Abstract
Microbial sensory rhodopsins are a family of membrane-embedded photoreceptors in prokaryotic and eukaryotic organisms. Structures of archaeal rhodopsins, which function as light-driven ion pumps or photosensors, have been reported. We present the structure of a eubacterial rhodopsin, which differs from those of previously characterized archaeal rhodopsins in its chromophore and cytoplasmic-side portions. Anabaena sensory rhodopsin exhibits light-induced interconversion between stable 13-cis and all-trans states of the retinylidene protein. The ratio of its cis and trans chromophore forms depends on the wavelength of illumination, thus providing a mechanism for a single protein to signal the color of light, for example, to regulate color-sensitive processes such as chromatic adaptation in photosynthesis. Its cytoplasmic half channel, highly hydrophobic in the archaeal rhodopsins, contains numerous hydrophilic residues networked by water molecules, providing a connection from the photoactive site to the cytoplasmic surface believed to interact with the receptor's soluble 14-kilodalton transducer.
Over the past 4 years, microbial genomics has revealed a large family of photoactive, seven-transmembrane-helix retinylidene proteins called microbial rhodopsins in phylogenetically diverse species, including haloarchaea, proteobacteria, cyanobacteria, fungi, and algae (1–4). The first members of this family were discovered in halophilic archaea: the light-driven ion pumps bacteriorhodopsin and halorhodopsin and the phototaxis receptors sensory rhodopsins I and II. These four related haloarchaeal pigments are among the best-characterized membrane proteins in terms of structure and function, and nearly all of our knowledge of the properties of microbial rhodopsins, such as isomeric configuration and conformation of their chromophore, photochemical reactions, light-induced conformational changes in the protein, and function, derives from the study of these four, including atomic resolution structures that have been obtained for three of them (5–9). Studies of non-haloarchaeal rhodopsins, of which >800 are known to exist (10, 11), are needed to examine the diversity of properties of this widespread family (12). Anabaena sensory rhodopsin, a recently discovered sensory representative outside of archaea (2), is well suited for exploration. It is the only bacterial sensory rhodopsin so far expressed in a photoactive form. Unlike the haloarchaeal sensory rhodopsins, which transmit signals to other integral membrane proteins, its function appears to involve modulation of a soluble cytoplasmic transducer, analogous to animal visual pigments (2).
In this study, we report the structure of the retinal-complexed protein at 2.0 Å resolution, obtained by X-ray diffraction of crystals grown in a cubic lipid phase (table S1). The overall membrane-embedded seven-helical structure is similar to those of the archaeal rhodopsins. However, distinct differences in the photoactive site prompted analysis of the isomeric configuration of the retinal and the photochemical reactions of the pigment.
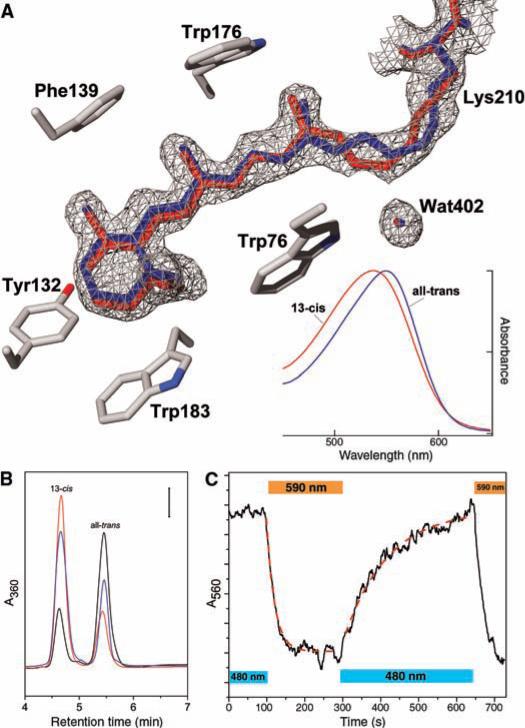
