Abstract
Fluctuations in attentional control can lead to failures of Working Memory (WM), in which the subject is no better than chance at reporting items from a recent display. In three experiments, we used a whole-report measure of visual WM to examine the impact of feedback on the rate of failures. In each experiment, subjects remembered an array of colored items across a blank delay and then reported the identity of items using a whole-report procedure. In Experiment 1, we gave subjects simple feedback about the number of items they correctly identified at the end of each trial. In Experiment 2, we gave subjects additional information about the cumulative number of items correctly identified within each block. Finally, in Experiment 3 we gave the subjects weighted feedback in which poor trials resulted in lost points and consistent successful performance received “streak” points. Surprisingly, simple feedback (Exp. 1) was ineffective at improving average performance or decreasing the rate of poor-performance trials. Simple cumulative feedback (Exp. 2) modestly decreased poor-performance trials (by 7%). Weighted feedback produced the greatest benefits, decreasing the frequency of poor-performance trials by 28% relative to baseline performance. This set of results demonstrates the usefulness of whole-report working memory measures for investigating the effects of feedback on working memory performance. Further, we showed that only a feedback structure that specifically discouraged lapses using negative feedback led to large reductions in working memory failures.
Keywords: visual working memory, cognitive and attentional control, feedback
Introduction
Failures of attention are frequent and have unintended consequences ranging in severity from variable reaction times on simple laboratory tasks to fatal car accidents in the real world (Reason, 2003; Robertson, Manly, Andrade, Baddeley, & Yiend, 1997). Given that ongoing attentional fluctuations lead to deficits in simple reaction time measures, the effect of impaired attention on demanding tasks can be even more exaggerated. Consistent with this idea, previous research has found a strong relationship between Working Memory (WM) capacity and propensity toward periods of mind wandering and failed executive attention (McVay & Kane, 2012). Here, we investigated the potential for feedback about task performance to reduce attentional failures during a difficult visual WM task.
Performance feedback might improve WM performance for a variety of reasons. First, subjects are relatively unaware of periods of inattention to the task at hand (Reichle, Reineberg, & Schooler, 2010; Schooler et al., 2011), but bringing subjects’ attentional state into awareness allows them to re-engage (deBettencourt, Cohen, Lee, Norman, & Turk-Browne, 2015). Performance feedback should alert subjects that their current attentional state is not sufficient to perform well. In addition to alerting subjects to failures, feedback may also improve performance by increasing subjects’ baseline motivation and arousal levels. Cognitive feedback can act as an extrinsic reward (Aron, 2004), and game-like visual feedback can increase subjects’ intrinsic motivation (Miranda & Palmer, 2013). If subjects are relatively unmotivated in typical lab settings, they may underperform their true ability; providing feedback could increase task engagement and overall performance levels.
To provide informative feedback to subjects, we must first have a reliable indicator of trial-by-trial fluctuations in performance. Performance fluctuations during simple attention tasks have been extensively studied (M. R. Cohen & Maunsell, 2011; Esterman, Noonan, Rosenberg, & DeGutis, 2013; Manly, 1999; Smallwood, Riby, Heim, & Davies, 2006; Unsworth & McMillan, 2014; Weissman, Roberts, Visscher, & Woldorff, 2006), but there are few observations of performance fluctuations during complex WM tasks (Adam, Mance, Fukuda, & Vogel, 2015). Trial-by-trial performance fluctuations are difficult to measure in WM tasks because of the partial-report nature of common WM measures. For example, in a typical change-detection task, subjects are asked to remember a large number of items (e.g. 6–8) and are tested randomly on one of the items. However, capacity is extremely limited, so subjects will remember only 3–4 items on average. As such, even if a subject was performing quite well (e.g., 3 items out of 6) on 100% of trials, they would receive feedback that they were incorrect on 50% of trials. Such unreliable feedback is unlikely to be informative to the subject.
