Abstract
A combination of the interrelated metabolic risk factors obesity, insulin resistance, dyslipidemia, and hypertension, often described as the “metabolic syndrome,” is known to increase the risk of developing cardiovascular disease and diabetes. Stearoyl-coenzyme A desaturase (SCD) activity has been implicated in the metabolic syndrome, but detailed studies of the beneficial metabolic effects of SCD deficiency have been limited. Here, we show that absence of the Scd1 gene product reduces plasma triglycerides and reduces weight gain in severely hyperlipidemic low density lipoprotein receptor (LDLR)-deficient mice challenged with a Western diet. Absence of SCD1 also increases insulin sensitivity, as measured by intraperitoneal glucose and insulin tolerance testing. SCD1 deficiency dramatically reduces hepatic lipid accumulation while causing more modest reductions in plasma apolipoproteins, suggesting that in conditions of sustained hyperlipidemia, SCD1 functions primarily to mediate lipid stores. In addition, absence of SCD1 partially ameliorates the undesirable hypertriglyceridemic effect of antiatherogenic liver X receptor agonists. Our results demonstrate that constitutive reduction of SCD activity improves the metabolic phenotype of LDLR-deficient mice on a Western diet.
Supplementary key words: monounsaturated fatty acids, very low density lipoprotein, high density lipoprotein, apolipoprotein B, mouse model, liver, atherosclerosis, ATP binding cassette transporter A1, hyperlipidemia, Western diet, low density lipoprotein receptor, coenzyme A
Susceptibility to cardiovascular disease and diabetes is associated with the metabolic abnormalities of obesity, insulin resistance, dyslipidemia, and hypertension (1–3). A key enzyme that has been implicated in these metabolic abnormalities is stearoyl-coenzyme A desaturase (SCD), which introduces a cis double bond in the Δ-9 position in its substrates, thereby converting palmitic acid (16:0) and stearic acid (18:0) to palmitoleic acid (16:1n7) and oleic acid (18:1n7), respectively (4, 5). These nutritionally and physiologically important MUFAs are the major fatty acids found in triglycerides (TGs) and cholesteryl esters (6).
A number of mammalian SCD genes have been reported, including a human SCD on 10q24 (7) and a cluster of four genes on mouse chromosome 19 [Scd1 (8), Scd2 (9), Scd3 (10), and Scd4 (11)]. These appear to have arisen from local duplications after the divergence of the primate and rodent genomes. An additional SCD gene, SCD5, which predates the separation of the primate and rodent lineages, was identified recently and is expressed in the brain and pancreas (12–14). The most prominent site of expression for human SCD is in adipose tissue (14), with lower expression in liver and brain (7). Like other lipogenic genes, mammalian SCD genes are highly regulated. Human SCD is repressed in cultured cells by polyunsaturated fatty acids and cholesterol via sterol-regulatory element binding protein-1 (SREBP-1) (15). Furthermore, SCD mRNA is increased in skeletal muscle of obese humans (16), and several but not all (17) observational studies in humans have shown an association between increased indices of SCD activity and components of the metabolic syndrome, including insulin resistance (18–21), obesity (16, 22–24), and hypertension (25). The expression of mouse Scd1 in adipose tissue and liver (8, 10) and its regulation by the lipogenic transcription factors SREBP-1 and liver X receptor (LXR) (26) define it as the most relevant murine ortholog for studying the metabolic function of SCD activity in humans.
Seven Scd1 mutant alleles have now been described in mice: four spontaneous mutations, BALB/c-Scd1ab (27), ABJ/Le-Scd1ab-J (28), DBA/1LacJ-Scd1ab-2J/J (29), and Kunming-Scd1Xyk (30); one chemically induced mutation, C57BL6/J-Scd1flk (31); and two targeted mutations, 129S-Scd1tm1Ntam (32) and B6129S1F2-Scd1tm1Wst (33). The asebia series of alleles (ab, ab-J, and ab-2J) has been studied most extensively. Homozygosity for each is associated with atrophic sebaceous glands, alopecia, and scaly skin, phenotypes that are also observed in mice carrying the targeted disruption of the gene (32).
SCD1-deficient mice have been observed to exhibit reduced plasma TG, increased insulin sensitivity, an increased metabolic rate, and resistance to diet-induced obesity (34, 35). However, the impact of SCD1 deficiency on hyperlipidemic mice fed a Western diet is unknown.
A recent study using homozygous 129S-Scd1tm1Ntam mice fed a chow diet has shown that SCD1 can influence the plasma lipid response to a synthetic LXR agonist (26), T0901317, which increases cholesterol efflux in hyperlipidemic mice (36, 37). However, LXR activation exacerbates hypertriglyceridemia (HTG) in hyperlipidemic mice (36, 37), and the role of SCD1 in regulating the severe LXR-induced HTG observed in hyperlipidemic mice has not yet been determined.
Familial hypercholesterolemia (FH; Online Mendelian Inheritance in Man accession 143890), characterized by markedly increased LDL-cholesterol levels and premature atherosclerosis, was the first genetic disease of lipid metabolism to be clinically and molecularly defined (38). The risk to FH patients of developing coronary disease is further increased by the presence of the metabolic syndrome (39) or its individual components, including low HDL-cholesterol (40–44), high TGs (40), and insulin resistance (45–47).
The low density lipoprotein receptor (LDLR)-deficient hyperlipidemic mouse mimics human FH and has now been used in numerous studies (48) as a model for the disrupted lipoprotein regulation and metabolic function that leads to diabetes and atherosclerosis. Unlike the most commonly used hyperlipidemic model, apolipoprotein E (apoE)-deficient mice (49), LDLR-deficient mice develop diet-induced diabetes and obesity when fed a Western diet and also develop a lipoprotein phenotype similar to that seen in FH (48, 50–53).
Earlier studies on the beneficial metabolic effects of SCD1 deficiency have been confined to normolipidemic mice (6, 26, 34, 54–58), and the influence on metabolic parameters in hyperlipidemic mice is unknown. Our results reveal that SCD1 deficiency reduces hepatic and plasma TG, inhibits diet-induced weight gain and insulin resistance, and reduces the hypertriglyceridemic effect of an LXR agonist in hyperlipidemic LDLR-deficient mice.
METHODS
Animals and diet
Mice carrying the Scd1ab-J (28) or Scd1ab-2J (29) null allele were backcrossed to C57BL/6 for five generations and then crossed to the B6.129S7-Ldlrtm1Her mutant strain (59). Mice carrying the Scd1ab-J allele were used in all experiments except those involving hepatocyte isolation, in which mice carrying the Scd1ab-2J allele were used. Animals received a standard laboratory rodent chow diet (LabDiet 5010 Autoclavable Rodent Diet; PMI Nutrition International, Richmond, IN) or a Western diet (TD.88137; Harlan Teklad, Madison, WI). For LXR agonist treatment, animals received T0901317 (60) (10 mg/kg body weight) daily by oral gavage for 3 days. The weight of the food contained in the food bin and any that had been spilled or buried in each cage (to the nearest 0.1 g) was recorded every 2–3 days for 8 consecutive days, and food intake for each mouse was averaged over the 8 days. All studies were approved by the University of British Columbia Animal Care Committee.
Adiposity measurements using MRI and relaxometry
For imaging, mice were anesthetized with isoflurane and imaged in a Bruker Biospec 70/30 7 Tesla MRI Scanner (Bruker Biospin, Ettlingen, Germany) with and without fat suppression. Images were acquired in the abdominal region in 1.5 mm transverse slices with a MSME T1-weighted pulse sequence, acquiring a field of view of 4 cm and a matrix size of 128 × 128. The echo time was 12 ms, and the repetition time was 300 ms. MR signals from the bodies of nonanesthetized mice were acquired with a quadrature volume RF coil tuned to 300 MHz. Absolute fat and lean mass were calculated from the NMR data as described by Kunnecke et al. (61).
Fat pad measurements and histology
Periuterine and periepididymal white adipose depots were dissected and weighed. For routine histology, similar areas from liver tissue from mice after 16 h of starvation were formalin-fixed, embedded, sectioned, and stained with Oil Red O (counter-stained with hematoxylin).
Lipid and lipoprotein analysis
Fast-protein liquid chromatography (FPLC) was performed to separate the three major lipoprotein classes, VLDL, LDL, and HDL, in pooled plasma. For hepatic lipid analysis, liver tissue was homogenized in PBS and total lipids were extracted using Folch solution (chloroform-methanol, 2:1), dried under N2, and resuspended in 2% Triton X-100. Unfractionated plasma, FPLC fractions, and tissue lipid extracts were assayed for cholesterol and TG concentrations by enzymatic assays with the use of commercially available reagents. Plasma HDL-cholesterol levels were determined after precipitation of apoB-containing lipoproteins with phosphotungstic acid/Mg (Wako Diagnostics, Richmond, VA). Lipoproteins in the density < 1.21 g/ml fraction obtained by preparative ultracentrifugation were analyzed by SDS-PAGE on gradient gels (4–16%) for the determination of apoB and apoE as described (62). Briefly, 10 μg of protein was added to each lane of the gel. Gels were stained with Coomassie blue, and bands corresponding to apoB and apoE were quantified by scanning using a densitometer. Unfractionated plasma levels of apoC-III were determined by immunonephelometry with the use of mouse-specific antibodies developed in rabbits. The distribution of lipids in plasma lipoproteins was assessed as described (63).
