Abstract
Transmission of HIV across mucosal barriers accounts for the majority of HIV infections worldwide. Thus, efforts aimed at enhancing protective immunity at these sites are a top priority, including increasing virus-specific antibodies (Abs) and antiviral activity at mucosal sites. Mucin proteins, including the largest cell-associated mucin, MUC16, help form mucus to provide a physical barrier to incoming pathogens. Here we describe a natural interaction between Abs and MUC16 that is enhanced in specific disease settings such as chronic HIV infection. Binding to MUC16 was independent of IgG subclass, but strongly associated with shorter Ab glycan profiles, with agalactosylated (G0) Abs demonstrating the highest binding to MUC16. Binding of Abs to epithelial cells was diminished following MUC16-knockdown, and the MUC16 N-linked glycans were critical for binding. Further, agalactosylated VRC01 captured HIV more efficiently in MUC16. These data point to a novel opportunity to enrich Abs at mucosal sites by targeting Abs to MUC16 through changes in Fc-glycosylation, potentially blocking viral movement and sequestering the virus far from the epithelial border. Thus, next-generation vaccines or monoclonal therapeutics may enhance protective immunity by tuning Ab glycosylation to promote the enrichment of Abs at mucosal barriers.
Keywords: Antibody glycosylation, mucin, mucus, HIV
INTRODUCTION
Sexual transmission of HIV across mucosal barriers accounts for the majority of new HIV infections each year. Women are at particular risk of infection, with young women twice as likely as young men to be infected with HIV via heterosexual transmission (1, 2). While an effective HIV vaccine remains elusive, enhancing protection at mucosal sites is key to providing protective immunity in next-generation vaccine strategies. Passive transfer of neutralizing antibodies (Abs) against HIV can provide protection against mucosal challenge (3, 4), and beyond neutralization, Fc-mediated Ab effector functions have been implicated in protection against HIV (5, 6). Recently, targeting of broadly neutralizing Abs (bNAbs) to mucosal compartments resulted in increased protection of non-human primates from mucosal challenge (7), suggesting that strategies aimed at increasing the concentration of virus-specific Abs at mucosal sites may provide enhanced protection from infection. However, as vaccination cannot induce mutations in the Fc, identifying natural Ab modifications that increase Ab concentration at mucosal sites represents a novel opportunity to enhance immunity against HIV and other mucosal pathogens.
All mucosal surfaces are lined with a thick layer of mucus that provides a protective physical barrier for the underlying epithelium by trapping pathogens and microbes. Anti-microbial peptides, immune proteins, and Abs are present within mucus, and can be bound to a lattice of heavily glycosylated mucin proteins that line the membranes (8). The specific mechanisms by which these immune proteins are bound in mucus are not fully understood but may hold the key to vaccine or therapeutic strategies aimed at enriching antiviral Abs along these vulnerable tissues. Cell-associated mucin proteins including the largest mucin, mucin 16 (MUC16), line the endocervix, endometrium, and fallopian tubes to provide an additional barrier for pathogens to overcome in order to reach the epithelium (9, 10). Because the endocervix is lined by a single layer of columnar epithelial cells that is highly susceptible to infection by HIV (11), the mucin barrier provides an additional protective layer against infectious agents.
In this study, we aimed to determine whether Abs could be selectively enriched at mucosal barriers, ultimately identifying novel means to promote higher concentrations of HIV-specific Abs at these sites. Here we identified an interaction between IgG and the mucin, MUC16, which is selectively enhanced in chronic HIV+ subjects. Specifically, particular Fc-glycosylation patterns, independent of Ab subclass, were associated with enhanced binding to MUC16, and manipulation of the glycan structure modulated MUC16 binding interactions, and subsequent capture of virus. Together, these data highlight a novel opportunity to promote HIV-specific Ab enrichment above mucosal membranes through altered Ab glycosylation that may immobilize incoming virus to provide enhanced protection from infection.
RESULTS
Abs from HIV+ patients preferentially bind to MUC16
Previous studies demonstrate increased levels of IgG1 and IgG3 Abs in the cervicovaginal secretions (CVS) of HIV+ compared to HIV-negative women (12). While the increased amounts of IgG in CVS likely results from hypergammaglobulinemia associated with HIV infection (13), we reasoned that there might be specific interactions between Abs from HIV+ individuals and mucus proteins that may allow them to retain high levels of Abs within mucus. Thus, to determine if specific proteins in mucus bind differentially to Abs during HIV infection, we examined the capacity of Abs isolated from chronic HIV+ patients or healthy controls to bind to a number of proteins that associate with epithelial cells at mucosal membranes. Among the proteins found at these sites that may interact with Abs (14), no differences were observed in Ab binding to galectin proteins Gal-1, Gal-3, Gal-7, and Gal-9 (Fig. 1A). Next we probed the capacity of Abs to interact with some of the most abundant proteins at the mucosal barrier in the female reproductive tract, the membrane-associated mucin proteins, MUC1 and MUC16. While Abs were able to bind to recombinant fragments of both mucins, limited differences were observed between the groups in binding to MUC1 (Fig. 1B, left). In contrast, Abs purified from subjects with chronic HIV infection exhibited significantly enhanced binding to MUC16 (Fig. 1B, right).
Fig. 1. Abs from HIV+ patients preferentially bind to MUC16.
(A-B) Bulk IgG from HIV+ patients, or healthy subjects were assayed for binding to galectins (A) or mucins (B) by ELISA.
(C) An endocervical explant from a donor was stained for MUC16 (FITC), cytokeratin 7 (blue; marks simple epithelium), and IgG (red) and imaged by fluorescence microscopy. The black and white inset shows overlap of IgG and MUC16. ROI i and ii show regions of cell-associated MUC16 (ROI i) as well as shed MUC16 (ROI ii).
(D) Bulk IgG from HIV+ patients or healthy subjects was assayed for binding to native MUC16 isolated from OVCAR3 cells by ELISA.
(E) Bulk IgG from chronic HIV+ patients were labeled with Cy5 and assayed for binding to either WT or MUC16 knockdown OVCAR-3 cells by flow cytometry.
(F) Bulk IgG from different HIV patient populations (elite (EC) or viremic controllers (VC); chronic progressor off ARV (UTx) or on ARV (Tx)) were assayed for MUC16 binding by ELISA.
(G) Bulk IgG from acute (0-3 or 6-12 months) or chronic HIV infection (12 months; >2 years) or healthy subjects were assayed for binding to MUC16 by ELISA.
(H) Bulk IgG from HIV+, HCV+, influenza patients or healthy subjects were assayed for binding to MUC16 by ELISA.
*p<0.05, **p<0.01, ***p<0.0005 by Mann-Whitney analysis (2 groups) or Kruskal-Wallis analysis (3 or more groups).