Unlike change-detection tasks, recall tasks allow for trial-by-trial feedback about the number of correctly recalled items. We took advantage of a whole-report visual WM task to test the effects of feedback. In this task, subjects report the identity of all items in the array. Because all items are tested, performance can be calculated for every trial. Additionally, by holding set-size constant across all trials, fluctuations in memory performance can be observed without the confounding factor of intermixed difficulty from multiple set-sizes. Using this task, Adam et al. (2015) found that performance in the whole-report task was highly predictive of typical partial-report capacity measures, and that performance strongly fluctuated from trial to trial. Importantly, these results revealed that nearly all subjects have substantial numbers of WM failure trials, in which they perform no better than chance for the set of 6 items.
Here, we designed a series of experiments to provide different amounts of information to subjects about their trial-by-trial performance. In Experiment 1, we provided a simple form of feedback: the number correct for the trial. Simple feedback of this type is commonly used by researchers with the rationale that the feedback will increase motivation and task compliance. However, the effectiveness of such feedback is often not quantified. In Experiment 2, we added a reminder of ongoing performance by tallying the number of items correct for all trials within a block. After completing the first block of trials, subjects tried to beat their personal “high score” on subsequent blocks. We hypothesized that giving subjects a long-term goal of improving their high score would further boost performance. Finally, in Experiment 3 we used a weighted points system instead of simple number of items correct on the trial. With this weighted points system, subjects lost points if they performed poorly and gained points if they performed consistently well. Note, however, that the subjects understood that these points were arbitrary and were not associated with any financial payout or other outcome. Unlike the other two feedback conditions, the weighted points system was designed to reinforce a particular strategy; to perform optimally, subjects needed to minimize the number of failure trials. In the other two feedback conditions, subjects could have instead attempted to maximize the number of stored items on good trials without necessarily reducing the number of poor trials. We predicted that specifically encouraging subjects to reduce the number of failure trials would maximally boost performance.
Materials and Methods
Participants
All participants gave written informed consent according to procedures approved by the University of Oregon institutional review board. Participants were compensated for participation with course credit or payment ($10/hour). 45 subjects (21 male) participated in Experiment 1, 44 (22 male) in Experiment 2 and 56 (23 male) in Experiment 3. Subjects were excluded from analyses for non-compliance with task instructions (one in Exp. 1, one in Exp. 2 and three in Exp. 3) or for leaving the experiment early (one in Exp. 1, and one in Exp. 3).
Stimuli
Stimuli were generated in MATLAB (The MathWorks, Natick, MA) using the Psychophysics toolbox (Brainard, 1997). Participants were seated approximately 60 cm from an 18-in CRT monitor; distances are approximate as subjects were not head-restrained. Stimuli were presented on a gray background (RGB values: 127.5 127.5 127.5), and subjects fixated a small dot (0.25° visual angle). In all experiments, colored squares (2.5°) served as memoranda. Each square could appear in one of nine colors, sampled without replacement (RGB values: Red=255 0 0; Green=0 255 0; Blue=0 0 255; Yellow=255 255 0; Magenta=255 0 255; Cyan=0 255 255; Orange=255 128 0; White=255 255 255; Black=1 1 1) within an area extending 12.8° horizontally and 9.6° vertically from fixation. At response, a 3×3 grid of all nine colors appeared at the location of each remembered item. After response, a feedback screen displayed information about task performance in size 24 Arial font.