Despite intense white-light illumination [light adaptation (13)] of the crystals before cryocooling and X-ray data collection, which results in a fully all-trans retinal configuration in bacteriorhodopsin, maps of the retinal and Schiff base region of Anabaena sensory rhodopsin show electron density incompatible with 100% all-trans retinal (Fig. 1A). Subsequent extractions and chemical structure determinations of retinal isomers from orange-illuminated (580-nm) and blue-illuminated (480-nm) Anabaena pigment showed light-induced shifts of the isomeric configuration. In the fully dark-adapted state, the all-trans form [absorption maximum (λmax) of 549 nm in detergent-solubilized membranes] predominates [>75%, (Fig. 1B)]. Orange illumination rapidly shifted the pigment to a stable >80% 13-cis state (λmax of 537 nm), and blue light rapidly increased the all-trans content toward the dark-adapted isomer ratio (Fig. 1B). Therefore, the relative amounts of Anabaena sensory rhodopsin with cis and trans chromophore configurations depend on the quality (color) of illumination and are shifted between the two forms by pulses of orange and blue illumination (Fig. 1C). This photochromic property provides a possible mechanism for single-pigment color sensing. Its two distinct groundstate species thermally interconvert with halftimes of ~100 min and ~300 min for the trans and cis forms, respectively; this is a fundamental difference from that of another color-sensitive microbial rhodopsin, the archaeal phototaxis receptor sensory rhodopsin I (14). Such relatively long-lasting color sensitivity is similar to that of the red/far-red photochromic states of phytochrome and may be used, in the Anabaena cell in analogy to phytochrome (15–17), to control expression of proteins required under either orange-light or blue-light illumination. The photochromic reactions are also similar to those between 11-cis and all-trans forms of invertebrate visual pigments, which have been suggested to reset the 11-cis state in a light-dependent manner (18).
Fig. 1.
The electron densities indicate a mixture of retinal isomers. (A) Annealed electron density omit map contoured at 1σ with 13-cis,15-syn (in red) and all-trans,15-anti (in blue) retinal models. The density suggests a mixture of all-trans,15-anti and 13-cis,15-syn retinal after white-light illumination (13). The conjugated π system of the retinylidene is more bent than in archaeal sensory rhodopsin II, with the distance from the Schiff base nitrogen to the β-ionone C1 reduced to 11.6 Å from 12.2 Å. The increased bent also causes an increase, by 0.6 Å, in the distance between the two tryptophan side chains (Trp76 and Trp176) that sandwich the retinal in its binding site. (Inset) Absorption spectra of the 13-cis and trans forms of the pigment calculated from the measured spectra of the orange-illuminated and dark-adapted states using the isomer ratios depicted in (B). (B) Extraction of retinal isomers from orange-illuminated (580 ± 5 nm, 5 min, red line), blue-illuminated (480 ± 5 nm, 5 min, blue line), and dark-adapted (black line) pigments in detergent-solubilized (0.1% dodecylmaltoside) Escherichia coli membranes revealed a decrease of the fraction of 13-cis,15-syn retinal from 82% (orange) to 24% (dark). The units of the A360 axis (absorbance at 360 nm) are 2.0 × 10−3, 1.2 × 10−3, and 1.2 × 10−3 absorption units for the orange-illuminated, blue-illuminated, and dark-adapted samples, respectively. (C) Photoconversion between cis- and trans-forms under continuous monochromatic illumination. Absorbance at 560 nm, greater in the all-trans form compared to the 13-cis form, is used to monitor the wavelength-sensitive spectral transitions. The photoconversions follow approximately first-order kinetics as shown by the single exponential fits (dashed red curves) to the transitions during illumination through 10-nm band-pass interference filters centered at 590 nm or 480 nm, as indicated.
