Abstract
Previous research has suggested a relationship between low-frequency power of heart rate variability (HRV; LF in normalized units, LFnu) and muscle sympathetic nerve activity (MSNA). However, investigations have not systematically controlled for breathing, which can modulate both HRV and MSNA. Accordingly, the aims of this experiment were to investigate the possibility of parallel responses in MSNA and HRV (LFnu) to selected acute stressors and the effect of controlled breathing. After data were obtained at rest, 12 healthy males (28 ± 5 yr) performed isometric handgrip exercise (30% maximal voluntary contraction) and the cold pressor test in random order, and were then exposed to hypoxia (inspired fraction of O2 = 0.105) for 7 min, during randomly assigned spontaneous and controlled breathing conditions (20 breaths/min, constant tidal volume, isocapnic). MSNA was recorded from the peroneal nerve, whereas HRV was calculated from ECG. At rest, controlled breathing did not alter MSNA but decreased LFnu (P < 0.05 for all) relative to spontaneous breathing. MSNA increased in response to all stressors regardless of breathing. LFnu increased with exercise during both breathing conditions. During cold pressor, LFnu decreased when breathing was spontaneous, whereas in the controlled breathing condition, LFnu was unchanged from baseline. Hypoxia elicited increases in LFnu when breathing was controlled, but not during spontaneous breathing. The parallel changes observed during exercise and controlled breathing during hypoxia suggest that LFnu may be an indication of sympathetic outflow in select conditions. However, since MSNA and LFnu did not change in parallel with all stressors, a cautious approach to the use of LFnu as a marker of sympathetic activity is warranted.
Keywords: microneurography, autonomic nervous system, hypoxia, cold pressor test, exercise
Microneurographic recordings of muscle sympathetic nerve activity (MSNA) offer a direct measurement of efferent post-ganglionic sympathetic nerve activity and, as such, are considered the gold-standard measurement of global sympathetic outflow to skeletal muscle (51). Physiological stressors such as exercise, the cold pressor test, and hypoxia elicit robust increases in MSNA (5, 7, 15, 22, 53). Resting levels of MSNA have been shown to be elevated with chronic disease (14, 17, 23, 24, 28), and decreases in basal MSNA have been shown following medical therapy (50). Studies have reported an exaggerated MSNA response to physiological stress such as isometric exercise (30) and hypoxia (17) in chronic disease compared with healthy controls. In contrast, patients display an MSNA response similar to that of healthy individuals to the cold pressor test (17, 29). Importantly, because of the invasiveness and technical complexity associated with this technique, microneurography is generally not practical for repeated measurements or large clinical studies.
Heart rate variability (HRV) uses the changes of beat-to-beat heart rate to assess autonomic control of the heart (1). Mathematical transformations of the time between each beat are used in a variety of clinical settings, including risk stratification in cardiac patients and assessment of diabetic neuropathy (47a). Spectral analysis of heart rate provides values of high-and low-frequency power (HF and LF) that are thought to be indicators of autonomic control of heart rate (32, 35). It has been suggested that HF is a measure of efferent cardiac parasympathetic activity, whereas LF represents both cardiac sympathetic and parasympathetic activity (2). HRV is noninvasive and technically straightforward, which makes this technique far more suitable for studies that involve large samples and/or repeated measures.
Whether HRV reflects cardiac sympathetic activity is a topic of much debate (26, 33). Previous studies have compared MSNA and low-frequency fluctuations of HRV at rest and shown no relationship in healthy individuals or in those with chronic disease (20, 31). Studies employing orthostatic stress or pharmacological manipulation of blood pressure have demonstrated significant relationships between MSNA and HRV (4, 9, 43). In healthy subjects, parallel increases in MSNA and the ratio of low- to high-frequency HRV (LF/HF) were observed with orthostatic challenge (9, 10), whereas experimental changes in blood pressure resulted in parallel responses in MSNA and LF (32, 43). These results suggest that MSNA and cardiac sympathetic markers of HRV may change in parallel in response to an autonomic challenge.
Breathing is a potential confounding factor in evaluating the relationship between MSNA and HRV, since both are modulated by the mechanics of breathing (47a, 8, 36, 46). Pulmonary stretch reflexes are implicated in modulation of MSNA within a breathing cycle (8, 44). MSNA decreases during inspiration and peaks during expiration, and this effect is augmented at larger lung volumes (44). The evidence for modulation of HRV by breathing is that the peak in the power spectrum curve changes with the respiratory frequency (13, 46), which may be secondary to stimulation of pulmonary stretch receptors (36). As an example, when subjects breathe at a respiratory frequency of 6 breaths/min (i.e., 0.1 Hz), the peak will entrain to this input, resulting in an increase in the power within the LF (0.04 – 0.15 Hz) band. Conversely, if a subject were to breathe at 12 breaths/min (i.e., 0.2 Hz), the power within the HF band (0.15–0.4 Hz) would increase.
Previous work examining the relationship between MSNA and HRV has not systematically controlled breathing, and therefore changes in breathing pattern in response to acute stress may have confounded both the MSNA and HRV response. Accordingly, the aims of the present study were to determine whether sympathetic markers of HRV (i.e., LF in normalized units, LFnu) parallel MSNA following exposure to selected acute stressors (handgrip exercise, cold pressor test, hypoxia) previously shown to markedly increase MSNA and to examine the effect of controlled breathing on these responses. We hypothesized that sympathetic markers of HRV would closely parallel changes in MSNA during handgrip exercise, the cold pressor test, and hypoxia. Furthermore, a controlled breathing condition would help to separate the effects of breathing from those of the perturbations themselves.
METHODS
Subjects
Twelve men volunteered to participate in this study. The mean (± SD) age, weight, and height were 27.8 (5.4) yr, 79.0 (11.7) kg, and 180 (6.7) cm, respectively. All were normotensive nonsmokers with no history of chronic disease, and none reported the use of any medication at the time of testing. At enrollment, written informed consent was obtained from all subjects, and the study was approved by the University of Alberta Health Research Ethics Board (Biomedical Panel).
Measurements
All measurements were recorded continuously throughout the experimental protocol with the exception of 5-min recovery periods between interventions. Data were recorded and integrated with a data acquisition system (Powerlab 16/30; ADInstruments, New South Wales, Australia) and analyzed offline using associated software (LabChart 6.0 Pro; ADInstruments). Raw MSNA input was sampled at a rate of 10,000 s−1; all other parameters were sampled at a rate of 1,000 s−1.
Apparatus
Throughout the procedures, subjects lay semisupine and breathed through a mouthpiece with the nose occluded. Inspired gas was humidified (HC 150; Fisher and Paykel Healthcare, Auckland, New Zealand) and delivered continuously using a flow-through system to prevent rebreathing of expired gas (flow: 0.5–1.0 l/s). Ventilation was measured by a pneumotachometer (3700 series; Hans Rudolph, Kansas City, MO) just distal to the mouthpiece. Expired CO2 and O2 (mmHg) were measured (CD-3A and S-3A; AEI Technologies, Naperville, IL) continuously from a small sample port off of the mouthpiece. Arterial oxygen saturation (SaO2) was estimated with pulse oximetry (N-595; Nellcor Oximax, Boulder, CO) using a forehead sensor. Heart rate was recorded with a single-lead ECG (lead II, Dual Bio Amp; ADInstruments), and blood pressure was monitored using finger photoplethysmography (Finometer model 2; Finapres, Amsterdam, The Netherlands). During the handgrip exercise trials, force output from a handgrip dynamometer (G100; Biometrics, Ladysmith, VA) was recorded.
Microneurography
Sympathetic nerve activity was recorded using tungsten microelectrodes (200-μm shaft, 1- to 3-μm tip, model UNA32F2S; FHC, Bowdoinham, ME) inserted into the peroneal nerve proximal to the bifurcation posterior to the fibular head. Raw MSNA was filtered (5-kHz low pass, 300-Hz high pass, 60-Hz notch, and mains), integrated (absolute value, time constant decay 0.1 s), and smoothed to obtain a mean voltage neurogram.
Confirmation that sympathetic activity was that of muscle, and not skin, was verified by the following observations: pulse synchronous bursts of MSNA, a twitch response of the muscle without skin paresthesia following weak electrical stimuli from the electrode, and mechanoreceptor discharge following tapping or stretching the muscle but not following gentle stroking of the skin. An acceptable neural recording was defined as a signal-to-noise ratio >3:1.
