Abstract
Recovery from emotional challenge and increased tolerance of negative affect are both hallmarks of mental health. Mindfulness training (MT) has been shown to facilitate these outcomes, yet little is known about its mechanisms of action. The present study employed functional MRI (fMRI) to compare neural reactivity to sadness provocation in participants completing 8 weeks of MT and waitlisted controls. Sadness resulted in widespread recruitment of regions associated with self-referential processes along the cortical midline. Despite equivalent self-reported sadness, MT participants demonstrated a distinct neural response, with greater right-lateralized recruitment, including visceral and somatosensory areas associated with body sensation. The greater somatic recruitment observed in the MT group during evoked sadness was associated with decreased depression scores. Restoring balance between affective and sensory neural networks—supporting conceptual and body based representations of emotion— could be one path through which mindfulness reduces vulnerability to dysphoric reactivity.
Keywords: sadness, mindfulness, fMRI, reactivity, depression
In Shakespeare’s Macbeth, a noble chastises a fearful messenger to “give sorrow words: the grief that does not speak; whispers the o’er-fraught heart and bids it break” (Oxford Version, 4.3.191). It seems a common wisdom that to deal with a negative emotion one must “give it voice,” to articulate emotional experience in a way that can temper its impact. Indeed, studies of emotion regulation have shown that initial reactions to negative affect can determine the intensity of subsequent distress, with important differences between the individual strategies evoked (Gross, 2002). For example, reappraisal, in which a negative feeling is recast more optimistically or viewed from a third person perspective, has been shown to effectively reduce the intensity of fear and anxiety (Ochsner et al., 2004). Yet regulatory reactions to emotion may also be maladaptive, as in the cases of rumination or self-focused attention, in which elaborating on negative affect ironically perpetuates the very mood it is intended to reduce (Nolen-Hoeksema, 2000; Watkins, Moberly, & Molds, 2008). When one’s best regulatory efforts only exacerbate the stress of an emotional challenge, how does one break the dysphoric cycle?
Neuroimaging investigations of emotion regulation have identified a constellation of prefrontal regions associated with active reappraisal of the emotional salience of events (see Ochsner & Gross, 2008, for a review). Studies of sadness provocation in both clinical and at-risk samples show altered activation in these same regions, notably the medial prefrontal, orbital frontal and subgenual cingulate cortices (Keightley et al., 2003; Liotti, Mayberg, McGinnis, Brannanm, & Jerabek, 2002). Critically, dysphoria-linked changes in the configurations of prefrontal engagement may signal compromised cognitive control over emotion (Johnstone, van Reekum, Urry, Kalin, & Davidson, 2007; Ramel et al., 2007), suggesting perseverative efforts to regulate negative emotion without commensurate relief. Taken together, clinical and experimental data suggest that chronic depressed mood or clinical depression may stem in part from an impaired ability to regulate emotion rather than a lack of reappraisal effort per se (Segal et al., 2006).
If dysphoric reactions following reappraisal failure explain why some individuals become overwhelmed by negative emotion, one approach to reducing dysphoric reactivity is to develop metacognitive skills for tolerating and approaching negative affect from a wider attentional frame (Teasdale et al., 2002). Supportive evidence comes from studies in which increased metacognitive awareness of emotions was associated with reduced endorsement of dysfunctional cognitions following sadness challenge (Fresco, Segal, Buis, & Kennedy, 2007), reduced cognitive processing of negative material in present moment awareness (Frewen, Evans, Maraj, Dozois, & Partridge, 2007), and an increased willingness to tolerate negative affect (Arch & Craske, 2006). Increases in meta-cognitive awareness have been reliably linked to the practice of mindfulness meditation, which is now taught through a number of clinical protocols for the treatment of depressive, anxiety, and chronic pain disorders (Kabat-Zinn, 1990; Segal, Williams, & Teasdale, 2002).
Critically, mindfulness training purports to regulate affect without altering the momentary subjective experience of emotion. Mindfulness may differ from traditional reappraisals, which are based on the view that affective distress is problematic and requires cognitive alteration. The distinction between the mindful and reappraisal approaches may be better understood with reference to pain research, in which distinct neural systems subserve the affective and sensory components of pain appraisal (e.g., Singer et al., 2004). Although the somatosensory cortices, thalamus, and the posterior insula provide discriminative information about nociceptive stimulus sensation, cortical midline structures such as the anterior cingulate support affective appraisals related to perceived unpleasantness (Craig, 2002). Applying this model, reappraisal processes focus on altering affective judgments and evaluation, whereas mindfulness practice may focus attention on emotions as transitory sensory responses. Following mindfulness meditation, negative emotions may be experienced more as fluctuations in body state sensations and less as affective mental states reflecting what is good or bad for the self. Thus, mindfulness training may reduce chronic reactivity by shifting attention away from subjective appraisals of affect, toward the incorporation of more sensory-based representations of emotions.