Hepatocyte isolation and radiolabeling with [3H]acetate
Primary hepatocytes were isolated as described (64). Briefly, mice fed a Western diet for 4 weeks were anesthetized by intra-peritoneal injection of Somnotol (22 μl/50 g body weight) and the livers were perfused with Hanks’ EGTA solution containing 1 mg/ml insulin followed by Hanks’ collagenase solution (100 U/ml) containing 1 mg/ml insulin. The hepatocytes were dispersed in Hanks’ collagenase solution and washed three times in DMEM, then suspended in medium containing 10% fetal bovine serum and plated on 60 mm collagen-coated dishes (1 × 106 cells/ml). Hepatocytes were incubated for up to 3 h with DMEM containing 25 μCi/ml [3H]acetate and then washed twice with DMEM. Lipids were extracted with chloroform-methanol (2:1, v/v) and then saponified by heating to 80°C in methanolic KOH. Nonsaponifiable lipids were removed by extraction with diethyl ether. The aqueous phase containing released fatty acids was acidified and the fatty acids were extracted with hexane. Incorporation of [3H]acetate into fatty acids was determined as 3H-labeled fatty acids per milligram of total cell protein.
Physiological analysis
Intraperitoneal glucose tolerance tests were performed on 12 h fasted mice injected with 1.5 g/kg glucose. Blood samples were taken at 0, 15, 30, 60, and 90 min, and blood glucose was measured with a glucometer (Lifescan, Milpitas, CA). Insulin tolerance tests were performed on 12 h fasted mice injected with 0.75 U/kg human recombinant insulin (Novo Nordisk, Princeton, NJ). We measured plasma insulin by ELISA (Crystal Chem, Downers Grove, IL).
Real-time PCR and immunoblotting
We extracted total RNA from liver using the TRIzol reagent according to the manufacturer’s instructions (Invitrogen Canada, Burlington, Ontario, Canada). One microgram of DNase-treated RNA was reverse-transcribed using SuperScript II (Invitrogen Canada) to generate RNase H-treated cDNA for real-time PCR using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) in an ABI Prism 7700 Sequence Detection System. We used Gapdh as the invariant control. mRNA levels in control mice were arbitrarily set at 1.
Western blotting was performed as described previously (65). Briefly, tissues were homogenized in low-salt lysis buffer containing complete protease inhibitor (Roche Diagnostics, Laval, Quebec, Canada), and protein concentration was determined by the assay of Lowry et al. (66). Equivalent amounts of total protein were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed with anti-ABCA1 (65) or anti-GAPDH antibody.
Statistical analysis
Data are presented as means ± SEM. Initial analyses were performed by the unpaired two-tailed Student’s t-test. If the data did not fit the constraints of this parametric test, data were analyzed with the Mann-Whitney test. Data from body weight, [3H]acetate incorporation, and tolerance tests were analyzed by two-way ANOVA (time, within subjects; genotype, between subjects) using repeated measures for body weight and tolerance tests, all followed by Bonferroni posttests. Areas under the glucose curves were calculated by the trapezoid rule. Statistical analysis was performed with GraphPad Prism software (GraphPad, San Diego, CA) and with the open-source R package (67). P < 0.05 was considered significant.
RESULTS
SCD1 deficiency reduces weight gain and adiposity in Ldlr−/− mice
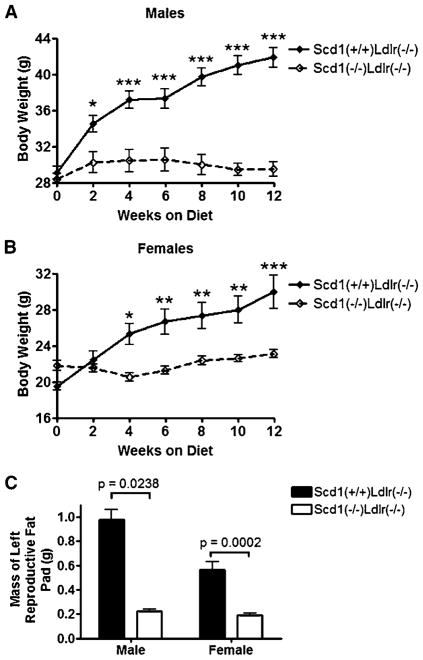
An existing mouse strain with a spontaneous deletion in Scd1 (B6.Cg-Scd1ab-J) and an existing dyslipidemic mouse model (B6.129S7-Ldlrtm1Her) (59) were crossed to generate mice with deficiencies of both LDLR (Ldlr−/−) and SCD1 (Scd1−/−). Mice at the age of 11–13 weeks were fed an atherogenic Western diet (68) for 12 weeks. Male Scd1−/− Ldlr−/− mice had similar weight to the control Scd1+/+ Ldlr−/− mice at the beginning of the diet study (28.4 vs. 29.1 g; P = 0.51), but they gained less weight after feeding a Western diet (Fig. 1A), despite tending to consume more food than controls (1.5 vs. 1.1 g/day; P = 0.11, n = 4). Female Scd1−/−Ldlr−/− mice also gained less weight than controls after feeding a Western diet (Fig. 1B). After 12 weeks on a Western diet, weights for male and female Scd1+/+ Ldlr−/− mice were 44% and 54% higher than initial values, respectively, whereas neither male nor female Scd1−/− Ldlr−/− mice showed a significant increase in body weight. Both male and female Scd1−/−Ldlr−/− mice had smaller periepididymal or periuterine fat pads than control Ldlr−/− mice (Fig. 1C).
Fig. 1.
Total body and fat pad weights of low density lipoprotein receptor-deficient (Ldlr−/−) mice lacking stearoyl-coenzyme A desaturase (SCD1). A, B: Body weight was measured in male (A) and female (B) Scd1+/+Ldlr−/− and Scd1−/−Ldlr−/− mice fed a Western diet starting at 12 weeks of age (males, P < 0.0001; females, P = 0.01; repeated-measures ANOVA). n = 6–12 mice per group. C: Fat pad weights are shown. Data shown are means ± SEM. * P < 0.05, ** P < 0.01, *** P < 0.001.
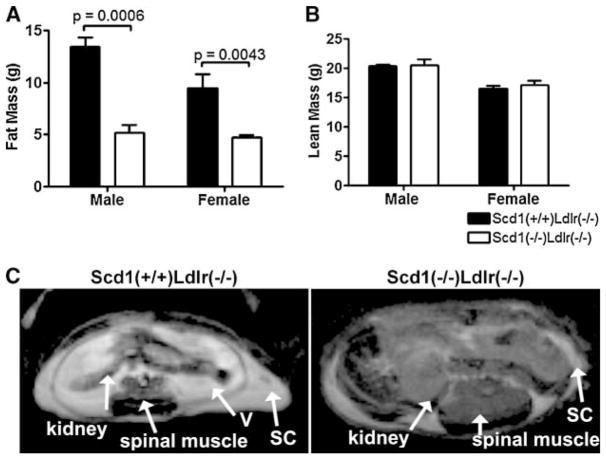
To evaluate weight gain in terms of adiposity, fat mass and lean mass were determined using MR relaxometry, a recently validated noninvasive method for the precise assessment of body composition (61) (Fig. 2A–C). Lean body mass was not different between SCD1-deficient mice and controls (Fig. 2B). However, SCD1-deficient mice had a significant 50% reduction in total fat mass compared with controls (males, P = 0.0006; females, P = 0.0043). Representative images in Fig. 2C show a decrease in both visceral and subcutaneous lipid in SCD1-deficient mice.
Fig. 2.
Adiposity in Ldlr−/− mice lacking SCD1. A, B: Total body fat mass (A) and total body lean mass (B) from mice fed a Western diet. n = 3–10 mice per group. Data shown are means ± SEM. C: Transverse abdominal cross-sections of an SCD1-deficient mouse and a control mouse obtained by magnetic resonance imaging. Slices (1.5 mm thick) at the kidneys were identified in sagittal images from each mouse. Fatty tissues are show as bright areas. Spinal muscle, kidneys, and subcutaneous (SC) and visceral (V) fat are indicated.
