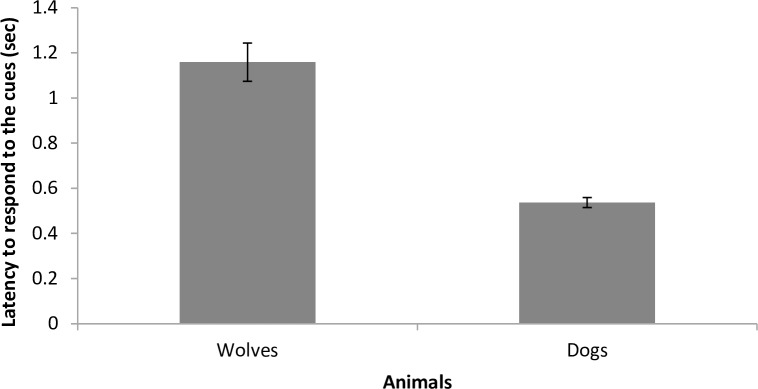
Dogs responded to a greater proportion of cues than wolves (79.4±1.2% versus 65.5±1.2%; t = -5.499, p < 0.001, Fig 4). Trainer identity explained 22.8% of the variability in performance of dogs and wolves. In addition, although both dogs and wolves responded to the cues promptly (mean latency 0.5±0.02 seconds in dogs and 1.2±0.1 seconds in wolves), dogs were significantly faster than wolves (t = 5.502, p < 0.001, Fig 5). The older the animal, the shorter the latency to respond to the cues (t = -3.510, p = 0.003) and the fewer repetitions were necessary to get a response, in both wolves and dogs (1.5±0.04 times, t = -2.878, p < 0.009). In dogs and wolves together, trainer identity explained 16.6% of the variability in latency for response to the cue and 16.4% of variability in cue repetition. The mixed models are available on S1 Table.
Fig 4. Mean proportion (±SE) of cues responded by dogs and wolves during the training sessions.
Fig 5. Mean latencies (±SE) of dogs and wolves to respond to the cues during the training sessions.
Discussion
Both wolves and dogs intensely attended the training in terms of proximity, orientation towards trainer and responses to the cues. In addition, we recorded a reduction in SC concentrations in both dogs and wolves, in response to the training sessions. These results, in connection with the low rates of NTB observed, suggest that the animals were motivated to participate in the training interactions and that these procedures may have positively affected their wellbeing. Dogs and wolves performed very similarly. However, dogs were slightly, but still significantly more attentive and responsive, and faster than wolves.
Training has been claimed to be an animal welfare promoter in some species since it affords learning, defined as a change in behaviour resulting from practice or experience [55, 84], as shown in chimpanzees [59], gorillas [26], baboons [30], dogs [29, 60] and African wild dogs [61]. This effect is connected to the understanding of the relationship between an action and a reward, which elicits a positive affective state [84]. In our study, however, all behaviours requested during the training sessions were already familiar to the animals. Once an animal has understood the connection between a cue and the correct behavioural answer to the cue, this is no longer a learning process. Therefore, our data allowed us to de-couple the effects of learning from the effects of the training interaction. Our results suggest that the measured decrease in SC levels was not due to a learning process but rather to mastering a task in a social setting together with a trainer, with whom the animals were well socialised.
Once an individual is rewarded upon the correct execution of certain behaviour on cue, it exerts control over its environment [95–96]. Control is claimed as psychologically and physiologically important [97] since the immediate psychological goal of behaviour is to exert control over a situation to which an individual is exposed (e.g. [98–99]). In the wild, animals are able to control the amount and quality of stimulations they receive, by performing various behaviours such as approaching, attacking or hiding from situations, until the stimulation they receive is at an acceptable level, or until their expectations of the stimulation are met. The matching of behaviours performed with their expected consequences are achievements known to be emotionally positive [100], a process possibly linked to the activation of the brain reward systems [84, 101–102]. Besides, the age effect on the quality of performances observed in our study parallels other studies on cooperation and a body of experience of people working with domestic dogs; generally, attentiveness and impulse control in dogs seem to improve particularly over the first two years of life [80, 103].
Beyond control over the environment, it appears that the relationship between the animals and their trainers and/or the manner in which the interaction is conducted—i.e. the tone of the trainers’ voice, the rhythm of the session and other aspects related to the personality of the trainer—also played a significant role in the relaxing effect of the interaction. Importantly, the effect of the identity of the trainer was not due to his/her stress level (measured by his/her SC concentration) at the time of the session. Being comfortable in the situation and having a good relationship with the trainers likely contributed to the fact that the animals participated in our training sessions voluntarily and intensely, and that their SC levels dropped during the sessions, in agreement with previous results [26, 30, 46, 59, 104].