Supporting this altered process of self-reflection, Farb et al. (2007) used fMRI to study how mindfulness trainees and wait-listed controls differed in their capacities for self-representation. While freely engaging in self-referential thought, individuals exhibited distinct engagement of cortical midline structures, including the pregenual dorsomedial prefrontal (dmPFC) and posterior cingulate cortices, regions associated with the affective appraisal of events as good or bad for the self (e.g., Northoff & Bermpohl, 2004; Ochsner et al., 2004). By contrast, mindfulness practitioners engaging in a metacognitive focus exhibited a pronounced shift away from midline cortical activation toward a right-lateralized network comprised of the ventral and dorsolateral PFC, as well as sensory representations in the insula and secondary somatosensory cortices. These regions may support more detached, objective interoceptive, and somatic awareness (Craig, 2002) that may serve as the primitive sensory representations of the “self.” Through repeated observation of affective states as “objects” of attention (Creswell, Way, Eisenberger, & Lieberman, 2007), participants learn that emotions have their own somatic signatures, whose fluctuations in intensity and duration, provide a continuous cue for refocusing attention (Brefczynski-Lewis, Lutz, & Schaefer, 2007; Jha, Krompinger, & Baime, 2007), especially when allocation of scarce mental resources to maladaptive, ruminative processing has been the default (Slagter et al., 2007).
The present study investigated the effects of mindfulness training (MT) on emotional experience and its neural expression. Although MT has been associated with increases in self-reported well-being (see Grossman, Niemann, Schmidt, & Walach, 2004, for a meta-analysis), it remains unclear whether and how such training influences the neural representations of emotional experience. Evidence of reduced self-referential activity in favor of integrating information from viscerosomatic cortices would suggest that through this training, practitioners acquire emotion regulation skills that reduce their affective reactivity to stressful or challenging experiences. To examine the neural effects of mindfulness training on emotional reactivity, we employed fMRI to compare neural responses to sadness provocation in participants who completed a Mindfulness-Based Stress Reduction Program and waitlisted controls.
Method
Participants
Participants were recruited on enrolment in the Mindfulness-Based Stress Reduction program (MBSR; Kabat-Zinn, 1990) at St. Joseph’s Health Centre in Toronto, Canada. All participants were right-handed volunteers that gave informed consent to procedures that were approved by the St. Joseph’s Health Centre and Sunny-brook and Women’s College Health Sciences Clinical Ethics Committees. There were 36 participants in total, of which 16 participants were randomly assigned to the waitlisted control group (controls) and were scanned before they attended MBSR, and 20 participants were randomly assigned to the mindfulness training group (MT) and were scanned after they completed MBSR. Participants were predominantly self-referred and were enrolled in the MBSR program following self-initiated registration with the Stress Reduction Clinic at St. Joseph’s Health Centre.
Measures
BDI–II
The Beck Depression Inventory–Second Edition (BDI–II, Beck et al., 1996) is a 21-item, 4-point Likert scale style questionnaire measuring depressive affect and associated behavioral symptoms, with high validity and reliability. Scores range from 0 to 63, with clinical cutoff of 17 to 20, with an internal reliability of alpha greater than .9 across both psychiatric and nonpsychiatric samples.
BAI
The Beck Anxiety Inventory (BAI, Beck & Steer, 1990) is a 21 item, 4-point Likert scale designed to assess multiple-behavioral and cognitive aspects of anxiety; scoring, validity, and reliability are all similar to the BDI.
SCL–90 –R
The Symptom Checklist 90 Revised (SCL–90 –R, Derogatis, 1994) is a 90-item, 5-point Likert scale used to broadly canvass participant psychopathology across multiple-positive symptom dimensions, with high (α > .8) internal reliability in most samples.
Training Protocol
MBSR was delivered by an instructor in eight weekly 2-hr group sessions involving up to 25 participants. Mindfulness was taught through lying, sitting, walking, or eating meditations and yoga. The program included daily homework of CD-guided and self-guided mindfulness practices, designed to increase moment-by-moment nonjudgmental awareness of bodily sensations, thoughts, and feelings, together with the application of awareness skills into daily life. Participants were encouraged to cultivate an acceptant mode of response to experience, in which they intentionally face difficulties and discomfort, and to develop a meta-cognitive perspective on thoughts and feelings, in which these are viewed as passing events in the mind.
Sadness Provocation
Participants viewed four sets of film clips, with audio. Each set of clips came from a different source; neutral clips from TV programs on gardening and woodworking, and sad clips from the films The Champ (1979) and Terms of Endearment (1983). Sets of clips were 3-min long and were edited into four 45-s clips. Clips were shown in their original order from each film with an interspersed 30-s reflection period between clips. At the end of each reflection period participants had 6 s to rate their level of sadness on a 5-point Likert scale (1 = not at all sad; 5 = extremely sad). Sets of film clips were always shown in the order neutral/sad/neutral/sad to limit the amount of emotional contagion between blocks.
Procedure
The experiment was conducted in two runs, with one set of neutral and sad films (composed of 4 clips each) presented in each run. A sample run would involve presentation of the four neutral film clips (45 s each) interleaved with rest periods between each clip (30 s each), followed by a resting state period for 36 s, then followed by four sad film clips interleaved with rest periods. Prior to each set of film clips a 10-s instruction screen appeared to orient the participant to the viewing task. The resting periods at the beginning, middle, and end of each run constituted the basis for the implicit baseline used to investigate the independent effects of sad and neutral films. Questionnaire data was collected in separate interviews at St. Joseph’s Health Centre.