SCD1 deficiency reduces hepatic steatosis in Ldlr−/− mice
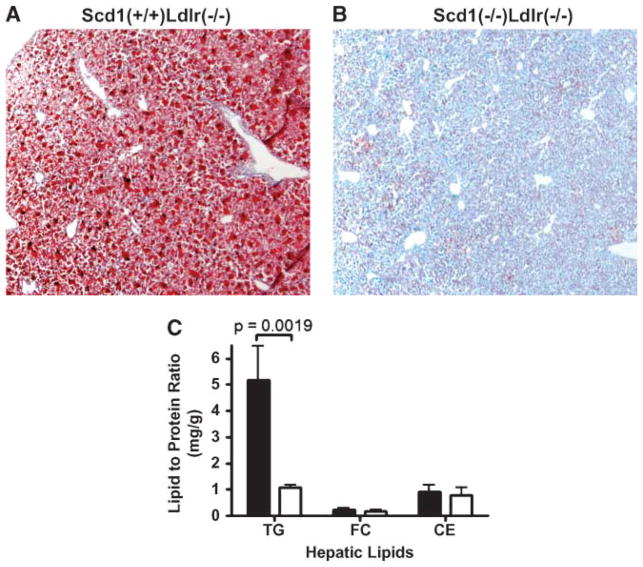
Nonadipose tissue also exhibited a marked decrease in lipid accumulation. Histological examination of the livers revealed protection from hepatic steatosis (Fig. 3A, B), and hepatic TG levels were 5-fold higher in control Scd1+/+ Ldlr−/− mice than in Scd1−/−Ldlr−/− mice (Fig. 3C).
Fig. 3.
Hepatic lipids in Ldlr−/− mice lacking SCD1. A, B: Livers of female Scd1+/+Ldlr−/− (A) and Scd1−/−Ldlr−/− (B) mice fed a Western diet stained with Oil Red O. C: Liver triglyceride (TG), free cholesterol (FC), and cholesteryl ester (CE) contents of Scd1+/+Ldlr−/− and Scd1−/−Ldlr−/− mice fed a Western diet. Data shown are means ± SEM. n = 8 mice per group.
SCD1 deficiency reduces plasma lipids and improves lipoprotein profiles in Ldlr−/− mice
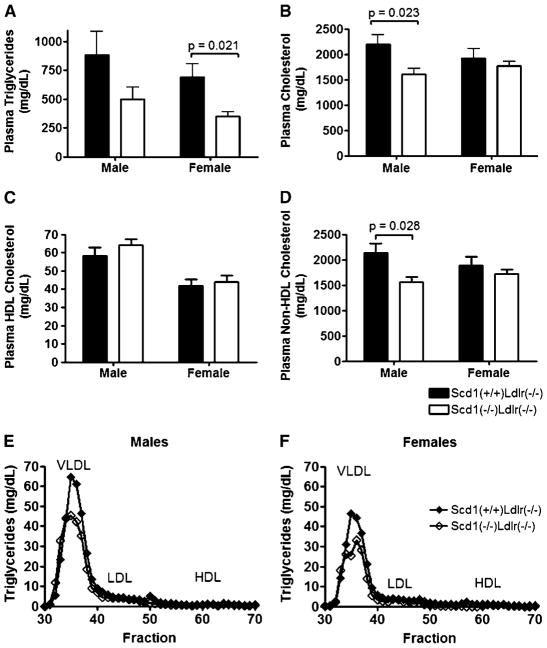
To determine whether the reduced levels of tissue lipids in hyperlipidemic SCD1-deficient mice are reflected in plasma lipoprotein levels, fasting plasma lipid concentrations and lipoprotein profiles for Scd1−/−Ldlr−/− mice and control Scd1+/+Ldlr−/− mice were evaluated (Fig. 4, Table 1). Total plasma TG was significantly reduced by ~51% in female SCD1-deficient mice (P = 0.021) (Fig. 4A). Plasma TG also tended to be reduced in male SCD1-deficient mice, but this difference was not statistically significant (P= 0.23). Plasma total cholesterol (TC) was significantly reduced by ~26% in SCD1-deficient male mice (P = 0.023) (Fig. 4B), but this trend was not observed in females. HDL-cholesterol was not significantly altered in SCD1-deficient mice, whereas non-HDL-cholesterol paralleled the reduction observed in plasma TC (Fig. 4C, D). FPLC analysis confirmed the decrease in VLDL-TG in SCD1-deficient mice, with a small increase noted in VLDL-cholesterol (Fig. 4E, F).
Fig. 4.
Plasma lipids and lipoprotein profiles in Ldlr−/− mice lacking SCD1. A–D: Plasma TG (A), TC (B), HDL-cholesterol (C), and non-HDL-cholesterol (D) contents of Scd1+/+Ldlr−/− and Scd1−/−Ldlr−/− mice fed a Western diet. n = 8–12 mice per group. Data shown are means ± SEM. E, F: Fast-protein liquid chromatography lipoprotein profiles of pooled plasma samples from Scd1+/+Ldlr−/− and Scd1−/−Ldlr−/− mice fed a Western diet. TG levels were determined for each fraction from male (E) and female (F) mice. The lipoprotein peaks for VLDL, LDL, and HDL are indicated.
TABLE 1.
Plasma lipid and apolipoprotein levels in LDL receptor-deficient mice lacking stearoyl-coenzyme A desaturase 1
| Lipid | Males
|
Females
|
||||
|---|---|---|---|---|---|---|
| Scd1+/+Ldlr−/− | Scd1−/−Ldlr−/− | P | Scd1+/+Ldlr−/− | Scd1−/−Ldlr−/− | P | |
| Triglyceride, mg/dl | 885 ± 673 (11) | 497 ± 294 (8) | 0.23 | 689 ± 414 (12) | 350 ± 114 (9) | 0.021 |
| Cholesterol, mg/dl | 2,192 ± 635 (11) | 1,614 ± 307 (8) | 0.023 | 1,927 ± 611 (12) | 1,769 ± 269 (9) | 0.86 |
| HDL-cholesterol, mg/dl | 58.5 ± 14.5 (11) | 64.4 ± 8.3 (7) | 0.34 | 41.7 ± 12.2 (12) | 44.0 ± 10.9 (9) | 0.66 |
| Non-HDL-cholesterol, mg/dl | 2,133 ± 627 (11) | 1,555 ± 306 (8) | 0.028 | 1,885 ± 612 (12) | 1,725 ± 268 (9) | 0.86 |
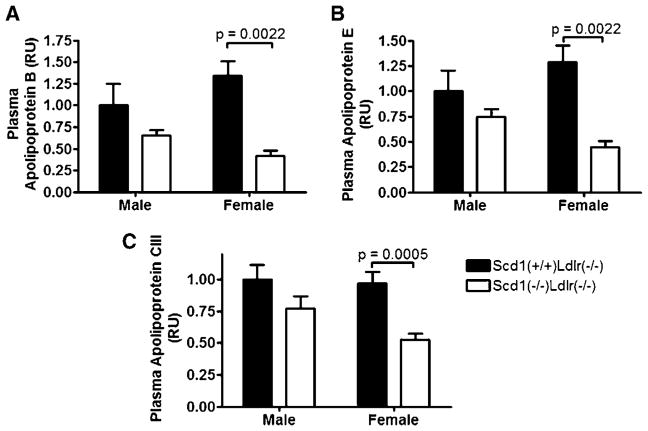
| ApoB | 1.00 ± 0.60 (6) | 0.66 ± 0.15 (6) | 0.18 | 1.35 ± 0.42 (6) | 0.41 ± 0.15 (6) | 0.0022 |
| ApoE | 1.00 ± 0.50 (6) | 0.75 ± 0.18 (6) | 0.24 | 1.29 ± 0.41 (6) | 0.45 ± 0.14 (6) | 0.0022 |
| ApoC-III | 1.00 ± 0.37 (11) | 0.77 ± 0.26 (7) | 0.17 | 0.97 ± 0.31 (12) | 0.53 ± 0.15 (10) | 0.0005 |
ApoB, apolipoprotein B. Apolipoprotein levels are expressed in relative units compared with the levels of male Scd1+/+Ldlr−/− mice. Data represent means ± SD. The number of animals in each subgroup is indicated in parentheses.
SCD1 deficiency reduces plasma apolipoproteins in Ldlr−/− mice
Reductions of plasma apoB (P = 0.0022), apoE (P = 0.0022), and apoC-III (P = 0.0005) in females (Fig. 5A–C), consistent with a reduction in VLDL-TG, were evident. Similar trends were observed in males, but these reductions in plasma apolipoproteins were not significant (Table 1).
Fig. 5.
Plasma apolipoproteins in Ldlr−/− mice lacking SCD1. Apolipoprotein B (apoB; A), apoE (B), and apoC-III (C) were measured in male and female Scd1+/+Ldlr−/− and Scd1−/−Ldlr−/− mice fed a Western diet. Apolipoprotein levels are expressed in relative units (RU) compared with the levels of male Scd1+/+ Ldlr−/− mice. Data shown are means ± SEM. n = 6–12 mice per group.
