Abstract
Toxic heavy metals have been implicated in the loss of spinal motoneurons in amyotrophic lateral sclerosis/motor neuron disease (ALS/MND). Motoneuron loss in the spinal anterior horn is severe in ALS/MND at the time of death, making this tissue unsuitable for examination. We therefore examined spinal cords of people without muscle weakness to look for any presence of heavy metals that could make these neurons susceptible to damage. Spinal cord samples from 50 individuals aged 1–95 y who had no clinical or histopathological evidence of spinal motoneuron loss were studied. Seven μm formalin-fixed paraffin-embedded sections were stained for heavy metals with silver nitrate autometallography (AMGHM) which detects intracellular mercury, silver or bismuth. Neurons in the spinal cord were classified as interneurons or α-motoneurons based on their site and cell body diameter. Spinal interneurons containing heavy metals were present in 8 of 24 people (33%) aged 61–95 y, but not at younger ages. These AMGHM interneurons were most numerous in the lumbar spinal cord, with moderate numbers in the caudal cervical cord, few in the rostral cervical cord, and almost none in the thoracic cord. All people with AMGHM interneurons had occasional AMGHM staining in α-motoneurons as well. In one man AMGHM staining was present in addition in dorsomedial nucleus and sensory neurons. In conclusion, heavy metals are present in many spinal interneurons, and in a few α-motoneurons, in a large proportion of older people. Damage to inhibitory interneurons from toxic metals in later life could result in excitotoxic injury to motoneurons and may underlie motoneuron injury or loss in conditions such as ALS/MND, multiple sclerosis, sarcopenia and calf fasciculations.
Introduction
The cause of the motoneuron loss that occurs in the neurodegenerative disorder amyotrophic lateral sclerosis/motor neuron disease (ALS/MND) remains largely unknown [1]. Possibilities include environmental, genetic, or epigenetic factors, or combinations of these, but so far no common cause for the sporadic form of the disease has emerged [2]. Similarly, the genetic or environmental causes of other age-related losses or dysfunctions of motoneurons that occur in normal aging [3,4], the muscle wasting of sarcopenia that occurs in later life [5], or benign fasciculation syndromes [6] are still not identified.
A major difficulty in finding environmental factors that could contribute to motoneuron loss in humans is that in the industrial age an almost limitless number of environmental toxins (toxicants) exist in air, water and soil. This has led to attempts to locate toxicants within the central nervous system in diseases such as ALS/MND, but judging if a patient died with, or from, a toxicant is a challenge. The toxicant could have entered the nervous system years before the disease become clinically apparent and could no longer be detectable at the time of post mortem examination. Furthermore, after death from ALS/MND a severe loss of motoneurons is usually present, and the remaining motoneurons may have survived because they did not contain the toxicant. Finally, a low level of toxicant may be present in only a few neurons within the tissue and so would be missed by chemical analyses [7].
In an attempt to overcome these problems we examined the spinal cords of people who had no evidence of motoneuron damage, to see if any groups of neurons took up toxicants that could cause later motoneuron dysfunction. We used a histochemical technique, autometallography, that visualises some heavy metals within cells [8]. Importantly, this technique detects mercury, a toxicant that has long been implicated in ALS/MND [7]. Unexpectedly, uptake of heavy metals was seen mostly in spinal interneurons, rather than in motoneurons. Inhibitory interneurons damaged by toxicants would lead to a prolonged excitotoxic insult to motoneurons [9], which could result in a range of motoneuron disorders.
Methods
Spinal cord samples
Paraffin tissue blocks of spinal cord were available from 50 individuals (24 male, 26 female) with an age range of 1–95 years, who had no clinical or histopathological evidence of spinal motoneuron loss. Individuals included were those where at least one paraffin block from either the cervical or lumbar spinal cord was available. Tissue was obtained from the Department of Forensic Medicine, Sydney, New South Wales, Australia (N = 29), and from the Multiple Sclerosis Research Australia Brain Bank (N = 21) (Table 1). Causes of death were trauma (N = 20), infection (N = 7), cancer (N = 6), cardiac (N = 4), drowning (N = 4), infection (N = 2), undernutrition (N = 2), choking (N = 2), and one each of asphyxia and drug overdose. One individual from the multiple sclerosis brain bank had neuromyelitis optica, and another was found not to have any neurological disorder (see later); of the 19 individuals with neuropathologically-confirmed multiple sclerosis, 17 had secondary progressive, 1 relapsing-remitting, and 1 primary progressive multiple sclerosis. The study was approved by the Human Research Committees of the Sydney Local Health District (Royal Prince Alfred Hospital Zone) and the University of Sydney, and the Office of the New South Wales Coroner. The institutional review board waived the need for written informed consent from relatives of participants since this was a de-identified retrospective study of post mortem tissue.
Table 1. Heavy Metal Staining in Spinal Interneurons and Alpha-motoneurons.