Individual bursts of sympathetic activity were identified using the Peak Analysis module of the software program (LabChart 6.0 Pro; ADInstruments). Briefly, a voltage threshold was set for each intervention within a subject. The program then detected bursts above the selected threshold. The same threshold was used within an intervention (baseline and intervention) across breathing conditions to facilitate within-intervention comparisons. Each detected burst was con-firmed manually by reviewing the discharge pattern on the raw neurogram to detect false bursts that resulted from transient artifacts. In addition, the timing of potential bursts was monitored relative to other physiological variables such as blood pressure obtained from Finapres. Sympathetic activity was quantified as burst frequency (bursts/min), burst incidence (bursts/100 heartbeats), and total activity (bursts/min × minute mean of burst height, arbitrary units).
Heart Rate Variability
Selection of units
Measurements of HRV are classified as either time- or frequency-domain parameters as a result of the processing method. Standard deviation of normal R-R intervals (SDNN) and the square root of the mean squared successive differences between adjacent intervals (RMSSD; time domain) are estimates of the overall variability or of short-term variations in heart rate, respectively (47a). LF and HF (frequency domain) are associated with autonomic control of heart rate, with HF dominated by vagal influences and LF a result of sympathetic and vagal modulations (47a). Because of the association of LF with sympathetic modulations, this measurement was the focus of the present analysis.
Processing
HRV data were collected following procedures outlined elsewhere (47a). Calculations of SDNN, RMSSD, LF (0.04 to <0.15) and HF power (0.15–0.4 Hz) in absolute and normalized units [absolute power × 100/(total power − very low frequency power)] and LF/HF were carried out using associated software (LabChart 6.0 Pro; ADInstruments). After detection of each heartbeat, the interval between beats (RR interval) was transformed into a continuous digitized signal by resampling at a rate of 1/mean RR interval. The mean RR interval was subtracted from the resampled signal to remove the direct-current frequency component before calculation of the power spectrum using the fast-Fourier transformation (size 256, Welch window, resolution <0.01 Hz/bin). To allow for an initial adjustment period at the onset of an intervention, we restricted data analysis to the last 5 min of each baseline or intervention period.
Experimental Protocol
At least 24 h before the experimental session, a familiarization session served to acquaint the subjects with the protocols. During this session each procedure was carried out, with the exception of micro-neurography. Cold pressor and handgrip exercise trials were done in random order, followed by the hypoxic interventions, to prevent any contamination from the potential prolonged effects of hypoxia. The order of breathing condition was always randomized within each intervention.
All experiments began between 8:00 and 9:00 AM. Subjects abstained from alcohol, exercise, and caffeine for 12 h before the experiment and ate a light breakfast (e.g., cereal and milk) before their arrival. After maximal handgrip testing, subjects were instrumented, and data collection commenced following verification of the MSNA signal.
Throughout the experiment, subjects were encouraged to lie quietly, relax, and refrain from moving, performing Valsalva maneuvers, or verbalizing effort. Steady-state eucapnic PCO2 was determined during the initial resting trial before the experimental interventions. End-tidal PCO2 was subsequently maintained at this isocapnic level throughout all controlled breathing trials by adding CO2 to the inspired gas.
During the resting trial, the subject breathed spontaneously for at least 5.5 min (longer if necessary to reach normal baseline levels) and then performed controlled breathing for 5.5 min. All subsequent trials consisted of 5.5 min of resting baseline in the appropriate breathing condition (i.e., spontaneous or controlled breathing), followed by 7 min of the intervention and, finally, a 5-min recovery period. During the recovery periods the subject could remove the mouthpiece, and the finger blood pressure cuff was adjusted as necessary between conditions.
Breathing Conditions
Subjects performed each intervention in the spontaneous (SB) and controlled breathing (CB) condition. No instructions were given for the SB condition, and subjects freely adjusted tidal volume and breathing rate. During controlled breathing trials, subjects breathed in time with a metronome at a rate of 20 breaths/min, with a Ti/Ttot ratio of .3. Within each controlled breathing intervention, the target VT was set at a specific volume based on the ventilation observed during the practice trial. The target VT ensured that minute ventilation during the controlled breathing condition would be at least equal to minute ventilation during the spontaneous breathing condition. Pilot work indicated that this was preferable to restricting tidal volume during interventions. The target VT was held for both the baseline period and the intervention. Oscillations of tidal volume were displayed on a monitor for the subject to use as a guide.
Interventions
Exercise
Maximal handgrip was measured with a hand dynamometer. The experimental trial consisted of a sustained isometric contraction at 30% of maximal voluntary contraction (MVC). Visual feedback of force output on a monitor allowed participants to adjust their effort as necessary.
Cold pressor test
The cold pressor test (CPT) consisted of immersion of the hand and arm up to the elbow in ice water (0–3°C).
Hypoxia
A mixture of 10.5% O2-balance N2 medical grade gas was administered.
Analysis
Subjects did not attempt interventions if the baseline microneurography recording was not satisfactory (i.e., signal to noise ratio <3:1 or nerve search time limit of 45 min was reached). Complete data were obtained from 12 subjects at rest; 11 subjects completed the CPT, 9 completed handgrip exercise, and 9 completed hypoxia. Later, on review, poor-quality recordings were omitted from analysis, which amounted to one subject from each intervention. Thus 12 subjects were analyzed at rest, 10 during cold pressor, 8 during handgrip, and 8 during hypoxia.
To allow for an initial adjustment period at the onset of an intervention, we took data from the last 5 min of each baseline or intervention period. Pilot studies indicated that this delay was sufficient to achieve stationarity. Individual 5-min averages were used for statistical analysis. Data were tested for normality using the Kolmogorov-Smirnov test for continuous variables (WinSTAT version 2007.1). We used t-tests for comparisons of spontaneous vs. controlled breathing at rest. Data were analyzed using a two-way (breathing × intervention) repeated-measures ANOVA. Statistical analysis was performed using STATISTICA statistical software (7.0 StatSoft, Tulsa, OK). Tukey’s post hoc test was used when significant F ratios were found. For all inferential analyses, the probability of a type I error was set at 0.05. Results are reported as means ± standard deviation unless otherwise noted.
RESULTS
Group mean and standard deviations of the dependent variables are presented in Tables 1–7. The term “rest” refers to data collected during the initial rest period of the two breathing conditions (SB, spontaneous breathing; CB, controlled breathing) before any interventions. Baseline refers to data from the 5.5-min period immediately preceding each intervention.
Rest
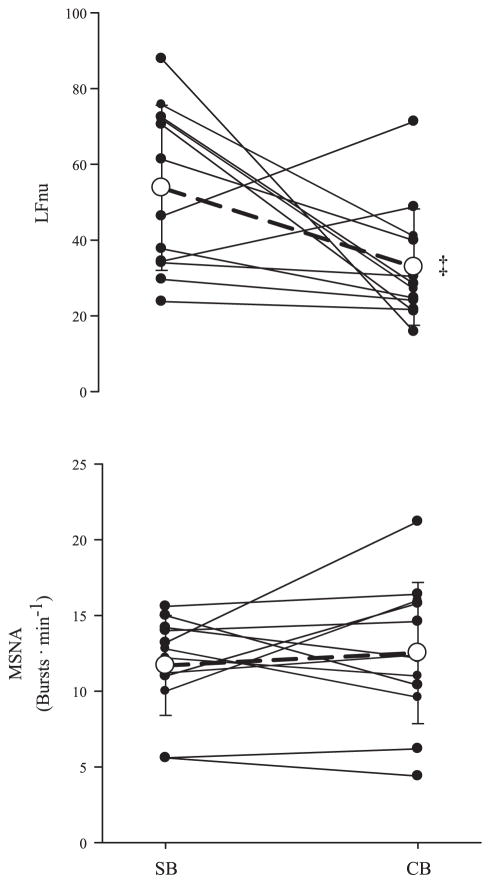
Resting cardiorespiratory, MSNA, and HRV data are shown in Table 1. There was no difference between the end-tidal PCO2 response to SB and CB. Mean arterial pressure (MAP) exhibited a slight but significant increase with CB (P < 0.01), whereas heart rate remained unchanged across breathing condition. End-tidal PO2 increased during CB from 99 ± 14 to 113 ± 11 mmHg (P < 0.01). SaO2 also changed from 97 ± 2% with spontaneous breathing to 99 ± 1% with controlled breathing. RMSSD was not altered with controlled breathing (77.9 ± 28.8, spontaneous; 84.8 ± 39.9, controlled), and SDNN decreased with controlled breathing (93.8 ± 28.8, spontaneous; 71.8 ± 29.8 controlled; P < 0.01). LF power (ms2 and nu) decreased with CB (P = 0.01, P = 0.04), whereas MSNA was unaffected by breathing condition (Fig. 1).