Our results suggest that controllable interactions in a cooperative setting with human partners—with whom the animals are closely socialised—may increase the welfare of wolves and dogs, in accordance with previous findings [46, 56, 88, 105–107]. In captive primates, desirable changes in the behaviour–i.e., reduction in the frequency of abnormal behaviour, and in physiological responses–levels of GC concentrations—have been found when the animals could exert control over aspects of their environment such as food delivery [108] or highly disturbing noise [109]. African wild dogs showed a reduction in the frequency of pacing in response to PRT, possibly because the requested behaviours replaced highly motivated species-typical behaviours–prevented by the captive environment, such as hunting—and, consequently, matched a behavioural need for expressing such behaviours [61].
An alternative explanation of the observed stress-reducing effects of training is the separation of the animals from the pack for the duration of the training sessions. In animal groups, during conflicts, individuals interact with each other aggressively, generating social stress. In these situations, even dominant animals may show signs of distress [110]. Aggressive interactions tend to be more frequent in captivity, where animals have limited possibilities of conflict reduction through spacing out [58, 111]. Considering the constraints of captivity, because the individuals would not be exposed to social tension for the time of the session, they might have experienced adrenal deactivation. However, this is unlikely, because the animals showed no reluctance to return to their packs after training.
Also, it is possible that the interest of the animals in the training sessions was strongly motivated by the food rewards provided during these sessions [112]. This hypothesis is supported by our results, as the proportion of time wolves spent oriented towards the trainer increased with the fasting period. However, previous work on African wild dogs refutes this hypothesis, as the time spent exhibiting pacing behaviour was significantly reduced after training sessions as compared to control sessions, in which the canids were fed without training [61]. Obtaining the food reward is certainly a factor for responding, but we suggest that control and social factors may play a role as well. This is supported by the fact that although all trainers have worked with food rewards, still a great variability (ranging from 5.3 to 22.8%) in virtually all tested parameters was caused by trainer identity-. As reported in other studies [113–114], attentiveness toward potential partners seems to vary according to the relationship the subject has with its partner. In other words, attentiveness is affected by a social interaction factor.
Our findings regarding the role of individualised animal-trainer relationships confirm that appropriate training procedures may be seen as positive, cooperative human-animal interactions [46]. Several studies on farm animals have shown that stockpersons differ in the way they interact with their animals, and this difference has an effect on the value of the relationship (negative, neutral or positive [46, 51]) between them and their animals. It is known that captive wild mammals also generally distinguish between different keepers and respond to them in a specific way [47–48]. Some evidence even indicates that the welfare of captive mammals scales with the time caretakers spend with them (chimpanzees [45]; callitrichids—Callithrix jacchus [104]; small felid species—Felis spp [115]), particularly if they engage in structured activities such as training (callitrichids [63, 116]).
The high degree of cooperation recorded in the sessions with wolves–i.e., the great proportion of correct responses, low latency to respond to the cues and low frequency of NTB—suggest a willingness of these animals for social interactions, even with partners from another species. These results corroborate other studies suggesting that the promptitude of dogs to cooperate with human beings might have evolved on the basis of wolf-wolf cooperation [103, 117–118]. That we found no fundamental difference between the readiness of dogs and wolves to comply with human instructions suggests domestication did not crucially contribute to the origins of the abilities of dogs to interact with human beings. Our findings show that regularly trained wolves that are intensively socialised with human beings can benefit from the relaxing effects of training to the same degree as dogs, and indicate that given proper socialisation, the cooperative attitude of wolves is sufficient to ensure response to human cues in exchange for food reward. This would not exclude, however, that domestication did affect responsiveness and attentiveness to human cues in dogs [53, 119], which may be reflected in the minor, but significant observed differences in training performances.
Non-human animals are an integral part of experimental research. The success of experiments with animals, i.e. the comparability and repeatability of results may crucially depend on keeping and handling procedures, and on how the animals are prepared to collaborate in the testing / training sessions–including the important stage of socialisation of the animals with human beings. Stress response may negatively affect the performance of animals in complex cognitive tasks [120], such as spatial memory [121–123]. This is where the quality of scientific results and animal welfare meet. Our results bear implications for animal welfare, pointing at training sessions to provide adequately socialised wild canids with positive interspecific interactions, contributing to reduce stress in captivity. Raising and maintaining animals in good physical and mental conditions, including friendly and relaxing interactions with human beings, have the potential not only to benefit welfare, but also to make the animals adequate partners for comparative studies. Our behavioural and physiological results highlight short-term effects of PRT on the stress levels of dogs and socialised wolves. Future research comparing trained and untrained canids, kept under the same conditions and evaluated for longer periods of time may shed more light in the matter.
Supporting Information
Non-significant effects of fixed effects and interactions between them are not shown because they were removed in the model selection process.