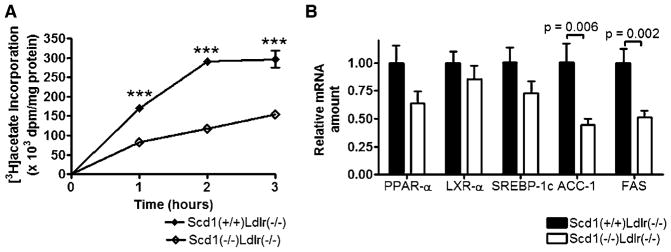
SCD1 deficiency reduces fatty acid synthesis in Ldlr−/− mice
Decreases in hepatic and plasma lipid levels could result from decreased lipogenesis. Therefore, to explore the mechanism by which SCD1 deficiency decreases hepatic and plasma lipids in hyperlipidemic SCD1-deficient mice, we evaluated the incorporation of [3H]acetate into saponifiable lipids in primary hepatocytes isolated from Scd1−/−Ldlr−/− mice and control Scd1+/+Ldlr−/− mice (Fig. 6A). The incorporation of [3H]acetate into fatty acids in the saponifiable lipid fraction over time was reduced in Scd1−/−Ldlr−/− hepatocytes relative to control Scd1+/+Ldlr−/− hepatocytes (P < 0.0001), and incorporation was significantly reduced by ~48–60% at all time points (P < 0.001).
Fig. 6.
Fatty acid synthesis and hepatic gene expression in Ldlr−/− mice lacking SCD1. A: Assessment of fatty acid synthesis in hepatocytes from Scd1+/+Ldlr−/− and Scd1−/−Ldlr−/− mice fed a Western diet. Primary hepatocytes were prepared from one mouse of each genotype and incubated for up to 3 h with [3H]acetate, after which lipids were saponified and 3H-labeled fatty acid content was measured (P < 0.0001; two-way ANOVA). Each value is the average of four independent dishes of hepatocytes, and error bars are included unless obscured by symbols. Data shown are means ± SEM. *** P < 0.001. B: Relative amount of various mRNAs in livers of Scd1+/+Ldlr−/− and Scd1−/−Ldlr−/− mice fed a Western diet. Each value represents the amount of mRNA relative to that in Scd1+/+Ldlr−/− mice (arbitrarily set at 1 for each transcript in these mice). ACC-1, acetyl-coenzyme A carboxylase-1; LXR-α, liver X receptor-α; PPAR-α, peroxisome proliferator-activated receptor α; SREBP-1c, sterol-regulatory element binding protein-1c. Data shown are means ± SEM. n = 6–17 mice per group.
Acetyl-coenzyme A carboxylase (ACC) and FAS are the two enzymes required for fatty acid synthesis (69, 70). Thus, we hypothesized that the reduced fatty acid synthesis in hepatocytes may result from reduced levels of transcripts encoding these enzymes as well as from reduced levels of the transcription factor SREBP-1c (71), an important regulator of these transcripts. We examined the hepatic expression of various lipid-sensitive mRNAs and observed significant reductions in the genes that encode ACC-1 (P = 0.0059) and FAS (P = 0.0020) in Scd1−/− Ldlr−/− mice relative to Scd1+/+Ldlr−/− mice (Fig. 6B). However, SCD1 deficiency did not significantly alter the hepatic mRNA levels of genes that encode the transcription factors peroxisome proliferator-activated receptor α, LXRα, and SREBP-1c in LDLR-deficient mice challenged with a Western diet, although trends toward reduction were seen, particularly for peroxisome proliferator-activated receptor α (P = 0.060) and SREBP-1c (P = 0.082).
SCD1 deficiency reduces insulin resistance in Ldlr−/− mice
The association between obesity, HTG, and diabetes is well documented (72, 73). To further investigate the effects of SCD1 on these parameters, the response to glucose challenge was assessed.
Male gender is a predisposing diabetes susceptibility factor in most mouse strains (74), so we were not surprised to observe a clear sexual dimorphism in diabetes susceptibility of Scd1+/+Ldlr−/− mice. At 7 and 11 weeks on a Western diet, male Scd1−/−Ldlr−/− mice were protected from the impaired glucose tolerance evident in male Scd1+/+Ldlr−/− mice (Fig. 7A, B). Female LDLR-deficient mice were more successful than males at controlling their blood glucose when fed a Western diet, but by 11 weeks female Scd1−/−Ldlr−/− mice showed improved glucose tolerance relative to female Scd1+/+Ldlr−/− mice (P = 0.03) (Fig. 7C).
Fig. 7.
Glucose tolerance and insulin resistance in Ldlr−/− mice lacking SCD1. A–C: Intraperitoneal glucose tolerance tests (1.5 g/kg) were performed on mice fasted overnight after being fed a Western diet for 7 weeks (A, males; P < 0.0001; repeated-measures ANOVA) or 11 weeks (B, males, P = 0.014; C, females, P = 0.03; repeated-measures ANOVA). Insets show areas under the glucose curves (AUCglucose; mg/dl/90 min). D: Intraperitoneal insulin tolerance tests (0.75 U/ml) were performed on male mice fasted overnight after being fed a Western diet for 9 weeks (P = 0.0085; repeated-measures ANOVA). Scd1+/+Ldlr−/− mice are indicated with solid lines, and Scd1−/−Ldlr−/− mice are indicated with dashed lines. * P < 0.05, ** P < 0.01, *** P < 0.001. E, F: Glucose was measured in blood (E) and insulin was measured in plasma (F) obtained from the saphenous vein of mice fasted overnight after being fed a Western diet for 9 weeks and 11 weeks, respectively. n = 3–10 mice per group. Data shown are means ± SEM.
Insulin sensitivity assays were performed on male mice fed a Western diet for 9 weeks and fasted overnight before intraperitoneal insulin injection (0.75 U/kg), and glucose was monitored over 90 min. Consistent with their lean phenotype, male Scd1−/−Ldlr−/− mice showed both an improved response at 15 min after insulin injection and reduced blood glucose (Fig. 7D). Measurement of fasting glucose levels indicated that male Scd1−/−Ldlr−/− mice were protected from the hyperglycemia that had developed in Scd1+/+Ldlr−/− controls by 9 weeks on a Western diet (Fig. 7E). Male Scd1−/−Ldlr−/− mice were also protected from the markedly increased fasting plasma insulin that had developed in Scd1+/+Ldlr−/− controls by 11 weeks on a Western diet (Fig. 7F). These data indicate that the improved glucose tolerance that was observed in mice lacking SCD1 is attributable in part to increased insulin sensitivity.
SCD1 mediates the plasma lipid response to LXR agonist treatment in Ldlr−/− mice
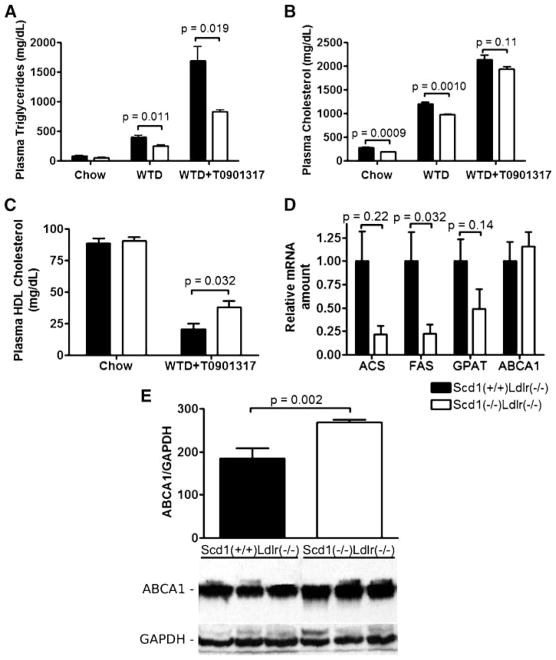
Treatment of mice with a synthetic LXR agonist, T0901317, has been shown to be antiatherogenic in hyper-lipidemic LDLR-deficient mice (36, 37), but its therapeutic utility has been limited by the accompanying severe HTG (36, 37) and hepatic steatosis (60, 75). Recent studies have shown that the lipogenic effect of LXR agonists is mediated through SCD1 (26), but the role of SCD1 in regulating the severe LXR-induced HTG observed in hyperlipidemic LDLR-deficient mice has not yet been determined. To determine whether SCD1 deficiency moderates the undesirable effects of LXR activation in hyperlipidemia, we fed female Scd1−/−Ldlr−/− mice and Scd1+/+Ldlr−/− controls a Western diet for 12 days and treated them with 10 mg/kg T0901317 by oral gavage daily for the final 3 days.
T0901317 treatment resulted in a 4.3-fold increase in plasma TG in LDLR-deficient mice (Fig. 8A). However, plasma TG was reduced by 48% in T0901317-treated Scd1−/−Ldlr−/− mice relative to Scd1+/+Ldlr−/− controls, a similar relative reduction to that induced by SCD1 deficiency in hyperlipidemic mice before treatment with an LXR agonist. Plasma TC was ~10–30% lower at all time points in SCD1-deficient mice compared with controls, primarily as a result of a reduction in non-HDL-cholesterol (Fig. 8B).
Fig. 8.