Table 1.
Cardiorespiratory, MSNA, and HRV responses during 5 min of SB and CB at rest
| Breathing Condition | PETCO2, mmHg | VT, liters | RR, breaths/min | V̇E, l/min | HR, beats/min | MAP, mmHg | MSNA
|
HRV
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Burst Incidence, burts/100 heartbeats | Total Activity, arbitrary units | LF, ms2 | HF, ms2 | HF, nu | LF/HF | |||||||
| SB | 44.2 (3.8) | 0.78 (0.19) | 12.5 (3.4) | 8.8 (2.1) | 62 (9) | 86 (9) | 19.7 (4.9) | 24.1 (11.1) | 3,420 (2,229) | 2,835 (1,995) | 43.6 (20.2) | 2.0 (2.1) |
| CB | 45.2 (3.8) | 0.80 (0.14) | 20.3 (0.4)† | 16.1 (2.9)† | 63 (9) | 89 (8)* | 18.8 (6.6) | 25.5 (13.5) | 1,327 (1,193)* | 2,375 (1,974) | 55.6 (17.6) | 0.80 (0.86) |
Values are means (SD); (n = 12). Responses were measured during spontaneous (SB) and controlled breathing (CB) at rest. PETCO2, end-tidal CO2 partial pressure; VT, tidal volume; RR, respiratory rate; V̇E, minute ventilation; HR, heart rate; MAP, mean arterial pressure; MSNA, muscle sympathetic nerve activity; HRV, heart rate variability [ms2, absolute power of low- (LF) or high-frequency (HF) HRV; nu, normalized units].
P < 0.05;
P < 0.01, significantly different from SB condition.
Fig. 1.
Individual and group (mean ± SD, n = 11) responses of low-frequency (LF in normalized units, LFnu) and muscle sympathetic nerve activity (MSNA; bursts/min) to spontaneous (SB) and controlled breathing (CB) conditions. Grouped response is indicated by the open circle and dashed line. ‡P < 0.01, significantly different from SB condition.
Handgrip Exercise
Despite visual feedback of force output and encouragement from the investigators, none of the participants was able to maintain 30% MVC for both handgrip trials (Table 2). However, force output was similar for the two trials (P = 0.41). Cardiorespiratory data are presented in Table 2. Handgrip exercise increased both MAP (P = 0.002, SB; P = 0.005, CB) and heart rate (P = 0.0003, SB; P = 0.003, CB). End-tidal PO2 did not change with exercise (SB baseline to exercise, 101 ± 21 to 107 ± 10 mmHg; CB baseline to exercise, 120 ± 6 to 116 ± 7 mmHg). Estimates of SaO2 remained at 98 ± 2% during both baseline and exercise with spontaneous breathing but increased during CB baseline to 99 ± 2% compared with SB baseline (P < 0.05) and did not change with exercise and CB (99 ± 2%).
Table 2.
Cardiorespiratory responses during 5 min of baseline and fatiguing isometric handgrip exercise with SB and CB
| Breathing Condition | PETCO2, mmHg | VT, liters | RR, breaths/min | V̇E, l/min | HR, beats/min | MAP, mmHg | Force Output, %MVC |
|---|---|---|---|---|---|---|---|
| SB baseline | 38.9 (5.0) | 0.72 (0.23) | 13.7 (2.6) | 12.2 (7.0) | 57 (8) | 83 (8) | |
| SB exercise | 36.9 (5.7) | 0.99 (0.39) | 19.7 (7.5)* | 19.3 (11.4) | 78 (14)* | 102 (6)* | 19.8 (4.7) |
| CB baseline | 41.7 (3.3) | 1.12 (0.22)* | 20.2 (0.1)* | 22.6 (4.4)* | 61 (8) | 86 (7) | |
| CB exercise | 42.2 (3.5) | 1.21 (0.22) | 20.5 (0.9) | 24.8 (4.7) | 74 (12)‡ | 99 (10)† | 17.8 (5.3) |
Values are mean (SD); n = 8. %MVC, percentage of maximal voluntary contraction.
P < 0.05, significantly different from SB baseline.
P < 0.05, significantly different from CB baseline.
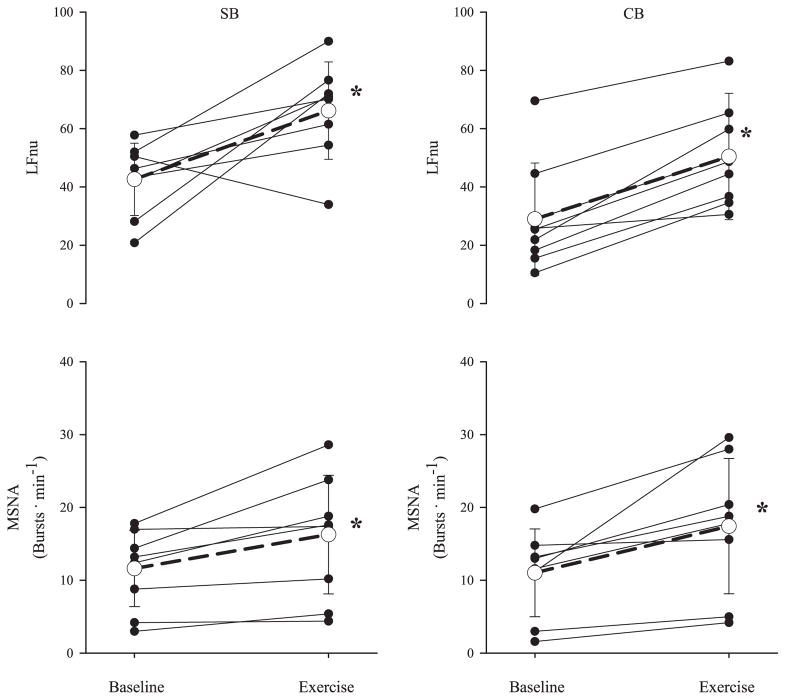
The responses of HRV and MSNA to exercise are presented in Table 3 and Fig. 2. Burst frequency (P = 0.008, SB; P = 0.001, CB) and total activity (P = 0.02, SB and CB) of MSNA increased with exercise in both breathing conditions, and burst incidence increased with exercise during CB (P = 0.04). RMSSD decreased with exercise in both breathing conditions (93.5 ± 34.5 to 42.4 ± 29.8, SB, P < 0.05; 116 ± 48.5 to 60.8 ± 41.6, CB, P < 0.05), and SDNN decreased only during spontaneous breathing (100 ± 20.1 to 69.6 ± 27.1, SB, P < 0.05; 85.1 ± 25.6 and 71.0 ± 25.5, CB). LFnu increased with exercise in both breathing conditions (P = 0.005, SB; P = 0.008, CB).
Table 3.
MSNA and HRV responses during 5 min of baseline and fatiguing isometric handgrip exercise with SB and CB
| Breathing Condition | MSNA
|
HRV
|
||||
|---|---|---|---|---|---|---|
| Burst Incidence, bursts/100 heartbeats | Total Activity, arbitrary units | LF, ms2 | HF, ms2 | HF, nu | LF/HF | |
| SB baseline | 19.5 (9.26) | 19.9 (11.8) | 2,698 (1,397) | 3,983 (2,783) | 53.7 (13.6) | 0.88 (0.39) |
| SB exercise | 19.9 (10.2) | 35.2 (20.9)* | 1,400 (894.2) | 958.8 (1,448)* | 27.6 (15.9)* | 3.5 (2.9)* |
| CB baseline | 18.3 (10.5) | 21.8 (15.3) | 1,499 (702.8) | 4,377 (3,369) | 62.5 (22.5) | 0.74 (0.99) |
| CB exercise | 23.32 (10.82)† | 38.49 (21.70)† | 1,467 (1,216) | 1,671 (2,294)† | 38.3 (18.0)† | 2.0 (1.8) |
Values are means (SD); n = 8.
P < 0.05, significantly different from SB baseline.
P < 0.05, significantly different from CB baseline.
Fig. 2.