Procedures
Subjects completed a whole-report memory task in Experiments 1, 2, and 3 (Adam et al., 2015). Trial events are shown in Figure 1a. Each trial began with a blank inter-trial interval of 1000 ms. An array containing six colored squares appeared for 250 ms followed by a blank retention interval of 1000 ms. To respond, subjects used the mouse to click on which color appeared at each of the six locations. Subjects were required to respond to all items before the trial would proceed. Trials were blocked within experiment into two conditions, “no feedback” and “feedback.” Condition order was counter-balanced across participants to prevent confounding order effects. As such, we will use the term “feedback first” to refer to subjects who received the feedback condition first, and “no feedback first” to refer to subjects who received the no-feedback condition first. Subjects received 100 trials of each condition in Experiment 1 (split into four within-condition blocks) and 150 trials of each condition in Experiments 2 & 3 (split into five within-condition blocks). In between blocks, subjects received a short break (30 seconds in Exp. 1 & 2, one minute in Exp. 3). In all experiments, subjects were not informed beforehand that there would be two experimental conditions. In Experiments 1 & 2, subjects completed 6 practice trials before beginning the first condition (2 trials each of set sizes 1, 2, and 6). In Experiment 3, subjects did not complete any separate practice trials.
Figure 1. Task illustration.
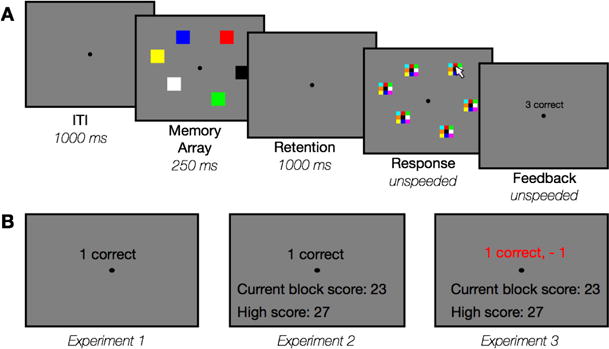
(A) Sequence of events for each trial. (B) Example feedback screen for each Experiment.
In the “no feedback” condition, subjects saw a blank gray screen after responding to all items then clicked the mouse to initiate the next trial. In the “feedback” condition, subjects saw a screen with text-based feedback about their performance. After viewing the feedback, subjects clicked the mouse to initiate the next trial. The main difference between the experiments was the content displayed on the feedback screen; an example of a typical feedback screen for each experiment is shown in Figure 1b.
In Experiment 1, subjects received simple feedback about their performance (e.g. “3 correct”). In Experiment 2, in addition to simple feedback about the current trial, subjects received information about their cumulative “score” within a block. The cumulative score was calculated as the total number of correct objects within the block. After the first block of the feedback condition, subjects also saw their “high score”, defined as the highest total performance achieved within any single block. Finally, in Experiment 3 subjects saw their current trial performance, a weighted number of “points”, and a cumulative score. The cumulative score was calculated as the total number of weighted points within each block. Subjects received −2 points for 0 correct, −1 for 1 correct, 0 for 2 correct +1 for 3 correct, +2 for 4 correct and +3 for 5 or 6 correct (Table 1). In addition, “streak” bonuses were added to the weightings to emphasize consistency. If subjects earned points (3 or more correct) on multiple trials in a row, a streak bonus was added to their score equaling the number of good trials. For example, if a subject got 3 items correct for the 5 trials in a row, they would receive 6 points in total on that trial (+1 for 3 correct, +5 for the current streak). As in Experiment 2, subjects saw their high score starting after the first block. In all three experiments, subjects received verbal instructions that the feedback information shown on the feedback screens in no way affected their payment or course credit.
Table 1.
Points assigned for trial outcomes (number of items correct)
| Trial Outcome | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| Simple Feedback | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Weighted Feedback | −2 | −1 | 0 | 1 | 2 | 3 | 3 |
Results
Experiment 1: Simple feedback
Subjects reported an average number of 2.77 (SD = .54) items correct in the no-feedback condition and 2.82 (SD = .50) items in the feedback condition, and the difference was not significant, t(42) = 1.90, p = .06, 95% CI [−.003, .11]. Next, we looked at the proportion of good- and poor-performance trials (defined as above−3 and below−3 correct, respectively). Subjects reported more than three items correct on 25.82% (SD = 16.88%) of trials in the no-feedback condition versus 28.44% (SD = 16.90%) of trials in the feedback condition. This difference was modest but significant, indicating that subjects had slightly more good-performance trials in the feedback condition, t(42) = 2.43, p = .01, 95% CI [.45 4.8]. On the other hand, there was no reduction in the proportion of poor-performance trials between the no-feedback (39.95%, SD = 19.50%) and feedback (38.05%, SD = 17.10%) conditions, t(42) = −1.52, p = .14, 95% CI [−4.4, .62]. Performance distributions are shown in Figure 2.
