Abstract
Recent studies have highlighted presence of endotoxin in indoor air and its role in respiratory morbidities. Burning of household fuels including unprocessed wood and dried animal dung could be a major source of endotoxin in homes. We measured endotoxin levels in different size fractions of airborne particles (PM10, PM2.5, and PM1), and estimated the deposition of particle-bound endotoxin in the respiratory tract. The study was carried out in homes burning solid biomass fuel (n = 35) and LPG (n = 35). Sample filters were analyzed for endotoxin and organic carbon (OC) content. Household characteristics including temperature, relative humidity, and carbon dioxide levels were also recorded. Multivariate regression models were used to estimate the contributing factors for airborne endotoxin. Respiratory deposition doses were calculated using a computer-based model. We found a higher endotoxin concentration in PM2.5 fractions of the particle in both LPG (median: 110, interquartile range, (IQR): 100-120 EU/m3) and biomass (median: 350, IQR: 315-430 EU/m3) burning homes. In the multivariate-adjusted model, burning of solid biomass fuel (β: 67; 95%CI: 10.5-124) emerged as the most significant predictor followed by OC (β: 4.7; 95%CI: 2.7-6.8), RH (β: 1.6; 95%CI: 0.76-2.4) and PM2.5 (β: 0.45; 95%CI: 0.11-0.78) for airborne endotoxin (p < 0.05). We also observed an interaction between PM organic carbon content and household fuel in predicting the endotoxin levels. The model calculations showed that in biomass burning homes, total endotoxin deposition was higher among infants (59%) than in adult males (47%), of which at least 10% of inhaled endotoxin is deposited in the alveolar region of the lung. These results indicate that fine particles are significant contributors to the deposition of endotoxin in the alveolar region of the lung. Considering the paramount role of endotoxin exposure, and the source and timing of exposure on respiratory health, additional studies are warranted to guide evidence-based public health interventions.
Keywords: Endotoxin, Household air pollution (HAP), LUDEP, Particulate matter, Respiratory deposition
INTRODUCTION
Household air quality is an important environmental determinant in public health and highlighted repeatedly in Global Burden of Disease (GBD) study1,2. The disease burden is a consequence of exposure to both chemical and biological components in the air. Among the potential triggers, biological components of airborne particulate matter pose an increasing level of health concerns3–13. Recent research shows presence of elevated levels of endotoxin in household air that burns solid biomass for cooking and heating3,10,14. Endotoxin is also found in the house dust and occurs naturally in the environment6,8,15–19. There is a growing literature on airborne endotoxin to be a risk factor for clinically symptomatic respiratory illnesses6,12,13,20,21. While the primary pathway may be the deposition of the particles (i.e., the initial and intimate contact with the respiratory tract), the mechanisms of endotoxin-induced respiratory health effects are not fully understood. As a first step, information on endotoxin levels in coarse, fine and ultrafine particle can help elucidate the underlying mechanisms. Although data on ambient endotoxin concentrations have been reported in the literature as part of occupational and ambient air studies, little is known about the size-segregated endotoxin levels in indoor air and respiratory deposition of inhaled particle-bound endotoxin. The current study was designed to characterize endotoxin levels in coarse, fine, and ultrafine particles, its predictors in household air, and respiratory deposition doses. In this study we also investigated the role of other environmental factors such as organic carbon, carbon dioxide, temperature, and relative humidity on the recorded endotoxin levels.
MATERIAL AND METHODS
Study households and study settings
This cross-sectional study was carried out in semi-urban and rural settings of Odisha, an eastern state in India and home to 41.9 million people. The state has one of the highest infant (57 per 1,000 live births) and maternal mortality (257 per 100,000 live births) in India22. A total of 70 homes representing a range of community settings were included for detailed household air pollution assessment. Since the study aimed to investigate the endotoxin concentration associated with household cooking fuel, we assigned the study households into two groups; biomass and LPG. In biomass user group (n=35) household cooking was primarily done in their homes using wood, cow dung, and agricultural refuse, such as straw, paddy husk, hay, dried leaves, and jute stick, on a traditional cooking stove called Chulha. Another group of households (n=35) from the same community settings, using LPG as cooking fuel was also included
Household characteristics
Information on home characteristics such as age and structure of the house, household cooking behavior and ownership of domestic animals were recorded by interviewing the family members of the study household. If the household did not have a mechanized ventilation system it was considered as a poorly ventilated house. When the roof of the house was made of straw and bamboo it was called a thatched-roofed house. Household ownership of animals such as cow, goat or dog was considered as houses with domestic animals.