(DOCX)
(DOCX)
Acknowledgments
We are grateful to Agatha Sacramento Rodrigues and Vinícius Frayze David for statistical advice. The Wolf Science Center was established by Kurt Kotrschal, Friederike Range and Zsófia Virányi in 2008 based on a private society; we are grateful for support by the Austrian Ministry of Science, by the University of Vienna, the Veterinary University Vienna and a number of private sponsors. We are particularly grateful to our team of trainers, Bea Belenyi, Marianne Heberlein, Christina Mayer, Marleen Hentrup, Rita Takacs, for the daily routine care, handling and training of the animals. We also thank two anonymous reviewers for their thorough work and their constructive comments on the manuscript. Dr. Ades passed away before the submission of the final version of this manuscript. Dr. Vasconcellos accepts responsibility for the integrity and validity of the data collected and analysed.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
ASV was supported by São Paulo Research Foundation (Project n. 2009/14258-4, http://www.fapesp.br/en) and Coordination for the Improvement of Higher Education Personnel (project grant BEX 5274/09-5, http://www.capes.gov.br). FR was financially supported by the Austrian Science Fund (project P21244-B17, https://www.fwf.ac.at/en/) and the European Research Council under the European Union's Seventh Framework Programme (FP/2007-2013, https://erc.europa.eu/) / ERC Grant Agreement n. 311870, ZV by the Austrian Science Fund (project I 1271-B24, https://www.fwf.ac.at/en/) and the Vienna Science and Technology Fund (project CS11-026, www.wwtf.at). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McDougall PT, Réale D, Sol D, Reader SM. Wildlife conservation and animal temperament: causes and consequences of evolutionary change for captive, reintroduced, and wild populations. Anim Conserv. 2006; 9: 39–48. [Google Scholar]
- 2.Mason G, Clubb R, Latham N, Vickery S. Why and how should we use environmental enrichment to tackle stereotypic behaviour? Appl Anim Behav Sci. 2007; 102: 163–188. [Google Scholar]
- 3.McBride SD, Cuddeford D. The putative welfare reducing effects of preventing equine stereotypic behaviour. Anim Welf. 2001; 10: 173–189. [Google Scholar]
- 4.Carpenter E. Animals and ethics: a report of the working party convened by Edward Carpenter. London: Watkins and Duverton; 1980. [Google Scholar]
- 5.Broom DM. Indicators of poor welfare. Br Vet J. 1986; 142: 524–526. [DOI] [PubMed] [Google Scholar]
- 6.Broom DM. Assessing welfare and suffering. Behav Processes. 1991; 25: 117–123. 10.1016/0376-6357(91)90014-Q [DOI] [PubMed] [Google Scholar]
- 7.Möstl E, Palme R. Hormones as indicators of stress. Domest Anim Endocrinol. 2002; 23: 67–74. [DOI] [PubMed] [Google Scholar]
- 8.Mason JW. A review of psychoendocrine research on the pituitary-adrenal cortical system. Psychosom Med. 1968; 30: 576–607. [PubMed] [Google Scholar]
- 9.Sapolsky RM. Adrenocortical function, social rank and personality among wild baboons. Biol Psychiatry. 1990; 28: 862–878 [DOI] [PubMed] [Google Scholar]
- 10.Broom DM. Animal welfare: concepts and measurements. J Anim Sci. 1991; 69: 4167–4175. [DOI] [PubMed] [Google Scholar]
- 11.McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995; 5: 205–216. [DOI] [PubMed] [Google Scholar]
- 12.Munck APM, Guyre PM, Holbrook NJ. Physiological functions of glucocorticosteroids in stress and their relation to pharmacological actions. Endocr Rev. 1984; 5: 25–44. [DOI] [PubMed] [Google Scholar]
- 13.Vining RF, McGinley RA, Maksvytis JJ, Ho KY. Salivary cortisol: a better measure of adrenal cortical function than serum cortisol. Ann Clin Biochem. 1983; 20, 329–335. [DOI] [PubMed] [Google Scholar]
- 14.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology. 1989; 22: 150–169. [DOI] [PubMed] [Google Scholar]
- 15.Vincent IC, Michell AR. Comparison of cortisol concentrations in saliva and plasma of dogs. Res Vet Sci. 1992; 53, 342–345. [DOI] [PubMed] [Google Scholar]
- 16.Hennessy MB, Williams MT, Miller DD, Douglas CW, Voith VL. Influence of male and female petters on plasma cortisol and behaviour: Can human interaction reduce the stress of dogs in a public animal shelter? Appl Anim Behav Sci. 1998; 61, 63–77. [Google Scholar]