| Age y | Sex | History | C1-T1 | T2-T12 | L1-S1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | INT | αMN | No. | INT | αMN | No. | INT | αMN | |||
| 95 | M | Dementia | 12 | 0 | 0 | 0 | na | na | 0 | na | na |
| 89 | F | Dementia | 16 | ++ | 0 | 5 | 0 | 0 | 6 | ++ | + |
| 85 | M | PD | 2 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| 84 | F | MS | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 |
| 83 | F | MS | 3 | ++ | + | 3 | 0 | 0 | 3 | ++ | + |
| 78 | M | Arthritis | 1 | ++ | 0 | 1 | 0 | + | 3 | ++ | + |
| 77 | F | PD | 0 | na | na | 2 | 0 | 0 | 6 | 0 | 0 |
| 74 | F | None | 3 | + | 0 | 4 | + | 0 | 3 | ++ | + |
| 72 | F | MS | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| 72 | F | PD | 7 | ++ | + | 0 | na | na | 0 | na | na |
| 71 | M | MS | 2 | + | 0 | 1 | 0 | 0 | 1 | + | 0 |
| 70 | M | MS | 2 | 0 | 0 | 0 | na | na | 0 | na | na |
| 69 | M | None | 4 | 0 | 0 | 13 | 0 | 0 | 0 | na | na |
| 69 | F | MS | 1 | 0 | 0 | 3 | 0 | 0 | 0 | na | na |
| 68 | F | MS | 4 | 0 | 0 | 4 | 0 | 0 | 2 | ++ | + |
| 68 | F | MS | 1 | 0 | 0 | 3 | 0 | 0 | 0 | na | na |
| 67 | F | Dementia | 1 | 0 | 0 | 0 | na | na | 0 | na | na |
| 66 | F | MS | 2 | 0 | 0 | 0 | na | na | 0 | na | na |
| 64 | M | NMO | 1 | 0 | 0 | 4 | 0 | 0 | 2 | 0 | 0 |
| 64 | M | MS | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 |
| 63 | M | MS | 2 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 |
| 62 | F | MS | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 |
| 62 | M | PD | 5 | 0 | 0 | 0 | na | na | 0 | na | na |
| 61 | F | BD | 11 | 0 | 0 | 3 | 0 | 0 | 4 | ++ | + |
| 61 | M | MSA | 2 | 0 | 0 | 0 | na | na | 0 | na | na |
| 60 | F | MS | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| 59 | F | MS | 0 | na | na | 0 | na | na | 2 | 0 | 0 |
| 57 | F | MS | 1 | 0 | 0 | 2 | 0 | 0 | 3 | 0 | 0 |
| 53 | M | None | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| 51 | F | MS | 3 | 0 | 0 | 0 | na | na | 2 | 0 | 0 |
| 50 | F | MS | 3 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 |
| 48 | M | MS | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 |
| 43 | F | Anorexia | 6 | 0 | 0 | 6 | 0 | 0 | 6 | 0 | 0 |
| 40 | M | None | 3 | 0 | 0 | 0 | na | na | 4 | 0 | 0 |
| 40 | M | MS | 2 | 0 | 0 | 0 | na | na | 1 | 0 | 0 |
| 38 | F | AN | 6 | 0 | 0 | 4 | 0 | 0 | 6 | 0 | 0 |
| 37 | F | None | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| 33 | M | None | 5 | 0 | 0 | 0 | na | na | 0 | na | na |
| 32 | F | None | 4 | 0 | 0 | 6 | 0 | 0 | 5 | 0 | 0 |
| 30 | M | None | 8 | 0 | 0 | 4 | 0 | 0 | 4 | 0 | 0 |
| 28 | M | Schizophrenia | 0 | na | na | 3 | 0 | 0 | 3 | 0 | 0 |
| 25 | M | None | 5 | 0 | 0 | 15 | 0 | 0 | 11 | 0 | 0 |
| 24 | M | None | 3 | 0 | 0 | 0 | na | na | 1 | 0 | 0 |
| 20 | M | Epilepsy | 4 | 0 | 0 | 4 | 0 | 0 | 4 | 0 | 0 |
| 18 | F | None | 4 | 0 | 0 | 5 | 0 | 0 | 4 | 0 | 0 |
| 9 | M | None | 12 | 0 | 0 | 2 | 0 | 0 | 0 | na | na |
| 4 | M | None | 5 | 0 | 0 | 11 | 0 | 0 | 10 | 0 | 0 |
| 2 | M | None | 2 | 0 | 0 | 3 | 0 | 0 | 7 | 0 | 0 |
| 2 | F | None | 6 | 0 | 0 | 6 | 0 | 0 | 4 | 0 | 0 |
| 1 | F | None | 0 | na | na | 1 | 0 | 0 | 2 | 0 | 0 |
Autometallography staining in the spinal cords of 50 individuals without motoneuron loss, arranged in declining order of age. αMN: α-motoneuron, BD: bipolar disorder, C: cervical cord, INT: interneuron, L: lumbar cord, MS: multiple sclerosis, MSA: multiple system atrophy, na: not applicable; NMO: neuromyelitis optica, No.: number of paraffin blocks, PD: Parkinson’s disease, S: sacral cord, T: thoracic cord, +: 1–2 AMGHM neurons per slide,++: 3 or more AMGHM neurons per slide.
Histochemical detection of heavy metals
Seven μm sections from each paraffin block were processed routinely and stained with silver nitrate autometallography, which under the protocol used in this study stains the sulphides or selenides of mercury, silver, or bismuth [8]. Briefly, sections were placed in physical developer containing gum arabic, citrate buffer, hydroquinone and silver nitrate at 26°C for 80 min in the dark, then washed in sodium thiosulphate to remove unbound silver. Sections were counterstained with mercury-free hematoxylin and viewed under bright-field microscopy. The silver-coated deposits of these metals in tissues are seen microscopically as black-staining grains, and referred to as autometallography-demonstrable heavy metals (AMGHM). In each staining run a positive control section was included of formalin-fixed paraffin-embedded mouse spinal motoneurons that contained mercury following intraperitoneal exposure to mercuric chloride [10].
Identification of spinal interneurons and motoneurons
Spinal interneurons were identified as neurons less than 24 μm in minimal diameter (measured at the nucleolar level) situated in the grey matter of the anterior horn ventral to the central canal, and α-motoneurons as those >32 μm in minimum diameter in the same region [3,11].