Plasma lipid response to LXR agonist treatment in Ldlr−/− mice lacking SCD1. A, B: Plasma TG (A) and TC (B) contents of Scd1+/+Ldlr−/− and Scd1−/−Ldlr−/− mice 10–12 months of age at baseline and after being fed a Western diet (WTD) for 9 days and a Western diet plus 10 mg/kg T0901317 for 3 additional days. C: Plasma HDL-cholesterol at baseline and after T0901317 feeding. n = 7–8 mice per group. D: Relative amount of mRNA encoding acetyl-CoA synthetase (ACS), FAS, glycerol-3-phosphate acyltransferase (GPAT), and ABCA1 in liver after T0901317 feeding. Each value represents the amount of mRNA relative to that in Scd1+/+Ldlr−/− mice (arbitrarily set at 1 for each transcript in these mice). n = 4–5 mice per group. E: Quantification and representative immunoblot of liver lysates from Scd1+/+Ldlr−/− and Scd1−/−Ldlr−/− mice with antibodies against ABCA1, with GAPDH as a loading control. n = 5–8 mice per group. Data shown are means ± SEM.
Interestingly, plasma HDL-cholesterol was increased by 73% in T0901317-treated Scd1−/−Ldlr−/− mice relative to Scd1+/+Ldlr−/− controls (Fig. 8C). To explore the molecular mechanism by which SCD1 influences plasma lipids in T0901317-treated hyperlipidemic mice, we assessed hepatic expression levels of various genes (Fig. 8D). We observed a 77% reduction in the level of FAS mRNA in SCD1-deficient mice (P = 0.0020). A reduction of FAS indicates that endogenous fatty acids are likely produced at a reduced rate and are less available for the generation of TGs for secretion into the plasma. Trends toward decreases in transcripts of other lipogenic genes [acetyl-CoA synthetase, 78% reduction (P = 0.22); glycerol-3-phosphate acyltransferase, 51% reduction (P = 0.14)] with SCD1 deficiency were also detected in mice fed the LXR agonist.
Hepatic ABCA1 protein expression was increased by 45% in T0901317-treated Scd1−/−Ldlr−/− mice relative to Scd1+/+Ldlr−/− controls (Fig. 8E) (P = 0.002), which might at least partly explain the increased HDL levels (76, 77). However, we did not observe a significant alteration in the level of Abca1 mRNA (Fig. 8D), suggesting that the increased ABCA1 protein level is not attributable to increased synthesis of ABCA1 but rather to alterations in posttranscriptional regulation.
DISCUSSION
We have shown that an absence of SCD1 improves the metabolic phenotype of a mouse model of FH on a Western diet. Absence of the Scd1 gene product reduces hepatic and plasma TG and strongly inhibits diet-induced weight gain in LDLR-deficient mice. Absence of SCD1 also provides striking protection from diet-induced insulin resistance, as measured by intraperitoneal glucose and insulin tolerance testing. Finally, we have demonstrated that absence of SCD1 partially reduces the undesirable hyper-triglyceridemic effect of antiatherogenic LXR agonists in hyperlipidemic mice.
Normolipidemic SCD1-deficient mice are known to be protected from insulin resistance and diet-induced obesity (34). The role of SCD1 in resistance to obesity has also been expanded to include the leptin-deficient model of obesity (35). We have now extended these findings and demonstrate that the absence of SCD1 provides significant protection from diet-induced obesity in the hyper-lipidemic LDLR-deficient model.
Liver TGs are reduced by 40–65% in SCD1-deficient mice (6, 54), and TG synthesis is also reduced (35, 54, 78). The most profound impact of absence of SCD1 on the metabolic features of LDLR-deficient mice in this study was a 5-fold reduction in hepatic steatosis, a greater relative reduction than the reductions of ~65% observed previously in chow-fed SCD1-deficient genetic models of obesity (35) and lipodystrophy (58).
Fatty liver is frequently observed in individuals with obesity, type 2 diabetes, and hyperlipidemia. Moreover, the degree of steatosis in nonalcoholic fatty liver disease is proportional to the degree of obesity (79), and insulin resistance is almost universally observed in nonalcoholic fatty liver disease (80, 81). Short term high-fat feeding in rodents in the absence of increases in peripheral fat accumulation previously demonstrated a causal role for intracellular hepatic fat accumulation in the pathogenesis of hepatic insulin resistance (82). Furthermore, hepatic fat accumulation is often accompanied by a chronic, subacute state of inflammation, which can increase insulin resistance (83, 84). Thus, the dramatic reduction in hepatic TGs that we observed in the absence of SCD1 may contribute in part to increasing insulin sensitivity. However, increased levels of saturated fatty acids (85, 86) and overexpression of SCD1 (87) decrease insulin signaling in muscle cells, suggesting that absence of SCD1 may also contribute to increased insulin sensitivity in skeletal muscle in addition to the liver.
The role of SCD1 in regulating plasma TG has been evaluated in several studies, and a reduction in plasma TG has not been consistently observed. Some studies have shown plasma TG reduced by >50% (6, 55, 88), but two studies have shown no significant differences (56, 57). It is not known whether the phenotypic differences could be attributed to the variations in age, sex, diet, fasting protocol, or genetic background of mice in the different studies. SCD1-deficient mice have a markedly reduced rate of VLDL-TG production (35), and the effect on plasma TG levels may be more apparent in hyperlipidemic mice. Our results show that absence of SCD1 reduces plasma lipids (TC and TG) and improves lipoprotein profiles in LDLR-deficient mice.
A potential mechanism for the reduction of hepatic and plasma lipids in SCD1-deficient mice is reduced lipogenesis. We found that SCD1 deficiency markedly reduces fatty acid synthesis in hepatocytes and reduces hepatic mRNA levels of the two SREBP-1c-regulated genes that encode enzymes required for long-chain fatty acid synthesis, ACC-1 and FAS. These data are consistent with previous studies that demonstrated reduced ACC-1 and FAS hepatic expression levels (34, 58, 89, 90) and reduced ACC activity (90) in SCD1-deficient chow-fed mice and indicate that a reduction in hepatic de novo fatty acid synthesis is likely to be a major contributor to the decreased hepatic and plasma lipids we observed in hyperlipidemic SCD1-deficient mice.
T0901317 is a synthetic LXR agonist that has been shown to be atheroprotective in hyperlipidemic mice (36, 37), an effect that is postulated to be mediated by stimulating cholesterol efflux in macrophages (36, 37, 75). However, LXR activation has been observed to lead to undesirable side effects, specifically HTG (36, 37) and hepatic steatosis. Our data demonstrate that absence of SCD1 significantly influences the plasma lipid response to antiatherogenic LXR agonist treatment, reducing non-HDL-cholesterol and increasing beneficial HDL-cholesterol. In addition, SCD1 deficiency is also able to partially improve LXR-induced HTG in the hyperlipidemic LDLR-deficient model. These data are consistent with data in a recent study of chow-fed mice showing that SCD1 can regulate the metabolic response to a synthetic LXR agonist (26).
Increased levels of SCD1 and its MUFA products inhibit cholesterol efflux mediated by ABCA1 (91–93) by a mechanism that may involve increased turnover rather than altered transcript levels (94). In addition, SCD1 deficiency can increase hepatic ABCA1 in SCD1-deficient mice fed a very-low-fat diet (95). Our data now provide evidence that the increased plasma HDL-cholesterol observed in hyperlipidemic LXR-treated mice is accompanied by increased levels of the ABCA1 protein but no significant alteration in the level of Abca1 mRNA, consistent with a mechanism involving alterations in posttranscriptional regulation.
In summary, our results establish the robust impact of SCD1 deficiency on the metabolic phenotype of the hyperlipidemic LDLR-deficient mouse model, including reduced hepatic and plasma TG, reduced diet-induced weight gain and insulin resistance, and a partially reduced hypertriglyceridemic response to an LXR agonist.
Acknowledgments
The authors thank Andrew C. Yung and Piotr Kozlowski at the University of British Columbia High Field MRI Centre for performing MRI analysis; Esra Asilmaz and Jeffrey M. Friedman at The Rockefeller University for generously providing incipient congenic mice carrying the Scd1ab-J allele; and Laura Hargreaves, Julie Chow, Brian W. Wong, and Emmanuelle Vallez for their expert technical assistance with hepatocyte isolation, histology, imaging, and lipoprotein analysis, respectively. The authors also thank Michael D. Winther at Xenon Pharmaceuticals for insightful comments and discussion. Special thanks to the staff of the Wesbrook Animal Unit for their expert care of mice. M.L.E.M. was the recipient of a Canadian Institutes of Health Research Canada Graduate Scholarship. M.R.H. holds the Canada Research Chair in Human Genetics.