As the endocervix is vulnerable to HIV infection, we next determined if both Abs and MUC16 are present in the same region of the reproductive tract. Thus, an endocervical explant from a healthy donor was stained for the presence of IgG and MUC16 and visualized by fluorescence microscopy. Consistent with previous studies demonstrating the presence of MUC16 lining the endocervix (8, 15), MUC16 staining was observed on the apical side of the columnar epithelium (Fig. 1C). The staining of the MUC16 at the epithelial border across the entire tissue section highlights the ideal localization of MUC16-mediated Ab enrichment to protect the underlying epithelium. Dispersed IgG staining was observed throughout the tissue section, consistent with prior studies demonstrating the presence of IgG within the reproductive tract (16, 17). Interestingly, an accumulation of IgG was observed at the apical side of the columnar epithelial cells, overlapping with surfaces occupied by MUC16. Thus, interactions between Abs and MUC16 have the potential to concentrate Abs within the glycocalyx covering the luminal surface of the endocervix, enhancing mucosal barrier function.
Native MUC16 within tissues is much larger and likely glycosylated differently than recombinant MUC16, thus we next determined whether Abs from HIV+ patients could also interact with naturally produced MUC16. Native MUC16 was purified from OVCAR3 cells and binding of Abs was evaluated. Consistent with our results using recombinant MUC16, HIV+ Abs demonstrated enhanced binding to native MUC16 compared to Abs from healthy controls (Fig. 1D). To determine whether purified MUC16 was conformationally distinct on the cell surface, potentially abrogating Ab binding, Abs from HIV+ patients were incubated with either wild-type (WT) OVCAR3 cells, which naturally express MUC16 (18), or MUC16-knockdown OVCAR3 cells. As shown in Fig. 1E, Ab binding was detected on WT OVCAR3 cells, but reduced on MUC16 knockdown cells. Together, these data demonstrate that Abs can interact with native shed and cell-associated MUC16.
Chronic HIV infection enhances the production of MUC16 binding Abs
To begin to identify features of Abs that confer enhanced binding to MUC16, we sought to determine if Abs with enhanced MUC16 binding are preferentially induced in subjects with different HIV associated clinical disease outcomes, such as spontaneous control of HIV infection, previously associated with the induction of unique Ab Fc-profiles (19). We compared Abs from HIV controllers and chronic HIV patients with and without antiretroviral treatment for binding to MUC16. Interestingly, no differences were observed in Ab binding to MUC16 between the different HIV patient groups, suggesting that HIV infection alone, rather than viremia, induces an increase in Abs with MUC16 binding abilities (Fig. 1F).
The Ab response during HIV infection is dynamic, and Abs generated during acute infection differ from those produced during chronic infection with regard to subclass, antiviral function, and epitope specificity (13, 20). Thus to determine at which point during infection Abs gain an enhanced capacity to bind to MUC16, Abs from acutely HIV infected patients (<1 year post-infection) was compared to Abs from chronically infected HIV+ patients (>2 years) and healthy controls for MUC16 binding. While limited binding was observed for Abs from acute infection, Abs from HIV patients infected for at least one year had enhanced MUC16 binding (Fig. 1G). As hypergammaglobulinemia arises within the first 3 months of infection (13), it is likely that qualitative changes in the Ab, rather than the total amount of Ab, must change over the first year of HIV infection resulting in the generation of Abs with enhanced MUC16 binding abilities.
To determine if Abs generated in other viral infections also exhibit amplified binding to MUC16, we examined the capacity of Abs induced during acute influenza infection or chronic hepatitis C virus (HCV) infection to bind to MUC16. Enhanced MUC16 binding was not observed in subjects with acute influenza infection or chronic HCV infection (Fig. 1H). Combined, these data demonstrate that there is an increase in the amounts of Ab with optimal capacity to bind to MUC16 induced in the context of chronic HIV infection.
Fc-mediated binding to MUC16
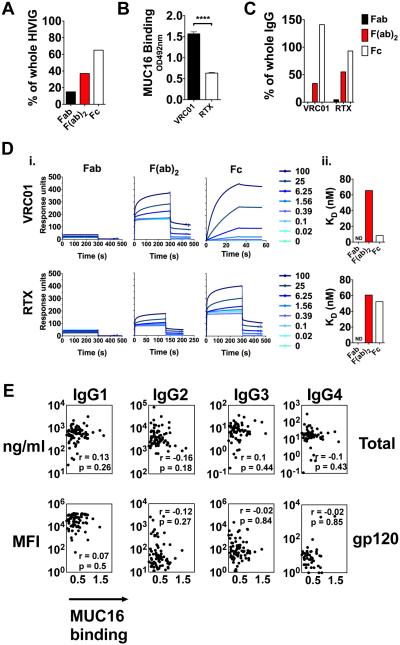
As Abs can be divided into two distinct functional domains - the variable Fab domain involved in antigen binding and the constant Fc domain involved in directing effector function - we next aimed to define which domain interacts preferentially with MUC16. Enzymatic digestion of a polyclonal pool of IgG from chronic HIV patients (HIVIG) was performed with papain or IdeS to produce Fab or F(ab)2, respectively, and Fc domains. Overall, the cleaved Fc domains from HIVIG demonstrated preferential binding to MUC16 compared to Fab and/or F(ab)2, with the Fc domain mediating approximately 60% of whole IgG binding to MUC16 (Fig. 2A). To confirm the role of the Fc domain in the Ab/MUC16 interaction, we used a pair of monoclonal Abs (mAbs), VRC01 and Rituximab (RTX), that exemplify the two extremes (low and high) of MUC16 binding, representing a consistent surrogate of polyclonal IgG to dissect Ab determinants of binding (Fig. 2B). VRC01 and RTX were cleaved to produce Fab or F(ab)2 and Fc domains, and binding to MUC16 was assessed. The Fc domain bound MUC16 at approximately equal levels as whole IgG, whereas the F(ab)2 domain bound at approximately half of the binding of whole IgG (Fig. 2C). As we observed F(ab)2 binding, but not Fab binding to MUC16, it is possible that the increased binding is attributable to the detection of two Fabs in the F(ab)2 cleavage condition by the anti-Fab detection reagent. Thus, we performed surface plasmon resonance (SPR) to determine the binding affinity of Fc, Fab, and F(ab)2 of VRC01 and RTX to MUC16. Consistent with our results in the ELISA, the Fc portion of the VRC01 bound to MUC16 with approximately eightfold greater affinity than F(ab)2, whereas binding of the Fab portion was undetectable (Fig. 2D). These data suggest that while some level of F(ab)2 binding to MUC16 can occur, a majority of binding is mediated via the Fc domain. In addition, both the Fc and F(ab)2 of RTX showed reduced binding affinity to MUC16 comparable to the level of VRC01 F(ab)2 binding, further supporting the hypothesis that the Fc domain of Abs mediate differential binding to MUC16.
Fig. 2. Fc-mediated binding to MUC16.
(A) HIVIG was cleaved with IdeS to generate F(ab)2 fragments, or papain to generate intact Fc or Fab fragments, and assayed for MUC16 binding. The percent binding compared to whole IgG is shown.
