Abstract
Independently, HIV infection and heavy alcohol use increase microbial translocation of gut products into systemic circulation. Microbial translocation and consequent immune response have been linked to chronic inflammation and a host of negative health outcomes in individuals living with HIV. However, previous research has not systematically investigated the immune correlates of heavy drinking specifically within the HIV-positive population. This pilot study investigated microbial translocation and immune activation as a function of alcohol use in 21 HIV-positive men who met NIAAA criteria for heavy drinking. Participants averaged 46.7 ± 8.5 (mean ± standard deviation) years of age, 12.2 ± 9.2 years since HIV diagnosis, 337 ± 158 CD4 nadir, and 643 ± 245 current CD4 count. All participants were virologically suppressed on antiretroviral therapy. Data on alcohol use and immune function were collected at baseline and three-month follow-up. Plasma concentrations of markers of microbial translocation and immune activation [lipopolysaccharide (LPS), soluble CD14 (sCD14), endotoxin core antibody immunoglobulin M (EndoCAb)] were measured using enzyme-linked immunosorbent assays. Generalized estimating equation models tested alcohol use variables as predictors of LPS, sCD14, and EndoCAb levels. Greater quantity and frequency of drinking significantly predicted higher sCD14 levels (p’s <.01). Conversely, longer duration of abstinence from alcohol significantly predicted lower sCD14 levels (p <.001). These results remained significant after controlling for age, HIV duration, smoking status, current CD4 count, CD4 nadir, and antiretroviral drug type. In addition, participants with ≥50% relative reduction in drinks per week showed a significant decrease (p <.05) in sCD14 from baseline to three-month follow-up. This pilot study provides preliminary evidence that heavy drinking may increase a key inflammatory marker in HIV-infected individuals with suppressed infection.
Keywords: sCD14, alcohol, heavy drinking, microbial translocation, HIV infection, inflammation
Introduction
Among the 1.2 million people living with HIV infection (PLWH) in the United States, 65% used alcohol in the past year, and 15% reported binge drinking in the past month (Centers for Disease Control and Prevention, 2014). Alcohol use is associated with HIV progression and increased mortality, yet the biological mechanisms have been difficult to elucidate (Hahn & Samet, 2010). Both heavy drinking and HIV infection promote microbial translocation (MT), the movement of gut microbial products into systemic circulation (Brenchley & Douek, 2012). In HIV infection, MT may be a consequence of gut CD4 cell depletion, altered microbiota composition, and/or epithelial damage (for review, see Marchetti, Tincati, & Silvestri, 2013). Gut immune dysfunction and MT are linked to chronic immune activation, a defining feature of HIV infection that predicts disease progression independent of viral load (Brenchley et al., 2006; Deeks et al., 2004). Chronic immune activation, characterized by elevated turnover of T cells and high levels of proinflammatory cytokines in circulation, leads to persistent systemic inflammation (Marchetti et al., 2013). At the same time, alcohol and its metabolites cause MT by increasing gut permeability and promoting oxidative stress (Szabo & Bala, 2010). This pilot study investigated associations among alcohol use, MT, and immune activation in PLWH.
A widely used measure of MT is plasma concentration of lipopolysaccharide (LPS). LPS is a component of cell walls of Gram-negative bacteria and an endogenous antigen for toll-like receptor 4 (TLR4) and its co-receptor, cluster of differentiation 14 (CD14) (Park & Lee, 2013). LPS in circulation stimulates monocytes to secrete soluble CD14 (sCD14), a protein that mediates inflammatory response to LPS (Kitchens, Thompson, Viriyakosol, O'Keefe, & Munford, 2001). Endotoxin core antibody immunoglobulin M (EndoCAb) is an antibody that binds to and clears LPS (Barclay, 1995). In short, both sCD14 and EndoCAb levels reflect MT-associated immune activation.
Perturbations of LPS, sCD14, and EndoCAb appear to exhibit similar profiles in HIV infection and heavy drinking. Compared to healthy controls, PLWH consistently show elevated plasma LPS and sCD14 and lower EndoCAb (Ancuta et al., 2008; Brenchley et al., 2006; Dinh et al., 2015). Antiretroviral treatment (ART) does not fully normalize these biomarkers, possibly due to ongoing T-cell depletion in the gut (Brenchley et al., 2004). Moreover, elevated LPS and sCD14 are induced by acute alcohol exposure and are reported in early alcohol withdrawal (Bala, Marcos, Gattu, Catalano, & Szabo, 2014; Frank, Witte, Schrodl, & Schutt, 2004; Leclercq et al., 2012). In a community sample, consuming ≥5 drinks one or more times per week was associated with lower EndoCAb (Kazbariene, Krikstaponiene, & Monceviciute-Eringiene, 2006).