Image Acquisition
Images were collected on a 3-T Signa MRI system (CV/i hard-warnme, LX8.3 software; General Electric Medical Systems, Waukesha, WI) with a standard quadrate birdcage head coil. An fMRI was conducted using T2*-weighted single-shot spiral in-out k-space trajectories optimized for sensitivity to the blood-oxygenation-level-dependent (BOLD) effect (TE/TR/flip angle = 30 ms/2000 ms/70 degrees, 20 cm field-of-view (FOV), 5 mm slice thickness, 64 by 64 matrix, 26 slices in axial oblique orientation.
Image Analysis
Data preprocessing and analysis were performed using Statistical Parametric Mapping (SPM5; Friston et al., 2007). Time series functional data was spatially coregistered and motion-corrected, and then normalized into a common stereotactic reference space (MNI) and spatially smoothed (FWHM = 6 mm). FMRI responses to each event were modeled by a canonical hemodynamic response function scaled to film clip duration. At the first level of analysis (within-subject), contrast images were calculated between sad and neutral film conditions; the ensuing contrast maps were then entered in a separate second-level random effects analysis for each group to investigate neural response to emotion challenge. Follow-up comparisons of each condition to a common baseline were made by comparing sad and neutral film clip activity to SPM’s implicit baseline, which includes all unmodeled time points, that is, task unrelated activity between film viewing periods. For the between-group differences in emotional challenge reactivity, participant sad versus neutral difference maps generated at the first level of analysis were subjected to a two-way mixed analysis of variance (ANOVA) between the control and MT groups and the neutral and sad film viewing conditions. Cluster thresholding was applied to increase power in identifying heavily recruited neural areas while maintaining a reasonably low Type-I error rate. Only clusters of activation yielding k > = 10 voxels at the uncorrected activation thresholds of p < .001 were considered, yielding a family-wise error rate of p < .05 (Forman et al., 1995).
To further characterize between-groups differences in emotional reactivity, functionally defined region of interest (ROI) clusters were identified from the between-groups independent samples t test. ROIs were constructed using a 6 mm radius sphere around the peak voxels from the ROI-identifying contrast, and extracting the mean signal from this sphere from both the sad and neutral film conditions. To relate altered neural activity to patient with patient symptom measures, task-related signal from each of these ROIs was tested for correlation with the BDI-II, BAI, and the SCL–90–R.
Results
Emotion Ratings
Mood ratings were collected over four time points for both neutral and sad films. Data was subjected to a 2 (control vs. MT) × 2 (sad vs. neutral film) × 4 (time point) mixed model ANOVA. Main effects of sadness, F(1, 34) = 206.73, p < .001, suggested that the sad films induced a reliably greater dysphoric mood than the neutral films. A main effect of time point was also found, F(3, 102) = 28.67, p < .001, which was qualified by an interaction between film type and time point, F(3, 102) = 33.36, p < .001; simple effect analyses suggested that sadness increased on viewing consecutive sad, but not neutral, film clips. Sadness ratings did not differ between the control and MT groups, F(1, 34) = .714, p = .403.
Clinical Effects of MT
Control and MT participants did not differ in age or gender distribution. All participants showed moderate levels of depression and anxiety, according to the clinical cut-offs on the BDI-II (Beck & Steer, 1987), BAI (Beck & Steer, 1990), and SCL–90–R (Derogatis, 1994), with no significant differences between the groups prior to MT. The MT group showed substantial reductions in depression, anxiety, and somatic distress following training (see Table 1). Thus, although both groups demonstrated equivalent and moderately high pretraining levels of self-reported psychopathology, the MT group showed significant training-related improvements on all three scales.
Table 1.
Levels of Depression and Anxiety in Control and MT Patients
| Variable | Age | (±SD) | Gender (M/F) | BDI–II | (±SD) | BAI | (±SD) | SCL–90R | (±SD) |
|---|---|---|---|---|---|---|---|---|---|
| Both groups at pretraininga | |||||||||
| Controlb | 42.00 | 4/12 | 20.56 | (13.10) | 13.38 | (8.49) | 79.88 | (50.41) | |
| MTc | 45.55 | 5/15 | 23.35 | (13.92) | 16.35 | (12.66) | 108.25 | (66.52) | |
| t(34) | 0.94 | ns | 0.62 | ns | 0.84 | ns | 1.45 | ns | |
| MT group training effects (pre- vs. post-MT) | |||||||||
| Posttraining | 6.58 | (5.67) | 9.79 | (9.82) | 55.63 | (50.13) | |||
| Difference score | −15.84 | (11.04) | −5.32 | (6.64) | −47.00 | (39.44) | |||
| t(19) | 6.25 | p < .001 | 3.49 | p < .003 | 5.19 | p < .001 | |||
Note. MT = mindfulness training; BDI–II = Beck Depression Inventory Revised; BAI = Beck Anxiety Inventory; SCL–90 –R = Symptom Checklist 90 Revised; F = female; M = male; ns = not significant.