(B) VRC01 or Rituximab (RTX) were assayed for MUC16 binding by ELISA.
(C) Fab, F(ab)2, or Fc fragments of VRC01 or RTX were assayed for MUC16 binding by ELISA. Percent binding of whole IgG is shown.
(D) Fab, F(ab)2, or Fc fragments of VRC01 or RTX were assayed for MUC16 binding by SPR. Raw SPR curves are shown in (i) and KD in (ii).
(E) Total IgG subclass (top panel) or gp120-specific subclass titers (bottom panel) in IgG from HIV patients were determined by multiplex analysis. A spearman correlation coefficient was used to determine correlations between MUC16 binding and Ab titers.
***p<0.0001 by unpaired t-test.
Ab subclass does not account for differential MUC16 binding
As the Fc-domain of Abs can be rapidly modulated during an immune response to drive differential Ab functionality via subclass selection and glycosylation (21), we next aimed to determine if specific subclasses preferentially enhanced binding to MUC16. No relationship was observed between bulk subclass Ab titers or gp120-specific subclass titers and MUC16 binding (Fig. 2E), pointing to a non-subclass mediated mechanism for enhanced MUC16 binding.
Elevated levels of agalactosylated Abs correlate with enhanced MUC16-binding
The Fc domain of IgG contains a glycosylation site at N297 (22), and beyond subclass selection differences, Ab Fc glycosylation can be tuned during an immune response, aimed at modulating Ab interactions with Fc-receptors and complement (23). Moreover, the Ab glycan varies widely with age, sex, and autoimmune diseases (24), and significant changes in IgG glycosylation have been observed in HIV infection, marked by the accumulation of high levels of agalactosylated Abs (19, 25), also observed in active rheumatoid arthritis (RA) flares (26). Given the similarity in Fc glycosylation in HIV and RA, we hypothesized that Abs from RA patients also exhibit enhanced binding to MUC16. Indeed, Abs from RA patients bound to MUC16 at higher levels, similar to those from HIV patients, and trended towards enhanced binding to MUC16 compared to Abs from healthy controls (Fig. 3A). As it is unlikely that Abs from RA patients and HIV patients share similar antigen-binding domains and subclass selection profiles, these data suggest that alterations in Fc glycosylation may play a role for the observed increase of MUC16 binding. Interestingly, Abs from influenza and HCV infection exhibit lower levels of agalactosylation (Fig. S1), supporting the hypothesis that differential Fc glycosylation may modulate enhanced MUC16 binding.
Fig. 3. Elevated levels of agalactosylated Abs correlate with enhanced MUC16-binding.
(A) Bulk IgG from HIV+or RA patients or healthy subjects were assayed for binding to MUC16 by ELISA.
(B) The relative abundance of glycan structures in bulk IgG from HIV+ patients (top panel) or healthy subjects (bottom panel) was determined by CE. Spearman correlation coefficients indicate a significant positive (red), significant negative (blue), or no correlation (white) between relative glycan abundance and MUC16 binding.
(C) The relative abundance of total G0, G1, and G2 glycan structures in VRC01 and RTX was determined by mass spectrometry.
(D) HIVIG was enriched for G0 Abs using ECL beads. Bound Abs were eluted and compared to unbound Abs for binding to MUC16 by ELISA. The data are expressed as percent binding to unenriched HIVIG Abs.
(E) HIVIG was treated with or without β-galactosidase and assayed for MUC16 binding by ELISA. **p<0.005 by unpaired t-test.
Ab glycan structures can be divided into classes based on the number of galactose molecules incorporated into the glycan, with G0 containing no galactose, G1 containing one galactose, and G2 containing two galactose molecules (22). As HIV-infected subjects and RA patients exhibit an enrichment of G0 Abs, the relationship between the abundance of G0, G1, and G2 structures and MUC16 binding was explored in a population of HIV+ patients with variable Ab glycosylation following glycan analysis by capillary electrophoresis (27). A significant positive correlation was observed between MUC16 binding and G0 glycan levels across the entire population (Fig. 3B). Conversely, a significant negative correlation was observed with the relative abundance of G2 containing Abs, as G2 and G0 are inversely correlated. The same glycan/MUC16 binding relationships were confirmed in a replication cohort of acute and chronic HIV patients, influenza patients, HCV patients, RA patients, and healthy subjects (Fig. S2).
While G0-containing Abs are enriched in HIV and RA patients compared to healthy subjects, G0-Abs are less abundant than G1 and G2 Abs in HIV+ subjects (Fig. S3), suggesting that G0-Abs preferentially bind to MUC16. Moreover, although healthy patients show less binding to MUC16 relative to HIV+ donors (Fig. 1B), the abundance of G0-containing Abs also correlates with enhanced MUC16 binding in a healthy cohort (Fig. 3B, bottom panel), consistent with a role for G0 glycosylation in the selective tuning of MUC16 binding, regardless of patient population. mAb glycosylation varies due to differences in the host expression cell line (28), and as VRC01 bound better than RTX to MUC16 (Fig. 2B), we determined the relative percentage of G0, G1, and G2 glycan structures on these mAbs. Interestingly, the enhanced MUC16 binding with VRC01 was linked to higher G0 glycosylation whereas lower MUC16 binding and lower G0 glycosylation with RTX (Fig. 3C). Similar results were observed with two additional HIV-specific mAbs, F240 and 2G12, where differential G0 content was linked to MUC16 binding (Fig. S4), further supporting a role for G0 glycosylation in preferential MUC16 binding, independent of antigen specificity.
To confirm the role of the G0 glycan in enhanced MUC16 binding, G0 Abs were enriched from HIVIG using the Erythina cristagalli lectin, which specifically binds to terminal β1,4-linked galactose (29), thus binding to G1/G2-containing Abs, and depleting these species, while enriching G0 structures in the unbound pool. G0-enriched Ab fractions bound MUC16 at approximately 80% of the amount of the input HIVIG, whereas G1/G2-enriched Ab fractions bound MUC16 at 20% of input HIVIG (Fig. 3D). Similarly, removal of the terminal galactose from HIVIG by enzymatic digestion with β1,4-galactosidase to generate G0-Abs, resulted in increased binding to MUC16 compared to undigested Abs (Fig. 3E), further supporting the hypothesis that G0 Abs have an enhanced capacity to bind to MUC16.