Abbreviations
- ACC-1
acetyl-coenzyme A carboxylase-1
- apoE
apolipoprotein E
- FH
familial hypercholesterolemia
- FPLC
fast-protein liquid chromatography
- HTG
hypertriglyceridemia
- LDLR
low density lipoprotein receptor
- LXR
liver X receptor
- SCD
stearoyl-coenzyme A desaturase
- SREBP-1
sterol-regulatory element binding protein-1
- TC
total cholesterol
- TG
triglyceride
References
- 1.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. I. Diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 2.Isomaa B. A major health hazard: the metabolic syndrome. Life Sci. 2003;73:2395–2411. doi: 10.1016/s0024-3205(03)00646-5. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM. Obesity, metabolic syndrome, and coronary atherosclerosis. Circulation. 2002;105:2696–2698. doi: 10.1161/01.cir.0000020650.86137.84. [DOI] [PubMed] [Google Scholar]
- 4.Miyazaki M, Ntambi JM. Role of stearoyl-coenzyme A desaturase in lipid metabolism. Prostaglandins Leukot Essent Fatty Acids. 2003;68:113–121. doi: 10.1016/s0952-3278(02)00261-2. [DOI] [PubMed] [Google Scholar]
- 5.Ntambi JM. Regulation of stearoyl-CoA desaturase by poly-unsaturated fatty acids and cholesterol. J Lipid Res. 1999;40:1549–1558. [PubMed] [Google Scholar]
- 6.Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J Biol Chem. 2000;275:30132–30138. doi: 10.1074/jbc.M005488200. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Ge L, Parimoo S, Stenn K, Prouty SM. Human stearoyl-CoA desaturase: alternative transcripts generated from a single gene by usage of tandem polyadenylation sites. Biochem J. 1999;340:255–264. [PMC free article] [PubMed] [Google Scholar]
- 8.Ntambi JM, Buhrow SA, Kaestner KH, Christy RJ, Sibley E, Kelly TJJ, Lane MD. Differentiation-induced gene expression in 3T3-L1 preadipocytes. Characterization of a differentially expressed gene encoding stearoyl-CoA desaturase. J Biol Chem. 1988;263:17291–17300. [PubMed] [Google Scholar]
- 9.Kaestner KH, Ntambi JM, Kelly TJJ, Lane MD. Differentiation-induced gene expression in 3T3-L1 preadipocytes. A second differentially expressed gene encoding stearoyl-CoA desaturase. J Biol Chem. 1989;264:14755–14761. [PubMed] [Google Scholar]
- 10.Zheng Y, Prouty SM, Harmon A, Sundberg JP, Stenn KS, Parimoo S. Scd3—a novel gene of the stearoyl-CoA desaturase family with restricted expression in skin. Genomics. 2001;71:182–191. doi: 10.1006/geno.2000.6429. [DOI] [PubMed] [Google Scholar]
- 11.Miyazaki M, Jacobson MJ, Man WC, Cohen P, Asilmaz E, Friedman JM, Ntambi JM. Identification and characterization of murine SCD4, a novel heart-specific stearoyl-CoA desaturase isoform regulated by leptin and dietary factors. J Biol Chem. 2003;278:33904–33911. doi: 10.1074/jbc.M304724200. [DOI] [PubMed] [Google Scholar]
- 12.Beiraghi S, Zhou M, Talmadge CB, Went-Sumegi N, Davis JR, Huang D, Saal H, Seemayer TA, Sumegi J. Identification and characterization of a novel gene disrupted by a pericentric inversion inv(4)(p13.1q21.1) in a family with cleft lip. Gene. 2003;309:11–21. doi: 10.1016/s0378-1119(03)00461-x. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S, Yang Y, Shi Y. Characterization of human SCD2, an oligomeric desaturase with improved stability and enzyme activity by cross-linking in intact cells. Biochem J. 2005;388:135–142. doi: 10.1042/BJ20041554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Yu L, Schmidt RE, Su C, Huang X, Gould K, Cao G. Characterization of HSCD5, a novel human stearoyl-CoA desaturase unique to primates. Biochem Biophys Res Commun. 2005;332:735–742. doi: 10.1016/j.bbrc.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Bené H, Lasky D, Ntambi JM. Cloning and characterization of the human stearoyl-CoA desaturase gene promoter: transcriptional activation by sterol regulatory element binding protein and repression by polyunsaturated fatty acids and cholesterol. Biochem Biophys Res Commun. 2001;284:1194–1198. doi: 10.1006/bbrc.2001.5102. [DOI] [PubMed] [Google Scholar]
- 16.Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, Thyfault JP, Stevens R, Dohm GL, Houmard JA, et al. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab. 2005;2:251–261. doi: 10.1016/j.cmet.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riserus U, Tan GD, Fielding BA, Neville MJ, Currie J, Savage DB, Chatterjee VK, Frayn KN, O’Rahilly S, Karpe F. Rosiglitazone increases indexes of stearoyl-CoA desaturase activity in humans: link to insulin sensitization and the role of dominant-negative mutation in peroxisome proliferator-activated receptor-gamma. Diabetes. 2005;54:1379–1384. doi: 10.2337/diabetes.54.5.1379. [DOI] [PubMed] [Google Scholar]
- 18.Corpeleijn E, Feskens E, Jansen E, Mensink M, Saris W, de Bruin T, Blaak E. Improvements in glucose tolerance and insulin sensitivity after lifestyle intervention are related to changes in serum fatty acid profile and desaturase activities: the SLIM study. Diabetologia. 2006;49:2392–2401. doi: 10.1007/s00125-006-0383-4. [DOI] [PubMed] [Google Scholar]
- 19.Stumvoll M. Control of glycaemia: from molecules to men. Minkowski Lecture 2003. Diabetologia. 2004;47:770–781. doi: 10.1007/s00125-004-1400-0. [DOI] [PubMed] [Google Scholar]
- 20.Lovejoy JC, Champagne CM, Smith SR, DeLany JP, Bray GA, Lefevre M, Denkins YM, Rood JC. Relationship of dietary fat and serum cholesterol ester and phospholipid fatty acids to markers of insulin resistance in men and women with a range of glucose tolerance. Metabolism. 2001;50:86–92. doi: 10.1053/meta.2001.19440. [DOI] [PubMed] [Google Scholar]
- 21.Antonini FM, Bucalossi A, Petruzzi E, Simoni R, Morini PL, D’Alessandro A. Fatty acid composition of adipose tissue in normal, atherosclerotic and diabetic subjects. Atherosclerosis. 1970;11:279–289. doi: 10.1016/0021-9150(70)90066-3. [DOI] [PubMed] [Google Scholar]
- 22.Warensjo E, Riserus U, Vessby B. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia. 2005;48:1999–2005. doi: 10.1007/s00125-005-1897-x. [DOI] [PubMed] [Google Scholar]
- 23.Warensjo E, Ohrvall M, Vessby B. Fatty acid composition and estimated desaturase activities are associated with obesity and lifestyle variables in men and women. Nutr Metab Cardiovasc Dis. 2006;16:128–136. doi: 10.1016/j.numecd.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Pan DA, Lillioja S, Milner MR, Kriketos AD, Baur LA, Bogardus C, Storlien LH. Skeletal muscle membrane lipid composition is related to adiposity and insulin action. J Clin Invest. 1995;96:2802–2808. doi: 10.1172/JCI118350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon JA, Fong J, Bernert JTJ. Serum fatty acids and blood pressure. Hypertension. 1996;27:303–307. doi: 10.1161/01.hyp.27.2.303. [DOI] [PubMed] [Google Scholar]
- 26.Chu K, Miyazaki M, Man WC, Ntambi JM. Stearoyl-coenzyme A desaturase 1 deficiency protects against hypertriglyceridemia and increases plasma high-density lipoprotein cholesterol induced by liver X receptor activation. Mol Cell Biol. 2006;26:6786–6798. doi: 10.1128/MCB.00077-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilkinson DI, Karasek MA. Skin lipids of a normal and mutant (asebic) mouse strain. J Invest Dermatol. 1966;47:449–455. doi: 10.1038/jid.1966.168. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Y, Eilertsen KJ, Ge L, Zhang L, Sundberg JP, Prouty SM, Stenn KS, Parimoo S. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet. 1999;23:268–270. doi: 10.1038/15446. [DOI] [PubMed] [Google Scholar]