- 17.Carlstead K. Effects of captivity on the behaviour of wild animals In: Kleiman DG, Allen ME, Thompson KV, Lumpkin S, Harris H, editors. Wild Animals in Captivity: Principles and Techniques. Chicago: University of Chicago Press; 1996. pp 317–333. [Google Scholar]
- 18.Shapiro SJ, Bloomsmith MA, Porter LM, Suarez SA. Enrichment effects on rhesus monkeys successively housed singly, in pairs, and in groups. Appl Anim Behav Sci. 1996; 48, 159–172. [Google Scholar]
- 19.Price EE, Stoinski S. Group size: Determinants in the wild and implications for the captive housing of wild mammals in zoos. Appl Anim Behav Sci. 2007; 103, 255–264. [Google Scholar]
- 20.Reimers M, Schwarzenberger F, Preuschoft S. Rehabilitation of research chimpanzees: stress and coping after long-term isolation. Horm Behav. 2007; 51, 428–435. [DOI] [PubMed] [Google Scholar]
- 21.Young LJ, Young AZM, Hammock EAD. Anatomy and neurochemistry of the pair bond. J Comp Neurol. 2005; 493, 51–57. [DOI] [PubMed] [Google Scholar]
- 22.De Rouck M, Nelissen M, Kitchener A, Law G. A comparative study of the influence of social housing conditions on the behaviour of captive tigers (Panthera tigris). Anim Welf. 2005; 14, 229–238. [Google Scholar]
- 23.Poole TB. Social behavior of a group of orangutans (Pongo pygmaeus) on an artificial island in Singapore Zoological Gardens. Zoo Biol. 1987; 6, 315–330. [Google Scholar]
- 24.Griffin JA, McCune S, Maholmes V, Hurley K. Human-animal interaction research: an introduction to issues and topics In McCardle PD, McCune S, Griffin JA, Maholmes VE, editors. How animals affect us: examining the influences of human-animal interaction on child development and human health. Washington DC: American Psychological Association; 2012. [Google Scholar]
- 25.Bayne K, Dexter SL, Strange G. The effects of food treat provisioning and human interaction on the behavioural well-being of rhesus monkeys (Macaca mulatta). Contemp Top Lab Anim Sci. 1993; 32: 6–9. [PubMed] [Google Scholar]
- 26.Carrasco L, Colell M, Calvo M, Abello MT, Velasco M, Posada S. Benefits of training/playing therapy in a group of captive lowland gorillas (Gorilla gorilla gorilla). Anim Welf. 2009; 18: 9–19. [Google Scholar]
- 27.Coleman K, Maier A. The use of positive reinforcement training to reduce stereotypic behaviour in rhesus macaques. Appl Anim Behav Sci. 2010; 124: 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tallet C, Veissier I, Boivin X. Temporal association between food distribution and human caregiver presence and the development of affinity to humans in lambs. Dev Psychobiol. 2008; 50: 147–159. 10.1002/dev.20254 [DOI] [PubMed] [Google Scholar]
- 29.Bergamasco L, Osella MC, Savarino P, Larosa G, Ozella L, Manassero M, et al. Heart rate variability and saliva cortisol assessment in shelter dog: human-animal interaction effects. Appl Anim Behav Sci. 2010; 125: 56–68. [Google Scholar]
- 30.O'Brien JK, Heffernan S, Thomson PC, McGreevy PD. Effect of positive reinforcement training in physiological and behavioural stress responses in the hamadryas baboon (Papio hamadryas). Anim Wef. 2008; 17: 125–138. [Google Scholar]
- 31.Hubrecht RC. A comparison of social and environmental enrichment methods for laboratory housed dogs. Appl Anim Behav Sci. 1993; 37: 345–361. [Google Scholar]
- 32.Baker KC, Bloomsmith M, Neu K, Griffis C, Maloney M, Brooke O, et al. Positive reinforcement training moderates only high levels of abnormal behaviour in singly housed rhesus macaques. J Appl Anim Welf Sci. 2009; 12: 236–252. 10.1080/10888700902956011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bloomsmith MA, Baker KC, Ross SK, Lambeth SP. Comparing animal training to non-training human interaction as environmental enrichment for chimpanzees. Am J Primatol. 1999; 49: 35–36. [Google Scholar]
- 34.Horvath Z, Doka A, Miklósi A. Affiliative and disciplinary behaviour of human handlers during play with their dog affects cortisol concentrations in opposite directions. Horm Behav. 2008; 54: 107–114. 10.1016/j.yhbeh.2008.02.002 [DOI] [PubMed] [Google Scholar]
- 35.Haubenhofer DK, Kirchengast S. Physiological arousal for companion dogs working with their owners in animal-assisted activities and animal-assisted therapy. J Appl Anim Welf Sci. 2006; 9: 165–172 [DOI] [PubMed] [Google Scholar]