Increased binding affinity to MUC16 is modulated by smaller Fc glycan structures
To quantitatively measure the impact of the Fc glycan on binding to MUC16, we performed SPR analysis of the HIV-specific mAbs VRC01 and 2G12, which showed high and low MUC16 binding, respectively (Fig. 2B, S4A), and polyclonal HIVIG after: 1) the enzymatic removal of sialic acid (SA) and galactose from the glycan, thus generating G0 glycoforms, or 2) after enzymatic removal of the entire glycan by PNGaseF. Consistent with the ELISA data demonstrating that removal of galactose increases binding to MUC16 (Fig. 3E), truncation of the glycan to G0 dramatically increased Ab affinity to MUC16 compared to undigested Abs (Fig. 4A). Unexpectedly, the affinity of VRC01 and HIVIG binding to MUC16 was also increased when the Fc glycan was removed completely by PNGaseF (Fig. 4A). Similarly, removal of the glycan increased the binding affinity of RTX to MUC16 compared to undigested Ab (Fig. 4B). Conversely, RTX binding to Protein A, an Fc glycan-independent interaction, was not significantly altered with removal of the glycan, whereas binding to FcγRIIIa, an Fc-glycan dependent interaction, was disrupted (Fig. 4B), as expected (30, 31). Of note, RTX does not have an N-linked glycosylation site in the Fab region, and only has an Fc glycan, thus the increased binding affinity following PNGaseF digestion supports the role of the Fc in mediating the interaction with MUC16 (Fig. 2B-C). Together with the G0-MUC16 association within patient cohorts (Fig. 3B, S2), these data provide compelling evidence that G0-containing Abs have greater affinity for MUC16. As G0 represents the smallest naturally occurring Fc glycan structure, these data suggest that smaller or no glycan structures are confer enhanced MUC16 binding.
Fig. 4. Increased binding affinity to MUC16 is modulated by smaller Fc glycan structures.
(A) VRC01 (top), 2G12 (middle), and HIVIG (bottom) were digested with enzymes to produce G0, or aglycosylated Abs and binding affinity to MUC16 was determined by SPR. Raw SPR curves and bar graphs of KD values for indicated groups are shown.
(B) RTX was digested with PNGaseF to produce aglycosylated Abs and binding affinity to MUC16 (top), protein A (middle) or FcγRIIIA (bottom) was determined by SPR. Raw SPR curves and bar graphs of KD values for indicated groups are shown.
(C) N-glycans on MUC16 were removed by digestion with PNGase F and binding affinity of indicated Abs to digested MUC16 was determined by SPR. Raw SPR curves are shown.
MUC16 glycosylation is required for Ab binding
Given the role of the Fc glycan in modulating Ab binding to MUC16, we hypothesized that the glycans on MUC16 may also modulate binding to the Abs. MUC16 is glycosylated with both O- and N-linked glycans that accounts for nearly 30% of the protein mass (32), To determine if MUC16 N-linked glycans modulate binding of Abs, MUC16 was digested with PNGaseF to remove N-linked glycans, and the binding affinity to the mAbs was measured by SPR. Strikingly, PNGaseF treatment of MUC16 resulted in complete loss of binding to all Abs (Fig. 4C), indicating that N-linked glycans on MUC16 are critical for Ab binding.
Fucosylation also impacts MUC16-binding
In addition to galactose, three additional sugars can be modified to alter Ab functionality: fucose reduces Ab Fc-binding to FcγRIIIa, thus reducing ADCC (33); bisecting GlcNAc enhances binding to FcγRIIIa thereby enhancing ADCC (34); and SA can dampen inflammation and ADCC (35). To gain greater resolution of the glycan modifications that preferentially interact with MUC16, Abs from HIV+ patients were incubated with beads coated with MUC16, and bound Abs were eluted prior to glycan characterization by mass spectrometry (Fig. S4). The frequency of specific Ab glycan structures in the total pool of Abs was compared to those in the MUC16-bound fraction to define the preferred structures that bound to MUC16. MUC16 pull-down captured the G0F glycan structure most abundantly, followed by G1F, G2F, G0FB, and G1FB (Fig. 5A). Calculation of the percentage enrichment of MUC16-bound Abs compared to the input HIVIG demonstrated that MUC16-bound Abs were enriched for G0F, G1F, G2F, G1FB, and G0FB but reduced for G0B, G1B, and G2B (Fig. 5B). Moreover, collective analysis of total G0, G1, and G2 glycans (including all substructures +/− fucose, SA, and bisecting GlcNAc) demonstrated an enrichment of G0 structures in MUC16 bound Abs whereas G1 and G2 structures were reduced compared to input levels in HIV+ Abs (Fig. 5C). Similarly, collective analysis of total glycan structures that include fucose and/or the bisecting GlcNAc highlight the preferential binding of all fucosylated Abs, but not afucosylated bisected glycosylated Abs (Fig. 5D). Because fucose and the bisecting GlcNAc are typically (but not exclusively) added in a reciprocal order (36), reduced bisecting glycan binding may be related to a preferential interaction between MUC16 and fucosylated Abs. Moreover, the relative abundance of G0F-containing Abs correlated with MUC16 binding in both HIV+ subject and healthy control populations (Fig. 5E, S5), supporting the hypothesis that G0 fucosylated structures promote MUC16 binding. Finally, to test if reducing fucosylation alters Ab binding to MUC16, the mAb VRC03 was produced either in wild type or stable FUT8 knock down (the enzyme that adds fucose) 293T cells, the latter demonstrating reduced MUC16 binding (Fig. 5F). Taken together, these data support a role for Ab fucosylation in promoting Ab/MUC16 interactions.
Fig. 5. Agalactosylated and fucosylated Abs are enriched in MUC16-bound Abs.
(A) MUC16-bound Abs were analyzed by mass spectrometry to determine glycan structure. The relative intensity values for input or MUC16-bound Abs for the indicated glycan are shown.
(B) The percent enrichment of the indicated glycan structure in the MUC16 bound Ab pool compared to the input Ab pool are shown.
(C-D) The percent enrichment of total G0, G1 or G2 glycan structures (C) or fucosylated (G0F, G1F, G2F), fucosylated and bisected (G0FB, G1FB) and bisected structures (G0B, G1B, G2B) (D) in the MUC16 bound Ab pool compared to the input Ab pool are shown.
(E) The relative abundance of fucosylated glycan structures in bulk IgG from HIV+ patients (top panel) or healthy subjects (bottom panel) was determined by CE. Spearman correlation coefficients indicate a significant positive (red), significant negative (blue), or no correlation (white) between relative glycan abundance and MUC16 binding.
(F) VRC03 monoclonal Ab produced in either wild-type or FUT8kd 293T cells to produce fucosylated or afucosylated Abs, respectively, were assayed for MUC16 binding by ELISA.