Household air pollution monitoring
Air sampling was conducted using a low-pressure three-stage cascade impactor (Dekati® PM10 impactor), with an aerodynamic diameter (dae) cut-off at 10, 2.5 and 1 μm. The impactor was operated at 10 L/min and collected particles on the quartz filter (Whatman International, Ltd., Maidstone, England). In the Dekati PM10 impactor particles >1 μm are collected on 25 mm substrates and particles <1 μm are collected on 47 mm filters23. Before mounting the filter paper, the serial number was recorded and the filter equilibrated overnight in ambient temperature and humidity. A calibrated microbalance (Mettler Instrument Corp., Hightstown, NJ) was used to weigh the filter papers with a precision of ±5 μg. A thorough quality inspection was conducted to identify any tears, folds, and other imperfections in the filter papers. About 10% of the filters were prepared for blanks (transport blank and laboratory blank) and was carried out as a control of contaminants check. Both blank and air sample filters were analyzed following the same protocol. Pre and post-weighing of the filters (including the blanks) were conducted under the same environmental conditions, in an air-conditioned room with humidity control. Instruments were positioned at the center of the living room and placed 1.5 m above the ground, and at least 0.5 m away from walls. After sampling, the particle loaded filter papers were placed in a sealed container and transported to the laboratory for further analysis. Total mass concentration of each filter were estimated by weighing the particle loaded filters. Carbon dioxide, temperature, and relative humidity were measured concurrently by a portable multi-gas air quality monitor (YES Plus, Canada). Sampling was carried out for twelve hours (8.0 am - 8.0 pm) during the month of February – April and was same across sites.
Endotoxin analysis
Samples were stored at −20°C and transported in batches for processing in the laboratory within 2-4 weeks of collection. Endotoxin concentrations of airborne particles were determined using the endotoxin-specific kinetic chromogenic LAL-assay (Pyrochrome, Associates of Cape Cod) described elsewhere18. Briefly, a part of sample filters were extracted in 5 mL pyrogen-free sterile water for 1h by sonication and intermittent shaking, followed by centrifugation at 3000g for 5 min. Blank filters were assayed in the same manner. Pyrogen-free polypropylene vials and centrifuge tubes were used for aliquoting and processing of filter extracts. Although filters were not depyrogenated, we analyzed the levels of endotoxin in media blank and field blank filters and found < LOD levels of endotoxin in our filters. A multi-point standard curve was generated ranging from 5 to 50 EU/mL (R2 > 0.992), using control standard endotoxin (Escherichia coli). The absorbance was measured photometrically at 405 nm. The analysis used time of onset needed to reach a predetermined absorbance or transmission of the reaction mixture and a standard curve, showing the linear correlation between the log onset time and the log concentration of standard endotoxin. All samples were run in triplicate and the detection limit was calculated from sample filters as well as in field blank filters. Endotoxin concentrations in the extracts were finally reported as endotoxin units (EU) per cubic meter of air collected.
Organic carbon (OC) analysis
The carbon content of PM loaded filters were analyzed using Chemo-Thermal Oxidation method and subsequent analysis on CHNS-O analyzer as described previously24,25. Briefly, two circular punches of 6 mm diameter were taken from the quartz filters. One of the pieces was pre-combusted in the furnace at 350°C for 24 h to remove OC. Both un-combusted and combusted pieces were then weighed with the microbalance and analyzed for carbon content using the CHNS-O analyzer (Thermo Scientific™ FLASH 2000 CHNS/O Analyzer), which was operated in the CHN mode with acetanilide (71.09% C, 6.71% H, 10.36% N) as calibration standard. Prior to analysis, carbonates were removed by HCl treatment. The OC content was obtained by subtracting elemental carbon (EC) from total carbon (TC).
Estimation of respiratory Deposition
Deposition of particle-bound airborne endotoxin in different regions of the human respiratory tract was calculated using a computer-based respiratory deposition model, the LUDEP (Lung Dose Evaluation Program; LUDEP 2.07; Health Protection Agency, London, WC1V 7PP, U.K.) with parameters representing typical adults and children of different age groups. LUDEP is a computer program, which implements the model of the human respiratory tract proposed by the International Commission on Radiological Protection26. The calculations were based on the endotoxin concentration data collected within specific particle size ranges (PM1). Breathing rates and time intervals that simulate typical indoor activities was used for the modeling process. The LUDEP model predicts the particle deposition into five regions in the respiratory tract: anterior nasal region (ET1), main extrathoracic region comprising the posterior nasal passages, larynx, pharynx, and mouth (ET2), bronchial region (BB), bronchiolar region (bb), and alveolar–interstitial region (AI). Detailed formulas for calculating deposition in each of the regions are presented in ICRP, 199426. The output from the model described the percent deposition in the entire respiratory tract, as well as in different regions separately.
Data analysis
A database was created using a custom-designed Epi-info platform. Descriptive statistics such as frequency, means, standard deviations, and IQR (inter quartile range) were calculated for all parameters and cross-tabulated with household fuel use. Group comparisons were performed using the chi-Square (χ2) test (for categorical variables). For continuous variables Mann-Whitney test or Student’s t-test was performed. Unadjusted and adjusted β Coefficients and 95%Cis were computed using OLS regression models to estimate the predictors of endotoxin levels in airborne particles. All multivariate regression models were performed using a priori hypotheses. The interactions between environmental factors and household cooking fuel in predicting the endotoxin was also tested. Results from all analyses were considered significant at an alpha of 0.05. All data analysis including production of tables and figures were performed using Stata® Software version SE 13.0 (College Station, TX, USA).