- 36.Haubenhofer DK, Kirchengast S. Dog handlers’ and dogs’ emotional and cortisol secretion responses associated with animal-assisted therapy sessions. Soc Anim. 2007; 15: 127–150. [Google Scholar]
- 37.Deldalle S, Gaunet F. Effects of 2 training methods on stress-related behaviours of the dog (Canis familiaris) and on the dog-owner relationship. J Vet Behav. 2014; 9: 58–65. [Google Scholar]
- 38.Hosey GR. Zoo animals and their human audiences: what is the visitor effect? Anim Welf. 2000; 9: 343–357. [Google Scholar]
- 39.Thomsen CE Eye contact by non-human primates toward a human observer. Anim Behav. 1974; 22: 144–149. [Google Scholar]
- 40.Anderson US, Benne M, Bloomsmith MA, Maple TL. Retreat space and human visitor density moderate undesirable behaviour in petting zoo animals. J Appl Anim Welf Sci. 2002; 5: 125–137. [DOI] [PubMed] [Google Scholar]
- 41.Hogan LA, Johnston SD, Lisle AT, Keeley T, Wong P, Nicolson V, et al. Behavioural and physiological responses of captive wombats (Lasiorhinus latifrons) to regular handling by humans. Appl Anim Behav Sci. 2011; 134: 217–228. [Google Scholar]
- 42.Maloney MA, Baker KC, Griffis C, Neu K, Bloomsmith MA, Martinez M. Behavioural responses by singly housed rhesus macaques (Macaca mulatta) during positive reinforcement training and human interaction. Am J Primatol. 2007; 69: 53–54. [Google Scholar]
- 43.Claxton AM. The potential of the human–animal relationship as an environmental enrichment for the welfare of zoo‐housed animals. Appl Anim Behav Sci. 2011; 133: 1–10. [Google Scholar]
- 44.Hosey G. Hediger revisited: how do zoo animals see us? J Appl Anim Welf Sci. 2013; 16: 338–359. 10.1080/10888705.2013.827916 [DOI] [PubMed] [Google Scholar]
- 45.Baker KC. Benefits of positive human interaction for socially housed chimpanzees. Anim Welf. 2004; 13: 239–245. [PMC free article] [PubMed] [Google Scholar]
- 46.Hosey G, Melfi V. Human-animal interactions, relationships and bonds: a review and analysis of the literature. Int J Comp Psychol. 2014; 27: 117–142. [Google Scholar]
- 47.Carlstead K A comparative approach to the study of keeper-animal relationships in the zoo. Zoo Biol. 2009; 28: 589–608. 10.1002/zoo.20289 [DOI] [PubMed] [Google Scholar]
- 48.Ward SJ, Melfi V. The implications of husbandry training on zoo animal response rates. Appl Anim Behav Sci. 2013; 147: 179–185. [Google Scholar]
- 49.Hemsworth PH, Barnett JL, Coleman GJ. The human-animal relationship in agriculture and its consequences for the animal. Anim Welf. 1993; 2: 33–51. [Google Scholar]
- 50.Rennie LJ, Bowell VA, Dearing JM, Haskell MJ, Lawrence AB. A study of three methods to assess stockmanship on commercial dairy farms: can these become effective welfare assessment techniques? Anim Welf. 2003; 12: 591–597. [Google Scholar]
- 51.Waiblinger S, Menke C, Foelsch DW. Influences on the avoidance and approach behaviour of dairy cows towards humans on 35 farms. Appl Anim Behav Sci. 2003; 84: 23–39. [Google Scholar]
- 52.Frank H, Frank MG. Inhibition training in wolves and dogs. Behav Processes. 1983; 8: 363–377. 10.1016/0376-6357(83)90024-4 [DOI] [PubMed] [Google Scholar]
- 53.Klinghammer E, Goodmann PA. Socialisation and management of wolves in captivity In Frank H, editor. Man and wolf: advances, issues and problems in captive wolf research. Dordrecht: W. Junk Publishers; 1987. pp. 31–60. [Google Scholar]
- 54.Hemetsberger J, Scheiber IBR, Weiss B, Frigerio D, Kotrschal K. Socially involved hand-raising makes Greylag geese which are cooperative partners in research, but does not affect their social behaviour. Interact Stud. 2010; 11, 380–395. [Google Scholar]
- 55.Melfi V Is training zoo animals enriching? Appl Anim Behav Sci. 2013; 147: 299–305. [Google Scholar]
- 56.Basset L, Buchanan-Smith HM Effects of predictability on the welfare of captive animals. Appl Anim Behav Sci. 2007; 102: 223–245. [Google Scholar]
- 57.Westlund K Training is enrichment–And beyond. Appl Anim Behav Sci. 2014; 152: 1–6. [Google Scholar]
- 58.Miklósi A. Dog behaviour, evolution, and cognition Oxford: Oxford University Press; 2007. [Google Scholar]
- 59.Pomerantz O, Terkel J. Effects of positive reinforcement training techniques on the psychological welfare of zoo-housed chimpanzees (Pan troglodytes). Am J Primatol. 2009; 71: 687–695. 10.1002/ajp.20703 [DOI] [PubMed] [Google Scholar]