MUC16-bound Abs captures HIV
Capture of infectious HIV at mucosal barriers could serve to block transmission by immobilizing/neutralizing incoming HIV, leading to protective immunity (37, 38). Given the apical localization of MUC16 above the epithelium (Fig. 1C, (8)), MUC16-bound Abs may be well positioned to capture incoming virions at a distance from the underlying epithelium, thereby helping prevent infection. To determine whether MUC16-bound Abs could capture virus, fluorescent HIV viral particles were incubated with MUC16-bound HIVIG (10μg/ml or 100μg/ml) or Abs from a healthy subject (100μg/ml), and the number of HIV particles trapped by MUC16-bound Abs was quantified by confocal microscopy. While Abs from healthy controls trapped HIV particles to similar levels as MUC16 alone, MUC16-bound HIVIG trapped significantly more HIV particles at the highest concentration (100μg/ml) of HIVIG only, likely related to the low abundance of HIV-specific Abs within the polyclonal HIVIG pool (39) (Fig. 6A). In addition to the visualization of viral capture by microscopy, viral capture was quantified by p24 ELISA. As G0 forms of Abs increases binding affinity to MUC16, the G0 glycoform of VRC01 was directly compared to the undigested VRC01. As shown in Fig. 6B, VRC01 G0 captured higher amounts of HIV compared to undigested VRC01, and significantly higher virus than the healthy Ab control, indicating that increased MUC16 binding affinity results in increased viral capture. Together, these functional data indicate that MUC16-bound Abs not only bind to MUC16, but can capture HIV virions, potentially resulting in the sequestration of the virus above the epithelial border.
Fig. 6. MUC16-bound Abs capture HIV.
(A) MUC16 was coated onto microscopy plates at 2μg/ml. Abs were incubated at 10μg/ml (HIVIG) or 100μg/ml (HIVIG and healthy IgG) prior to washing and incubation with a fluorescent HIV (HIV-RFP). Trapped HIV were imaged by confocal microscopy (left) and the number of trapped virus was quantified using Image J (right).
(B) Indicated Abs (1μg/ml) were incubated with plate-bound MUC16, followed by incubated with 10ng/ml of HIV (SF162). After 1hr, plates were washed and the amount of virus captured was determined by p24 ELISA.
DISCUSSION
Reducing transmission of HIV across mucosal barriers is critical to ending the HIV epidemic, and identifying interactions that enhance mucosal protection will be key to providing sterilizing immunity. Approaches aimed at naturally inducing Ab enrichment at mucosal barriers through vaccination or therapeutics could have a profound impact on limiting infection. Here, we demonstrate an interaction between the cell-associated mucin protein, MUC16, and Abs. Moreover, this interaction was preferentially enhanced in the context of chronic HIV infection, allowing us to identify a specific glycoform that confers enhanced binding and affinity to MUC16 and subsequent viral capture.
While mucus alone slows the transit of viruses, the antiviral function of virus-specific Abs in mucus has been suggested in the context of herpes simplex virus-2 (HSV-2) where the presence of HSV-2-specific Abs within mucus decreases movement of that virus, correlating with reduced vaginal infection in mice (40). The synergy of Abs and mucus is likely mediated through multiple mechanisms including increasing the size of the pathogen by immune complex formation, and higher affinity interactions with particular mucus components such as mucins or additional Fc-interacting proteins (17, 40, 41). In the case of HSV-2, Ab glycosylation on HSV-2 specific Abs was critical in slowing virus transit through mucus (40), yet the mucus proteins engaged in this HSV-2 slowing process and specific Ab modifications that resulted in enhanced viral trapping are unclear. However, the results raised the exciting possibility that strategies aimed at increasing Ab abundance within mucus could effectively reduce pathogen infection across mucosal membranes. Here we demonstrate a preferential interaction of Abs and MUC16 that can be enhanced by altering Ab glycosylation to shorter, agalactosylated fucosylated Abs that allows for capture of virus.
Generation of Abs with enhanced MUC16 binding capacity was not specific to HIV infection and was detectable in the setting of active RA (Fig. 3A), but not in influenza or HCV infection, suggesting that specific inflammatory profiles, rather than infection per se, drive Ab modifications that enhance Ab binding to MUC16. Of note, while we only evaluated plasma Abs in this study, locally produced mucosal Abs may have distinct glycan profiles that allow for differential binding to mucosal proteins and will be probed in future studies. However, plasma Abs can access mucosal sites (42), allowing for enrichment of systemic Abs with enhanced MUC16 binding activity at MUC16-lined surfaces.
ELISA binding data, mass spectrometry analysis, and associations in patients point to the preferential binding of G0-containing Abs to MUC16 (Fig. 3, 5C). These data were validated by SPR studies demonstrating that truncation of the Ab glycan to G0 on both polyclonal and monoclonal Abs dramatically increased the affinity of Ab binding to MUC16 (Fig. 4A). Intriguingly, removal of the glycan by PNGaseF digestion also increased binding affinity of Abs to MUC16 (Fig. 4A). The presence of different glycan structures (e.g. G0 vs sialylated) or removal of glycan alters the CH2 domain (31, 43-45), potentially impacting the flexibility/shape of the Ab, with G0 glycans representing the shortest naturally occurring structures, suggesting a possible mechanism of interaction between these two proteins. Alternatively, as the MUC16 N-linked glycans are critical for Ab binding (Fig. 4C), it is possible that larger Fc glycans with multiple galactose and SA residues may antagonize or hinder binding to MUC16 through glycan/glycan interactions, similar to the mechanism by which fucose hinders interaction with FcγRIIIA (33). Future structural and glycosylation analyses may point to the specific mechanism of interaction, but together, our data demonstrate that Ab affinity to MUC16 can be tuned via alteration in galactosylation to potentiate viral capture.
In addition to the importance of G0 to improve MUC16 binding, natural Abs that preferentially bind to MUC16 tend towards enhanced fucosylation and reduced bisection. Enhanced fucosylation (33) and reduced bisection (34) are both independently linked to diminished ADCC activity, and thus the preference of fucosylated Abs by MUC16 may offer a non-inflammatory advantage at mucosal membranes, as virus-immune complexes decorated with fucosylated Abs would limit highly-inflammatory cytolytic activity and pathology if detected by NK cells at mucosal membranes.
Methods to glyco-engineer Abs to produce particular glycan structures have been established (46), rendering it feasible to produce bNAbs with an enhanced capacity to bind to MUC16. We demonstrate here that HIV-specific mAbs can be modified to enhance MUC16 binding (Fig. 4A), indicating that existing monoclonal therapeutics can be optimized to take advantage of enhanced MUC16 binding capacity to protect the underlying mucosa. Further, it is likely that particular vaccine vectors/adjuvants may selectively skew and tune Ab glycosylation to produce Abs with shorter glycans. Thus, next-generation vaccine design efforts may be able to modulate Ab/MUC16 interactions to trap virus within mucus, leading to enhanced protection from infection.
Beyond enrichment, enhancing Ab binding to MUC16 resulted in improved viral capture (Fig. 6B). Viral capture has been associated with protection and reduced transmitted viruses (38, 47), but Ab trapping of virus at the mucosa by non-neutralizing Abs may provide the virus with an enhanced capacity to infect. While targeting neutralizing Abs to FcRn led to enhanced protection from infection, enrichment of non-neutralizing Abs on FcRn may result in more transcytosis of virus, and infection (48). MUC16 extends up to 300nm into the lumen, forming a dense sheet of proteins that protect the underlying epithelium (49), thus virus trapped by MUC16-bound Abs will be sequestered above the epithelial surface and FcRn, and any capacity to transcytose. Moreover, MUC16 has a protease cleavage site that enables the ectodomain to be shed from the epithelial surface into mucus (49, 50). Thus, it is plausible that upon immune complex mediated cross-linking of one or more MUC16 proteins, the complexes would be shed far from the surface in a larger complex with soluble MUC16 for removal in mucus.