Figure 2. Distributions of performance across experiments.
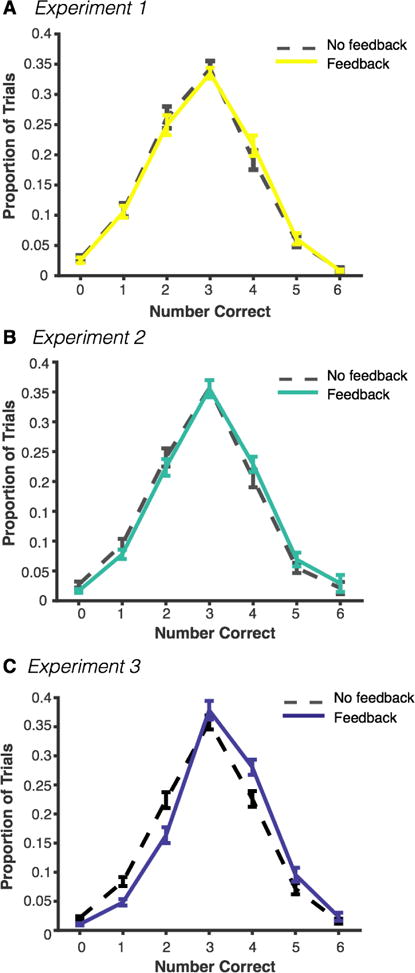
Dotted lines represent performance in the feedback condition, solid lines represent performance in the no-feedback condition in (a) Experiment 1, (b) Experiment 2, and (c) Experiment 3. All error bars represent standard error of the mean.
We also calculated a “percent change” score for each subject to better visualize the impact of feedback on performance since there were large individual differences in the baseline rate of trial types in the no-feedback condition (Figure 3). Percent change was calculated as the difference in the frequency of trials between the feedback and no-feedback conditions divided by the frequency in the no-feedback condition. The average percent change in good-performance trials was +20.73% (SD = 39.80%) relative to baseline, and the percent change in poor-performance trials was −.48% (SD = 26.2%). Histograms of the full range of individuals’ change scores are shown in Figure S1 for Experiment 1 and all following experiments.
Figure 3. Average change in performance between the no-feedback and feedback conditions.
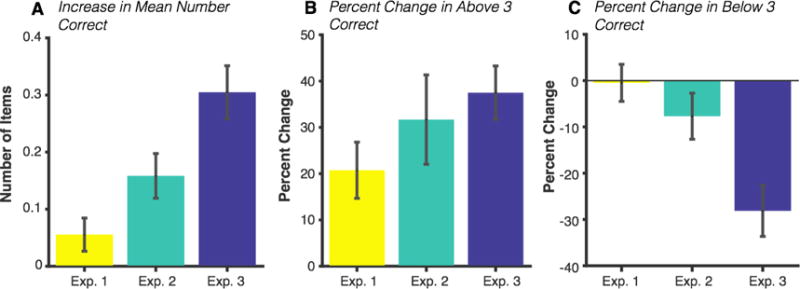
(a) Change in the mean number of items correct. (b) Percent change in the prevalence of good-performance trials in the feedback condition relative to prevalence in the no-feedback condition. (c) Percent change in the prevalence of poor-performance trials in the feedback condition relative to relative to prevalence in the no-feedback condition. All error bars represent standard error of the mean.