Individual and group (mean ± SD, n = 8) responses of LFnu and MSNA to isometric handgrip exercise during SB and CB conditions. Grouped response is indicated by the open circle and dashed line. *P < 0.05, significantly different from baseline. †P < 0.05, significantly different from SB condition.
Cold Pressor Test
Cardiorespiratory responses to the CPT are presented in Table 4. With SB, minute ventilation increased (P = 0.004) during the CPT and end-tidal PCO2 fell below baseline levels (P = 0.007). MAP increased from baseline in both breathing conditions (P = 0.00008, SB; P = 0.002, CB). When breathing was spontaneous, end-tidal PO2 increased from 101 ± 28 mmHg at baseline to 105 ± 26 mmHg during forearm immersion (P < 0.05). A different response was observed with CB. The PO2 values were elevated (P < 0.05) from those observed during SB, both at baseline and during immersion. However, immersion did not result in any change (118 ± 12 mmHg at baseline and 119 ± 9 mmHg during immersion). SaO2 did not change from baseline in either breathing condition; however, SaO2 was higher (P < 0.05) with CB (98 ± 2% for SB vs. 99 ± 1% for CB).
Table 4.
Cardiorespiratory responses during 5 min of baseline and the cold pressor test with SB and CB
| Breathing Condition | PETCO2, mmHg | VT, liters | RR, breaths/min | V̇E, l/min | HR, beats/min | MAP, mmHg |
|---|---|---|---|---|---|---|
| SB baseline | 41.5 (3.8) | 0.70 (0.22) | 13.2 (4.2) | 8.5 (2.3) | 60 (10) | 89 (8) |
| SB cold pressor test | 37.1 (4.9)* | 0.87 (0.43) | 14.5 (3.6) | 11.6 (4.1)* | 63 (11) | 103 (13)* |
| CB baseline | 42.9 (3.5) | 0.85 (0.25) | 20.2 (0.2)* | 17.2 (5.1)* | 60 (12) | 91 (9) |
| CB cold pressor test | 43.0 (3.2)† | 0.87 (0.23) | 20.2 (0.1)† | 17.5 (4.6)† | 64 (12) | 100 (10)†‡ |
Values are means (SD); n = 11.
P < 0.05, significantly different from SB baseline.
P < 0.05, significantly different from SB cold pressor test.
P < 0.05, significantly different from CB baseline.
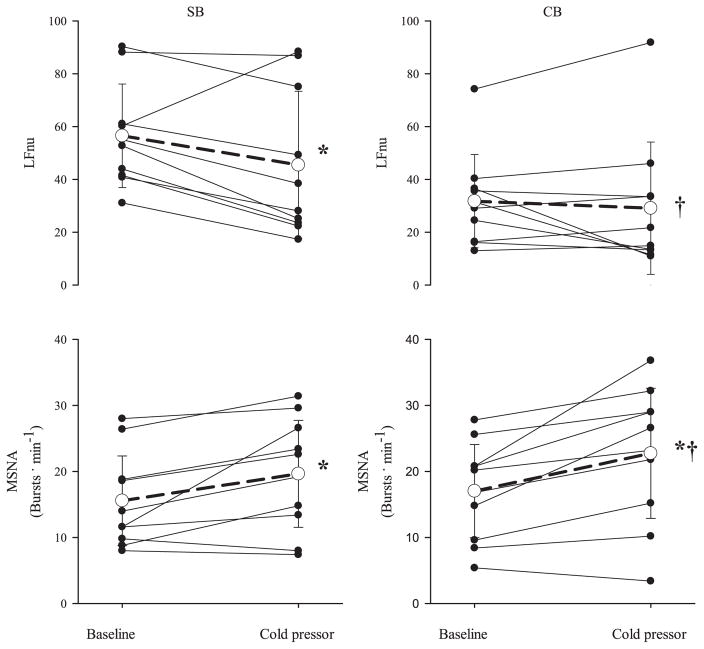
Table 5 presents the responses of HRV and MSNA to the CPT, and Fig. 3 displays group and individual responses of LFnu and MSNA (bursts/min). The CPT elicited an increase in burst frequency (P = 0.008, SB; P = 0.0008, CB), burst incidence (P = 0.005, SB; P = 0.0007, CB), and total activity in both conditions (P = 0.03, SB and CB). Further increases with CB during the CPT above those observed with SB during the CPT were seen with burst frequency (P = 0.04) and burst incidence (P = 0.02). RMSSD was unchanged in both breathing conditions with the CPT (74.3 ± 28.3 to 77.0 ± 39.8, SB; 88.8 ± 45.1 to 82.3 ± 46.9, CB), and SDNN decreased only during spontaneous breathing (97.8 ± 42.3 to 78.4 ± 28.7, SB, P < 0.05; 76.9 ± 28.5 and 65.7 ± 26.6, CB). LFnu decreased with the CPT with SB and did not change during CB (P = 0.02).
Table 5.
MSNA and HRV responses during 5 min of baseline and the cold pressor test with SB and CB
| Breathing Condition | MSNA
|
HRV
|
||||
|---|---|---|---|---|---|---|
| Burst Incidence, burts/100 heartbeats | Total Activity, arbitrary units | LF, ms2 | HF, ms2 | HF, nu | LF/HF | |
| SB baseline | 26.5 (13.5) | 27.9 (21.2) | 4,194 (5,057) | 1,919 (1,284) | 40.8 (19.0) | 2.7 (3.6) |
| SB cold pressor test | 32.2 (15.6)* | 38.7 (24.4)* | 2,524 (2,825) | 2,525 (2,596) | 47.3 (27.9) | 2.4 (3.2) |
| CB baseline | 28.9 (13.9) | 31.4 (16.8)† | 1,023 (726.6)* | 2,687 (2,080) | 62.4 (20.3) | 0.8 (1.2) |
| CB cold pressor test | 36.6 (18.4)†‡ | 40.7 (20.9)‡ | 607.3 (332.6)† | 2,305 (2,000) | 62.6 (29.5)† | 2.3 (6.0) |
Values are means (SD); n = 11.
P < 0.05, significantly different from SB baseline.
P < 0.05, significantly different from SB cold pressor test.
P < 0.05, significantly different from CB baseline.
Fig. 3.
Individual and group (mean ± SD, n = 10) responses of LFnu and MSNA to the cold pressor test during SB and CB conditions. Grouped response is indicated by the open circle and dashed line. *P < 0.05, significantly different from baseline. †P < 0.05, significantly different from SB condition.
Hypoxia
Cardiorespiratory data for hypoxia are presented in Table 6. End-tidal PO2 and SaO2 decreased during both hypoxic trials (P = 0.0002, SB and CB); however, both SaO2 and end-tidal PO2 were greater in CB hypoxia vs. SB hypoxia (P = 0.01 and P = 0.0006, respectively). With spontaneous breathing, tidal volume increased (P = 0.004) and end-tidal PCO2 decreased during hypoxia (P = 0.0003). Heart rate increased in both breathing conditions with hypoxia (P = 0.0002 for both), and MAP increased during hypoxia with CB (P = 0.03).
Table 6.
Cardiorespiratory responses during 5 min of baseline and hypoxia with SB and CB
| Breathing Condition | PETCO2, mmHg | PETO2, mmHg | SaO2, % | VT, liters | RR, breaths/min | V̇E, l/min | HR, beats/min | MAP, mmHg |
|---|---|---|---|---|---|---|---|---|
| SB baseline | 40.6 (4.0) | 98.7 (11) | 98 (1) | 0.67 (0.19) | 13.8 (3.0) | 8.8 (2.0) | 54 (6) | 90 (9) |
| SB hypoxia | 33.2 (3.6)* | 42.8 (15)* | 83 (4)* | 0.91 (0.40)* | 15.4 (3.5) | 13.2 (3.7) | 71 (5)* | 88 (11) |
| CB baseline | 42.8 (3.2) | 117 (8) | 99 (1) | 0.87 (0.13) | 20.0 (0.62) | 17.3 (2.1) | 55 (7) | 91 (10) |
| CB hypoxia | 42.9 (3.5)† | 50 (15)†‡ | 87 (2)†‡ | 0.95 (0.17) | 20.4 (0.35)† | 19.3 (3.9) | 69 (5)‡ | 93 (10)† |
Values are means (SD); n = 9. PETO2, end-tidal O2 partial pressure; SaO2, estimated arterial hemoglobin saturation.
P < 0.05, significantly different from SB baseline.