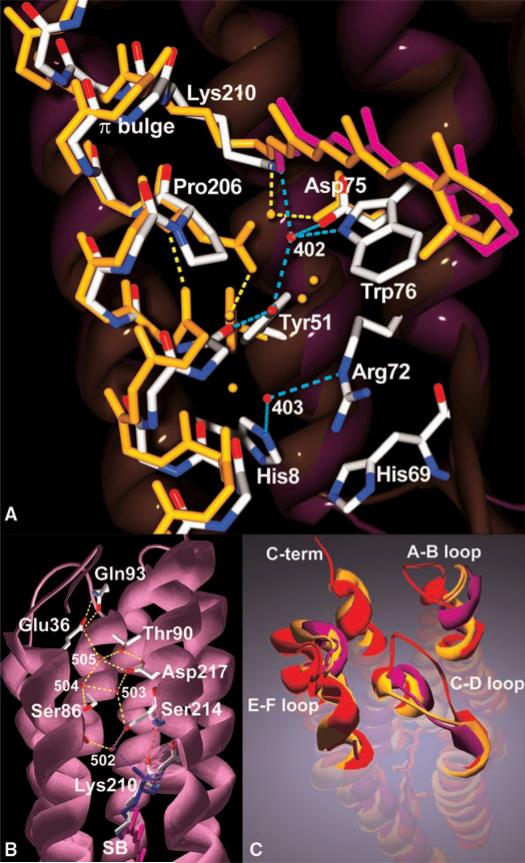
Further detailed structural analysis of the active site revealed two alternate conformations for Lys210. In one, its carbonyl oxygen forms a regular a-helical hydrogen bond with the peptide of Ser214; in the other, its hydrogen bond donor is a nearby water (Wat502) (Fig. 2B). Wat502 also connects helices B and G by bridging the hydroxyl of Ser214 with the backbone carbonyl of Ala40. Multiple conformations of the residue-210 peptide may be facilitated by the presence of a π bulge at residues Ser209, Lys210, and Val211, which is believed to soften the otherwise relatively rigid α helix (5, 19). A further reduction of the α-helical character of this region stems from the replacement of the aspartic residue at position 206 (anionic Asp212, which is part of the complex counterion in bacteriorhodopsin), highly conserved in archaeal rhodopsins, with a proline, Pro206 (Fig. 2A). Although the a helix on both sides of Pro206 is undisturbed by the loss of the peptide amide of the proline, the main-chain carbonyl of residue 202 accepts a hydrogen bond from the hydroxyl of reoriented Tyr51. In other microbial rhodopsin structures, the Tyr51 hydroxyl forms a strong hydrogen bond with the anionic aspartate carboxyl. The rearrangement also results in a 1.3 Å movement of Wat402, the water that bridges the protonated Schiff base and its counterion (5, 7, 20), toward the β-ionone ring of the retinal. Wat402 receives hydrogen bonds from the Schiff base (3.0 Å versus 2.6 Å in sensory rhodopsin II) and from the Trp76 indole while donating hydrogen bonds to the OD2 of Asp75 and, weakly, to the hydroxyl of Tyr51. Further toward the extracellular side, the flexible guanidinium side chain of Arg72 points away from the Schiff base and toward the extracellular side, as in archaeal sensory rhodopsin II (7); however, here Arg72 is flanked by two histidines (His69 and His8).
Fig. 2.