Experiment 2: Cumulative simple feedback
Subjects reported an average number of 2.87 (SD = .51) items correct in the no-feedback condition and 3.02 (SD = .53) items in the feedback condition, and subjects performed significantly better in the feedback condition, t(42) = 4.27, p < .001, 95% CI [.08, .22]. The proportion of good-performance trials was 27.90% (SD = 16.10%) in the no-feedback condition and 32.57% (SD = 16.72%) in the feedback condition. This difference was significant, indicating that subjects had slightly more good-performance trials in the feedback condition, t(42) = 3.62, p < .001, 95% CI [2.07 7.28]. Similarly, there was a small reduction in the proportion of poor-performance trials between the no-feedback (35.69%, SD = 15.44%) and feedback (31.72%, SD = 13.89%) conditions, t(42) = −2.94, p = .005, 95% CI [−6.7 −1.2]. Performance distributions are shown in Figure 2. The average percent change in good-performance trials was +30.57% (SD = 56.27%), and the percent change in poor-performance trials was −7.23% (SD = 29.07%; Figure 3).
Experiment 3: Cumulative weighted feedback
Subjects reported an average number of 2.95 (SD = .47) items correct in the no-feedback condition and 3.26 (SD = .49) items in the feedback condition, and subjects performed significantly better in the feedback condition, t(51) = 6.70, p < .001, 95% CI [.21, .40]. The average proportion of high performance trials was 31.47% (SD = 16.85%) in the no-feedback condition and 40.22% (SD = 18.98%) in the feedback condition. This difference was significant, indicating that subjects had more good-performance trials in the feedback condition, t(51) = 5.92, p < .001, 95% CI [5.78 11.72]. Similarly, there was a large reduction in the proportion of poor-performance trials between the no-feedback (32.85%, SD = 15.11%) and feedback (22.26%, SD = 13.37%) conditions, t(51) = −7.27, p < .001, 95% CI [−13.52 −7.67]. Change scores relative to baseline rates are shown in Figure 3 (full range in Figure S1). The average percent change in good-performance trials was +37.56% (SD = 40.9%), and the percent change in poor-performance trials was -27.91% (SD = 39.1%).
Given the general shift in subjects’ performance away from poor-performance trials, we wanted to further examine the prevalence of extreme lapses(0 or 1 correct). During lapses, subjects are at chance levels of performance for this task (Adam et al., 2015), so detecting and correcting such trials would be particularly impactful in applied settings. In particular, we wanted to investigate (1) how reliably individual subjects reduced failure rates and (2) whether a reduction in failures was consistent across all feedback blocks. Subjects had lapses during 10.49% (SD = 7.04) of trials in the no-feedback condition and 5.86% (SD = 4.97) of trials in the feedback condition, and this difference is significant, t(51) = −6.19, p <.001, 95% CI [−6.13, −3.13]. Thus, subjects showed on average a 33.56% reduction (SD = 52.38) in their lapse rate (0 or 1 correct). Figure 4a shows that almost all subjects showed a lower rate of lapses during the feedback than in the no-feedback condition, indicating that feedback interventions are effective for both high- and low-performing subjects. In addition, because the lowest-performing subjects had the highest rate of lapses, the degree of lapse reduction was negatively correlated with overall task performance, r = −.48, p < .001, 95% CI [−.24, −.67], Figure 4b. However, this significant correlation was likely driven by the near-floor rates of lapses for the high capacity subjects. Previously, it was found that motivation and feedback manipulations affected high- and low-capacity subjects equally (Heitz, Schrock, Payne, & Engle, 2007). Finally, we found that performance is stable for blocks of the same condition (all Cronbach’s alpha values > .78.) After a slight learning effect from block 1 to 2, performance was stable from blocks 2 to 5 with a marked shift when the condition changed in block 6 (Figure 5).
Figure 4. Change in lapse rate for individual subjects in Experiment 3.