Results
Interneurons
In eight individuals aged between 61 and 95 years (33% of people in this age range) AMGHM staining was present in the cytoplasm of spinal interneurons (Table 1). AMGHM interneurons were situated between, or within, groups of α-motoneurons (Figs 1 and 2). The number of AMGHM interneurons per section varied from 1 to 5. In one individual an AMGHM interneuron was seen in the grey matter commissure adjacent to the central canal (Fig 3). Three individuals with AMGHM interneurons had a history of multiple sclerosis and one each had had dementia, Parkinson’s disease, bipolar disorder, arthritis, and no previous illness.
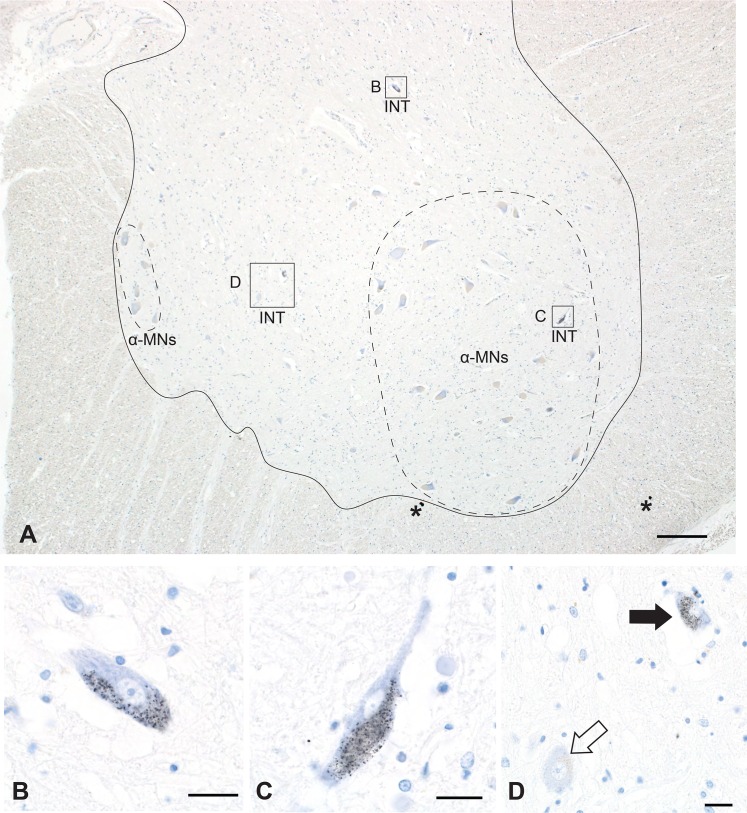
Fig 1. Interneuron Heavy Metal Staining.
A. Caudal lumbar spinal cord showing AMGHM-stained interneurons (INT) either peripheral to (B, D) or within (C) groups of unstained α-motoneurons (α-MN, within dashed outlines; lateral group right, medial group left) within the grey matter of the anterior horn (the area within the solid outline). Asterisks mark small black dots of artefactual silver staining. Bar = 200 μm. B, C. AMGHM staining (small black grains) is seen in the cytoplasm of two interneurons. Bar = 20 μm. D. Not all interneurons stain with AMGHM, as can be seen with this non-stained interneuron (open arrow) adjacent to a stained interneuron (closed arrow). Bar = 20 μm. AMG and hematoxylin.
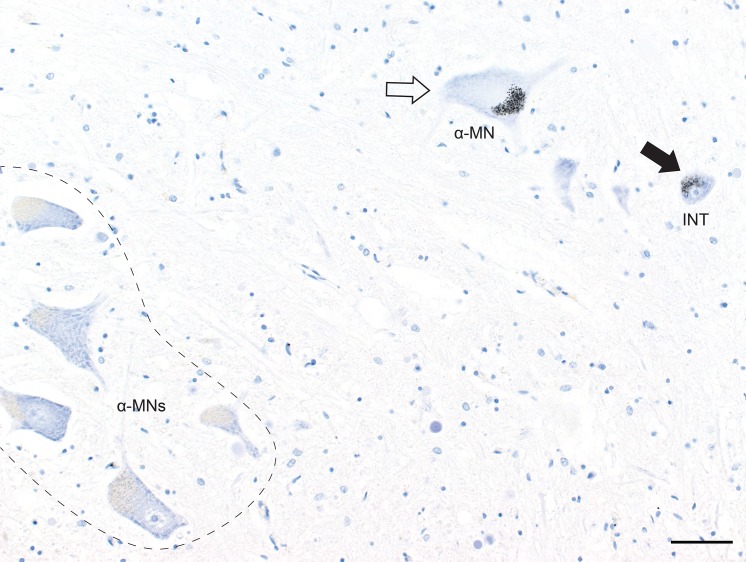
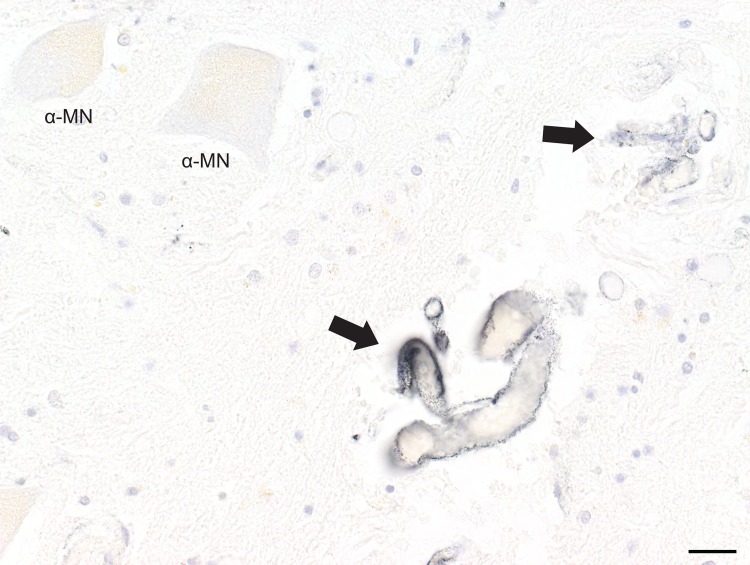
Fig 2. Interneuron and Alpha-motoneuron Heavy Metal Staining.