Ethical approval
Ethical approval for the study was obtained from the Ethical Review Committee of the Asian Institute of Public Health (AIPH). Consent was taken from the head of the households after full information about the study was given to them by trained personnel. They were free to withdraw from the study at any point. The survey team received cultural competency and confidentiality training prior to administering the questionnaires by a qualified trainer.
RESULTS
The study investigated the endotoxin-laden airborne particulate matter released during fuel combustions, which can increase the respiratory deposition doses in subjects. The characteristics of the study households including endotoxin levels stratified by household fuel use are presented in Table 1. Of the study variables, only RH and ownership of domestic animals were not significantly different among the biomass and LPG groups. The airborne particles and endotoxin in three different size fractions (PM10, PM2.5, and PM1) in indoor air of homes burning biomass fuel and LPG are presented in Table 1. In both types of households, PM10 fraction of the airborne particles was found to be present in higher concentrations. However, higher concentration of endotoxin was found in PM2.5 fractions of the airborne particles. Endotoxin concentration was significantly higher in all size fractions in biomass burning homes compared to LPG burning homes (p<0.01). In this study, PM2.5 median concentrations of endotoxin from biomass user households (350.0 EU/m3) was found to be 3-fold higher than LPG using households (110 EU/m3).
Table 1.
Descriptive statistics of household characteristics for LPG and biomass fuel user groups*. [N= 70]
| Characteristics | LPG, median (IQR) [n = 35] |
Biomass, median (IQR) [n =35] |
P-value |
|---|---|---|---|
| Age of the house (years) | 15.0 (10.0-15.0) | 25.0 (20.0-30.0) | <0.001 |
| Type of house - Thatched [n, %] |
7 (20.0) | 21 (60.0) | 0.001 |
| House with mud walls and floor [n, %] |
15 (42.8) | 34 (97.1) | 0.001 |
| House with poor ventilation, Yes [n, %] |
13 (37.1) | 23 (65.7) | 0.017 |
| House having domestic animals [n, %] |
15 (42.8) | 13 (37.1) | 0.626 |
| Person Occupancy | 5 (5-6) | 6 (5-7) | 0.001 |
| Temperature (°C) | 29.6 (28.0-30.8) | 31.8 (30.0-35.2) | 0.002 |
| Relative Humidity (%) | 65.7 (60.0-73.5) | 65.7 (60.0-78.9) | 0.719 |
| Carbon Dioxide (PPM) | 950 (870-1040) | 1320 (1185-1400) | <0.001 |
| Organic Carbon (μg/m3) | 15.2 (11.7-22.4) | 35.8 (32.4-41.2) | <0.001 |
| PM10 (μg/m3) | 128.5 (120-138.2) | 320 (292.4-340) | <0.001 |
| PM2.5 (μg/m3) | 100.2 (90.5-114.5) | 285.5 (246.9-312.2) | <0.001 |
| PM1 (μg/m3) | 70.1 (52.4-80.0) | 168.6 (140.0-182.4) | <0.001 |
| Endotoxin in PM10 (EU/m3) | 92.0 (85.0-97.0) | 300.0 (280.0-325.0) | <0.001 |
| Endotoxin in PM2.5 (EU/m3) | 110.0 (100.0-120.0) | 350.0 (315.0-430.0) | <0.001 |
| Endotoxin in PM1 (EU/m3) | 22.0 (18.0-27.0) | 130.0 (107.0-160.0) | <0.001 |
Reported p-values are from Mann-Whitney test for differences in medians and Pearson’s chi squared tests for categorical variables. Two-sided test (p<0.05).
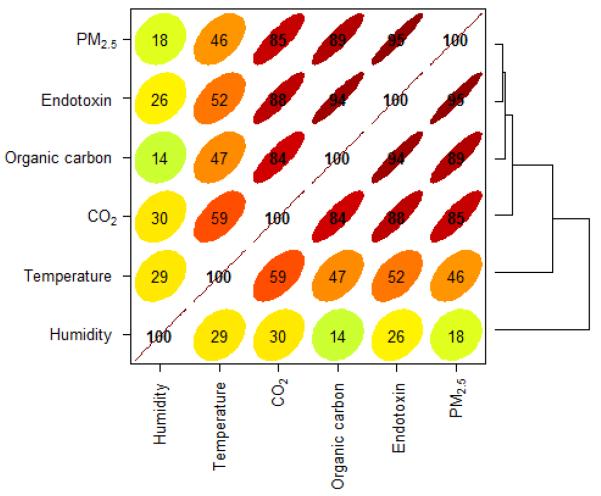
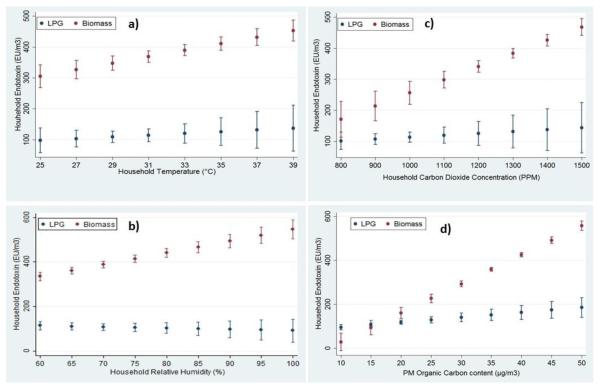
Figure 1 shows the correlation coefficients between environmental parameters. Figure 2 shows the adjusted predictions of endotoxin concentration in PM2.5 fractions with household a) temperature; b) relative humidity; c) carbon dioxide and d) particle organic carbon content. In biomass burning homes, the predicted airborne endotoxin concentrations are significantly higher with higher concentration of OC and CO2 than its counterparts.