- 60.Rooney NJ, Cowan S. Training methods and owner-dog interactions: Links with dog behaviour and learning ability. Appl Anim Behav Sci. 2011; 132: 169–177. [Google Scholar]
- 61.Shyne A, Block M. The effects of husbandry training on stereotypic pacing in captive African wild dogs (Lycaon pictus). J Appl Anim Welf Sci. 2010; 13: 56–65. 10.1080/10888700903372069 [DOI] [PubMed] [Google Scholar]
- 62.McCormick W. How enriching is training? Proceedings of the 5th Annual Symposium on Zoo Research. Winchester: Federation of Zoological Gardens of Great Britain and Ireland; 2003. pp. 9–19
- 63.McKinley J, Buchanan-Smith HM, Bassett L, Morris K. Training common marmosets (Callithrix jacchus) to cooperate during routine laboratory procedures: Ease of training and time investment. J Appl Anim Welf Sci. 2003; 6: 209–220. [DOI] [PubMed] [Google Scholar]
- 64.Young RJ, Cipreste CF. Applying animal learning theory: training captive animals to comply with veterinary and husbandry procedures. Anim Welf. 2004; 13: 225–232. [Google Scholar]
- 65.Lambeth SP, Hau J, Perlman JE, Martino M, Schapiro SJ. Positive reinforcement training affects hematologic and serum chemistry values in captive chimpanzees (Pan troglodites). Am J Primatol. 2006; 68: 245–256. [DOI] [PubMed] [Google Scholar]
- 66.Luescher AU, Medlock RT. The effects of training and environmental alterations on adoption success of shelter dogs. Appl Anim Behav Sci. 2009; 117: 63–68. [Google Scholar]
- 67.Normando S, Corain L, Salvadoretti M, Meers L, Valsecchi P. Effects of an enhanced human interaction program on shelter dogs’ behaviour analysed using a novel nonparametric test. Appl Anim Behav Sci. 2009; 116: 211–219. [Google Scholar]
- 68.Owen J, Amory JR. A case study employing operant condition to reduce stress of capture for red-bellied tamarins (Saguinus labiatus). J Appl Anim Welf Sci. 2011; 14: 124–137. 10.1080/10888705.2011.551625 [DOI] [PubMed] [Google Scholar]
- 69.Minier DE, Tatum L, Gottlieb DH, Cameron A, Snarr J, Elliot R et al. Human-directed contra-aggression training using positive reinforcement with single and multiple trainers for indoor-housed rhesus macaques. Appl Anim Behav Sci. 2011; 132: 178–186. [Google Scholar]
- 70.Herzog H. Some we love, some we hate, some we eat: why it's so hard to think straight about animals New York: Harper Collins; 2010. [Google Scholar]
- 71.Kotrschal K. Wolf–Hund–Mensch. Die Geschichte einer jahrtausende alten Beziehung. Wien: Brandstätter; 2012. [Google Scholar]
- 72.Boitani L. Action Plan for the conservation of wolves in Europe (Canis lupus). Nature and Environment Series, no. 113: Convention on the Conservation of European Wildlife and Natural Habitats. Concil of Europe, Strasbourg; 2003. 81 pp.
- 73.Ginsburg BE. The wolf pack as a socio-genetic unit In: Frank H, editor. Man and wolf: advances, issues and problems in captive wolf research. Dordrecht: Dr. W. Junk Publishers; 1987. pp. 401–413. [Google Scholar]
- 74.Packard JM. Wolf behavior: reproductive, social, and intelligent In: Mech LD, Boitani L, editors. Wolves: Behavior, Ecology, and Conservation. Chicago: The University Chicago Press; 2003. pp. 35–65. [Google Scholar]
- 75.Clubb R, Mason GJ. Captivity effects on wide-ranging carnivores. Nature. 2003; 425: 473 [DOI] [PubMed] [Google Scholar]
- 76.Mech D, Boitani L. Wolves: Behavior, Ecology, and Conservation. Chicago: The University of Chicago Press; 2003. 448 pp. [Google Scholar]
- 77.Miklósi A, Topál J. What does it take to become ‘best friends’? Evolutionary changes in canine social competence. Trends Cogn Sci. 2013; 17, 287–294. 10.1016/j.tics.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 78.Cafazzo S, Bonanni R, Valsecchi P, Natoli E. Social variables affecting mate preferences, copulation and reproductive outcome in a pack of free-ranging dogs. PLoS One. 2014; 9, e98594 10.1371/journal.pone.0098594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Range F, Ritter C, Virányi Z. Testing the myth: tolerant dogs and aggressive wolves. Proc. R. Soc. B. 2015; 282: 20150220 10.1098/rspb.2015.0220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gácsi M, Gyoöri B, Virányi Z, Kubinyi E, Range F, Belényi B. Explaining dog wolf differences in utilizing human pointing gestures: selection for synergistic shifts in the development of some social skills. PLoS One. 2009; 4: e6584 10.1371/journal.pone.0006584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Udell MAR, Dorey NR, Wynne CDL. Wolves outperform dogs in following human social cues. Anim Behav. 2008; 76: 1767–1773. [Google Scholar]