AMGHM staining is seen in the cytoplasm of an α-motoneuron (α-MN) and a nearby interneuron (INT) in the lumbar cord. Most α-motoneurons (within the dashed outline) do not stain for heavy metals. Bar = 50 μm. B.
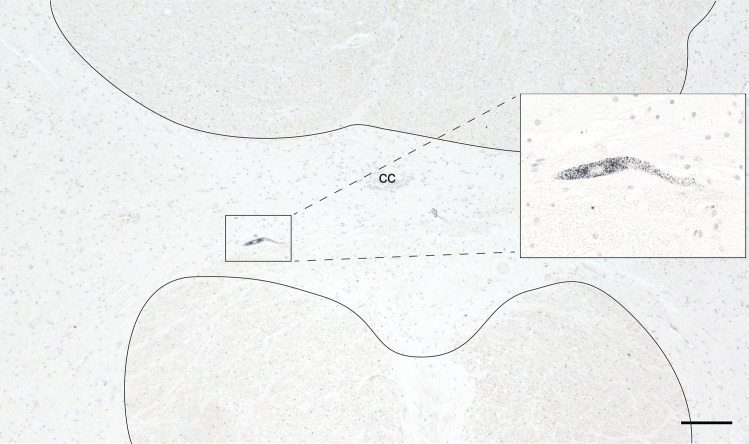
Fig 3. Commissural Neuron Heavy Metal Staining.
An occasional AMGHM-stained interneuron (within box, magnified in the insert) is seen in the spinal grey matter commissure (the midline region within the solid outlines) in the cervical cord. Bar = 200 μm. AMG and hematoxylin. CC: central canal.
In some sections AMGHM interneurons were present on only one side of the spinal cord. The number of AMGHM interneurons also varied at different rostro-caudal levels of the spinal cord; often no AMGHM interneurons were seen in a block immediately adjacent to a block containing AMGHM interneurons. Overall, AMGHM interneurons tended to be seen more often in the lumbar compared to the cervical spinal cord. In only one section was an AMGHM interneuron seen in the T2-T12 thoracic spinal cord.
In two individuals where serial blocks had been sampled from C3 to T1, AMGHM interneurons were seen frequently at caudal levels of the cervical cord, as well as in T1, but infrequently at levels rostral to C6. In one individual who had serial blocks sampled from L1-S1 no differences in AMGHM interneuron numbers were apparent between different levels.
Alpha motoneurons
In individuals where AMGHM interneurons were present, in some sections AMGHM staining was also seen in the cytoplasm of a few spinal α-motoneurons (Fig 2, Table 1). AMGHM stained α-motoneurons were however not present in most sections that contained AMGHM interneurons. In sections where both types of neurons contained heavy metals, numbers of AMGHM interneurons were always greater than numbers of AMGHM stained α-motoneurons.
Multiple neuronal types and endothelial cells
In one 78 year-old man AMGHM staining was present in spinal interneurons and a few α-motoneurons, as well as in a few posterior horn sensory neurons and in all neurons of the dorsomedial nucleus (Clarke’s column) in the caudal thoracic and rostral lumbar cord (Fig 4). AMGHM staining was also present in the endothelial cells of some small spinal cord blood vessels (Fig 5). Early in life there had been a suggestion that he might have multiple sclerosis, but he was later found to have rheumatoid arthritis. Post mortem brain MRI and a neuropathological examination of his brain and spinal cord showed no evidence of CNS pathology.
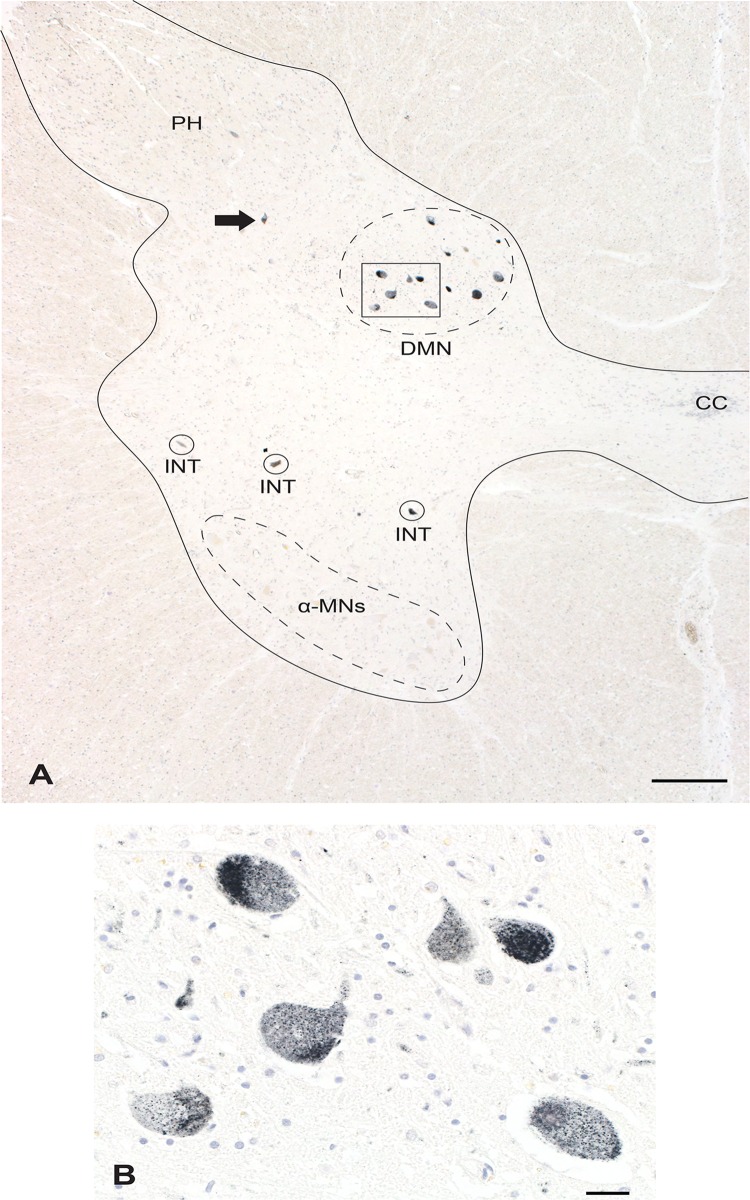
Fig 4. Widespread Heavy Metal Staining.