- 29.Sundberg JP, Boggess D, Sundberg BA, Eilertsen K, Parimoo S, Filippi M, Stenn K. Asebia-2J (Scd1(ab2J)): a new allele and a model for scarring alopecia. Am J Pathol. 2000;156:2067–2075. doi: 10.1016/S0002-9440(10)65078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Y, Bu L, Zhou S, Jin M, Sundberg JP, Jiang H, Qian M, Shi Y, Zhao G, Kong X, et al. Scd1ab-Xyk: a new asebia allele characterized by a CCC trinucleotide insertion in exon 5 of the stearoyl-CoA desaturase 1 gene in mouse. Mol Genet Genomics. 2004;272:129–137. doi: 10.1007/s00438-004-1043-3. [DOI] [PubMed] [Google Scholar]
- 31.Georgel P, Crozat K, Lauth X, Makrantonaki E, Seltmann H, Sovath S, Hoebe K, Du X, Rutschmann S, Jiang Z, et al. A Toll-like receptor 2-responsive lipid effector pathway protects mammals against skin infections with Gram-positive bacteria. Infect Immun. 2005;73:4512–4521. doi: 10.1128/IAI.73.8.4512-4521.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J Nutr. 2001;131:2260–2268. doi: 10.1093/jn/131.9.2260. [DOI] [PubMed] [Google Scholar]
- 33.Binczek E, Jenke B, Holz B, Günter RH, Thevis M, Stoffel W. Obesity resistance of the stearoyl-CoA desaturase-deficient (scd1(−/−)) mouse results from disruption of the epidermal lipid barrier and adaptive thermoregulation. Biol Chem. 2007;388:405–418. doi: 10.1515/BC.2007.046. [DOI] [PubMed] [Google Scholar]
- 34.Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci USA. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, Sharma R, Hudgins LC, Ntambi JM, Friedman JM. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 2002;297:240–243. doi: 10.1126/science.1071527. [DOI] [PubMed] [Google Scholar]
- 36.Terasaka N, Hiroshima A, Koieyama T, Ubukata N, Morikawa Y, Nakai D, Inaba T. T-0901317, a synthetic liver X receptor ligand, inhibits development of atherosclerosis in LDL receptor-deficient mice. FEBS Lett. 2003;536:6–11. doi: 10.1016/s0014-5793(02)03578-0. [DOI] [PubMed] [Google Scholar]
- 37.Levin N, Bischoff ED, Daige CL, Thomas D, Vu CT, Heyman RA, Tangirala RK, Schulman IG. Macrophage liver X receptor is required for antiatherogenic activity of LXR agonists. Arterioscler Thromb Vasc Biol. 2005;25:135–142. doi: 10.1161/01.ATV.0000150044.84012.68. [DOI] [PubMed] [Google Scholar]
- 38.Hobbs HH, Brown MS, Goldstein JL. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Hum Mutat. 1992;1:445–466. doi: 10.1002/humu.1380010602. [DOI] [PubMed] [Google Scholar]
- 39.Rana JS, Jansen AC, Zwinderman AH, Nieuwdorp M, van Aalst-Cohen ES, Jukema JW, Trip MD, Kastelein JJP. Metabolic syndrome and risk of coronary, cerebral, and peripheral vascular disease in a large Dutch population with familial hypercholesterolemia. Diabetes Care. 2006;29:1125–1127. doi: 10.2337/diacare.2951125. [DOI] [PubMed] [Google Scholar]
- 40.Hill JS, Hayden MR, Frohlich J, Pritchard PH. Genetic and environmental factors affecting the incidence of coronary artery disease in heterozygous familial hypercholesterolemia. Arterioscler Thromb. 1991;11:290–297. doi: 10.1161/01.atv.11.2.290. [DOI] [PubMed] [Google Scholar]
- 41.Ferrières J, Lambert J, Lussier-Cacan S, Davignon J. Coronary artery disease in heterozygous familial hypercholesterolemia patients with the same LDL receptor gene mutation. Circulation. 1995;92:290–295. doi: 10.1161/01.cir.92.3.290. [DOI] [PubMed] [Google Scholar]
- 42.Junyent M, Cofán M, Núñez I, Gilabert R, Zambón D, Ros E. Influence of HDL cholesterol on preclinical carotid atherosclerosis in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2006;26:1107–1113. doi: 10.1161/01.ATV.0000218507.95149.42. [DOI] [PubMed] [Google Scholar]
- 43.Hirobe K, Matsuzawa Y, Ishikawa K, Tarui S, Yamamoto A, Nambu S, Fujimoto K. Coronary artery disease in heterozygous familial hypercholesterolemia. Atherosclerosis. 1982;44:201–210. doi: 10.1016/0021-9150(82)90114-9. [DOI] [PubMed] [Google Scholar]
- 44.Real JT, Chaves FJ, Martínez-Usó I, García-García AB, Ascaso JF, Carmena R. Importance of HDL cholesterol levels and the total/HDL cholesterol ratio as a risk factor for coronary heart disease in molecularly defined heterozygous familial hypercholesterolaemia. Eur Heart J. 2001;22:465–471. doi: 10.1053/euhj.2000.2408. [DOI] [PubMed] [Google Scholar]
- 45.Yanagi K, Yamashita S, Kihara S, Nakamura T, Nozaki S, Nagai Y, Funahashi T, Kameda-Takemura K, Ueyama Y, Jiao S, et al. Characteristics of coronary artery disease and lipoprotein abnormalities in patients with heterozygous familial hypercholesterolemia associated with diabetes mellitus or impaired glucose tolerance. Atherosclerosis. 1997;132:43–51. doi: 10.1016/s0021-9150(97)00076-2. [DOI] [PubMed] [Google Scholar]
- 46.Vuorio AF, Turtola H, Piilahti KM, Repo P, Kanninen T, Kontula K. Familial hypercholesterolemia in the Finnish north Karelia. A molecular, clinical, and genealogical study. Arterioscler Thromb Vasc Biol. 1997;17:3127–3138. doi: 10.1161/01.atv.17.11.3127. [DOI] [PubMed] [Google Scholar]
- 47.Gaudet D, Vohl MC, Perron P, Tremblay G, Gagné C, Lesiège D, Bergeron J, Moorjani S, Després JP. Relationships of abdominal obesity and hyperinsulinemia to angiographically assessed coronary artery disease in men with known mutations in the LDL receptor gene. Circulation. 1998;97:871–877. doi: 10.1161/01.cir.97.9.871. [DOI] [PubMed] [Google Scholar]
- 48.Getz GS, Reardon CA. Diet and murine atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:242–249. doi: 10.1161/01.ATV.0000201071.49029.17. [DOI] [PubMed] [Google Scholar]
- 49.Schreyer SA, Vick C, Lystig TC, Mystkowski P, LeBoeuf RC. LDL receptor but not apolipoprotein E deficiency increases diet-induced obesity and diabetes in mice. Am J Physiol Endocrinol Metab. 2002;282:E207–E214. doi: 10.1152/ajpendo.2002.282.1.E207. [DOI] [PubMed] [Google Scholar]
- 50.Reardon CA, Blachowicz L, Lukens J, Nissenbaum M, Getz GS. Genetic background selectively influences innominate artery atherosclerosis: immune system deficiency as a probe. Arterioscler Thromb Vasc Biol. 2003;23:1449–1454. doi: 10.1161/01.ATV.0000079793.58054.2E. [DOI] [PubMed] [Google Scholar]
- 51.Towler DA, Bidder M, Latifi T, Coleman T, Semenkovich CF. Diet-induced diabetes activates an osteogenic gene regulatory program in the aortas of low density lipoprotein receptor-deficient mice. J Biol Chem. 1998;273:30427–30434. doi: 10.1074/jbc.273.46.30427. [DOI] [PubMed] [Google Scholar]
- 52.Merat S, Casanada F, Sutphin M, Palinski W, Reaven PD. Western-type diets induce insulin resistance and hyperinsulinemia in LDL receptor-deficient mice but do not increase aortic atherosclerosis compared with normoinsulinemic mice in which similar plasma cholesterol levels are achieved by a fructose-rich diet. Arterioscler Thromb Vasc Biol. 1999;19:1223–1230. doi: 10.1161/01.atv.19.5.1223. [DOI] [PubMed] [Google Scholar]
- 53.Coenen KR, Gruen ML, Chait A, Hasty AH. Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue. Diabetes. 2007;56:564–573. doi: 10.2337/db06-1375. [DOI] [PubMed] [Google Scholar]
- 54.Miyazaki M, Kim YC, Ntambi JM. A lipogenic diet in mice with a disruption of the stearoyl-CoA desaturase 1 gene reveals a stringent requirement of endogenous monounsaturated fatty acids for triglyceride synthesis. J Lipid Res. 2001;42:1018–1024. [PubMed] [Google Scholar]
- 55.Attie AD, Krauss RM, Gray-Keller MP, Brownlie A, Miyazaki M, Kastelein JJ, Lusis AJ, Stalenhoef AF, Stoehr JP, Hayden MR, et al. Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J Lipid Res. 2002;43:1899–1907. doi: 10.1194/jlr.m200189-jlr200. [DOI] [PubMed] [Google Scholar]
- 56.Kang K, Miyazaki M, Ntambi JM, Pariza MW. Evidence that the anti-obesity effect of conjugated linoleic acid is independent of effects on stearoyl-CoA desaturase1 expression and enzyme activity. Biochem Biophys Res Commun. 2004;315:532–537. doi: 10.1016/j.bbrc.2004.01.087. [DOI] [PubMed] [Google Scholar]
- 57.Miyazaki M, Dobrzyn A, Man WC, Chu K, Sampath H, Kim H, Ntambi JM. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J Biol Chem. 2004;279:25164–25171. doi: 10.1074/jbc.M402781200. [DOI] [PubMed] [Google Scholar]