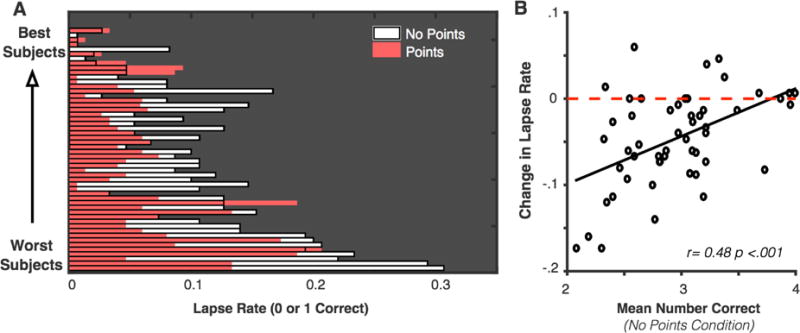
(a) Proportion of lapses (x-axis) is shown for individual subjects. Subjects are sorted on the y-axis by their overall performance in the no-feedback condition. White bars and black outlines depict the proportion of lapses in the no-feedback condition, and shaded bars represent proportion of lapses in the feedback condition. Thus, if lapse rate is reduced in the feedback condition, the corresponding bar appears white on the right-hand side. If lapse rate is not reduced, then the corresponding bar is entirely shaded. Subjects in both the top and bottom of the overall performance distribution show a large reduction of lapses in the feedback condition relative to baseline lapse rates. (b) The change in lapse rate across conditions is correlated with overall task performance, likely because high-performing subjects have lapse rates near floor in both conditions.
Figure 5. Proportion of lapse trials (0 or 1 correct) over each block of Experiment 3.
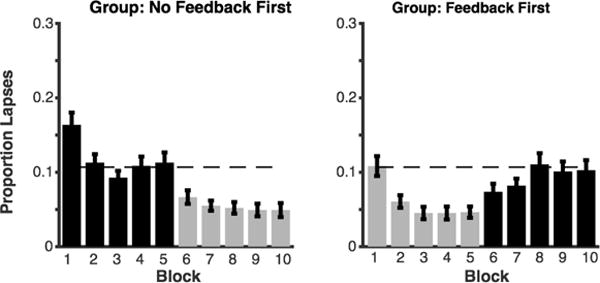
Black bars represent blocks in the no-feedback condition; gray bars represent blocks in the feedback condition. The left panel shows the group of subjects who received no feedback first; the right panel shows the group of subjects who received feedback first. The dotted line shows the average lapse rate for blocks 2–5 during the no-feedback condition in the left panel.
Between Experiments Analyses
To compare the change in performance across experiments, we calculated change scores (feedback – no feedback) for each subject and ran a one-way ANOVA using Experiment as a between-subjects factor. First, we looked at the change in mean number of items correct and found a significant effect of Experiment, F(2,135) = 11.02, p < .001, η2p = .14. Post hoc tests (Tukey’s HSD) revealed that the change in mean performance was larger for Experiment 3 than for either Experiment 1 (p < .001) or Experiment 2 (p = .014). However, the change in mean performance between feedback conditions was equivalent for Experiments 1 and 2 (p = .211).
Similarly, we examined the change in the proportion of good- and poor-performance trials (calculated as proportion in feedback condition minus the proportion in the no-feedback condition). We found a significant effect of Experiment on the proportion of good-performance trials, F(2,135) = 5.77, p = .004, η2p = .08. Tukey’s HSD tests revealed a significant difference between Experiment 1 and Experiment 3 (p = .003) but no significant difference between Experiments 1 and 2 (p = .54) or between Experiments 2 and 3 (p = .08). Finally, we found a significant effect of Experiment on the proportion of poor-performance trials, F(2,135) = 11.45, p < .001, η2p = .15. Post hoc tests (Tukey’s HSD) revealed that the feedback in Experiment 3 led to a greater reduction in poor-performance trials than either Experiment 1 (p < .001) or Experiment 2 (p = .002). However, there was no difference between Experiments 1 and 2 (p = .56).