N = 36.
n = 16.
n = 20.
Neural Correlates of Sadness Provocation
We first examined the neural correlates of sadness in the control group (Figure 1A). Sadness provocation revealed a midline network of activation associated with ruminative and self-reflective processing (Supplementary Table 1); including the ventral medial prefrontal cortex (vmPFC, BA 11), dorsal medial prefrontal cortex (dmPFC, BA 9/32), and a wide expanse of the posterior cingulate extending from the retrosplenial cortex (BA 29) dorsally through to the precuneus (BA 23). Induced sadness was also associated with predominantly left-sided activations, including the left dorsolateral prefrontal cortex extending into the left operculum including Broca’s area (BA 9/10/46/47), left midtemporal gyrus (BA 21), and in bilateral caudate, as well as on the right hippocampus, cerebellum, and temporal parietal junction (BA 41/42).
Figure 1.
Regional activation (a) and deactivation (b) in response to sadness provocation in the control group (sad vs. neutral within-group contrast). Panel C provides some examples of both activation (Precuneus and mPFC) and deactivation (right insula) in the control group relative to a resting state baseline; activation/ deactivation is computed as the difference score between sad and neutral film clip activity. Pcn = precuneus; mPFC = medial prefrontal cortex; Opc = operculum; STS = superior temporal sulcus; Som = primary and secondary somatosensory cortices; rLPFC = right lateral prefrontal cortex; mCing = middle cingulate gyrus; sPar = superior parietal lobule; Ins = insula; Fus = fusiform; red clusters = sad > neutral (neu); blue clusters = neutral > sad.
Mood provocation also led to robust cortical deactivations (reductions relative to the neutral film condition) in the right viscerosomatic cortices, peaking in the right insula and extending rostrally into the anterior right amygdala and subgenual anterior cingulate cortical (subgenual ACC) regions (BA 25), and caudally into the right superior temporal gyrus (BA 22) (Figure 1B). Deactivations were also found bilaterally in superior parietal regions (BA 7/40), extending rostrally and medially into the midcingulate (BA 24) and somatosensory cortices (BA 3/4/5). Several other deactivated clusters were detected, including the dorsal and ventral extents of the right lateral prefrontal cortex (BA 45– 47), left dorsolateral prefrontal cortex (BA 46), the bilateral parahippocampal gyrus (BA 36/37) extending on the left side through the fusiform gyrus (BA27), and in the right superior temporal gyrus (BA 21). Several deactivations in bilateral motor and supplementary motor regions were also observed (BAs 4 & 6).
Effects of MT on the Neural Correlates of Sadness Provocation
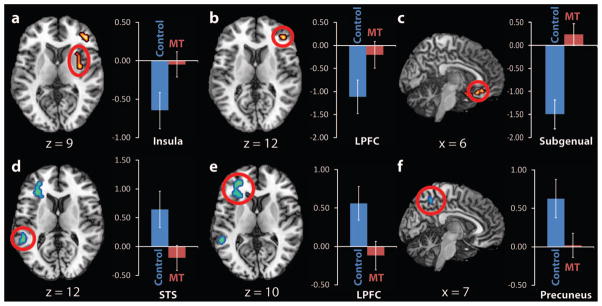
To examine the effects of MT on induced sadness, the sad film versus neutral film viewing differences were contrasted between the control and MT groups (see Supplementary Table 2 for areas significantly related to sadness in the MT group). These analyses revealed areas reliably linked to sadness provocation, in which reactivity was also sensitive to mindfulness training (Table 2, Figure 2). Primarily two classes of between group difference were observed, both of which indicated reduced reactivity to the sadness challenge: (a) areas deactivating with sadness provocation (deactivating regions) in controls, but not in the MT group and (b) areas activating with sadness provocation (activating regions) in controls, but not in the MT group.
Table 2.
Differences in Regional Activation and Deactivation Between Control and MT Groups in Response to Sadness Provocation
| Anatomic region | BA | Side | Cluster size | Peak Z | x | y | z (mm) |
|---|---|---|---|---|---|---|---|
| MT > control (training-related increases) | |||||||
| Insula | 48 | R | 43 | 4.07 | 33 | −6 | −6 |
| Lateral prefrontal cortex | 46 | R | 13 | 3.90 | 42 | 42 | 9 |
| Subgenual ACC/gyrus rectus | 11 | R | 11 | 3.87 | 6 | 15 | −24 |
| Subgenual ACC/gyrus rectus | 25 | R | 13 | 3.57 | 3 | 27 | −18 |
| Thalamus (pulvinar) | L | 11 | 3.35 | −12 | −27 | 6 | |
| Control > MT (training-related decreases) | |||||||
| Superior temporal sulcus | 22 | L | 32 | 4.26 | −54 | −42 | 12 |
| Lateral prefrontal cortex | 10 | L | 13 | 3.87 | −30 | 45 | 9 |
| Middle temporal gyrus | 21 | L | 18 | 3.65 | −45 | −21 | −9 |
| Precuneus | 7 | R | 15 | 3.57 | 6 | −51 | 51 |
Note. MT = mindfulness training; R = right; L = left.