A. In this rostral lumbar spinal cord AMGHM staining is seen in interneurons (INT), in posterior horn sensory neurons (arrow), and in neurons in the dorsomedial nucleus (DMN, Clarke’s column). No α-motoneurons are stained in this section. The grey matter of the cord is within the solid outline. Bar = 500 μm. CC: central canal. B. Higher magnification of the boxed region in A showing heavy AMGHM staining of all dorsomedial nucleus neurons. Bar = 20 μm. AMG and hematoxylin.
Fig 5. Endothelial cell Heavy Metal Staining.
AMGHM staining is seen in the endothelial cells lining two small blood vessels (arrows) in the same spinal lumbar cord as in Fig 4. Two α-motoneurons show no AMGHM staining. Bar = 20 μm. AMG and hematoxylin.
Discussion
Heavy metals appear to be commonly taken up by spinal interneurons in advancing age in people who to have no evidence of motoneuron dysfunction. The particular heavy metal present here is likely to be mercury, which is increasingly widespread in air, water and soil due to industrial sources that emit mercury which then enters the mercury cycle [12,13]. Human exposure to the other two heavy metals stained with autometallography, silver and bismuth, is uncommon. Protective mechanisms against heavy metal toxicity, such as binding to metallothionein or selenium in the case of mercury, would protect most individuals against intracellular accumulations of toxic metals. However, those with genetic, epigenetic or environmental susceptibilities to heavy metals [14] could suffer damage to heavy metal-containing interneurons.
Alpha-motoneurons are inhibited by a number of spinal interneurons, either Renshaw cells which inhibit the same α-motoneuron, or 1a interneurons which inhibit α-motoneurons supplying antagonist muscles [15]. Any damage to inhibitory interneurons could result in excitotoxicity to α-motoneurons, a mechanism suggested to underlie motoneuron death in ALS/MND [9]. Neurons containing metal toxins can have decreased physiological function but still survive [10], which could explain why in ALS/MND α-motoneuron numbers may decrease before those of interneurons [16], though others have reported that both sets of neurons degenerate at the same time. The number of interneurons with heavy metals in any one section was small, but interneurons synapse with many α-motoneurons. For example, the ratio of Renshaw cells to α-motoneurons is 1:5 [17], so damage to even a few interneurons could result in widespread α-motoneuron dysfunction.
Heavy metals in interneurons causing excitotoxic damage to α-motoneurons could explain a number of puzzles in ALS/MND. (1) The finding of heavy metals in the interneurons of older individuals fits with the known later age of onset of ALS/MND [18]. (2) The preponderance of interneurons with heavy metals in the lumbar and caudal cervical spinal cord, regions of greatest motoneuron activity during exercise, could be responsible for the ALS/MND postulated risk factor of exercise [19] since active interneurons would be inclined to take up more circulating toxicants. (3) The rarity of a respiratory onset of ALS/MND [20] accords with our finding of the infrequency of heavy-metal containing interneurons in the thoracic spinal cord. (4) Extraocular muscles are rarely affected in ALS/MND [21], and their motor neurons have a different system of inhibitory interneurons which are at some distance from the extraocular nuclei [22]. (5) ALS/MND appears to start at a focal neuroanatomical site and spread from there to adjacent motoneurons [23]. The number of initiation sites could determine the rate of diseases progression [2], and having more interneurons containing heavy metals could underlie increased rates of progression.
Heavy metal staining was widespread in the spinal cord of one individual, involving interneurons, motor neurons, sensory neurons, and neurons in the dorsomedial nucleus (Clarke’s column). Heavy metal staining in endothelial cells suggests ongoing exposure to the metal, though the individual had no clinical features of heavy metal toxicity. Clarke’s column neurons have long been known to be involved in ALS/MND [24], and pathological TDP43 inclusions are seen in this nucleus in one-third of patients [25]. Sensory abnormalities can often be found on nerve conduction studies in ALS/MND, consistent with our finding of heavy metals in some posterior horn neurons [26]. It seems possible therefore that toxicants could determine which types of neurons are affected in ALS/MND and could therefore underlie some of the variability in neuroanatomical phenotype that is typical for the disease [27].
Of interest was the finding of an occasional α-motoneuron with cytoplasmic heavy metal staining, often with a nearby interneuron showing similar staining. The dual stress of excitotoxicity and toxicant load could well be too much for the α-motoneurons to bear, and cell damage and death could result, especially if some susceptibility to metal toxicity were present. This could initiate the spread of disease, particularly since the metal in question is likely to be mercury. Mercury has many of the toxic properties which are suspected to underlie ALS/MND [28]. These include an ability to activate retroelements [29], some of which, such as human endogenous retrovirus-K, have been implicated in the spread of ALS/MND [30].