P < 0.05, significantly different from SB hypoxia.
P < 0.05, significantly different from CB baseline.
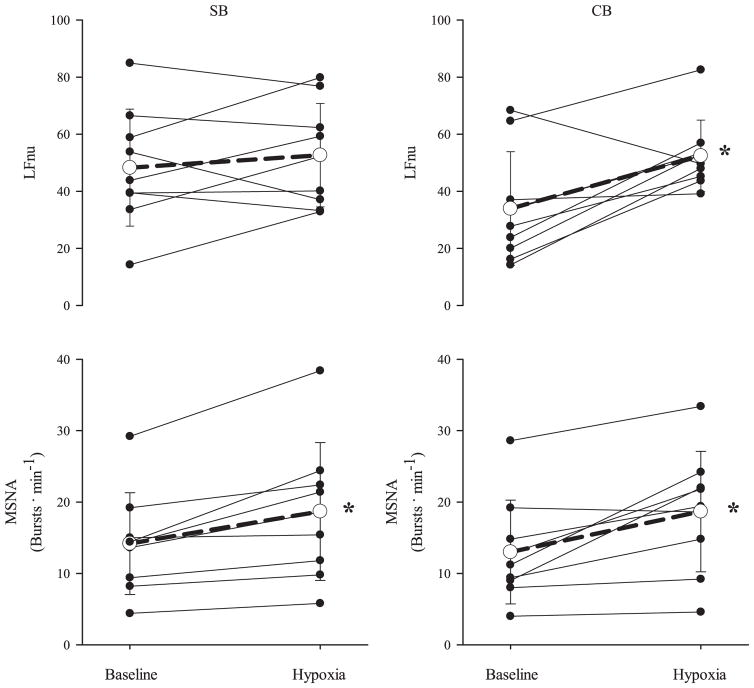
MSNA and HRV responses are presented in Table 7. Figure 4 highlights the response of LFnu and MSNA (bursts/min) to hypoxia. Burst frequency of MSNA increased with hypoxia (P = 0.01, SB; P = 0.002, CB). Both conditions decreased RMSSD during hypoxia (86.4 ± 25.3 to 47.1 ± 25.6, SB, and 97.7 ± 36.7 to 58.1 ± 14.1, CB, P < 0.05), and SDNN decreased only with spontaneous breathing (99.2 ±35.8 to 63.1 ±25.5, SB, P <0.05; 78.2 ± 15.2 to 60.4 ± 17.8, CB). LF (normalized units) increased with CB (P = 0.05).
Table 7.
MSNA and HRV responses during 5 min of baseline and hypoxia with SB and CB
| Breathing Condition | MSNA
|
HRV
|
||||
|---|---|---|---|---|---|---|
| Burst Incidence, bursts/100 hearbeats | Total Activity, arbitrary units | LF, ms2 | HF, ms2 | HF, nu | LF/HF | |
| SB baseline | 26.3 (14.5) | 26.7 (17.3) | 3,658 (4,021) | 3,014 (1,915) | 50.2 (20.3) | 1.5 (1.7) |
| SB hypoxia | 26.1 (13.9) | 33.0 (21.9) | 1,178 (1,168)* | 813.6 (705.7)* | 37.5 (14.2) | 1.8 (1.3) |
| CB baseline | 23.5 (13.8) | 25.0 (17.0) | 1,132 (521.8) | 3,024 (2,099) | 62.9 (21.5) | 0.80 (0.88) |
| CB hypoxia | 20.4 (9.4) | 34.3 (24.4) | 980.9 (354.1) | 765.4 (397.8)† | 37.5 (11.5) | 1.9 (2.1)† |
Values are means (SD); n = 9.
P < 0.05, significantly different from SB baseline.
P < 0.05, significantly different from CB baseline.
Fig. 4.
Individual and group (mean ± SD, n = 9) responses of LFnu and MSNA to hypoxia during SB and CB conditions. Grouped response is indicated by the open circle and dashed line. *P < 0.05, significantly different from baseline.
DISCUSSION
Whether measures of HRV can be used to infer a valid estimate of changes in sympathetic activity remains a topic of much debate (26, 33). The purpose of this study was, first, to determine whether changes in the normalized LF component of HRV, frequently chosen as a sympathetic marker, parallel changes in MSNA following exposure to acute stressors; and second, to examine the effect of controlled breathing on these responses. The major findings were, first, that changes in LF HRV (normalized units, LFnu) occur in parallel with increases in burst frequency of MSNA during isometric handgrip exercise; second, that LFnu does not change in parallel with the MSNA response to the CPT regardless of breathing condition; and third, that increases in LFnu with hypoxia parallel that of MSNA only when breathing is controlled. The MSNA and LFnu response to stress was not consistent across sympathoexcitatory interventions, suggesting that LFnu is not a universal indicator of the MSNA response to stress. The parallel response observed during exercise and controlled breathing hypoxia would suggest that the LFnu response may be an indication of sympathetic outflow in these select conditions.
Rest
In the present study, careful control of breathing had no effect on MSNA at rest. Breathing cycle has been shown to influence the timing of MSNA throughout a given breath without affecting burst frequency or total activity (44, 45). Similarly, the inclusion of a consistent tidal volume and maintenance of end-tidal CO2 in addition to paced breathing did not alter basal MSNA. We observed an increase in MAP with controlled breathing with no change in MSNA or heart rate, suggesting a breathing-induced alteration in baroreceptor sensitivity. Previous research has shown that slow breathing enhances baroreflex sensitivity (37). Conversely, the increase in breathing frequency with paced breathing may reduce baroreceptor sensitivity and explain the increase in MAP with no change in MSNA or heart rate. Other studies also have found that paced breathing does not change HR (13, 46) but decreases LFnu (13, 36, 46), which indicates that the observed decrease in LFnu with controlled breathing cannot be attributed to changes in heart rate, per se. The decrease in LFnu with a change from spontaneous to controlled breathing may be due to the increase in breathing rate to 20 breaths/min (0.33 Hz), which effectively shifts any respiratory modulation of heart rate into the HF band (i.e., >0.15 Hz). The decrease in LFnu during controlled breathing occurred in the absence of changes in heart rate or MSNA, which indicates that LFnu is not a universal indicator of MSNA.
Handgrip Exercise
The increases in burst frequency of MSNA in the present study are similar to those obtained previously with a comparable protocol (7 min of isometric handgrip at 30% MVC) (55). The increases in LFnu and LF observed in the present study are directionally consistent with previous examples of the response of HRV to isolated limb exercise (16, 21). The LFnu response may be secondary to a decrease in HFnu as a result of vagal withdrawal during exercise or, alternately, increased cardiac sympathetic nerve activity. During exercise, increases in LFnu parallel increases in burst frequency of MSNA regardless of breathing condition.
The increase in MSNA in response to isometric handgrip exercise (30% MVC) typically exhibits a 1- to 2-min delay following the onset of muscle contraction. This time delay is thought to be due to the accumulation of metabolites necessary to stimulate metaboreflex-mediated increase of MSNA (15, 40). In contrast, heart rate increases within the first minute in response to the same exercise (15) and has been attributed to central command (53). Present findings are consistent with other reports (15, 53) of studies where heart rate and MSNA remained elevated for the duration of muscle contraction.
The time delay between MSNA and heart rate would suggest that the MSNA and heart rate response to handgrip may be unrelated; however, research on central command implies otherwise. Previous work has documented an increase in both heart rate and MSNA during attempted handgrip exercise with muscular paralysis (53). An increase in MSNA with maximal effort, but no muscular contraction, in the absence of metaboreflex or mechanoreflex stimulation during attempted handgrip suggests that with maximal effort, central command can contribute to the activation of MSNA (53). Similarly to previous studies (55), participants in the present experiment were unable to maintain the target force output for 7 min, indicating that effort (i.e., central command) likely increased throughout the exercise trial. Thus, in addition to the metaboreflex-mediated increase in MSNA, the observed increase in LFnu and MSNA during fatiguing handgrip exercise may also be secondary to central command, thus explaining the parallel response.