Discussion
We have demonstrated that a behavioral feedback manipulation can lead to global improvement in WM performance for supra-capacity arrays. Critically, however, not all forms of feedback led to the same level of improvement. These results are an important reminder to test the effects of feedback manipulations. While feedback is often assumed by researchers to always be beneficial, feedback interventions can sometimes lead to no improvement, or even to a decline in performance (Kluger & DeNisi, 1996). Moreover, the present results also suggest that estimates of maximum performance may be slightly underestimated under baseline motivational levels.
Somewhat surprisingly, providing subjects simple feedback about performance (Experiment 1) did not improve average performance, though this effect approached conventional significance (p = .06). The Experiment 1 feedback manipulation was similar to the feedback condition in Heitz et al. (2008), who similarly found only a small effect of feedback on reading-span performance. In the present dataset, the marginal difference in performance was driven exclusively by an increased proportion of good-performance trials. Similarly, the increase in good-performance trials in Experiment 2 was much larger than the reduction in poor-performance trials. This asymmetry suggests that subjects attempted a sub-optimal strategy of maximizing the number of items held in mind on good trials without attempting to reduce the frequency of failure trials. Overall, the persistence of lapse trials in Experiments 1 and 2 suggests that lapses of attention remain frequent and persistent in some cases where subjects are explicitly made aware of poor performance.
The weighted feedback manipulation in Experiment 3 was the most effective, reducing poor-performance trials by 27%. Why was this manipulation so much more effective than others? Streak bonuses and the conjunction of positive and negative feedback were unique design features in Experiment 3, and they could both affect performance dramatically. Miranda and Palmer (2013) found that a visual feedback system with streaks and negative feedback increased subjects’ subjective ratings of intrinsic motivation during a visual search task. Increased intrinsic motivation as a mechanism of improvement dovetails nicely with previous findings that feedback serves as an extrinsic reward (Aron et al., 2004). The conjunction of positive and negative feedback, in particular, may increase the effectiveness of feedback manipulations by engaging both pathways of the dopaminergic reward system (Frank, Seeberger, & O’reilly, 2004). However, given the present data, we can not say whether the addition of streak bonuses (emphasizing positive feedback) or negative feedback (punishing lapses) was most critical for performance improvement in Experiment 3. These two types of feedback are intertwined in the current design; successful streaks are perfectly anti-predictive of negative feedback. Future experiments are needed to disentangle the relative impact of each.
We would also like to emphasize the potential importance of providing an attainable performance goal in Experiment 3. Our prior work revealed that nearly all subjects are capable of accurately reporting at least 3 items (Adam et al 2015). By setting a performance goal of 3 items, we encouraged subjects to perform consistently over a series of trials, rather than to maximize the number of items stored on individual trials. Indeed, an inappropriate performance goal could undermine the motivational benefits of feedback. If the goal was too easy (1 item correct), then subjects would have incentive to underachieve their capacity. Alternatively, if the goal was too hard (6 items correct), then subjects may become frustrated and similarly underperform.
Finally, our results raise some interesting questions that could be addressed by future studies. First, the observed feedback benefit dissipates shortly after the feedback is taken away (Figure S2). However, more extensive training with feedback may help subjects learn to implement a lapse-reduction strategy without ongoing feedback. Given that behavioral feedback is relatively unobtrusive and inexpensive, there is potential for such interventions in real-world settings. Second, it will be important to disentangle the relative contributions of positive feedback, negative feedback, and performance goals to WM improvement. Finally, markers such as pupil dilation (Unsworth & Robison, 2014) and frontal theta power in EEG (Adam et al., 2015) may identify mechanisms underlying the reduction of lapses, including changes in arousal and consistency in the deployment of controlled attention.