These parallels point to the possibility for heavy drinking to potentiate MT and immune activation in PLWH, yet previous research on this topic is sparse. Ancuta and colleagues (2008) found higher plasma LPS in PLWH who had alcohol use disorders than PLWH without substance use disorders. Cioe and colleagues (2015) reported that smoking, but not heavy drinking, was associated with elevated plasma sCD14 in PLWH. However, systematic investigation of MT and immune activation in relation to alcohol use has not been conducted among PLWH with virologic suppression (i.e., undetectable viral load). This pilot study investigated LPS, sCD14, and EndoCAb levels over a three-month interval in heavy-drinking men with treated HIV infection.
Method
Participants
Participants were 21 men who have sex with men, enrolled in a randomized, controlled trial of a brief alcohol intervention. Briefly, male heavy drinkers living with HIV were recruited from an urban health center with extensive experience providing HIV clinical care (for details see Kahler et al., 2014). Participants were randomized either to treatment-as-usual or to a brief motivational intervention to reduce alcohol use plus treatment-as-usual. Behavioral and biomedical outcomes were measured at several points over a twelve-month follow-up period. Inclusion criteria were (1) male; (2) ≥18 years of age; (3) heavy drinking in the past month, following NIAAA guidelines [≥5 drinks per day, on one or more days; or >14 drinks per week, on average (Dawson, 2011)]; (4) confirmed diagnosis of HIV; (5) self-reported sex (insertive or receptive, oral or anal) with a male partner in the past 12 months. Participants on ART were stable on their current regimen for at least three months. Exclusion criteria were (1) current intravenous drug use; (2) current psychosis, suicidality, or mania, determined with the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (First, Spitzer, Gibbon, & Williams, 1995); (3) treatment within past three months for an HIV-related opportunistic infection; (4) current psychotherapeutic or pharmacologic treatment for alcohol or drug use; (5) score >7 on Clinical Institute Withdrawal Assessment for Alcohol [CIWA-Ar (Sullivan, Sykora, Schneiderman, Naranjo, & Sellers, 1989)]. Participants were asked to abstain from alcohol for 24 hours before assessment, and zero breath alcohol content was confirmed using a handheld digital breath analyzer. Participants provided informed consent in a format approved by Institutional Review Boards.
The current analysis retrospectively selected a subsample of participants from the parent study for biomarker testing on cryogenically preserved plasma samples. Selection was based on alcohol use at three-month follow-up [actual days to follow-up =90±16 (mean±standard deviation)]. Participants with differential drinking outcomes were selected using clinical and empirical guidelines for significant change in alcohol use (European Medicines Agency, 2010; Falk, Litten, Anton, Kranzler, & Johnson, 2014). Specifically, the analysis utilized plasma samples from participants who either had maintained heavy drinking (Maintainers, n=10) or reported ≥50% relative reduction in drinks per week (Reducers, n=11).
Alcohol use assessment
The Timeline Followback Interview [TLFB (Sobell & Sobell, 1992)] was used to assess the number of standard alcoholic drinks (12 oz. of beer, 5 oz. of wine, 1.5 oz. 80-proof liquor) consumed each day and other drug use.
Plasma assays
Blood samples were collected into endotoxin-free tubes, processed, and stored at −80 °C. Commercially available enzyme-linked immunosorbent assays for LPS (MyBiosource), sCD14 (Enzo LifeSciences), and EndoCAb (Hycult) were performed in duplicate following manufacturer instructions. Data were available for 18/21 participants for LPS and 20/21 participants for sCD14 and EndoCAb.
Statistical analysis
We hypothesized that heavier and more recent alcohol consumption would be linked with higher LPS, higher sCD14, and lower EndoCAb at both time points. LPS was non-normally distributed and was log-transformed. Generalized estimating equations [GEE (Zeger, Liang, & Albert, 1988)] in SPSS v.22 (IBM Corp, Released 2013)) tested whether the time-varying effect of alcohol use was associated with biomarker outcomes, while accounting for non-independence of observations within individuals. Alcohol use variables (average drinks per week, number of drinking days, number of heavy drinking days, drinks per drinking day, and days since last drink) were tested in separate models. Age, years since HIV diagnosis, current CD4, nadir CD4, smoking status, and ART type [protease inhibitor (PI)-based vs. PI-sparing] also were tested as predictors. Correlations among biomarkers were tested using non-parametric Spearman’s rho.
Differences between Maintainer and Reducer groups at follow-up were evaluated using the Mann-Whitney U test for LPS and independent t-tests for sCD14 and EndoCAb. Within-group changes were analyzed with Wilcoxon signed rank tests on LPS and paired t-tests on sCD14 and EndoCAb.