Figure 1.
Corelation plot for endotoxin and environmental parameters.
Figure 2.
Interaction plots for adjusted predictions of endotoxin concentration in PM2.5 fractions with household a) temperature; b) relative humidity; c) carbon dioxide and d) particle organic carbon content.
Table 2 describes the multivariable model with predictors of indoor airborne endotoxin concentrations. The adjusted model indicated that biomass fuel contributed the most to the indoor endotoxin levels (β: 67; 95%CI: 10.5-124), followed by organic carbon (β: 5; 95%CI: 2.7-6.8), relative humidity (β: 2; 95%CI: 0.8-2.5), and higher load of PM2.5 (β: 0.4; 95%CI: 0.1-0.8).
Table 2.
Multivariable Model showing predictors of Airborne Endotoxin Concentration
| Factors | β Coef. [95% CI] Unadjusted |
β Coef. [95% CI] Adjusted+ |
|---|---|---|
| Biomass Cooking Fuel | 272.31 [243.49-301.13] | 67.23 [10.52-123.93]* |
| PM2.5 | 1.49 [1.37-1.61] | 0.45 [0.11-0.78]* |
| Organic Carbon | 11.98 [10.89-13.07] | 4.79 [2.72-6.86]* |
| Temperature | 21.86 [13.19-30.54] | 2.49 [−0.27-5.27] |
| Relative Humidity | 3.74 [0.44-7.05] | 1.62 [0.76-2.49]* |
| Carbon Dioxide | 0.63 [0.55-0.71] | 0.02 [−0.05-0.11] |
| No ventilation | 137.12 [7.77-149.56] | 3.45 [−21.54-28.45] |
| Age of the House | 15.41 [11.68-19.14] | 0.19 [−1.66-2.04] |
| Thatched house | 150.14 [86.35-213.92] | 1.43 [−19.84-22.71] |
| Mud walls and floor | 202.97 [141.77-264.17] | 6.08 [−16.40-28.57] |
| Domestic Animal | 61.40 [32.63-90.17] | 12.25 [−5.43-29.95] |
| Person Occupancy | 0.67 [−72.72-74.08] | 2.89 [−5.30-11.09] |
p<0.05
All analyses also control for the variables in the table as it was measured at the household level.
Table 3 shows the estimation of the deposition of inhaled of ≤1 μm particle-bound endotoxin in the human respiratory tract using the LUDEP (lung dose evaluation program) computer program. The model shows the regional deposition pattern of the inhaled endotoxin in specific regions of the respiratory tract. Our results indicate that endotoxin present in sub-micrometer fragments, which significantly contributes to pulmonary dose of all tested categories of human subjects including infants. The model calculations showed that in biomass burning homes, total endotoxin deposition was higher among infants (59%) than in an adult male (47%), of which at least 10% of inhaled endotoxin is deposited in the alveolar region of the lung.
Table 3.
Deposition Fluxes to the Respiratory Tract of Particulate bound Endotoxin (Aerodynamic size: 1μm) in the Biomass and LPG Burning Homes
| Adult male | Adult female | Boy-15 years | Girl-15 years | Child- 5 years | Infant - 1 year | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomass* | LPG | Biomass* | LPG | Biomass* | LPG | Biomass* | LPG | Biomass* | LPG | Biomass* | LPG | |
| ET1 | 14.59 | 1.62 | 15.50 | 1.72 | 14.75 | 1.64 | 15.22 | 1.69 | 16.87 | 1.88 | 20.55 | 2.29 |
| ET2 | 18.36 | 2.04 | 19.64 | 2.18 | 18.60 | 2.07 | 19.27 | 2.14 | 22.54 | 2.51 | 26.54 | 2.95 |
| BB | 0.60 | 0.07 | 0.62 | 0.07 | 0.59 | 0.07 | 0.59 | 0.07 | 0.54 | 0.06 | 0.54 | 0.06 |
| Bb | 1.13 | 0.13 | 1.16 | 0.13 | 1.10 | 0.12 | 1.10 | 0.12 | 1.05 | 0.12 | 0.95 | 0.11 |
| AI | 12.22 | 1.36 | 11.33 | 1.26 | 11.26 | 1.25 | 11.07 | 1.23 | 10.66 | 1.19 | 10.16 | 1.13 |
| Total | 46.9 | 5.22 | 48.25 | 5.36 | 46.3 | 5.15 | 47.25 | 5.25 | 51.66 | 5.76 | 58.74 | 6.54 |
Abbreviations: ET1: anterior nasal region; ET2: main extrathoracic region; BB: bronchial region; bb: bronchiolar region; AI: alveolar interstitial region
Significant at (p<0.001)
DISCUSSION
The study presents indoor concentrations of airborne particles in coarse, fine and ultrafine sizes as well as its associated endotoxin levels in biomass and LPG burning households. Endotoxin concentrations found in PM1 was evaluated using the LUDEP model to estimate the regional depositions in the lungs. The study also predicted predominant contributors of airborne endotoxin in indoor air using linear models. The ventilation condition of each home is an important variable influencing endotoxin concentrations. There were no HVAC systems installed in these homes and homes were naturally ventilated. The kitchens, however, were relatively less ventilated than other areas in the home and were mostly without fans. Getting accurate information on the duration when windows and doors were open in these houses was not feasible making it very difficult to estimate air exchanges. Lacking air exchange estimates in these homes, we took the indoor CO2 levels as a surrogate measure of ventilation conditions27.