MUC16 is present in multiple mucosal tissues, including the reproductive and respiratory tracts, thus approaches to enhance Ab binding to MUC16 offers a unique opportunity to coat mucosal surfaces to provide protection from additional viral, bacterial, and parasitic infections. Moreover, alternate Ab interactions likely exist for other mucin proteins, such as MUC5AC/MUC5B in the respiratory tract or MUC2 in the large intestine (51), offering additional opportunities and strategies to selectively program Abs to line specific mucosal barriers and improve anti-pathogen activity and protective efficacy. In summary, we describe a novel interaction between a mucosal protein, MUC16, and Abs that is enhanced through modulation of the Fc-glycan, highlighting a novel opportunity to increase protection against virus transmission by enriching mucosal surfaces with HIV-specific Abs.
METHODS
Patient Abs and mAbs
Plasma samples from HIV+, HCV, influenza, RA patients, and healthy subjects were obtained with MGH Institutional review board approval, and all patients provided written informed consent. Bulk IgG was purified and quantified as described in the Supplementary Methods (SM). mAbs were purchased as indicated in the SM.
Binding ELISA
Recombinant galectins (R&D Systems), MUC1 (aa1-264; Origene), MUC16 (aa21005-21992; R&D Systems), or native MUC16 isolated from OVCAR3 cells (see SM for details) were immobilized onto ELISA plates, and Abs were assayed for binding using detecting using α-human IgG Fc (MP Cappel).
Microscopy
Cervical tissue was isolated from patients undergoing hysterectomies at Northwestern Memorial Hospital and explants of endocervix were preserved in OCT media (Study #00025456). Tissue sections were stained as detailed in SM.
Cell-based Ab-MUC16 binding assay
Wild type or OVCAR3 cells stably expressing an shRNA targeting MUC16 were incubated with Cy5-labeled Abs from chronic HIV+ patients as detailed in SM. Binding of Abs to cells, defined by mean fluorescence intensity, was measured by flow cytometry.
Glycan analysis
The relative abundance of Ab glycan structures were quantified by capillary electrophoresis or mass spectrometry as previously described (27), detailed in SM.
Enzymatic digestions and glycan modification
Enzymatic digestion was used to modify glycans as described in the SM. IgG was digested into Fab, F(ab)2, and Fc using IdeS (Genovis) and papain (Thermo Scientific). Glycans were digested with PNGase F (NEB), neuraminidase (NEB), and β1,4-galactosidase (EMD Millipore). G0 Abs were enriched using ECL-agarose beads (Vectors Labs) as described in SM.
SPR analysis
The binding affinity of digested Abs to recombinant MUC16 (R&D), FcγRIIIA (R&D), and Protein A (Sigma Aldrich) was determined by SPR as detailed in the SM.
MUC16 capture assay
MUC16 was bound to magnetic beads and incubated with pre-cleared HIVIG. Beads were washed, and bound Abs eluted in 6M guanidine HCl prior to analysis.
Afucosylated Ab production
293T cells stably expressing FUT8 shRNA were generated and transfected with a plasmid expressing the heavy and light chains of IgG1 VRC03. Abs were purified by Protein A and evaluated for MUC16 binding as described above.
Viral capture assays
The amount of HIV capture by MUC16-bound Abs was determined by microscopy and p24 ELISA as detailed below and in SM.
Microscopy-based viral capture
MUC16 was bound to a poly-d-lysine-coated microscopy plate (MatTek) and Abs were bound prior to addition of fluorescent HIV (52). Captured virions were imaged by confocal microscopy and enumerated using ImageJ.
ELISA-based viral capture
MUC16 was immobilized onto an ELISA plate and Abs were bound prior to the addition of HIV SF162. Captured HIV was determined by p24 ELISA (Perkin Elmer).
Statistical Analysis
Data was analyzed for statistical significance using GraphPad Prism as detailed in SM.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Bill and Melinda Gates Foundation (BMGF) OPP1031734 (TJH). HIV+ samples were collected under the BMGF OPP1066973 (BDW). Ab glycan analysis was developed under the BMGF OPP1114729 (GA). HCV samples were collected under the NIH U19 A166345 (GL). Co-first authors contributed equally to this work including the identification of the Ab-MUC16 interaction (MS), performance of experiments (BG/JS/MS), and all figure and paper preparation (BG/JS). We would like to thank Daniel Stieh for helpful advice pertaining to experimental design.