Figure 2.
Differences in regional activation and deactivation between control and mindfulness training (MT) groups in response to sadness provocation (the interaction between-groups and sad vs. neutral within-group contrasts). Top panel: sadness-related deactivations in the control group with nonsignificant activity in the MT group (red clusters = MT > control); bottom panel: sadness-related activations in the control group with nonsignificant activity in the MT group (blue clusters = control > MT). Error bars are 95% confidence intervals. LPFC = lateral prefrontal cortex; STS = superior temporal sulcus/Wernicke’s area.
Deactivating regions
The MT group showed greater signal intensity relative to controls in the right basal ganglia extending dorsolaterally into the right insula and claustrum, the right subgenual ACC (BA 25) and gyrus rectus (BA 11) extending into the ventromedial prefrontal cortex, the right ventrolateral prefrontal cortex (BA 45), and the right superior frontal gyrus (BA 9). Follow up ROI analyses revealed that in areas in which sadness evoked deactivations in controls, there was little neural reactivity in the MT group, that is, there were few areas with distinct levels of activation during sad relative to neutral film clip viewing. This indicated that regions of relatively increased activity in MT represent a recovery from the deactivations related to sadness found in controls (see Figure 2).
Subsequent analyses of deactivating regions using the implicit baseline (composed of resting state activity between film clips) demonstrated that variance from the resting state in areas such as the right insula was much greater in the control group than in the MT group, with both reliable activations and deactivations from the global mean during sad film clip viewing in the control group, t(15) > 2.19, p < .05, but with no reliable changes in the MT group (Figure 1, Panel C). Similar profiles of higher neutral film activation and sad film deactivation versus the global mean were also observed in the subgenual and ventrolateral cortices. These results support the notion that the deactivating regions observed were driven by generalized greater reactivity in the control group, as the source of this reactivity seems to stem from both elevated activation in the neutral film clip condition, and deactivations in the sad film clip condition.
Activating regions
The MT group also showed reductions relative to controls in posterior cortical midline areas such as the right precuneus/posterior cingulate (BA 7/31), and left-lateralized reductions in the prefrontal cortex (BA 45) and frontal operculum/ Broca’s area (BA 47), superior temporal Sulcus/Wernicke’s area (BA 42/22) and inferior temporal gyrus (BA 21). ROI analyses revealed that the deactivations in MT represent a reduction of activations related to sadness (see Figure 2). Subsequent analyses of activating regions using the global mean revealed that although neutral films led to equivalent deactivation relative to the resting state between the two groups, t(34) = 0.2, ns, sad films reliably activated areas such as the precuneus and mPFC, and the degree of activation was reliably greater in the control than the MT group, t(34) = 3.40, p < .002, suggesting that it is primarily the sad film condition driving the reactivity scores (Figure 1, Panel C for the control group).
Post hoc comparisons
To more sensitively assess training-related changes, a more liberal post hoc analysis (p < .05) was conducted restricted to the reliable regions of reactivity in the control group (control ROIs; Supplementary Table 1) to determine whether these established regions of emotional reactivity were differentially recruited in the MT group. In addition to the areas described in the between-groups analysis above (see Table 2), several other left-lateralized activating control ROIs demonstrated reduced activity in the MT group, including Broca’s area (BA 47/48), the left ventromedial prefrontal cortex (BA 11), right temporal junction (BA 41/42), and right cerebellum. Of the deactivating control ROIs, the somatosensory/middle cingulate clusters (BA 3/4/5/40) bilaterally demonstrated reduced deactivations, as well as some additional right-lateralized clusters such as the supplementary motor area (BA 6), and superior temporal (BA 21) clusters. By contrast, none of the motor or parahippocampal clusters showed between-groups differences in reactivity.
To assess the presence of novel reactivity following mindfulness training, the same ROI-based between-groups comparison was performed on clusters of reactivity in the MT group (MT ROIs; Supplementary Table 2). Relatively few of the MT ROIs demonstrated altered activity between groups compared to the number of control ROIs. Only the left hippocampus and right inferior orbitofrontal (BA 47) clusters demonstrated reliably greater reactivity in the MT group, and only the left inferior frontal gyrus (BA 9) and right cerebellum demonstrated greater deactivations in the MT group.