A study in Malawian and Nepalese homes showed that the median concentrations of total inhalable endotoxin were 24 EU/m3 in charcoal-burning homes and 40 EU/m3 wood-burning homes in Malawi10. The median endotoxin values were 43 EU/m3 in wood-burning homes and 365 EU/m3 in dung-burning homes in Nepal10. Another study on airborne endotoxin from biomass fuel in the Ladakh region of India reported endotoxin level of 190 EU/m3 in a rural community settings28. Our results in the range of 315-430 EU/m3 in biomass burning homes and are in line with the findings of these studies suggesting burning of biomass fuels to be associated with airborne endotoxin. Another recent study17 done in Canadian homes reported higher level of endotoxin in coarse particles in outdoor (median: 6.7 μg/m3, IQR: 3.4-1.2 μg/m3) compared to indoor air (median: 3.4 μg/m3, IQR: 1.6-5.7 μg/m3).
A study in Boston, United States, suggested multiple home characteristics to be major predictors of airborne endotoxin and explained 42% of the variability in a multivariate model29. The study tested 49 home characteristics and found the presence of a dog to be the strongest predictor of increased level of airborne endotoxin (partial R2 12.8%)29. In our study, most homes had animals and although presence of domestic animals emerged as the second most (12%) contributor in the multivariate model, their presence did not show statistical significance (p= 0.103), may be due to small sample size. However, burning of biomass fuel explains 67% (p = 0.013) of variability in predicting indoor airborne endotoxin in our study settings.
In our study, we observed a significant positive correlation between endotoxin level and temperature (r=0.5) and a weak correlation with humidity (r=0.2) (Fig.1). This is similar to an Italian study where authors showed statistically significant positive correlation between endotoxin and temperature (r = 0.5 p < 0.01), but relative humidity had a negative correlation (r = −0.4 p < 0.01)29. The effect of temperature and humidity on endotoxin concentration may be a result of the increased growth of bacteria on the biomass combustion particles30, an aspect we could not address in this study. Interestingly, we found an interaction between fuel use in households and organic carbon in particulate matter (PM 2.5) in predicting indoor endotoxin levels (Fig. 2). Such interactions have not been reported in previous studies.
Establishing an approximate aerodynamic particle size distribution of airborne endotoxin is an important factor in determining endotoxin toxicity and its health effects. However, relatively few empirical data regarding airborne endotoxin concentrations and particle size distribution in homes. A study showed that on an average 66% of airborne endotoxin was found in 0.56–3.2 μm size range31. To the contrary, another study from Germany observed endotoxin content in the coarse fraction to be ten times higher compared to the fine mass fractions (1.2 EU/mg)33. In our study we observed relatively high levels of endotoxin in both fine (PM 2.5) and ultrafine (PM 1) particles. The airborne endotoxin at fine particle sizes might be more damaging to the health than endotoxin at larger particle size ranges. Due to their small sizes, fine particles have longer residence time in the air and penetrate deeper into the respiratory system.
However, the deposition site and pattern of inhaled particles is a key factor in assessment of the association between lung dose and health effects, although the estimation of such patterns is complex. Several approaches have been used to estimate particle deposition in different regions of the human respiratory tract as a function of particle size32. Liao and colleagues used a probabilistic approach to quantitatively assess the potential inhalation risk of airborne endotoxin in homes11,33. Using similar approach, we evaluated the deposition pattern using the LUDEP model and estimated at least 10% of inhaled endotoxins to be deposited in the alveolar region of the lungs and this deposition pattern also appears to be higher in infants than adults in our model. The clearance rate of such fine particles from the alveolar region to lymph nodes is reported to be as low as 0.00002 d−1 compared to 1 d−1 obtained for few micrometer size particles in the nasal region26 , and hence, carries a very important risk in the pathogenesis of respiratory diseases.