Structural differences with archaeal rhodopsins. (A) The extracellular half of Anabaena sensory rhodopsin is shown as purple ribbon with CPK-colored atoms, magenta retinal near the top right, red water molecules, and turquoise hydrogen bonds, with residue numbering according to its sequence. The extracellular surface is near the bottom. The largest differences appear around the Asp-to-Pro mutation at position 206. For comparison, archaeal sensory rhodopsin II is shown in orange throughout with yellow hydrogen bonds. (B) The cytoplasmic half of the protein is markedly more hydrophilic than those of other microbial rhodopsins. The cytoplasmic surface is located at the top of the image and the retinal near the bottom. The peptide plane between residues 210 and 211 displays two alternate conformations. In one, Lys210 C=O accepts a regular intrahelical hydrogen bond from residue 214 N-H (Lys210 shown in blue; hydrogen bonds are shown as red dashed lines). In the other, it accepts a hydrogen bond from Wat502 (hydrogen bonds are shown as blue dashed lines). This alternate conformation results in an ~55° change in the orientation of the 210 peptide bond C=O vector, with a movement of the Lys210 carbonyl oxygen by 1.8 Å. Only the latter conformation completes a hydrogen bond chain that leads from Lys210 C=O at the active site via Wat502, Ser214, OH, and three more ordered waters (Wat503, Wat504, and Wat505) held in place by the side chains of Asp217, Ser86 (two alternate side-chain conformations, only one of which is shown for clarity), and Thr90 to the cytoplasmic surface near Glu36 of helix B and Gln93 in the C-D loop. (C) A comparison of the loop structures that define the respective cytoplasmic surfaces reveals large differences between the surfaces of archaeal sensory rhodopsin II (orange) and bacteriorhodopsin (purple) and the surface of Anabaena sensory rhodopsin (red), which is thought to interact with its soluble transducer. In particular, the A-B and C-D loops of the Anabaena protein are packed entirely differently, with relative backbone movements of 10 Å and 7 Å, respectively. The C-D loop contains surface-exposed Phe94/Ile95, Lys96/Lys97, and Trp99 side chains, and because of a four-residue insertion relative to sensory rhodopsin II, the loop protrudes 6 Å further into the cytoplasmic space.
Comparison of the cytoplasmic half of Anabaena sensory rhodopsin with those of other microbial rhodopsins reveals markedly increased hydrophilicity in this region (Fig. 2B). The active site near the middle of the bilayer is connected to the cytoplasm via a hydrophilic path that contains at least four water molecules. A number of hydrophilic side chains interact with these water molecules to form an almost continuous hydrogen-bonded network from the Lys210 carbonyl to the cytoplasm over a distance of 19 Å: Lys210 – Wat502 – Ser214 – Asp217 of helix G; Ser86, Thr90, and Gln93 of helix C and the C-D loop; and Glu36 of helix B (Fig. 2B). In contrast, the cytoplasmic region of the haloarchaeal sensory rhodopsin II is entirely hydrophobic (7). Most notably, Phe86 in the archaeal protein occupies the space occupied by three water molecules and Ser86 in the center of the hydrophilic path of the Anabaena protein. This difference is consistent with the fundamentally different transducer interactions of the Anabaena photoreceptor (soluble transducer) and haloarchaeal photoreceptor (membrane-embedded transducer) (2). For the latter, the cubic lipid phase crystal structure was used to predict the membrane-embedded surface of transducer interaction (7), later confirmed by the crystal structure of the receptor bound to a transducer fragment (9). The soluble Anabaena transducer (2) is thought to interact through the receptor's cytoplasmic surface. In the Anabaena photosensor, this surface is highly ordered, and all three loops that connect the transmembrane α helices (the A-B, C-D, and E-F loops) are structurally well defined, with conformations substantially different from those of bacteriorhodopsin and sensory rhodopsin II (Fig. 2C). Specifically, Gln93 is part of a four-residue insertion in the C-D loop relative to the archaeal receptor that results in an enlarged yet well-ordered cytoplasmic loop near the end of the hydrophilic path, a region likely to interact with the transducer.
As with most other membrane protein crystals prepared from the cubic lipid phase, long, tubular electron densities could be interpreted as lipid tails that form ordered, stacked bilayers in the crystal. Judging from the 13 lipid tails that could be built into electron density, it appears that, in contrast to earlier studies of cubic lipid phase crystals, this bilayer is not planar in Anabaena sensory rhodopsin crystals but rather undulates as a result of specific protein-protein interactions within and between bilayers (fig. S1).