Spinal motor neuron numbers decline with age [3], and this loss is most readily apparent after the age of 60 years, when motoneuron counts can be up to 50% lower than in early adult life [4]. Small neurons in the dorsomedial portion of the spinal anterior horn are most severely lost in aging, more so than large α-motoneurons [31]. These small neurons are in the same region of the spinal cord as many of the heavy metal-staining interneurons in our study. Age-related losses of interneurons and of α-motoneurons may underlie the muscle wasting of sarcopenia, since muscle denervation is a prominent feature of this condition [5]. Of note, spinal reciprocal inhibition decreases with age [32], as might be expected with damaged interneurons. Furthermore, in sarcopenia the decrease in muscle mass is greater in the lower than the upper limbs, which is counterintuitive since the lower limbs are usually exercised more [33]. There was a tendency in our study to see more heavy metal-containing spinal interneurons in the lumbar than the cervical spinal cord, which could account for this disproportionate decrease in lower limb muscle mass in sarcopenia.
Fasciculations, spontaneous intermittent contractions of a portion of a muscle, are a clinical hallmark of ALS/MND, but can be seen as a benign condition in later age when restricted to muscles below the knee [6]. Fasciculations are thought to be generated by abnormal activity of α-motoneurons, possibly from axonal damage, though the temporary increase in fasciculations produced by intense exercise suggests a more central mechanism may be in operation [6]. Such a mechanism may be toxicant-induced reduced interneuron inhibition of α-motoneurons, since our data show heavy metal uptake into interneurons is age-related and that interneurons in the lumbar spinal cord are particularly affected.
Spinal α-motoneuron and interneuron loss is not uncommon in multiple sclerosis, even in regions distant from plaques [34,35]. People with multiple sclerosis can have focal asymmetric wasting of muscles, especially in the hand [36]. Occasionally, multiple sclerosis and ALS/MND co-exist, raising the possibility of a shared pathogenesis for motoneuron loss in these conditions [37]. Our finding of heavy metals in spinal interneurons raises the possibility that toxicant uptake in these interneurons may be the factor underlying motoneuron loss in both these conditions, though α-motoneuron loss in multiple sclerosis does not appear to be age-related [36].
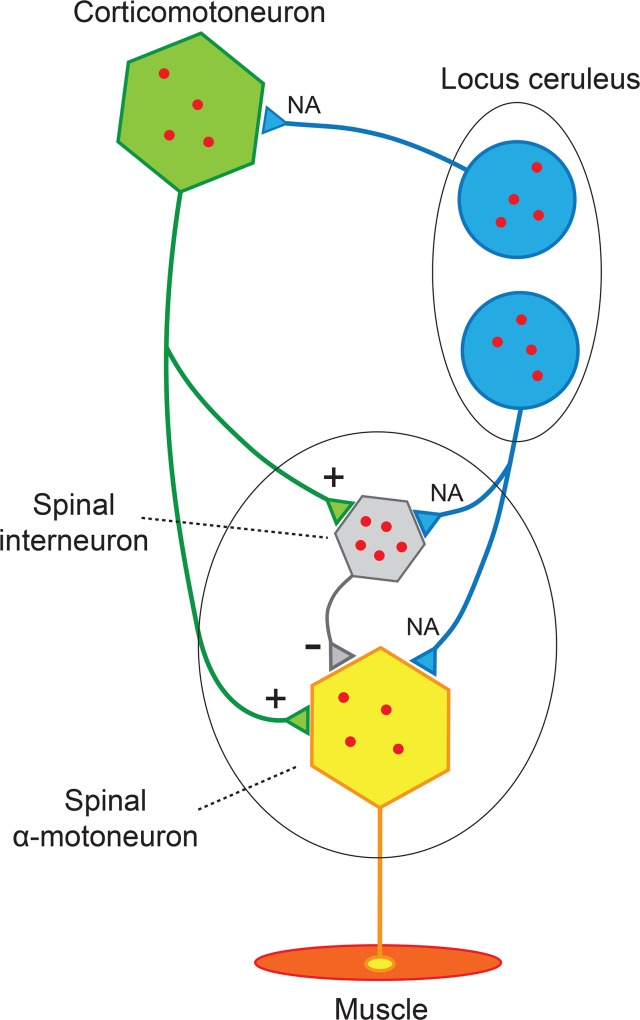
Spinal interneurons can now be added to three other types of neurons (α-motoneurons, corticomotoneurons, and locus ceruleus neurons) which human studies using autometallography have indicated are prone to take up heavy metals [38] (Fig 6). These neurons are part of (or in the case of locus ceruleus neurons, have a major influence on [39]) spinal and cortical motoneurons. We propose that exposures to single or multiple toxicants with uptake of these into different neurons, combined with susceptibilities to the toxicants, could underlie a spectrum of disorders of the motor system. Differences in susceptibility to mercury toxicity, for example, could arise due to genetic polymorphisms affecting the mercury-binding protein metallothionein [40], epigenetic differences [14], or low selenium levels [41], any of which could stimulate mercury neurotoxicity.
Fig 6. Neurons of the Motor System Predisposed to Heavy Metal Uptake.
Human spinal interneurons, α-motoneurons, corticomotoneurons, and locus ceruleus neurons are prone to take up circulating heavy metals. Differences in toxicant uptake between these neurons could explain some of the phenotypic variability of ALS/MND, and underlie a spectrum of conditions that include motoneuron loss in normal aging, sarcopenia, benign fasciculation syndrome, and amyotrophy in multiple sclerosis.