REFERENCES
- 1.UNAIDS Global Report: UNAIDS report of the global AIDS epidemic 2013. 2013 [Google Scholar]
- 2.Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nature reviews Microbiology. 2003 Oct;1(1):25–34. doi: 10.1038/nrmicro729. PubMed PMID: 15040177. [DOI] [PubMed] [Google Scholar]
- 3.Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nature medicine. 2009 Aug;15(8):951–4. doi: 10.1038/nm.1974. PubMed PMID: 19525965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007 Sep 6;449(7158):101–4. doi: 10.1038/nature06106. PubMed PMID: 17805298. [DOI] [PubMed] [Google Scholar]
- 5.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. Broadly Neutralizing Anti-HIV-1 Antibodies Require Fc Effector Functions for In Vivo Activity. Cell. 2014 Sep 11;158(6):1243–53. doi: 10.1016/j.cell.2014.08.023. PubMed PMID: 25215485. Pubmed Central PMCID: 4167398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nag P, Kim J, Sapiega V, Landay AL, Bremer JW, Mestecky J, et al. Women with cervicovaginal antibody-dependent cell-mediated cytotoxicity have lower genital HIV-1 RNA loads. The Journal of infectious diseases. 2004 Dec 1;190(11):1970–8. doi: 10.1086/425582. PubMed PMID: 15529262. Pubmed Central PMCID: 3119045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko SY, Pegu A, Rudicell RS, Yang ZY, Joyce MG, Chen X, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014 Aug;:13. doi: 10.1038/nature13612. PubMed PMID: 25119033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gipson IK, Blalock T, Tisdale A, Spurr-Michaud S, Allcorn S, Stavreus-Evers A, et al. MUC16 is lost from the uterodome (pinopode) surface of the receptive human endometrium: in vitro evidence that MUC16 is a barrier to trophoblast adherence. Biology of reproduction. 2008 Jan;78(1):134–42. doi: 10.1095/biolreprod.106.058347. PubMed PMID: 17942799. [DOI] [PubMed] [Google Scholar]
- 9.Blalock TD, Spurr-Michaud SJ, Tisdale AS, Heimer SR, Gilmore MS, Ramesh V, et al. Functions of MUC16 in corneal epithelial cells. Investigative ophthalmology & visual science. 2007 Oct;48(10):4509–18. doi: 10.1167/iovs.07-0430. PubMed PMID: 17898272. [DOI] [PubMed] [Google Scholar]
- 10.Govindarajan B, Menon BB, Spurr-Michaud S, Rastogi K, Gilmore MS, Argueso P, et al. A metalloproteinase secreted by Streptococcus pneumoniae removes membrane mucin MUC16 from the epithelial glycocalyx barrier. PloS one. 2012;7(3):e32418. doi: 10.1371/journal.pone.0032418. PubMed PMID: 22412870. Pubmed Central PMCID: 3296694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nature reviews Immunology. 2008 Jun;8(6):447–57. doi: 10.1038/nri2302. PubMed PMID: 18469831. Pubmed Central PMCID: 2587276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raux M, Finkielsztejn L, Salmon-Ceron D, Bouchez H, Excler JL, Dulioust E, et al. IgG subclass distribution in serum and various mucosal fluids of HIV type 1-infected subjects. AIDS research and human retroviruses. 2000 Apr 10;16(6):583–94. doi: 10.1089/088922200309007. PubMed PMID: 10777149. [DOI] [PubMed] [Google Scholar]
- 13.Dugast AS, Stamatatos L, Tonelli A, Suscovich TJ, Licht AF, Mikell I, et al. Independent evolution of Fc- and Fab-mediated HIV-1-specific antiviral antibody activity following acute infection. European journal of immunology. 2014 Oct;44(10):2925–37. doi: 10.1002/eji.201344305. PubMed PMID: 25043633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argueso P, Guzman-Aranguez A, Mantelli F, Cao Z, Ricciuto J, Panjwani N. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. The Journal of biological chemistry. 2009 Aug 21;284(34):23037–45. doi: 10.1074/jbc.M109.033332. PubMed PMID: 19556244. Pubmed Central PMCID: 2755710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabawat SE, Bast RC, Jr., Bhan AK, Welch WR, Knapp RC, Colvin RB. Tissue distribution of a coelomic-epithelium-related antigen recognized by the monoclonal antibody OC125. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 1983;2(3):275–85. doi: 10.1097/00004347-198303000-00005. PubMed PMID: 6196309. [DOI] [PubMed] [Google Scholar]
- 16.Kutteh WH, Hatch KD, Blackwell RE, Mestecky J. Secretory immune system of the female reproductive tract: I. Immunoglobulin and secretory component-containing cells. Obstetrics and gynecology. 1988 Jan;71(1):56–60. PubMed PMID: 3336542. [PubMed] [Google Scholar]
- 17.Fahrbach KM, Malykhina O, Stieh DJ, Hope TJ. Differential binding of IgG and IgA to mucus of the female reproductive tract. PloS one. 2013;8(10):e76176. doi: 10.1371/journal.pone.0076176. PubMed PMID: 24098437. Pubmed Central PMCID: 3788792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kui Wong N, Easton RL, Panico M, Sutton-Smith M, Morrison JC, Lattanzio FA, et al. Characterization of the oligosaccharides associated with the human ovarian tumor marker CA125. The Journal of biological chemistry. 2003 Aug 1;278(31):28619–34. doi: 10.1074/jbc.M302741200. PubMed PMID: 12734200. [DOI] [PubMed] [Google Scholar]
- 19.Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. The Journal of clinical investigation. 2013 May 1;123(5):2183–92. doi: 10.1172/JCI65708. PubMed PMID: 23563315. Pubmed Central PMCID: 3637034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomaras GD, Haynes BF. HIV-1-specific antibody responses during acute and chronic HIV-1 infection. Current opinion in HIV and AIDS. 2009 Sep;4(5):373–9. doi: 10.1097/COH.0b013e32832f00c0. PubMed PMID: 20048700. Pubmed Central PMCID: 3133462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forthal D, Hope TJ, Alter G. New paradigms for functional HIV-specific nonneutralizing antibodies. Current opinion in HIV and AIDS. 2013 Sep;8(5):393–401. doi: 10.1097/COH.0b013e328363d486. PubMed PMID: 23924999. Pubmed Central PMCID: 4097845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annual review of immunology. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. PubMed PMID: 17029568. [DOI] [PubMed] [Google Scholar]
- 23.Jefferis R. Isotype and glycoform selection for antibody therapeutics. Archives of biochemistry and biophysics. 2012 Oct 15;526(2):159–66. doi: 10.1016/j.abb.2012.03.021. PubMed PMID: 22465822. [DOI] [PubMed] [Google Scholar]
- 24.Huhn C, Selman MH, Ruhaak LR, Deelder AM, Wuhrer M. IgG glycosylation analysis. Proteomics. 2009 Feb;9(4):882–913. doi: 10.1002/pmic.200800715. PubMed PMID: 19212958. [DOI] [PubMed] [Google Scholar]
- 25.Moore JS, Wu X, Kulhavy R, Tomana M, Novak J, Moldoveanu Z, et al. Increased levels of galactose-deficient IgG in sera of HIV-1-infected individuals. Aids. 2005 Mar 4;19(4):381–9. doi: 10.1097/01.aids.0000161767.21405.68. PubMed PMID: 15750391. [DOI] [PubMed] [Google Scholar]
- 26.Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985 Aug 1-7;316(6027):452–7. doi: 10.1038/316452a0. PubMed PMID: 3927174. [DOI] [PubMed] [Google Scholar]