- 58.Miyazaki M, Dobrzyn A, Sampath H, Lee S, Man WC, Chu K, Peters JM, Gonzalez FJ, Ntambi JM. Reduced adiposity and liver steatosis by stearoyl-CoA desaturase deficiency are independent of peroxisome proliferator-activated receptor-alpha. J Biol Chem. 2004;279:35017–35024. doi: 10.1074/jbc.M405327200. [DOI] [PubMed] [Google Scholar]
- 59.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kunnecke B, Verry P, Benardeau A, von Kienlin M. Quantitative body composition analysis in awake mice and rats by magnetic resonance relaxometry. Obes Res. 2004;12:1604–1615. doi: 10.1038/oby.2004.200. [DOI] [PubMed] [Google Scholar]
- 62.Tailleux A, Bouly M, Luc G, Castro G, Caillaud JM, Hennuyer N, Poulain P, Fruchart JC, Duverger N, Fievet C. Decreased susceptibility to diet-induced atherosclerosis in human apolipoprotein A-II transgenic mice. Arterioscler Thromb Vasc Biol. 2000;20:2453–2458. doi: 10.1161/01.atv.20.11.2453. [DOI] [PubMed] [Google Scholar]
- 63.Singaraja RR, Fievet C, Castro G, James ER, Hennuyer N, Clee SM, Bissada N, Choy JC, Fruchart JC, McManus BM, et al. Increased ABCA1 activity protects against atherosclerosis. J Clin Invest. 2002;110:35–42. doi: 10.1172/JCI15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kulinski A, Vance JE. Lipid homeostasis and lipoprotein secretion in Niemann-Pick C1-deficient hepatocytes. J Biol Chem. 2007;282:1627–1637. doi: 10.1074/jbc.M610001200. [DOI] [PubMed] [Google Scholar]
- 65.Wellington CL, Walker EKY, Suarez A, Kwok A, Bissada N, Singaraja R, Yang Y, Zhang L, James E, Wilson JE, et al. ABCA1 mRNA and protein distribution patterns predict multiple different roles and levels of regulation. Lab Invest. 2002;82:273–283. doi: 10.1038/labinvest.3780421. [DOI] [PubMed] [Google Scholar]
- 66.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 67.R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2006. [Google Scholar]
- 68.Khan-Merchant N, Penumetcha M, Meilhac O, Parthasarathy S. Oxidized fatty acids promote atherosclerosis only in the presence of dietary cholesterol in low-density lipoprotein receptor knockout mice. J Nutr. 2002;132:3256–3262. doi: 10.1093/jn/132.11.3256. [DOI] [PubMed] [Google Scholar]
- 69.Wakil SJ. Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry. 1989;28:4523–4530. doi: 10.1021/bi00437a001. [DOI] [PubMed] [Google Scholar]
- 70.Wakil SJ, Stoops JK, Joshi VC. Fatty acid synthesis and its regulation. Annu Rev Biochem. 1983;52:537–579. doi: 10.1146/annurev.bi.52.070183.002541. [DOI] [PubMed] [Google Scholar]
- 71.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 73.Pratley RE. Gene-environment interactions in the pathogenesis of type 2 diabetes mellitus: lessons learned from the Pima Indians. Proc Nutr Soc. 1998;57:175–181. doi: 10.1079/pns19980029. [DOI] [PubMed] [Google Scholar]
- 74.Leiter EH. The genetics of diabetes susceptibility in mice. FASEB J. 1989;3:2231–2241. doi: 10.1096/fasebj.3.11.2673897. [DOI] [PubMed] [Google Scholar]
- 75.Joseph SB, McKilligin E, Pei L, Watson MA, Collins AR, Laffitte BA, Chen M, Noh G, Goodman J, Hagger GN, et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci USA. 2002;99:7604–7609. doi: 10.1073/pnas.112059299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clee SM, Kastelein JJ, van Dam M, Marcil M, Roomp K, Zwarts KY, Collins JA, Roelants R, Tamasawa N, Stulc T, et al. Age and residual cholesterol efflux affect HDL cholesterol levels and coronary artery disease in ABCA1 heterozygotes. J Clin Invest. 2000;106:1263–1270. doi: 10.1172/JCI10727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wellington CL, Yang Y, Zhou S, Clee SM, Tan B, Hirano K, Zwarts K, Kwok A, Gelfer A, Marcil M, et al. Truncation mutations in ABCA1 suppress normal upregulation of full-length ABCA1 by 9-cis-retinoic acid and 22-R-hydroxycholesterol. J Lipid Res. 2002;43:1939–1949. doi: 10.1194/jlr.m200277-jlr200. [DOI] [PubMed] [Google Scholar]
- 78.Dobrzyn A, Dobrzyn P, Miyazaki M, Sampath H, Chu K, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency increases CTP:choline cytidylyltransferase translocation into the membrane and enhances phosphatidylcholine synthesis in liver. J Biol Chem. 2005;280:23356–23362. doi: 10.1074/jbc.M502436200. [DOI] [PubMed] [Google Scholar]
- 79.Wanless IR, Lentz JS. Fatty liver hepatitis (steato-hepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 80.Loria P, Lonardo A, Carulli N. Should nonalcoholic fatty liver disease be renamed? Dig Dis. 2005;23:72–82. doi: 10.1159/000084728. [DOI] [PubMed] [Google Scholar]
- 81.Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 82.Samuel VT, Liu Z, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 83.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 85.Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999;274:24202–24210. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- 86.Chavez JA, Knotts TA, Wang L, Li G, Dobrowsky RT, Florant GL, Summers SA. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem. 2003;278:10297–10303. doi: 10.1074/jbc.M212307200. [DOI] [PubMed] [Google Scholar]
- 87.Voss MD, Beha A, Tennagels N, Tschank G, Herling AW, Quint M, Gerl M, Metz-Weidmann C, Haun G, Korn M. Gene expression profiling in skeletal muscle of Zucker diabetic fatty rats: implications for a role of stearoyl-CoA desaturase 1 in insulin resistance. Diabetologia. 2005;48:2622–2630. doi: 10.1007/s00125-005-0025-2. [DOI] [PubMed] [Google Scholar]
- 88.Sampath H, Miyazaki M, Dobrzyn A, Ntambi J. Stearoyl CoA desaturase-1 mediates the pro-lipogenic effects of dietary saturated fat. J Biol Chem. 2007;282:2483–2493. doi: 10.1074/jbc.M610158200. [DOI] [PubMed] [Google Scholar]
- 89.Flowers JB, Rabaglia ME, Schueler KL, Flowers MT, Lan H, Keller MP, Ntambi JM, Attie AD. Loss of stearoyl-CoA desaturase-1 improves insulin sensitivity in lean mice but worsens diabetes in leptin-deficient obese mice. Diabetes. 2007;56:1228–1239. doi: 10.2337/db06-1142. [DOI] [PubMed] [Google Scholar]
- 90.Dobrzyn P, Dobrzyn A, Miyazaki M, Cohen P, Asilmaz E, Hardie DG, Friedman JM, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proc Natl Acad Sci USA. 2004;101:6409–6414. doi: 10.1073/pnas.0401627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun Y, Hao M, Luo Y, Liang CP, Silver DL, Cheng C, Maxfield FR, Tall AR. Stearoyl-CoA desaturase inhibits ATP-binding cassette transporter A1-mediated cholesterol efflux and modulates membrane domain structure. J Biol Chem. 2003;278:5813–5820. doi: 10.1074/jbc.M208687200. [DOI] [PubMed] [Google Scholar]
- 92.Wang Y, Oram JF. Unsaturated fatty acids inhibit cholesterol efflux from macrophages by increasing degradation of ATP-binding cassette transporter A1. J Biol Chem. 2002;277:5692–5697. doi: 10.1074/jbc.M109977200. [DOI] [PubMed] [Google Scholar]
- 93.Uehara Y, Engel T, Li Z, Goepfert C, Rust S, Zhou X, Langer C, Schachtrup C, Wiekowski J, Lorkowski S, et al. Polyunsaturated fatty acids and acetoacetate downregulate the expression of the ATP-binding cassette transporter A1. Diabetes. 2002;51:2922–2928. doi: 10.2337/diabetes.51.10.2922. [DOI] [PubMed] [Google Scholar]
- 94.Wang Y, Kurdi-Haidar B, Oram JF. LXR-mediated activation of macrophage stearoyl-CoA desaturase generates unsaturated fatty acids that destabilize ABCA1. J Lipid Res. 2004;45:972–980. doi: 10.1194/jlr.M400011-JLR200. [DOI] [PubMed] [Google Scholar]
- 95.Flowers M, Groen A, Tebon OA, Keller M, Choi Y, Schueler K, Richards O, Lan H, Miyazaki M, Kuipers F, et al. Cholestasis and hypercholesterolemia in SCD1-deficient mice fed a low-fat, high-carbohydrate diet. J Lipid Res. 2006;47:2668–2680. doi: 10.1194/jlr.M600203-JLR200. [DOI] [PubMed] [Google Scholar]