In conclusion, heavy metals appear to accumulate during aging in human spinal interneurons, as well as in a few α-motoneurons. Toxicant-induced dysfunction of spinal interneurons could lead, especially in susceptible individuals, to damage or loss of α-motoneurons. This could manifest in a spectrum of motoneuron disorders which include ALS/MND, sarcopenia, benign fasciculation syndrome, and amyotrophy in multiple sclerosis.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the Aimee Stacey Memorial and Ignacy Burnett Bequests. The Multiple Sclerosis Research Australia Brain Bank at the Brain and Mind Centre in Sydney, Australia is supported by Multiple Sclerosis Research Australia, the University of Sydney, the NSW Office for Health and Medical Research, Royal Prince Alfred Hospital Sydney, the Trish MS Research Foundation, the Levy Foundation, the Collier Charitable Fund, the Medical Advances Without Animals Trust, and the FIL Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377: 942–955. 10.1016/S0140-6736(10)61156-7 [DOI] [PubMed] [Google Scholar]
- 2.Al-Chalabi A, Calvo A, Chio A, Colville S, Ellis CM, Hardiman O, et al. Analysis of amyotrophic lateral sclerosis as a multistep process: a population-based modelling study. Lancet Neurology. 2014;13: 1108–1113. 10.1016/S1474-4422(14)70219-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawamura Y, O'Brien P, Okazaki H, Dyck PJ Lumbar motoneurons of man II: the number and diameter distribution of large- and intermediate-diameter cytons in "motoneuron columns" of spinal cord of man. J Neuropathol Exp Neurol. 1977;36: 861–870. [DOI] [PubMed] [Google Scholar]
- 4.Tomlinson BE, Irving D The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci. 1977;34: 213–219. [DOI] [PubMed] [Google Scholar]
- 5.Lexell J, Taylor CC Variability in muscle fibre areas in whole human quadriceps muscle: effects of increasing age. J Anat. 1991;174: 239–249. [PMC free article] [PubMed] [Google Scholar]
- 6.Fermont J, Arts IM, Overeem S, Kleine BU, Schelhaas HJ, Zwarts MJ Prevalence and distribution of fasciculations in healthy adults: Effect of age, caffeine consumption and exercise. Amyotroph Lateral Scler. 2009: 1–6. [DOI] [PubMed]
- 7.Kasarskis EJ, Ehmann WD, Markesbery WR Trace metals in human neurodegenerative diseases. Prog Clin Biol Res. 1993;380: 299–310. [PubMed] [Google Scholar]
- 8.Stoltenberg M, Danscher G Histochemical differentiation of autometallographically traceable metals (Au, Ag, Hg, Bi, Zn): protocols for chemical removal of separate autometallographic metal clusters in Epon sections. Histochem J. 2000;32: 645–652. [DOI] [PubMed] [Google Scholar]
- 9.Turner MR, Kiernan MC Does interneuronal dysfunction contribute to neurodegeneration in amyotrophic lateral sclerosis? Amyotroph Lateral Scler. 2012;13: 245–250. 10.3109/17482968.2011.636050 [DOI] [PubMed] [Google Scholar]
- 10.Pamphlett R, Png FY Shrinkage of motor axons following systemic exposure to inorganic mercury. J Neuropathol Exp Neurol. 1998;57: 360–366. [DOI] [PubMed] [Google Scholar]
- 11.Eisen AA (1997) Electrophysiological Investigation of Disorders of the Spinal Cord In: Critchely E, Eisen A, editors. Spinal Cord Disease Basic Science, Diagnosis and Management. 1 ed. London: Spinger-Verlag; pp. 175–202. [Google Scholar]
- 12.Selin NE, Jacob DJ, Yantosca RM, Strode S, Jaegle L, Sunderland EM Global 3-D land-ocean-atmosphere model for mercury: Present-day versus preindustrial cycles and anthropogenic enrichment factors for deposition. Global Biogeochem Cy. 2008;22: Issue 2. [Google Scholar]
- 13.Pacyna EG, Pacyna JM, Sundseth K, Munthe J, Kindbom K, Wilson S, et al. Global emission of mercury to the atmosphere from anthropogenic sources in 2005 and projections to 2020. Atmos Environt. 2010;44: 2487–2499. [Google Scholar]
- 14.Callaghan B, Feldman D, Gruis K, Feldman E The association of exposure to lead, mercury, and selenium and the development of amyotrophic lateral sclerosis and the epigenetic implications. Neurodegener Dis. 2011;8: 1–8. 10.1159/000315405 [DOI] [PubMed] [Google Scholar]
- 15.Pearson K, Gordon J (2013) Spinal Reflexes In: Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ, editors. Principles of Neural Science. New York: McGraw Hill; pp. 790–811. [Google Scholar]
- 16.Oyanagi K, Ikuta F, Horikawa Y Evidence for sequential degeneration of the neurons in the intermediate zone of the spinal cord in amyotrophic lateral sclerosis: a topographic and quantitative investigation. Acta Neuropathol. 1989;77: 343–349. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez-Jarquin UN, Lazo-Gomez R, Tovar YRLB, Tapia R Spinal inhibitory circuits and their role in motor neuron degeneration. Neuropharmacology. 2014;82: 101–107. 10.1016/j.neuropharm.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 18.Aragones JM, Altimiras J, Roura-Poch P, Homs E, Bajo L, Povedano M, et al. Amyotrophic lateral sclerosis: a higher than expected incidence in people over 80 years of age. Amyotroph Lateral Scler Frontotemporal Degener. 2016: 1–6. [DOI] [PubMed] [Google Scholar]