- 27.Mahan AE, Tedesco J, Dionne K, Baruah K, Cheng HD, De Jager PL, et al. A method for high-throughput, sensitive analysis of IgG Fc and Fab glycosylation by capillary electrophoresis. Journal of immunological methods. 2015 Feb;417:34–44. doi: 10.1016/j.jim.2014.12.004. PubMed PMID: 25523925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frenzel A, Hust M, Schirrmann T. Expression of recombinant antibodies. Frontiers in immunology. 2013;4:217. doi: 10.3389/fimmu.2013.00217. PubMed PMID: 23908655. Pubmed Central PMCID: 3725456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cummings RD, Etzler ME. Antibodies and Lectins in Glycan Analysis. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, et al., editors. Essentials of Glycobiology. 2nd. Cold Spring Harbor; NY: 2009. [PubMed] [Google Scholar]
- 30.Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry. 1981 Apr 28;20(9):2361–70. PubMed PMID: 7236608. [PubMed] [Google Scholar]
- 31.Krapp S, Mimura Y, Jefferis R, Huber R, Sondermann P. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. Journal of molecular biology. 2003 Jan 31;325(5):979–89. doi: 10.1016/s0022-2836(02)01250-0. PubMed PMID: 12527303. [DOI] [PubMed] [Google Scholar]
- 32.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. The Journal of biological chemistry. 2001 Jul 20;276(29):27371–5. doi: 10.1074/jbc.M103554200. PubMed PMID: 11369781. [DOI] [PubMed] [Google Scholar]
- 33.Ferrara C, Grau S, Jager C, Sondermann P, Brunker P, Waldhauer I, et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proceedings of the National Academy of Sciences of the United States of America. 2011 Aug 2;108(31):12669–74. doi: 10.1073/pnas.1108455108. PubMed PMID: 21768335. Pubmed Central PMCID: 3150898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies J, Jiang L, Pan LZ, LaBarre MJ, Anderson D, Reff M. Expression of GnTIII in a recombinant anti-CD20 CHO production cell line: Expression of antibodies with altered glycoforms leads to an increase in ADCC through higher affinity for FC gamma RIII. Biotechnology and bioengineering. 2001 Aug 20;74(4):288–94. PubMed PMID: 11410853. [PubMed] [Google Scholar]
- 35.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006 Aug 4;313(5787):670–3. doi: 10.1126/science.1129594. PubMed PMID: 16888140. [DOI] [PubMed] [Google Scholar]
- 36.Longmore GD, Schachter H. Product-identification and substrate-specificity studies of the GDP-L-fucose:2-acetamido-2-deoxy-beta-D-glucoside (FUC goes to Asn-linked GlcNAc) 6-alpha-L-fucosyltransferase in a Golgi-rich fraction from porcine liver. Carbohydrate research. 1982 Mar 1;100:365–92. doi: 10.1016/s0008-6215(00)81049-6. PubMed PMID: 7083256. [DOI] [PubMed] [Google Scholar]
- 37.Liu P, Yates NL, Shen X, Bonsignori M, Moody MA, Liao HX, et al. Infectious virion capture by HIV-1 gp120-specific IgG from RV144 vaccinees. Journal of virology. 2013 Jul;87(14):7828–36. doi: 10.1128/JVI.02737-12. PubMed PMID: 23658446. Pubmed Central PMCID: 3700223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watkins JD, Sholukh AM, Mukhtar MM, Siddappa NB, Lakhashe SK, Kim M, et al. Anti-HIV IgA isotypes: differential virion capture and inhibition of transcytosis are linked to prevention of mucosal R5 SHIV transmission. Aids. 2013 Jun 1;27(9):F13–20. doi: 10.1097/QAD.0b013e328360eac6. PubMed PMID: 23775002. Pubmed Central PMCID: 4084966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris L, Binley JM, Clas BA, Bonhoeffer S, Astill TP, Kost R, et al. HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. The Journal of experimental medicine. 1998 Jul 20;188(2):233–45. doi: 10.1084/jem.188.2.233. PubMed PMID: 9670036. Pubmed Central PMCID: 2212446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang YY, Kannan A, Nunn KL, Murphy MA, Subramani DB, Moench T, et al. IgG in cervicovaginal mucus traps HSV and prevents vaginal Herpes infections. Mucosal immunology. 2014 Sep;7(5):1036–44. doi: 10.1038/mi.2013.120. PubMed PMID: 24496316. Pubmed Central PMCID: 4122653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen A, McKinley SA, Wang S, Shi F, Mucha PJ, Forest MG, et al. Transient antibody-mucin interactions produce a dynamic molecular shield against viral invasion. Biophysical journal. 2014 May 6;106(9):2028–36. doi: 10.1016/j.bpj.2014.02.038. PubMed PMID: 24806935. Pubmed Central PMCID: 4017286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russell MW, Mestecky J. Humoral immune responses to microbial infections in the genital tract. Microbes and infection / Institut Pasteur. 2002 May;4(6):667–77. doi: 10.1016/s1286-4579(02)01585-x. PubMed PMID: 12048036. [DOI] [PubMed] [Google Scholar]
- 43.Buck PM, Kumar S, Singh SK. Consequences of glycan truncation on Fc structural integrity. mAbs. 2013 Nov-Dec;5(6):904–16. doi: 10.4161/mabs.26453. PubMed PMID: 24492344. Pubmed Central PMCID: 3896604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mimura Y, Church S, Ghirlando R, Ashton PR, Dong S, Goodall M, et al. The influence of glycosylation on the thermal stability and effector function expression of human IgG1-Fc: properties of a series of truncated glycoforms. Molecular immunology. 2000 Aug-Sep;37(12-13):697–706. doi: 10.1016/s0161-5890(00)00105-x. PubMed PMID: 11275255. [DOI] [PubMed] [Google Scholar]
- 45.Hanson QM, Barb AW. A perspective on the structure and receptor binding properties of immunoglobulin g fc. Biochemistry. 2015 May;1954(19):2931–42. doi: 10.1021/acs.biochem.5b00299. PubMed PMID: 25926001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dalziel M, Crispin M, Scanlan CN, Zitzmann N, Dwek RA. Emerging principles for the therapeutic exploitation of glycosylation. Science. 2014 Jan 3;343(6166):1235681. doi: 10.1126/science.1235681. PubMed PMID: 24385630. [DOI] [PubMed] [Google Scholar]
- 47.Santra S, Tomaras GD, Warrier R, Nicely NI, Liao HX, Pollara J, et al. Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques. PLoS pathogens. 2015 Aug;11(8):e1005042. doi: 10.1371/journal.ppat.1005042. PubMed PMID: 26237403. Pubmed Central PMCID: 4523205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta S, Gach JS, Becerra JC, Phan TB, Pudney J, Moldoveanu Z, et al. The Neonatal Fc receptor (FcRn) enhances human immunodeficiency virus type 1 (HIV-1) transcytosis across epithelial cells. PLoS pathogens. 2013;9(11):e1003776. doi: 10.1371/journal.ppat.1003776. PubMed PMID: 24278022. Pubmed Central PMCID: 3836734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annual review of physiology. 2008;70:431–57. doi: 10.1146/annurev.physiol.70.113006.100659. PubMed PMID: 17850209. [DOI] [PubMed] [Google Scholar]
- 50.Blalock TD, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Release of membrane-associated mucins from ocular surface epithelia. Investigative ophthalmology & visual science. 2008 May;49(5):1864–71. doi: 10.1167/iovs.07-1081. PubMed PMID: 18436821. Pubmed Central PMCID: 2622730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGuckin MA, Linden SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nature reviews Microbiology. 2011 Apr;9(4):265–78. doi: 10.1038/nrmicro2538. PubMed PMID: 21407243. [DOI] [PubMed] [Google Scholar]
- 52.Chukkapalli V, Hogue IB, Boyko V, Hu WS, Ono A. Interaction between the human immunodeficiency virus type 1 Gag matrix domain and phosphatidylinositol-(4,5)-bisphosphate is essential for efficient gag membrane binding. Journal of virology. 2008 Mar;82(5):2405–17. doi: 10.1128/JVI.01614-07. PubMed PMID: 18094158. Pubmed Central PMCID: 2258911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.