Thus, once fine particles enter the lower respiratory tract and reside in the lungs for longer periods, they affect the endothelial and pulmonary mucosal immune system4. Experimental and epidemiological studies have reported that endotoxin associated with fine particles may increase inflammation and alter macrophage responses as well as contribute to an increase in chronic obstructive pulmonary disease, pneumonia, and an overall decrease in lung function13,34. In this context, our report on the presence of endotoxin in fine and ultrafine particles carries important health implications especially in populations that continue to use biomass fuel.
In this study, we did not have the opportunity to examine the health impacts of the endotoxin exposure observed in the households. In the west, such exposure in the childhood has been considered to be protective against development of allergic diseases in some settings and animal feed/grains to be the driver of this protective response35. While some studies have suggested protection due to microbial exposure against asthma or allergy only in childhood36, others have described lifelong benefit of childhood exposure to farming exposures37. To the contrary, Caudri and colleagues reported failure of protection in daycare settings due to specific exposures by age eight, and increasing airway symptoms during early childhood38. Genetic susceptibility and environmental influences are also important contributors to the development of asthma and atopic diseases. Epigenetic mechanisms may facilitate gene by environment interactions in these diseases. Some studies have implicated early life endotoxin exposure in DNA methylation and subsequent development of asthma and allergy39. Future evaluation of respiratory health in different age groups in our population with diverse exposure to environmental endotoxin will shed important light in the pathogenesis of respiratory diseases. In the current study, we could not address the role of bacteria (Gram negatives contributing the endotoxin, vs. Gram positives). Kujundzic et al. have shown the diversity between endotoxin and airborne bacteria in a home setting in Boulder, Colorado31. While outdoor airborne endotoxin varied significantly with season in this study, no seasonal variation was seen for indoor airborne endotoxin. Total airborne bacteria, however, indoors and outdoors significantly varied with seasons31.
We acknowledge some limitations to our study. First, the study is cross-sectional in nature and we could not account for seasonal effects and ventilation rates. Second, with a relatively small sample size it is difficult to generalize the results as in other larger studies. However, even in the small scale, our data clearly demonstrated that biomass fuel burning is significantly associated with increased endotoxin exposures and future large scale studies will be undertaken in large rural and urban population cohort to follow up this finding associated risks of respiratory diseases. Although we might have missed residual and other unmeasured confoundings, we adjusted for a variety of potential predictors of household endotoxin and our analyses indicated that our results were robust.
Nevertheless, with our results for the first time from India, it is now important to consider the emerging role of non-endotoxin components of Gram-positive airborne bacteria as well. Toll-like receptor 4 (TLR4), TLR2 and intracellular nucleotide oligomerization domain-like receptors are responsible for mediating the inflammatory response in the host. While repeated organic dust exposures may modulate innate and adaptive immune function resulting in protection against allergy, multiple exposures can cause lung parenchymal inflammation and decline in lung function over time40. In developing world settings such as India, where children and adults are exposed to a heavy load of environmental bacteria. While childhood atopic diseases may not be of significant concern at present, a plethora of respiratory ailments plague the pediatric, adult and elderly community in India. Ongoing and future work under our IMMENSE research platform addressing multiple environmental exposures and host response will provide invaluable insights into the role of air pollution and endotoxin in health and disease.
CONCLUSION
This study, for the first time, has investigated endotoxin levels in three different size-segregated airborne particulate matter in indoor air and its association with household cooking fuels in India. Biomass burning appears to be the significant contributor of indoor endotoxin in the studied homes. Our results also demonstrate interactions of household fuel use with organic carbon, temperature and humidity in predicting endotoxin levels. These factors may be favoring the growth of bacteria resulting in the observed high endotoxin levels.
Since most vulnerable groups (children, women, and the elderly) spend more than 80% of their time indoors, the respiratory deposition of endotoxin could be a potent risk factor for development of respiratory illness in this community. Research endeavors to identify major sources of endotoxin in household air and design of appropriate interventions to reduce such exposure can have an immense impact on population health. The current data have important practical implications and should help shape new national programs such as “Swachh Bharat” recently launched by the Prime Minister of India.