- 19.Lacorte E, Ferrigno L, Leoncini E, Corbo M, Boccia S, Vanacore N Physical activity, and physical activity related to sports, leisure and occupational activity as risk factors for ALS: A systematic review. Neurosci Biobehav Rev. 2016;66: 61–79. 10.1016/j.neubiorev.2016.04.007 [DOI] [PubMed] [Google Scholar]
- 20.Gautier G, Verschueren A, Monnier A, Attarian S, Salort-Campana E, Pouget J ALS with respiratory onset: clinical features and effects of non-invasive ventilation on the prognosis. Amyotroph Lateral Scler. 2010;11: 379–382. 10.3109/17482960903426543 [DOI] [PubMed] [Google Scholar]
- 21.Harvey DG, Torack RM, Rosenbaum HE Amyotrophic lateral sclerosis with ophthalmoplegia. A clinicopathologic study. Arch Neurol. 1979;36: 615–617. [DOI] [PubMed] [Google Scholar]
- 22.Sparks DL The brainstem control of saccadic eye movements. Nat Rev Neurosci. 2002;3: 952–964. [DOI] [PubMed] [Google Scholar]
- 23.Ravits JM, La Spada AR ALS motor phenotype heterogeneity, focality, and spread: deconstructing motor neuron degeneration. Neurology. 2009;73: 805–811. 10.1212/WNL.0b013e3181b6bbbd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi H, Oyanagi K, Ohama E, Ikuta F Clarke's column in sporadic amyotrophic lateral sclerosis. Acta Neuropathol. 1992;84: 465–470. [DOI] [PubMed] [Google Scholar]
- 25.Brettschneider J, Arai K, Del Tredici K, Toledo JB, Robinson JL, Lee EB, et al. TDP-43 pathology and neuronal loss in amyotrophic lateral sclerosis spinal cord. Acta Neuropathol. 2014;128: 423–437. 10.1007/s00401-014-1299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pugdahl K, Fuglsang-Frederiksen A, de Carvalho M, Johnsen B, Fawcett PR, Labarre-Vila A, et al. Generalised sensory system abnormalities in amyotrophic lateral sclerosis: a European multicentre study. J Neurol Neurosurg Psychiatry. 2007;78: 746–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner MR, Swash M The expanding syndrome of amyotrophic lateral sclerosis: a clinical and molecular odyssey. J Neurol Neurosurg Psychiatry. 2015;86: 667–673. 10.1136/jnnp-2014-308946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson FO, Atchison WD The role of environmental mercury, lead and pesticide exposure in development of amyotrophic lateral sclerosis. Neurotoxicology. 2009;30: 761–765. 10.1016/j.neuro.2009.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kale SP, Moore L, Deininger PL, Roy-Engel AM Heavy metals stimulate human LINE-1 retrotransposition. Int J Environ Res Public Health. 2005;2: 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W, Lee MH, Henderson L, Tyagi R, Bachani M, Steiner J, et al. Human endogenous retrovirus-K contributes to motor neuron disease. Sci Transl Med. 2015;7: 307ra153 10.1126/scitranslmed.aac8201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terao S, Sobue G, Hashizume Y, Li M, Inagaki T, Mitsuma T Age-related changes in human spinal ventral horn cells with special reference to the loss of small neurons in the intermediate zone: a quantitative analysis. Acta Neuropathol. 1996;92: 109–114. [DOI] [PubMed] [Google Scholar]
- 32.Hortobagyi T, Devita P Mechanisms responsible for the age-associated increase in coactivation of antagonist muscles. Exerc Sport Sci Rev. 2006;34: 29–35. [DOI] [PubMed] [Google Scholar]
- 33.Narici MV, Maffulli N Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull. 2010;95: 139–159. 10.1093/bmb/ldq008 [DOI] [PubMed] [Google Scholar]
- 34.Vogt J, Paul F, Aktas O, Muller-Wielsch K, Dorr J, Dorr S, et al. Lower motor neuron loss in multiple sclerosis and experimental autoimmune encephalomyelitis. Ann Neurol. 2009;66: 310–322. 10.1002/ana.21719 [DOI] [PubMed] [Google Scholar]
- 35.Gilmore CP, DeLuca GC, Bo L, Owens T, Lowe J, Esiri MM, et al. Spinal cord neuronal pathology in multiple sclerosis. Brain Pathol. 2009;19: 642–649. 10.1111/j.1750-3639.2008.00228.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shefner JM, Mackin GA, Dawson DM Lower motor neuron dysfunction in patients with multiple sclerosis. Muscle Nerve. 1992;15: 1265–1270. [DOI] [PubMed] [Google Scholar]
- 37.Ismail A, Cooper-Knock J, Highley JR, Milano A, Kirby J, Goodall E, et al. Concurrence of multiple sclerosis and amyotrophic lateral sclerosis in patients with hexanucleotide repeat expansions of C9ORF72. J Neurol Neurosurg Psychiatry. 2013;84: 79–87. 10.1136/jnnp-2012-303326 [DOI] [PubMed] [Google Scholar]
- 38.Pamphlett R, Kum Jew S Uptake of inorganic mercury by human locus ceruleus and corticomotor neurons: implications for amyotrophic lateral sclerosis. Acta Neuropathol Commun. 2013;1: 13 10.1186/2051-5960-1-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fung SJ, Barnes CD Membrane excitability changes in hindlimb motoneurons induced by stimulation of the locus coeruleus in cats. Brain Res. 1987;402: 230–242. [DOI] [PubMed] [Google Scholar]
- 40.Morahan JM, Yu B, Trent RJ, Pamphlett R Genetic susceptibility to environmental toxicants in ALS. Am J Med Genet B Neuropsychiatr Genet. 2007;144: 885–890. [DOI] [PubMed] [Google Scholar]
- 41.Peters TL, Beard JD, Umbach DM, Allen K, Keller J, Mariosa D, et al. Blood levels of trace metals and amyotrophic lateral sclerosis. Neurotoxicology. 2016;54: 119–126. 10.1016/j.neuro.2016.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.