Effects of MT on neural reactivity and clinical symptoms
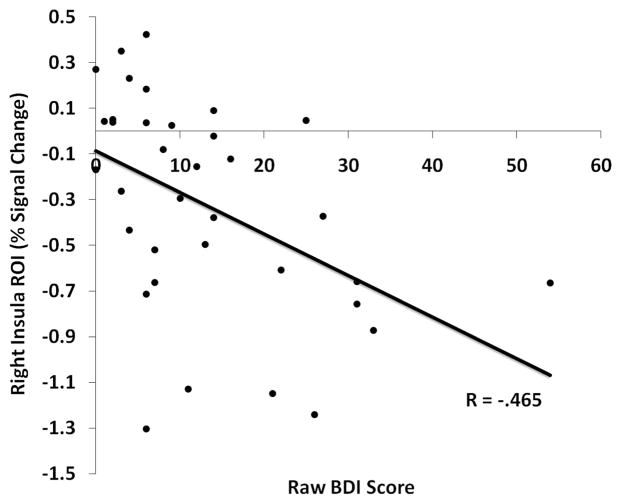
Correlation analyses was run using each participant’s mean signal scores from the reactivity ROIs and their BDI, BAI, and SCL–90 –R scores, thus capturing between-groups variance in clinical outcome. One participant was excluded from the MT group for not providing posttraining outcome scores. Of the three scales, only the BDI was reliably correlated with the ROIs, demonstrating a negative correlation with the right insula ROI, r(34) = −.465, p < .005, and the right LPFC, r(34) = −.406, p < .02, such that higher BDI scores were related to greater deactivations in these regions (see Figure 3). A positive correlation was also detected between BDI scores and left superior temporal sulcus, r(34) = .542, p < .001, in an area corresponding to Wernicke’s area. Notably, the right insula and right LPFC ROIs, both sadness-deactivating regions, were negatively correlated with activity in the Wernicke’s ROI, a sadness-activating region, r(34) > −.501, p < .001, suggesting an opponent relationship between left-sided language and right-sided interoceptive areas. No ROIs demonstrated correlations with symptom change scores in the MT group, nor with pretraining scale scores.
Figure 3.
Correlation plot between the right insula percentage signal change from all participants (control and mindfulness training [MT]) and their raw Beck Depression Inventory (BDI) scores. Greater BDI-II scores at time of functional MRI scan predict greater reductions of activity in the right insula region of interest (ROI).
Discussion
This study represents the first neuroimaging investigation of changes in emotion regulation associated with MT. Sadness provocation was associated with elevated self-reported ratings of sadness, accompanied by activation along the posterior and anterior regions of the cortical midline, as well as in left-lateralized language and conceptual processing centers. These cortical areas are characteristic of cognitive elaboration, increased self-focus, and ruminative problem solving that would be typical of reappraisal processes (Ochsner & Gross, 2008; Ray, Wilhelm, & Gross, 2008). Sadness provocation was also associated with widespread deactivations in posterior parietal attentional regions, somatosensory cortex, right insula and right ventral, and dorsolateral prefrontal regions, as well as the subgenual anterior cingulate (ACC), areas that may contribute information important to the primary appraisal of emotion (Craig, 2002). In the context of work on different forms of self-focus (Farb et al., 2007), such findings are consistent with the notion of a cognitively evaluative neural network responding to emotion challenge, accompanied by the simultaneous deactivation of a viscerosomatic-centered experiential network (see Figure 1).
Examining the effects of MT, we found that despite similar levels of self-reported dysphoria, the MT group demonstrated less neural reactivity to sadness provocation than the control group. Although both groups showed some recruitment of sadness-related activation along midline cortical areas associated with self-referential processing, the MT group demonstrated reduced reactivity both medial and lateral regions. In particular, the MT group demonstrated reduced activation in the cortical midline regions associated with autobiographical memory retrieval and self-referential processing (Cavanna & Trimble, 2006), and in classical language areas like the left posterior superior temporal gyrus (Wernicke’s area) and the left frontal operculum (Broca’s area). The MT group also demonstrated reduced deactivations, in that the lower BOLD response during sad relative to neutral films was significant in the control group, but not in the MT group. The presence of deactivating regions suggests that part of the neural response to emotional challenge may involve the down regulation of these regions. Specifically, the reduced deactivation in the insula during dysphoric challenge may therefore be associated with increased interoceptive awareness (Critchley et al., 2004), whereas altered subgenual ACC activity, a region of strong anatomical connections with the insular cortex (Barbas, Saha, Rempel-Clower, & Ghashghaei, 2003), has also been observed in the recovery of patients with mood disorders (Goldapple et al., 2004; Figure 2). Although there are several plausible accounts for reduced neural reactivity in the MT group, similar self-reports of sadness across both groups makes it unlikely that these effects are simply due to a blunting of emotional experience or impairment in affective processing, but instead suggests a more fundamental change in regulatory response. The equivalence of sadness ratings between groups also undermines the suggestion of demand characteristics at play, implying that the change in regulatory response is not simply a product of participant expectations. Recent research has associated mindfulness training with reductions in emotional interference following the viewing of affective pictures (Ortner, Kilner, & Zelazo, 2007). The neural patterns observed in the present study suggest that the reduced emotional interference associated with mindfulness may stem from the objectification of emotion as innocuous sensory information rather than as an affect-laden threat to self requiring a regulatory response. A plausible mechanism of action for mindfulness effects may include the development of metacognitive skills for detached viewing of emotions, rather than the elaboration of emotional content through cognitive reappraisal.