ACKNOWLEDGMENTS
The authors acknowledge the cooperation of the households during air sampling. We thank Karunakar Panda of AIPH, for assistance with household PM measurements. This study was supported in part by NICHD grant R01 HD 53719 and by the University of Nebraska Foundation through the IMMENSE (Impact of maternal, environmental socio-demographic and economic factors on Child health and development) study. We sincerely acknowledge the State Pollution Control Board (SPCB), Odisha for providing laboratory support.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015 doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sussan TE, Ingole V, Kim J-H, McCormick S, Negherbon J, Fallica J, et al. Source of biomass cooking fuel determines pulmonary response to household air pollution. Am J Respir Cell Mol Biol. 2014;50:538–48. doi: 10.1165/rcmb.2013-0201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Min K-B, Min J-Y. Exposure to household endotoxin and total and allergen-specific IgE in the US population. Environ Pollut. 2015;199C:148–154. doi: 10.1016/j.envpol.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Gordon SB, Bruce NG, Grigg J, Hibberd PL, Kurmi OP, Lam K-BH, et al. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir Med. 2014;2:823–860. doi: 10.1016/S2213-2600(14)70168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norbäck D, Markowicz P, Cai G-H, Hashim Z, Ali F, Zheng Y-W, et al. Endotoxin, ergosterol, fungal DNA and allergens in dust from schools in Johor Bahru, Malaysia- associations with asthma and respiratory infections in pupils. PLoS One. 2014;9:e88303. doi: 10.1371/journal.pone.0088303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawson JA, Dosman JA, Rennie DC, Beach JR, Newman SC, Crowe T, et al. Endotoxin as a determinant of asthma and wheeze among rural dwelling children and adolescents: a case-control study. BMC Pulm Med. 2012;12:56. doi: 10.1186/1471-2466-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs JH, Krop EJM, Borras-Santos A, Zock J-P, Taubel M, Hyvarinnen A, et al. Endotoxin levels in settled airborne dust in European schools: the HITEA school study. Indoor Air. 2014;24:148–57. doi: 10.1111/ina.12064. [DOI] [PubMed] [Google Scholar]
- 9.Delfino RJ, Staimer N, Tjoa T. Personal endotoxin exposure in a panel study of school children with asthma. Environ Health. 2011;10:69. doi: 10.1186/1476-069X-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semple S, Devakumar D, Fullerton DG, Thorne PS, Metwali N, Costello A, et al. Airborne endotoxin concentrations in homes burning biomass fuel. Environ Health Perspect. 2010;118:988–91. doi: 10.1289/ehp.0901605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao VH-C, Chio C-P, Chou W-C, Ju Y-R, Liao C-M. Modeling human health risks of airborne endotoxin in homes during the winter and summer seasons. Sci Total Environ. 2010;408:1530–7. doi: 10.1016/j.scitotenv.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Smit L a M, Heederik D, Doekes G, Blom C, van Zweden I, Wouters IM. Exposure-response analysis of allergy and respiratory symptoms in endotoxin-exposed adults. Eur Respir J. 2008;31:1241–8. doi: 10.1183/09031936.00090607. [DOI] [PubMed] [Google Scholar]
- 13.Dales R, Miller D, Ruest K, Guay M, Judek S. Airborne Endotoxin Is Associated with Respiratory Illness in the First 2 Years of Life. Environ Health Perspect. 2005;114:610–614. doi: 10.1289/ehp.8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNamara M, Thornburg J, Semmens E, Ward T, Noonan C. Coarse particulate matter and airborne endotoxin within wood stove homes. Indoor Air. 2013;23:498–505. doi: 10.1111/ina.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavilonis BT, Anthony TR, O’Shaughnessy PT, Humann MJ, Merchant JA, Moore G, et al. Indoor and outdoor particulate matter and endotoxin concentrations in an intensely agricultural county. J Expo Sci Environ Epidemiol. 2013 doi: 10.1038/jes.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams DAL, McCormack MC, Matsui EC, Diette GB, McKenzie SE, Geyh AS, et al. Cow allergen (Bos d2) and endotoxin concentrations are higher in the settled dust of homes proximate to industrial-scale dairy operations. J Expo Sci Environ Epidemiol. 2014:1–6. doi: 10.1038/jes.2014.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bari MA, MacNeill M, Kindzierski WB, Wallace L, Héroux M-È, Wheeler AJ. Predictors of coarse particulate matter and associated endotoxin concentrations in residential environments. Atmos Environ. 2014;92:221–230. [Google Scholar]
- 18.Adhikari A, Lewis JS, Reponen T, Degrasse EC, Grimsley LF, Chew GL, et al. Exposure matrices of endotoxin, (1→3)-β-d-glucan, fungi, and dust mite allergens in flood-affected homes of New Orleans. Sci Total Environ. 2010;408:5489–98. doi: 10.1016/j.scitotenv.2010.07.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazique D, Diette GB, Breysse PN, Matsui EC, McCormack MC, Curtin-Brosnan J, et al. Predictors of airborne endotoxin concentrations in inner city homes. Environ Res. 2011;111:614–7. doi: 10.1016/j.envres.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hadina S, Weiss JP, McCray PB, Kulhankova K, Thorne PS. MD-2-dependent pulmonary immune responses to inhaled lipooligosaccharides: effect of acylation state. Am J Respir Cell Mol Biol. 2008;38:647–54. doi: 10.1165/rcmb.2007-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perzanowski MS, Miller RL, Thorne PS, Barr RG, Divjan A, Sheares BJ, et al. Endotoxin in inner-city homes: associations with wheeze and eczema in early childhood. J Allergy Clin Immunol. 2006;117:1082–9. doi: 10.1016/j.jaci.2005.12.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odisha - National Health Mission [accessed 30 Nov2014]; http://nrhm.gov.in/nrhm-in-state/state-wise-information/odisha.html#state_profile.