Cold Pressor Test
The CPT has been shown to elicit an increase in MSNA that peaks during the second minute of immersion (22, 49). The increase in sympathetic activity has been accompanied by a decrease in vascular resistance (18), demonstrating the complexity of the response to the CPT. Although the bulk of previous research has detailed the response during the first 1–3 min of the CPT (5, 27, 49, 52), the present investigation employed a longer protocol (7 min). Consistent with the results from shorter protocols, we found significant increases in MSNA during minutes 2–7 of immersion. The increase in heart rate in response to the CPT has been eliminated with the administration of propranolol, indicating that the response of heart rate to the CPT is a result of sympathetic activation and not simply parasympathetic withdrawal (52). We did not observe a robust HRV response to the CPT. Similar protocols (2–4.5 min) have produced increases (49) or no change (56) in LFnu, and both decreases (57) and increases (56) of LF, when breathing at an assigned rate. The decrease in LFnu during the CPT with spontaneous breathing in the present study is contrary to our hypothesis that LFnu would parallel MSNA in response to the CPT. This is a novel finding given that previous research has not included a spontaneous breathing condition (49, 56, 57).
A number of previous investigations have demonstrated a separation of the heart rate and MSNA response to the CPT. First, heart rate and MSNA typically show a different time course in response to CPT (52). Second, stroke patients demonstrate an attenuated MSNA response to CPT despite a normal tachycardic HR response (27). Finally, although both MSNA and heart rate are modulated by the baroreflex during the CPT (5), only the sensitivity of baroreflex control of MSNA is increased during the CPT (5). Collectively, these results demonstrate independence of the heart rate and MSNA responses to CPT and indicate that indexes of HRV do not predict the MSNA response to the CPT.
Hypoxia
Stimulation of the carotid chemoreceptor with hypoxia results in increased MSNA and heart rate (12, 19, 47), as was observed in the present investigation. Our data were consistent with previous findings (3), where hypoxia [inspired fration of O2 (FIO2) = 0.115] with spontaneous breathing did not change LFnu. Previous work showed that when breathing frequency was set at 12 breaths/min while PETCO2 and VT were uncontrolled, hypoxia (FIO2 = 0.11) resulted in an increase in LFnu (38). Collectively, these findings indicate that the response of LFnu to hypoxia parallels that of MSNA only when breathing frequency is controlled and is independent of changes in PETCO2.
The inclusion of a controlled breathing condition in this experiment removes any interaction of the hypoxia-induced hyperpnea (i.e., increased breathing frequency and VT, and associated reduction in PETCO2) from the MSNA response to hypoxia. VT can modulate MSNA secondarily to feedback from lung stretch (8). The interaction among chemoreceptor stimulation, lung stretch, and sympathetic nervous activity is complex. Work in animals has shown that when the carotid chemoreceptors are stimulated while breathing is controlled, the net response is profound vasoconstriction, whereas allowing breathing to increase with chemoreceptor stimulation can cause vasodilation (41). In the present study we saw similar responses in MSNA to hypoxia in both conditions (14.2 to 18.7 bursts/min during SB, baseline, and hypoxia; 13.0 to 18.7 bursts/min during CB, baseline, and hypoxia) despite a higher PETO2 in controlled vs. spontaneous breathing. Of note, an increase in blood pressure secondary to hypoxia would act to limit the net MSNA response to hypoxia because of baroreceptor inhibition. We observed a hypertensive effect in the controlled breathing trial, whereas there was no change in blood pressure with spontaneous breathing. The similar MSNA response to hypoxia despite a reduced stimulus and hypertensive effect (i.e., higher PETO2 and baroreceptor stimulation) in the CB hypoxic trial would indicate that the normal hyperpnoea during SB in hypoxia acts to reduce the net MSNA and blood pressure response.
The Effect of Breathing
The inclusion of spontaneous and controlled breathing conditions in the present experiment provides a level of control not seen in previous comparisons of MSNA and HRV. The breathing rate and VT during the controlled trials were set high enough that there would be no increase with the various interventions. The subjects were therefore “overbreathing” during the baseline controlled breathing condition; however, since CO2 was added to maintain end-tidal CO2, subjects were not, by definition, hyperventilating. This approach was designed to effectively remove the influence of changes in breathing within an intervention. An alternate approach would have been to control breathing by having the subject maintain breathing rate and VT at baseline values (i.e., prevent the increase in V̇E in response to the intervention); however, this likely would have caused significant discomfort and altered the cardiovascular/autonomic response. The response of MSNA was consistent across breathing condition in all interventions except for the CPT, where the increase in burst frequency with controlled breathing was greater than that with spontaneous breathing. At rest, the observed decrease in LFnu with controlled breathing is likely associated with the upward shift of respiratory frequency, as has been observed previously (13, 32). Control of breathing was not necessary to observe a parallel relationship of LFnu and MSNA during isometric handgrip exercise; however, control of breathing was necessary to observe a similar parallel response to hypoxia. This relationship during hypoxia may not require isocapnea, since similar responses of LFnu have been observed with only paced breathing (38); however, further research is necessary to clarify the importance of specific respiratory parameters to the response of LFnu during hypoxia.
Limitations
A clear understanding of the scope of the techniques employed is essential for interpretation of the results of this experiment. As the name implies, MSNA is a direct measurement of sympathetic nervous activity to muscle. MSNA can be modulated by a number of central and peripheral mechanisms, including baroreceptors, mechanoreceptors, chemoreceptors, and nociceptors (54), with integration of afferent feedback occurring within the rostral ventrolateral medulla and nucleus tractus solitarius (11). HRV is the result of mathematical transformations of heart rate, which has both sympathetic and parasympathetic influences (39). Although aspects of these transformations may relate to changes prompted by alterations in autonomic (i.e., sympathetic) function, this does not imply that measurements of HRV are a direct assessment of autonomic function. Our findings during the CPT and hypoxia with spontaneous breathing are consistent with supine autonomic blockade studies showing that during some conditions, HRV and sympathetic activity are divergent (35, 48). This supports the assertion that the LF band is determined by a complex interaction between the sympathetic and parasympathetic autonomic system. As with previous work (9, 43), we compared HRV and MSNA responses to question whether HRV could be used as a surrogate for MSNA, but we did not assume that HRV and MSNA are equivalent.
Previous research indicates that between-test variability of HRV is commonly reported as 30% (42). In the present experiment, variation of LFnu in the baseline periods preceding each intervention was lower than the reported value with both spontaneous (25%) and controlled breathing (15%). Of note, there is some indication that LF and LFnu are the most reliable measurements of HRV (34). The relatively high variability in HRV must be considered as a limitation in the application of this method. To minimize the effect of this variation, we did not compare results across more than one specific intervention.
The interventions selected for the present investigation were chosen to produce predictable increases in MSNA and were not expected to elicit equivalent responses in MSNA. As such, each intervention was considered separately, and comparisons between interventions were avoided. During the interventions, heart rate did not exceed 100 beats/min. Below this rate, withdrawal of vagal tone is traditionally thought to account for increases in heart rate, whereas increases beyond this rate are a result of increased sympathetic drive to the sinus node (39). However, others have demonstrated sympathetic influence at heart rates below 100 beats/min, specifically during isometric exercise (25). The breathing rate of 20 breaths/min was selected to ensure adequate ventilation during all interventions. However, we acknowledge that this rate was above the breathing frequency seen during free breathing cold pressor and hypoxia and that this rapid rate may have limited the within-breath modulation of heart rate and MSNA. Another limitation of the present study is the potential contamination of the hypoxic trials by the continued effect of hypoxia on MSNA following return to normoxia (7). Importantly, hypoxia was the last intervention for all subjects, and breathing condition was randomized to control for any possible carryover effect.
Choice of HRV Variables
The emphasis on LFnu as a marker of sympathetic activity in the present study was not intended to suggest that it is a superior measurement of HRV; therefore, for the sake of completeness, commonly used time and frequency domain parameters are also reported. LFnu was selected for primary analysis in light of the following: 1) LFnu has been suggested to be a marker of sympathetic outflow to the heart (20, 32); 2) a relationship between LF and MSNA has been previously reported (4, 43); and 3) interventions in this experiment were selected to augment muscle sympathetic nerve activity; therefore, HF, a marker of parasympathetic influence, would not be expected to parallel changes in MSNA. Importantly, the selection of autonomic challenges does not imply the expectation of an equivalent response of heart and muscle sympathetic activity to physiological stress.