Supplementary Material
Acknowledgments
Research was supported by NIH grant 2R01 MH087214-06A1 and Office of Naval Research grant N00014-12-1-0972. Datasets for all experiments are available online on Open Science Framework at https://osf.io/nu8jd/.
Footnotes
Contributions: K.A. and E.V. designed the experiments and wrote the manuscript. K.A. collected the data and performed analyses.
Conflicts of Interest: none
References
- Adam KCS, Mance I, Fukuda K, Vogel EK. The Contribution of Attentional Lapses to Individual Differences in Visual Working Memory Capacity. Journal of Cognitive Neuroscience. 2015:1601–1616. doi: 10.1162/jocn_a_00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Cohen MR, Maunsell JHR. When Attention Wanders: How Uncontrolled Fluctuations in Attention Affect Performance. Journal of Neuroscience. 2011;31(44):15802–15806. doi: 10.1523/JNEUROSCI.3063-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deBettencourt MT, Cohen JD, Lee RF, Norman KA, Turk-Browne NB. Closed-loop training of attention with real-time brain imaging. Nature Neuroscience. 2015;18(3):470–475. doi: 10.1038/nn.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterman M, Noonan SK, Rosenberg M, DeGutis J. In the Zone or Zoning Out? Tracking Behavioral and Neural Fluctuations During Sustained Attention. Cerebral Cortex. 2013;23(11):2712–2723. doi: 10.1093/cercor/bhs261. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O’reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science (New York, NY) 2004;306(5703):1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Heitz RP, Schrock JC, Payne TW, Engle RW. Effects of incentive on working memory capacity: Behavioral and pupillometric data. Psychophysiology. 2007;45(1):119–129. doi: 10.1111/j.1469-8986.2007.00605.x. [DOI] [PubMed] [Google Scholar]
- Kluger AN, DeNisi A. The effects of feedback interventions on performance: A historical review, a meta-analysis, and a preliminary feedback intervention theory. Psychological Bulletin. 1996;119(2):254–284. http://doi.org/10.1037/0033-2909.119.2.254. [Google Scholar]
- Manly T. The absent mind: further investigations of sustained attention to response. Neuropsychologia. 1999;37(6):661–670. doi: 10.1016/s0028-3932(98)00127-4. [DOI] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Drifting from slow to “d’oh!”: Working memory capacity and mind wandering predict extreme reaction times and executive control errors. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2012;38(3):525–549. doi: 10.1037/a0025896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reason J. Lapses of Attention in Everyday Life. 2003:515–549. [Google Scholar]
- Reichle ED, Reineberg AE, Schooler JW. Eye movements during mindless reading. Psychological Science. 2010;21(9):1300–1310. doi: 10.1177/0956797610378686. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Manly T, Andrade J, Baddeley BT, Yiend J. ‘Oops!’: Performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia. 1997;35(6):747–758. doi: 10.1016/s0028-3932(97)00015-8. [DOI] [PubMed] [Google Scholar]
- Schooler JW, Smallwood J, Christoff K, Handy TC, Reichle ED, Sayette MA. Meta-awareness, perceptual decoupling and the wandering mind. Trends in Cognitive Sciences. 2011;15(7):319–326. doi: 10.1016/j.tics.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Riby L, Heim D, Davies JB. Encoding during the attentional lapse: Accuracy of encoding during the semantic sustained attention to response task. Consciousness and Cognition. 2006;15(1):218–231. doi: 10.1016/j.concog.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Unsworth N, McMillan BD. Fluctuations in pre-trial attentional state and their influence on goal neglect. Consciousness and Cognition. 2014;26(C):90–96. doi: 10.1016/j.concog.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Robison MK. Individual differences in the allocation of attention to items in working memory: Evidence from pupillometry. 2014 doi: 10.3758/s13423-014-0747-6. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nature Neuroscience. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