- 23.Dekati® PM10 Impactor ∣ DEKATI [accessed 12 Mar2015]; http://www.dekati.com/products/Fine Particle Measurement/Dekati%C2%AE PM10 Impactor.
- 24.See SW, Balasubramanian R. Chemical characteristics of fine particles emitted from different gas cooking methods. Atmos Environ. 2008;42:8852–8862. [Google Scholar]
- 25.Zappoli S, Andracchio A, Fuzzi S, Facchini MC, Gelencsér A, Kiss G, et al. Inorganic, organic and macromolecular components of fine aerosol in different areas of Europe in relation to their water solubility. Atmos Environ. 1999;33:2733–2743. [Google Scholar]
- 26.ICRP Human Respiratory Tract Model for Radiological Protection. Ann ICRP. 1994:1–3. ICRP Publi. [PubMed] [Google Scholar]
- 27.Petersen S, Jensen KL, Pedersen ALS, Rasmussen HS. The effect of increased classroom ventilation rate indicated by reduced CO 2 concentration on the performance of schoolwork by children. Indoor Air. 2015 doi: 10.1111/ina.12210. n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 28.Rosati JA, Yoneda KY, Yasmeen S, Wood S, Eldridge MW. Respiratory health and indoor air pollution at high elevation. Arch Environ Occup Health. 60:96–105. doi: 10.3200/AEOH.60.2.96-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Traversi D, Alessandria L, Schilirò T, Chiadò Piat S, Gilli G. Meteo-climatic conditions influence the contribution of endotoxins to PM10 in an urban polluted environment. J Environ Monit. 2010;12:484–90. doi: 10.1039/b913314c. [DOI] [PubMed] [Google Scholar]
- 30.Frankel M, Bekö G, Timm M, Gustavsen S, Hansen EW, Madsen AM. Seasonal variations of indoor microbial exposures and their relation to temperature, relative humidity, and air exchange rate. Appl Environ Microbiol. 2012;78:8289–97. doi: 10.1128/AEM.02069-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kujundzic E, Hernandez M, Miller SL. Particle size distributions and concentrations of airborne endotoxin using novel collection methods in homes during the winter and summer seasons. Indoor Air. 2006;16:216–226. doi: 10.1111/j.1600-0668.2005.00419.x. [DOI] [PubMed] [Google Scholar]
- 32.Sarangapani R. Modeling Particle Deposition in Extrathoracic Airways. Aerosol Sci Technol. 2000;32:72–89. [Google Scholar]
- 33.Liao VH-C, Chou W-C, Chio C-P, Ju Y-R, Liao C-M. A probabilistic approach to quantitatively assess the inhalation risk for airborne endotoxin in cotton textile workers. J Hazard Mater. 2010;177:103–8. doi: 10.1016/j.jhazmat.2009.11.151. [DOI] [PubMed] [Google Scholar]
- 34.Shi J, Mehta AJ, Hang J qing, Zhang H, Dai H, Su L, et al. Chronic lung function decline in cotton textile workers: Roles of historical and recent exposures to endotoxin. Environ Health Perspect. 2010;118:1620–1624. doi: 10.1289/ehp.0901178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacNeill SJ, Sozanska B, Danielewicz H, Debinska A, Kosmeda A, Boznanski A, et al. Asthma and allergies: is the farming environment (still) protective in Poland? The GABRIEL Advanced Studies. Allergy. 2013;68:771–779. doi: 10.1111/all.12141. [DOI] [PubMed] [Google Scholar]
- 36.Sordillo JE, Hoffman EB, Celedón JC, Litonjua AA, Milton DK, Gold DR. Multiple microbial exposures in the home may protect against asthma or allergy in childhood. Clin Exp Allergy. 2010;40:902–910. doi: 10.1111/j.1365-2222.2010.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eriksson J, Ekerljung L, Lötvall J, Pullerits T, Wennergren G, Rönmark E, et al. Growing up on a farm leads to lifelong protection against allergic rhinitis. Allergy. 2010;65:1397–1403. doi: 10.1111/j.1398-9995.2010.02397.x. [DOI] [PubMed] [Google Scholar]
- 38.Caudri D, Wijga A, Scholtens S, Kerkhof M, Gerritsen J, Ruskamp JM, et al. Early Daycare Is Associated with an Increase in Airway Symptoms in Early Childhood but Is No Protection against Asthma or Atopy at 8 Years. Am J Respir Crit Care Med. 2009;180:491–498. doi: 10.1164/rccm.200903-0327OC. [DOI] [PubMed] [Google Scholar]
- 39.Michel S, Busato F, Genuneit J, Pekkanen J, Dalphin J-C, Riedler J, et al. Farm exposure and time trends in early childhood may influence DNA methylation in genes related to asthma and allergy. Allergy. 2013;68:355–364. doi: 10.1111/all.12097. [DOI] [PubMed] [Google Scholar]
- 40.Poole JA, Romberger DJ. Immunological and inflammatory responses to organic dust in agriculture. Curr Opin Allergy Clin Immunol. 2012;12:126–132. doi: 10.1097/ACI.0b013e3283511d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]