The data shown here reveal two photochromic states of Anabaena sensory rhodopsin determined by the color of ambient light. The physiological function of the receptor is not yet known, but in cyanobacteria several physiological processes depend on light in the region of its absorption (2). For example, cyanobacteria adjust the pigment composition of their photosynthetic light-harvesting complexes based on the color of available light, a phenomenon called chromatic adaptation. Action spectra for chromatic adaptation show that orange light stimulates synthesis of phycocyanin, whereas shorter wavelength blue-green light activates synthesis of phycoerythrin (21–23). This color-sensitive pigment synthesis is generally assumed to be based on participation of two competitive receptor pigments with orange versus blue-green absorption maxima. However, the photochromic property of the Anabaena pigment shows that it is possible that such color sensing could be achieved by a single photoreceptor, namely the pigment in its two photo-interconvertible groundstates. The signaling mechanism could make use either of the ratio of the two stable groundstate forms or photochemical reaction of one of the forms, because in both cases the photointerconversion between the cisand trans-forms of the pigment depends on the light quality.
Supplementary Material
Acknowledgments
Supported by NIH grant nos. R01-GM59970 (H.L.), R01-GM067808 (H.L.) and R37-GM27750 (J.L.S.); NSF grant no. 0091287 (J.L.S.); a Welch Investigator Award (J.L.S.); and a Bessel Award from the Alexander-von-Humboldt Foundation (H.L.). The atomic coordinates and structure factors of Anabaena sensory rhodopsin are available at the Protein Data Bank with code 1XIO.
Footnotes
Supporting Online Material
www.sciencemag.org/cgi/content/full/1103943/DC1
Materials and Methods
Fig. S1
Table S1
References and Notes
References and Notes
- 1.These pigments, also known as type 1 rhodopsins, are found in each of the three domains of life (i.e., Archaea, Bacteria, and Eucarya), including haloarchaea, proteo-bacteria, cyanobacteria such as Anabaena, fungi, and algae (2–4, 24).
- 2.Jung KH, Trivedi VD, Spudich JL. Mol. Microbiol. 2003;47:1513. doi: 10.1046/j.1365-2958.2003.03395.x. [DOI] [PubMed] [Google Scholar]
- 3.Béjà O, et al. Science. 2000;289:1902. doi: 10.1126/science.289.5486.1902. [DOI] [PubMed] [Google Scholar]
- 4.Sineshchekov OA, Jung KH, Spudich JL. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8689. doi: 10.1073/pnas.122243399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luecke H, Schobert B, Richter HT, Cartailler JP, Lanyi JK. J. Mol. Biol. 1999;291:899. doi: 10.1006/jmbi.1999.3027. [DOI] [PubMed] [Google Scholar]
- 6.Kolbe M, Besir H, Essen LO, Oesterhelt D. Science. 2000;288:1390. doi: 10.1126/science.288.5470.1390. [DOI] [PubMed] [Google Scholar]
- 7.Luecke H, Schobert B, Lanyi JK, Spudich EN, Spudich JL. Science. 2001;293:1499. doi: 10.1126/science.1062977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Royant A, et al. Proc. Natl. Acad. Sci. U.S.A. 2001;98:10131. doi: 10.1073/pnas.181203898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordeliy VI, et al. Nature. 2002;419:484. doi: 10.1038/nature01109. [DOI] [PubMed] [Google Scholar]
- 10.Venter JC, et al. Science. 2004;304:66. doi: 10.1126/science.1093857. [DOI] [PubMed] [Google Scholar]
- 11.Spudich JL, Jung KH. In: Handbook of Photosensory Receptors. Briggs W, Spudich JL, editors. Wiley; Weinheim, Germany: in press. [Google Scholar]
- 12.Sineshchekov OA, Spudich JL. Photochem. Photobiol. Sci. 2004;3:548. doi: 10.1039/b316207a. [DOI] [PubMed] [Google Scholar]
- 13.The traditional notion of light and dark adaptation of microbial rhodopsins (LA and DA, illumination with intense white light and relaxation to thermodynamic equilibrium in the dark, respectively) stems from decades of research on bacteriorhodopsin. For wild-type bacteriorhodopsin, LA produces nearly 100% all-trans retinal, whereas DA yields È40% all-trans and 60% 13-cis,15-syn retinal. Because for Anabaena sensory rhodopsin, neither LA nor DA may represent a physiologically relevant state, we prefer to refer to orange-illuminated or blue-illuminated chromophores. The former encompasses cases in which the incident light is within the pigment’s absorption range but of substantially longer wavelength than the lmax of the chromophore and the latter cases where the light is of substantially shorter wavelength.