- 82.Hare B, Brown M, Williamson C, Tomasello M. The domestication of social cognition in dogs. Science. 2002; 298 (5598): 1634–1636. [DOI] [PubMed] [Google Scholar]
- 83.Coppola CL, Grandin T, Enns RM. Human interaction and cortisol: can human contact reduce stress for shelter dogs? Phys Behav. 2006; 87, 537–541. [DOI] [PubMed] [Google Scholar]
- 84.McGowan RTS, Rehn T, Norling Y, Keeling LJ. Positive affect and learning: exploring the “Eureka Effect” in dogs. Anim Cogn. 2014; 17: 577–587. 10.1007/s10071-013-0688-x [DOI] [PubMed] [Google Scholar]
- 85.Shiverdecker MD, Schiml PA, Hennessy MB. Human interaction moderates plasma cortisol and behavioral responses of dogs to shelter housing. Physiol Behav. 2013; 109, 75–79. 10.1016/j.physbeh.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 86.Ligout S. Reliability of salivary cortisol measures in dogs in a training context. J Vet Behav. 2010; 5: 49. [Google Scholar]
- 87.Jones AC, Josephs RA. Interspecies hormonal interactions between man and the domestic dog (Canis familiaris). Horm Behav. 2006; 50: 393–400. [DOI] [PubMed] [Google Scholar]
- 88.Beerda B, Schilder MBH, van Hooff JARAM, de Vries HW, Mol JA. Behavioural, saliva cortisol and heart rate responses to different types of stimuli in dogs. Appl Anim Behav Sci. 1998; 58: 365e381. [Google Scholar]
- 89.Schalke E, Stichnoth J, Ott S, Jones-Baade R. Clinical signs caused by the use of electric training collars on dogs in everyday life situations. Appl Anim Behav Sci. 2007; 105: 369e380. [Google Scholar]
- 90.Haverbeke A, Laporte B, Depiereux E, Giffroy J-M, Diederich C. Training methods of military dog handlers and their effects on the team’s performances. Appl Anim Behav Sci. 2008; 113, 110–122. [Google Scholar]
- 91.Patzl M. Entwicklung eines Biotin-Streptavidin-Enzymimmunoassays zur Bestimmung von Cortisol in Blut und Speichel von Hunden. Diss., Vet. Med. Univ. Wien 1990.
- 92.Norusis MJ. Linear mixed models In Norusis MJ. IBM SPSS Statistics 19 Advanced Statistical Procedures Companion. Boston: Addison Wesley; 2010. pp. 35–47. [Google Scholar]
- 93.Bräuer J, Schönefeld K, Call J. When do dogs help humans? Appl Anim Behav Sci. 2013; 148: 138–149. [Google Scholar]
- 94.Riemer S, Müller C, Virányi Z, Huber L, Range F. The predictive value of early behavioural assessments in ped dogs–a longitudinal study from neonates to adults. PLoS One. 2014; 9: e101237 10.1371/journal.pone.0101237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Overmier JB, Patterson J, Wielkiewicz RM. Environmental contingencies as sources of stress in animals In: Levine S, Ursin H, editor. Coping and Health. New York: Plenum Press; 1980. pp. 1–38. [Google Scholar]
- 96.Sambrook TD, Buchanan-Smith HM. Control and complexity in novel object enrichment. Anim Welf. 1997; 6: 207–216. [Google Scholar]
- 97.Chamove AS, Anderson JR. Examining environmental enrichment In: Segal EF, editor. Housing, care and psychological well-being of captive and laboratory primates. New Jersey: Noyes Publications; 1989. pp. 183–199. [Google Scholar]
- 98.Inglis IR. Towards a cognitive theory of exploratory behaviour In: Archer J, Birke L, editors. Exploration in Animals and Humans. London: Van Nostrand Reinhold; 1983. pp. 72–116. [Google Scholar]
- 99.Salzen EA. Imprinting and fear. Symp Zool Soc Lond. 1962; 8: 199–218. [Google Scholar]
- 100.Burman OHP, Parker RMA, Paul ES, Mendl M. Sensitivity to reward loss as an indicator of animal emotion and welfare. Biol Lett. 2008; 4: 330–333. 10.1098/rsbl.2008.0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hagen K, Broom DM. Emotional reactions to learning in cattle. Appl Anim Behav Sci. 2004; 85: 203–213. [Google Scholar]
- 102.Kalbe C, Puppe B. Long-term cognitive enrichment affects opioid receptor expression in the amygdala of domestic pigs. Genes Brain Behav. 2010; 9: 75–83. 10.1111/j.1601-183X.2009.00536.x [DOI] [PubMed] [Google Scholar]