Conclusions
The results of the present study indicate that MSNA and LFnu response to stress was not consistent across the interventions. During isometric handgrip exercise (30% MVC), there were parallel increases in MSNA and LFnu that were independent of breathing frequency, VT, and PETCO2. During hypoxia (PETO2 = 42.8–50 mmHg), increases in LFnu paralleled increases in MSNA only when breathing frequency, VT, and PETCO2 were controlled. Conversely, MSNA and LFnu responses to the cold pressor test were not consistent. The parallel responses observed during exercise and with hypoxia when breathing was controlled suggest that the LFnu response may be an indication of sympathetic outflow in some, but not all, sympathoexcitatory conditions.
Perspectives and Significance
Consistency in VT, respiratory rate, and end-tidal CO2 in the present study provided a level of control that has not been attained in previous comparisons of HRV and MSNA. As expected, the selected interventions elicited increases in MSNA regardless of breathing condition. In contrast, LFnu increased in both breathing conditions only during handgrip exercise and varied with breathing condition during hypoxia. These findings highlight the importance of the interaction of breathing in the exploration of a relationship between MSNA and HRV. Further investigation may help to clarify the nature of this relationship during both exercise and hypoxia. The nature of the protocol dictated that only one level of intensity for each intervention was studied (i.e., 10.5% O2, 30% MVC); future investigations should relate the findings of this study to observations throughout a range of exercise intensities or FIO2. The present investigation provides a thorough description of the HRV and MSNA response to acute stress; future work may expand on this through a more diverse subject pool and the use of modeling methods to define (rather than describe) the relationship between MSNA and HRV.
Acknowledgments
We thank Lindsay Hubenig and Tim Hartley for assistance.
GRANTS
This research was supported by a grant from the Department of National Defence. Dr. M. K. Stickland was supported by a Canadian Institutes of Health Research New Investigator Award.
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- 1.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 2.Britton AH, Harry . Heart rate variability in healthy populations: correlates and consequences. In: Malik MC, John A, editors. Dynamic Electrocardiography. Elmsford, NY: Blackwell Futura; 2004. pp. 90–111. [Google Scholar]
- 3.Buchheit M, Richard R, Doutreleau S, Lonsdorfer-Wolf E, Brandenberger G, Simon C. Effect of acute hypoxia on heart rate variability at rest and during exercise. Int J Sports Med. 2004;25:264–269. doi: 10.1055/s-2004-819938. [DOI] [PubMed] [Google Scholar]
- 4.Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KU, Eckberg DL. Human responses to upright tilt: a window on central autonomic integration. J Physiol. 1999;517:617–628. doi: 10.1111/j.1469-7793.1999.0617t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui J, Wilson TE, Shibasaki M, Hodges NA, Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during posthandgrip muscle ischemia in humans. J Appl Physiol. 2001;91:1679–1686. doi: 10.1152/jappl.2001.91.4.1679. [DOI] [PubMed] [Google Scholar]
- 7.Cutler MJ, Swift NM, Keller DM, Wasmund WL, Smith ML. Hypoxia-mediated prolonged elevation of sympathetic nerve activity after periods of intermittent hypoxic apnea. J Appl Physiol. 2004;96:754–761. doi: 10.1152/japplphysiol.00506.2003. [DOI] [PubMed] [Google Scholar]
- 8.Dempsey JA, Sheel AW, St Croix CM, Morgan BJ. Respiratory influences on sympathetic vasomotor outflow in humans. Respir Physiol Neurobiol. 2002;130:3–20. doi: 10.1016/s0034-5687(01)00327-9. [DOI] [PubMed] [Google Scholar]
- 9.Floras JS, Butler GC, Ando SI, Brooks SC, Pollard MJ, Picton P. Differential sympathetic nerve and heart rate spectral effects of nonhypotensive lower body negative pressure. Am J Physiol Regul Integr Comp Physiol. 2001;281:R468–R475. doi: 10.1152/ajpregu.2001.281.2.R468. [DOI] [PubMed] [Google Scholar]
- 10.Furlan R, Porta A, Costa F, Tank J, Baker L, Schiavi R, Robertson D, Malliani A, Mosqueda-Garcia R. Oscillatory patterns in sympathetic neural discharge and cardiovascular variables during orthostatic stimulus. Circulation. 2000;101:886–892. doi: 10.1161/01.cir.101.8.886. [DOI] [PubMed] [Google Scholar]
- 11.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 12.Halliwill JR, Morgan BJ, Charkoudian N. Peripheral chemoreflex and baroreflex interactions in cardiovascular regulation in humans. J Physiol. 2003;552:295–302. doi: 10.1113/jphysiol.2003.050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayano J, Mukai S, Sakakibara M, Okada A, Takata K, Fujinami T. Effects of respiratory interval on vagal modulation of heart rate. Am J Physiol Heart Circ Physiol. 1994;267:H33–H40. doi: 10.1152/ajpheart.1994.267.1.H33. [DOI] [PubMed] [Google Scholar]
- 14.Heindl S, Lehnert M, Criee CP, Hasenfuss G, Andreas S. Marked sympathetic activation in patients with chronic respiratory failure. Am J Respir Crit Care Med. 2001;164:597–601. doi: 10.1164/ajrccm.164.4.2007085. [DOI] [PubMed] [Google Scholar]
- 15.Ichinose M, Saito M, Kondo N, Nishiyasu T. Time-dependent modulation of arterial baroreflex control of muscle sympathetic nerve activity during isometric exercise in humans. Am J Physiol Heart Circ Physiol. 2006;290:H1419–H1426. doi: 10.1152/ajpheart.00847.2005. [DOI] [PubMed] [Google Scholar]
- 16.Iellamo F, Pizzinelli P, Massaro M, Raimondi G, Peruzzi G, Legramante JM. Muscle metaboreflex contribution to sinus node regulation during static exercise: insights from spectral analysis of heart rate variability. Circulation. 1999;100:27–32. doi: 10.1161/01.cir.100.1.27. [DOI] [PubMed] [Google Scholar]
- 17.Imadojemu VA, Mawji Z, Kunselman A, Gray KS, Hogeman CS, Leuenberger UA. Sympathetic chemoreflex responses in obstructive sleep apnea and effects of continuous positive airway pressure therapy. Chest. 2007;131:1406–1413. doi: 10.1378/chest.06-2580. [DOI] [PubMed] [Google Scholar]
- 18.Jacob G, Costa F, Shannon J, Robertson D, Biaggioni I. Dissociation between neural and vascular responses to sympathetic stimulation: contribution of local adrenergic receptor function. Hypertension. 2000;35:76–81. doi: 10.1161/01.hyp.35.1.76. [DOI] [PubMed] [Google Scholar]
- 19.Kara T, Narkiewicz K, Somers VK. Chemoreflexes—physiology and clinical implications. Acta Physiol Scand. 2003;177:377–384. doi: 10.1046/j.1365-201X.2003.01083.x. [DOI] [PubMed] [Google Scholar]
- 20.Kingwell BA, Thompson JM, Kaye DM, McPherson GA, Jennings GL, Esler MD. Heart rate spectral analysis, cardiac norepinephrine spillover, and muscle sympathetic nerve activity during human sympathetic nervous activation and failure. Circulation. 1994;90:234–240. doi: 10.1161/01.cir.90.1.234. [DOI] [PubMed] [Google Scholar]
- 21.Kluess HA, Wood RH. Heart rate variability and the exercise pressor reflex during dynamic handgrip exercise and postexercise arterial occlusion. Am J Med Sci. 2005;329:117–123. doi: 10.1097/00000441-200503000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Kregel KC, Seals DR, Callister R. Sympathetic nervous system activity during skin cooling in humans: relationship to stimulus intensity and pain sensation. J Physiol. 1992;454:359–371. doi: 10.1113/jphysiol.1992.sp019268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambert EA, Schlaich MP. Reduced sympathoneural responses to the cold pressor test in individuals with essential hypertension and in those genetically predisposed to hypertension. No support for the “pressor reactor” hypothesis of hypertension development. Am J Hypertens. 2004;17:863–868. doi: 10.1016/j.amjhyper.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Leimbach WN, Jr, Wallin BG, Victor RG, Aylward PE, Sundlof G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation. 1986;73:913–919. doi: 10.1161/01.cir.73.5.913. [DOI] [PubMed] [Google Scholar]