Volitional emotion regulation is traditionally predicated on cognitive strategies for reappraising aversive events in unemotional terms, or behavioral strategies for suppression of emotion-expressive behavior (Goldin, McRae, Ramel, & Gross, 2008; Schmitz & Johnson, 2007). However, a number of studies now have pointed to compromised medial frontal modulation of limbic circuitry as underlying ineffective regulation of negative emotion (Johnstone et al., 2007; Ressler & Mayberg, 2007). Compared to controls, effortful down regulation of sadness via reappraisal is rated as more difficult by patients with mood disorder (Keightley et al., 2003) and is shown to be less successful in decreasing limbic activation (Craig, 2002). In the presence of these disrupted neural circuits, especially in at-risk populations, strategies for generating positive affective appraisals may paradoxically reactivate existing negative appraisals. The use of MT to shift regulation strategies from medial and left-lateralized cortical regions supporting cognitive-affective representations of the self to more lateral viscerosomatic representations of body state may be one such example. In the present study, left-lateralized language areas were activated during mood challenge in controls, and such activation was negatively correlated with right insula recruitment. The MT group by contrast, showed reduced reactivity in both regions, resulting in an attenuation of the negative relationship between the two regions. Disengaging reappraisal of negative affective content, in favor of engaging attention toward sensory integration, would allow for the generation of novel affective appraisals rather than attempting to manipulate an existing negative cognitive set (Garland, 2007).
The idea that interoceptive recovery is important for successful emotion regulation is strongly supported by the negative correlation observed between BDI scores and right insula activation: This finding suggests that the participants experiencing the least dysphoria actually possessed the highest interoceptive activation in context of a dysphoric emotional challenge (see Figure 3). Furthermore, right insula and right LPFC activity was negatively correlated with activity in Wernicke’s area, suggesting that there may be some tradeoff between language and interoception-based reactions to mood challenge, with more language-laden regulatory efforts predicting higher BDI scores. Mindfulness training may reduce reliance on secondary appraisal systems in response to dysphoric challenge, an imbalance that predicts depressive symptoms.
Several limitations are apparent in the interpretation of the present data. Although the present findings are based on comparisons among randomly assigned pre- and posttreatment participant groups, testing the same individuals before and after treatment would have warranted stronger conclusions regarding the temporal effects of mindfulness training, but at the expense of controlling for carryover effects. In addition, gauging the frequency of mindfulness practice over the 8-week program would have permitted testing the relationship between mindfulness practice and the establishment of neural balance in response to affective challenge. The variance in participant practice over an 8-week period was, however, not large enough to be used as a predictor of training outcomes as most participants reported similar levels of practice during the course, approximately 75% adherence to assigned homework hours each week. Although the present results help to explain the effects of mindfulness on affective reactivity in a mixed community sample, recruiting a carefully diagnosed clinical sample would have enabled us to answer specific questions about emotion regulation in clinical depression or other disorders. Similarly, comparisons against an active control group would be needed to tease apart alternative explanations of enhanced emotion regulation, such as group support and destigmatization, which are unrelated to MT. Nevertheless, this study is the first of its kind to implicate specific neural mechanisms of emotion regulation as potentially underlying the effects of mindfulness training, and can inform these future efforts.
Affective reactivity, seen in the combination of cortical midline and language area recruitment and viscerosomatic suppression, characterized participants undergoing dysphoric mood provocation. By balancing participants’ regulatory responses to sadness with coordinated monitoring of less valenced and more sensory visceral information, mindfulness may represent one neural path for reducing affective reactivity and disorder vulnerability.
Supplementary Material
Acknowledgments
Supported by NIMH Grant MH66992, the Ontario Mental Health Foundation, and the Canadian Institutes for Health Research. Helen Mayberg received financial support from the Advanced Neuromodulation Systems, Consultant Intellectual Property Licensing.
Footnotes
Presented in part at the annual meeting of the Association for Behavioral and Cognitive Therapies in Philadelphia, November 2007.
There were no conflicts of interest for any authors.
Supplemental materials: http://dx.doi.org/10.1037/a0017151.supp
Contributor Information
Norman A. S. Farb, University of Toronto
Adam K. Anderson, University of Toronto
Helen Mayberg, Emory University.
Jim Bean, St. Joseph’s Health Centre, Toronto, Canada.
Deborah McKeon, St. Joseph’s Health Centre, Toronto, Canada.