- 14.Sensory rhodopsin I produces an attractant signal in response to orange-light–induced trans-to-cis isomerization, and its photocycle contains a transient 13-cis blue-shifted photointermediate. This intermediate’s cisto-trans photoreaction from near-ultraviolet (UV) light generates a repellent signal (25). Sensory rhodopsin I therefore detects the presence of near-UV light in an orange-light background over the few seconds’ duration of its photocycle. Anabaena sensory rhodopsin, in contrast, exhibits two distinct dark groundstate spectral species, each of which is stable for several orders of magnitude longer than their flash-induced photocycles (26).
- 15.Phytochromes exhibit red-absorbing and far-red–absorbing forms that control a variety of phenomena in plants, such as flowering and circadian rhythms. As described for Anabaena sensory rhodopsin here, the two forms of phytochrome are each stable in the dark over long periods and are rapidly photointer-converted, properties that provide color-sensitive physiological responses (16, 17, 27).
- 16.Wang H, Deng XW. In: The Arabidopsis Book. Somerville CR, Meyerowitz EM, editors. American Society of Plant Biologists; Rockville, MD: 2002. pp. 1–28. [Google Scholar]
- 17.Gyula P, Schäfer E, Nagy F. Curr. Opin. Plant Biol. 2003;6:446. doi: 10.1016/s1369-5266(03)00082-7. [DOI] [PubMed] [Google Scholar]
- 18.Gärtner W. In: Handbook of Biological Physics. Stavenga DG, de Grip WJ, Pugh EN Jr., editors. Vol. 3. Elsevier; Amsterdam: 2000. pp. 297–388. [Google Scholar]
- 19.Cartailler JP, Luecke H. Structure. 2004;12:133. doi: 10.1016/j.str.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Luecke H, Richter HT, Lanyi JK. Science. 1998;280:1934. doi: 10.1126/science.280.5371.1934. [DOI] [PubMed] [Google Scholar]
- 21.MacColl R. J. Struct. Biol. 1998;124:311. doi: 10.1006/jsbi.1998.4062. [DOI] [PubMed] [Google Scholar]
- 22.Grossman AR, Bhaya D, He Q. J. Biol. Chem. 2001;276:11449. doi: 10.1074/jbc.R100003200. [DOI] [PubMed] [Google Scholar]
- 23.Singh B, Chauhan VS, Singh S, Bisen PS. Curr. Microbiol. 2001;43:265. doi: 10.1007/s002840010299. [DOI] [PubMed] [Google Scholar]
- 24.Spudich JL, Yang CS, Jung KH, Spudich EN. Annu. Rev. Cell Dev. Biol. 2000;16:365. doi: 10.1146/annurev.cellbio.16.1.365. [DOI] [PubMed] [Google Scholar]
- 25.Spudich JL, Bogomolni RA. Nature. 1984;312:509. doi: 10.1038/312509a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sineshchekov OA, et al. in preparation.
- 27.Wagner G. In: ESP Review Series on Photobiology. Häder D-P, Lebert M, editors. Elsevier; Amsterdam: 2001. pp. 421–448. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.