- 25.Maciel BC, Gallo L, Junior, Marin Neto JA, Martins LE. Autonomic nervous control of the heart rate during isometric exercise in normal man. Pflügers Arch. 1987;408:173–177. doi: 10.1007/BF00581348. [DOI] [PubMed] [Google Scholar]
- 26.Malliani A. Cardiovascular variability is/is not an index of autonomic control of circulation. J Appl Physiol. 2006;101:684–688. doi: 10.1152/japplphysiol.00562.2006. [DOI] [PubMed] [Google Scholar]
- 27.Mizushima T, Tajima F, Nakamura T, Yamamoto M, Lee KH, Ogata H. Muscle sympathetic nerve activity during cold pressor test in patients with cerebrovascular accidents. Stroke. 1998;29:607–612. doi: 10.1161/01.str.29.3.607. [DOI] [PubMed] [Google Scholar]
- 28.Narkiewicz K, Pesek CA, Kato M, Phillips BG, Davison DE, Somers VK. Baroreflex control of sympathetic nerve activity and heart rate in obstructive sleep apnea. Hypertension. 1998;32:1039–1043. doi: 10.1161/01.hyp.32.6.1039. [DOI] [PubMed] [Google Scholar]
- 29.Narkiewicz K, Pesek CA, van de Borne PJ, Kato M, Somers VK. Enhanced sympathetic and ventilatory responses to central chemoreflex activation in heart failure. Circulation. 1999;100:262–267. doi: 10.1161/01.cir.100.3.262. [DOI] [PubMed] [Google Scholar]
- 30.Notarius CF, Atchison DJ, Floras JS. Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. Am J Physiol Heart Circ Physiol. 2001;280:H969–H976. doi: 10.1152/ajpheart.2001.280.3.H969. [DOI] [PubMed] [Google Scholar]
- 31.Notarius CF, Butler GC, Ando S, Pollard MJ, Senn BL, Floras JS. Dissociation between microneurographic and heart rate variability estimates of sympathetic tone in normal subjects and patients with heart failure. Clin Sci (Lond) 1999;96:557–565. [PubMed] [Google Scholar]
- 32.Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell’Orto S, Piccaluga E. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympathovagal interaction in man and conscious dog. Circ Res. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 33.Parati G, Mancia G, Di Rienzo M, Castiglioni P. Point: cardiovascular variability is/is not an index of autonomic control of circulation. J Appl Physiol. 2006;101:676–682. doi: 10.1152/japplphysiol.00446.2006. [DOI] [PubMed] [Google Scholar]
- 34.Pitzalis MV, Mastropasqua F, Massari F, Forleo C, Di Maggio M, Passantino A, Colombo R, Di Biase M, Rizzon P. Short- and long-term reproducibility of time and frequency domain heart rate variability measurements in normal subjects. Cardiovasc Res. 1996;32:226–233. doi: 10.1016/0008-6363(96)00086-7. [DOI] [PubMed] [Google Scholar]
- 35.Pomeranz B, Macaulay RJ, Caudill MA, Kutz I, Adam D, Gordon D, Kilborn KM, Barger AC, Shannon DC, Cohen RJ. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol Heart Circ Physiol. 1985;248:H151–H153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 36.Poyhönen M, Syvaoja S, Hartikainen J, Ruokonen E, Takala J. The effect of carbon dioxide, respiratory rate and tidal volume on human heart rate variability. Acta Anaesthesiol Scand. 2004;48:93–101. doi: 10.1111/j.1399-6576.2004.00272.x. [DOI] [PubMed] [Google Scholar]
- 37.Radaelli A, Raco R, Perfetti P, Viola A, Azzellino A, Signorini MG, Ferrari AU. Effects of slow, controlled breathing on baroreceptor control of heart rate and blood pressure in healthy men. J Hypertens. 2004;22:1361–1370. doi: 10.1097/01.hjh.0000125446.28861.51. [DOI] [PubMed] [Google Scholar]
- 38.Roche F, Reynaud C, Garet M, Pichot V, Costes F, Barthelemy JC. Cardiac baroreflex control in humans during and immediately after brief exposure to simulated high altitude. Clin Physiol Funct Imaging. 2002;22:301–306. doi: 10.1046/j.1475-097x.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 39.Rowell LB. Human Cardiovascular Control. New York: Oxford University Press; 1993. [Google Scholar]
- 40.Rowell LB, O’Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol. 1990;69:407–418. doi: 10.1152/jappl.1990.69.2.407. [DOI] [PubMed] [Google Scholar]
- 41.Rutherford JD, Vatner SF. Integrated carotid chemoreceptor and pulmonary inflation reflex control of peripheral vasoactivity in conscious dogs. Circ Res. 1978;43:200–208. doi: 10.1161/01.res.43.2.200. [DOI] [PubMed] [Google Scholar]
- 42.Sandercock GR, Bromley PD, Brodie DA. The reliability of short-term measurements of heart rate variability. Int J Cardiol. 2005;103:238–247. doi: 10.1016/j.ijcard.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Saul JP, Rea RF, Eckberg DL, Berger RD, Cohen RJ. Heart rate and muscle sympathetic nerve variability during reflex changes of autonomic activity. Am J Physiol Heart Circ Physiol. 1990;258:H713–H721. doi: 10.1152/ajpheart.1990.258.3.H713. [DOI] [PubMed] [Google Scholar]
- 44.Seals DR, Suwarno NO, Dempsey JA. Influence of lung volume on sympathetic nerve discharge in normal humans. Circ Res. 1990;67:130–141. doi: 10.1161/01.res.67.1.130. [DOI] [PubMed] [Google Scholar]
- 45.Seals DR, Suwarno NO, Joyner MJ, Iber C, Copeland JG, Dempsey JA. Respiratory modulation of muscle sympathetic nerve activity in intact and lung denervated humans. Circ Res. 1993;72:440–454. doi: 10.1161/01.res.72.2.440. [DOI] [PubMed] [Google Scholar]
- 46.Song HS, Lehrer PM. The effects of specific respiratory rates on heart rate and heart rate variability. Appl Psychophysiol Biofeedback. 2003;28:13–23. doi: 10.1023/a:1022312815649. [DOI] [PubMed] [Google Scholar]
- 47.Stickland MK, Morgan BJ, Dempsey JA. Carotid chemoreceptor modulation of sympathetic vasoconstrictor outflow during exercise in healthy humans. J Physiol. 2008;586:1743–1754. doi: 10.1113/jphysiol.2007.147421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47a.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 48.Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–555. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- 49.Tulppo MP, Kiviniemi AM, Hautala AJ, Kallio M, Seppanen T, Makikallio TH, Huikuri HV. Physiological background of the loss of fractal heart rate dynamics. Circulation. 2005;112:314–319. doi: 10.1161/CIRCULATIONAHA.104.523712. [DOI] [PubMed] [Google Scholar]
- 50.Usui K, Bradley TD, Spaak J, Ryan CM, Kubo T, Kaneko Y, Floras JS. Inhibition of awake sympathetic nerve activity of heart failure patients with obstructive sleep apnea by nocturnal continuous positive airway pressure. J Am Coll Cardiol. 2005;45:2008–2011. doi: 10.1016/j.jacc.2004.12.080. [DOI] [PubMed] [Google Scholar]
- 51.Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- 52.Victor RG, Leimbach WN, Jr, Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension. 1987;9:429–436. doi: 10.1161/01.hyp.9.5.429. [DOI] [PubMed] [Google Scholar]
- 53.Victor RG, Pryor SL, Secher NH, Mitchell JH. Effects of partial neuromuscular blockade on sympathetic nerve responses to static exercise in humans. Circ Res. 1989;65:468–476. doi: 10.1161/01.res.65.2.468. [DOI] [PubMed] [Google Scholar]
- 54.Wallin BG, Charkoudian N. Sympathetic neural control of integrated cardiovascular function: insights from measurement of human sympathetic nerve activity. Muscle Nerve. 2007;36:595–614. doi: 10.1002/mus.20831. [DOI] [PubMed] [Google Scholar]
- 55.Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R, Jennings G. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol. 1992;453:45–58. doi: 10.1113/jphysiol.1992.sp019217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weise F, Laude D, Girard A, Zitoun P, Siche JP, Elghozi JL. Effects of the cold pressor test on short-term fluctuations of finger arterial blood pressure and heart rate in normal subjects. Clin Auton Res. 1993;3:303–310. doi: 10.1007/BF01827331. [DOI] [PubMed] [Google Scholar]
- 57.Wirch JL, Wolfe LA, Weissgerber TL, Davies GA. Cold pressor test protocol to evaluate cardiac autonomic function. Appl Physiol Nutr Metab. 2006;31:235–243. doi: 10.1139/h05-018. [DOI] [PubMed] [Google Scholar]