Zindel V. Segal, Centre for Addiction and Mental Health, Toronto, Canada and University of Toronto
References
- Arch J, Craske M. Mechanisms of mindfulness: Emotion regulation following a focused breathing induction. Behaviour Research and Therapy. 2006;44:1849–1858. doi: 10.1016/j.brat.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neuroscience. 2003;4:25. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Steer R. Manual for the revised Beck Depression Inventory. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Beck A, Steer RA, Brown GK. Manual for the Beck Anxiety Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Brefczynski-Lewis J, Lutz A, Schaefer H. Neural correlates of attentional expertise in long-term mediation practitioners. Proceedings for the National Academy of Sciences USA. 2007;104:11483–11488. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A, Trimble M. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Craig A. How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Creswell J, Way B, Eisenberger N, Lieberman M. Neural correlates of dispositional mindfulness during affect labeling. Psychosomatic Medicine. 2007;69:560–565. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Derogatis L. SCL–90: Administration, scoring and procedures manual for the revised version. Baltimore, MD: Johns Hopkins University School of Medicine; 1994. [Google Scholar]
- Farb N, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, Anderson A. Attending to the present: Mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007;2:313–322. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman S, Cohen J, Fitzgerald M, Eddy W, Mintun M, Noll D. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnet Resonance in Medicine. 1995;33:636– 647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fresco D, Segal ZV, Buis T, Kennedy S. Relationship of posttreatment decentering and cognitive reactivity to relapse in major depression. Journal of Consulting and Clinical Psychology. 2007;75:447–455. doi: 10.1037/0022-006X.75.3.447. [DOI] [PubMed] [Google Scholar]
- Frewen P, Evans E, Maraj N, Dozois D, Partridge K. Letting go: Mindfulness and negative automatic thinking [Electronic version] Cognitive Therapy and Research. 2007;32:758–774. [Google Scholar]
- Garland EL. The meaning of mindfulness: A second-order cybernetics of stress, metacognition, and coping. Complementary Health Practice Review. 2007;12(15):15–30. [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H. Modulation of cortical-limbic pathways in major depression: Treatment-specific effects of cognitive behavior therapy. Archives of General Psychiatry. 2004;61:64–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Goldin P, McRae K, Ramel W, Gross J. The neural basis of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–291. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits: A meta-analysis. Journal of Psychosomatic Medicine. 2004;57:35– 43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- Jha A, Krompinger J, Baime M. Mindfulness training modifies subsystems of attention. Cognitive, Affective and Behavioral Neuroscience. 2007;7:109–119. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum C, Urry H, Kalin N, Davidson R. Failure to regulate: Counterproductive recruitment of top-down prefrontal subcortical circuitry in major depression. Journal of Neuroscience. 2007;27:8877– 8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full catastrophe living. New York, NY: Delacorte Press; 1990. [Google Scholar]
- Keightley M, Seminowicz D, Bagby R, Costa P, Fossati P, Mayberg H. Personality influences limbic-cortical interactions during sad mood induction. NeuroImage. 2003;20:2031–2039. doi: 10.1016/j.neuroimage.2003.08.022. [DOI] [PubMed] [Google Scholar]
- Liotti M, Mayberg H, McGinnis S, Brannanm S, Jerabek P. Unmasking disease-specific cerebral blood flow abnormalities: Mood challenge in patients with remitted unipolar depression. American Journal of Psychiatry. 2002;159:1830–1840. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology. 2000;109:504–511. [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structure and the self. Trends in Cognitive Science. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Ochsner K, Gross J. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K, Ray R, Cooper J, Robertson E, Chopra S, Gabrieli J, Gross J. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ortner CMN, Kilner S, Zelazo PD. Mindfulness meditation and emotional interference in a simple cognitive task. Motivation and Emotion. 2007;31:271–283. [Google Scholar]
- Ramel W, Goldin P, Eykel L, Brown G, Gotlib I, McQuaid J. Amygdala reactivity and mood-congruent memory in individuals at risk for depressive relapse. Biological Psychiatry. 2007;61:231–239. doi: 10.1016/j.biopsych.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Ray RD, Wilhelm FH, Gross JJ. All in the mind’s eye? Anger rumination and reappraisal. Journal of Personality and Social Psychology. 2008;94:133–145. doi: 10.1037/0022-3514.94.1.133. [DOI] [PubMed] [Google Scholar]
- Ressler K, Mayberg H. Targeting abnormal neural circuits in mood and anxiety disorders: From the laboratory to the clinic. Nature Neuroscience. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz T, Johnson S. Relevance to self: A brief review and framework of neural systems underlying appraisal. Neuroscience & Biobehavioral Reviews. 2007;31:585–596. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal Z, Kennedy S, Gemar M, Hood K, Pedersen R, Buis T. Cognitive reactivity to sad mood provocation and the prediction of depressive relapse. Archives of General Psychiatry. 2006;63:749–755. doi: 10.1001/archpsyc.63.7.749. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Williams M, Teasdale J. Mindfulness-based cognitive therapy for depression: A new approach to preventing relapse. New York: Guilford Press; 2002. [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Slagter H, Lutz A, Greischar L, Francis A, Nieuwenhuis S, Davis J, Davidson R. Mental training affects distribution of limited brain resources. PLoS Biol. 2007;5(6):e138. doi: 10.1371/journal.pbio.0050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale J, Moore R, Hayhurst R, Pope M, Williams S, Segal Z. Metacognitive awareness and prevention of relapse in depression: Empirical evidence. Journal of Consulting and Clinical Psychology. 2002;70:275–287. doi: 10.1037//0022-006x.70.2.275. [DOI] [PubMed] [Google Scholar]
- Watkins E, Moberly NJ, Moulds ML. Processing mode causally influences emotional reactivity: Distinct effects of abstract versus concrete construal on emotional response. Emotion. 2008;8:364–378. doi: 10.1037/1528-3542.8.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.