- 103.Range F, Virányi Z. Tracking the evolutionary origins of dog-human cooperation: the “Canine Cooperation Hypothesis”. Front Psychol. 2015; 5: 1582 10.3389/fpsyg.2014.01582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Manciocco A, Chiarotti F, Vitale A. Effects of positive interaction with caretakers on the behaviour of socially housed marmosets (Callithrix jacchus). Appl Anim Behav Sci. 2009; 120: 100–107. [Google Scholar]
- 105.Veissier I, Boissy A. Stress and welfare: two complementary concepts that are intrinsically related to the animal’s point of view. Physiol Behav. 2007; 92: 429–433. [DOI] [PubMed] [Google Scholar]
- 106.Greiveldinger L, Veissier I, Boissy A. Emotional experience in sheep: predictability of a sudden event lowers subsequent emotional responses. Physiol Behav. 2007; 92: 675–683. [DOI] [PubMed] [Google Scholar]
- 107.Alliger AA, Moller P. The effects of environmental control on cognition in rats (Rattus novergicus). J Appl Anim Welf Sci. 2011; 14: 271–285. 10.1080/10888705.2011.600153 [DOI] [PubMed] [Google Scholar]
- 108.Line SW, Clarke AS, Markowitz H, Ellman G. Responses of female rhesus macaques to an environmental enrichment apparatus. Lab Anim. 1990; 24: 213–220. [DOI] [PubMed] [Google Scholar]
- 109.Hanson JO, Larson ME, Snowdon CT. The effects of control over high intensity noise on plasma cortisol levels in rhesus monkeys. Genes Brain Behav. 1976; 16: 333–340. [DOI] [PubMed] [Google Scholar]
- 110.Sapolsky RM. Endocrine aspects of social instability in the olive baboon (Papio anubis). Am J Primatol. 1983; 5, 365–379. [DOI] [PubMed] [Google Scholar]
- 111.Creel S. Social dominance and stress hormones. Trends Ecol Evolut. 2001; 16, 491–497. [Google Scholar]
- 112.Rochais C, Henry S, Sankey C, Nassur F, Goracka-Bruzda A, Hausberger M. Visual attention, an indicator of human-animal relationships? A study of domestic horses (Equus caballus). Front Psychol. 2014; 5: 108 10.3389/fpsyg.2014.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Range F, Huber L. Attention in common marmosets: implications for social-learning experiments. Anim. Behav. 2007; 73: 1033–1041. [Google Scholar]
- 114.Horn L, Range F, Huber L. Dogs’ attention towards humans depends on their relationship, not only on social familiarity. Anim Cogn. 2013; 16: 435–443. 10.1007/s10071-012-0584-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mellen JD. Factors influencing reproductive success in small captive exotic felids (Felis spp): a multiple regression analysis. Zoo Biol. 1991; 10: 95–110. [Google Scholar]
- 116.Savastano G, Hanson A, McCann C. The development of an operant conditioning training program for New World primates at the Bronx Zoo. J Appl Anim Welf Sci. 2003; 6: 247–261. [DOI] [PubMed] [Google Scholar]
- 117.Range F, Virányi Z. Social learning from humans or conspecifics: differences and similarities between wolves and dogs. Front Psychol. 2013; 4: 868 10.3389/fpsyg.2013.00868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Range F, Virányi Z. Wolves are better imitators of conspecifics than dogs. PLoS One. 2014; 9: e86559 10.1371/journal.pone.0086559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Scott JP, Fuller JL. Genetics and the social behaviour of the dog Chicago: University of Chicago Press; 1965. [Google Scholar]
- 120.Cibulski L, Wascher CA, Weiss BM, Kotrschal K. Familiarity with the experimenter influences the performance of Common ravens (Corvus corax) and Carrion crows (Corvus corone corone) in cognitive tasks. Behav Processes. 2014; 103: 129–137. 10.1016/j.beproc.2013.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bodnoff SR, Humphreys AG, Lehman CJ, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and middle-aged rats. J Neurosci. 1995; 15: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Conrad CD, Galea LAM, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y-maze, and this effect is blocked by Tianeptine pretreatment. Behav Neurosci. 1996; 110: 1321–1334. [DOI] [PubMed] [Google Scholar]
- 123.Bardgett ME, Newcomer JW, Taylor GT. The effects of chronic corticosterone on memory performance in the platform maze task. Physiol Behav. 1996; 59: 1111–1115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Non-significant effects of fixed effects and interactions between them are not shown because they were removed in the model selection process.
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.