Results
Descriptive characteristics
Participants averaged 46.7±8.5 years of age and 12.2±9.2 years since HIV diagnosis. Participants were virologically suppressed on ART (HIV RNA ≤75 copies/ml) and had moderately high current and nadir CD4 counts. Two-thirds reported smoking. One participant had hepatitis C. Table 1 presents full clinical, demographic, and biomarker data. Regarding relationships across biomarkers (Table 2), LPS was significantly correlated with EndoCAb.
Table 1.
Demographic and clinical characteristics of men living with HIV (N = 21)
| Baseline (M ± SD or %) |
3-Month Follow-up (M ± SD) |
|
|---|---|---|
| Age | 46.7 ± 8.5 | ---- |
| Years since HIV diagnosis | 12.2 ± 9.2 | ---- |
| CD4 nadir cell count | 337 ± 158 | ---- |
| CD4 current cell count | 643 ± 245 | ---- |
| Viral load ≤ 75 copies/ml | 100% | ---- |
| ART type | ||
| PI-based | 48% | ---- |
| NRTI plus non-NRTI | 33% | ---- |
| NRTI plus INSTI | 10% | ---- |
| Type not reported | 10% | ---- |
| Hepatitis C positive | 5% | ---- |
| Race | ||
| White | 52% | ---- |
| African American | 24% | ---- |
| Multiracial | 14% | ---- |
| Race not reported | 10% | ---- |
| Ethnicity | ||
| Hispanic or Latino | 24% | ---- |
| Not Hispanic or Latino | 76% | ---- |
| Current smoker | 67% | ---- |
| Other drug use* | ||
| Cannabis | 43% | ---- |
| Methamphetamine | 5% | ---- |
| Cocaine | 19% | ---- |
| Poppers/inhalants | 14% | ---- |
| Alcohol use | ||
| Average drinks per week | 22.1 ± 16.0 | 17.1 ± 24.9 |
| Number of drinking days | 17.2 ± 7.8 | 12.3 ± 10.7 |
| Number of heavy drinking days | 8.0 ± 5.8 | 5.2 ± 7.8 |
| Drinks per drinking day | 6.2 ± 4.1 | 5.3 ± 3.3 |
| Days since last drink | 2.3 ± 3.2 | 5.0 ± 6.3 |
| Liver enzymes | ||
| Alanine aminotransferase (ALT, units/L) | 28.8 ± 8.9 | |
| Aspartate aminotransferase (AST, units/L) | 28.7 ± 12.2 | |
| Biomarker concentrations | ||
| LPS (pg/ml) | 36.2 ± 14.7 | 42.2 ± 25.6 |
| sCD14 (ng/ml) | 8285.5 ± 2162.0 | 7580.3 ± 2493.1 |
| EndoCAb (MMU/ml) | 45.1 ± 19.1 | 47.2 ± 22.1 |
Percent of sample reporting any use on 30-day TLFB.
Table 2.
Correlations of plasma biomarkers
| LPS - baseline |
LPS - follow-up |
EndoCAb - baseline |
EndoCAb - follow-up |
sCD14 - baseline |
sCD14 - follow-up |
|
|---|---|---|---|---|---|---|
| LPS - baseline |
---- | ---- | ---- | ---- | ---- | ---- |
| LPS - follow-up |
.48* | ---- | ---- | ---- | ---- | ---- |
| EndoCAb - baseline |
.59* | n.s. | ---- | ---- | ---- | ---- |
| EndoCAb - follow-up |
.58* | n.s. | .94** | ---- | ---- | ---- |
| sCD14 - baseline |
n.s. | n.s. | n.s. | n.s. | ---- | ---- |
| sCD14 - follow-up |
n.s. | n.s. | n.s. | n.s. | .84** | ---- |
p < .05;
p < .01;
n.s. = non-significant
Associations of alcohol use with biomarkers
In GEE models, past 30-day alcohol quantity, frequency, and abstinence duration significantly predicted sCD14 levels (Table 3; Figure 1). Controlling for clinical and demographic variables did not change results. Alcohol use did not predict LPS or EndoCAb.
Table 3.
Results of generalized estimating equation (GEE) models of sCD14 levels as a function of past 30-day alcohol use (Model Set 1) and alcohol use plus clinical and demographic control variables (Model Set 2).
| Model Set 1: Alcohol use only |
Alcohol use B (SE) |
p- value |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average drinks per week |
32.56 (9.50) |
.001 | ||||||||||
| Drinking days |
79.98 (23.01) |
.001 | ||||||||||
| Heavy drinking days |
111.64 (21.83) |
< .001 | ||||||||||
| Drinks per drinking day |
83.41 (116.87) |
.475 | ||||||||||
| Days since last drink |
−118.03 (24.73) |
< .001 | ||||||||||
|
Model Set 2: Alcohol use and control variables |
Alcohol use B (SE) |
p- value |
Age B (SE) |
p- value |
HIV duration B (SE) |
p- value |
Smoking B (SE) |
p- value |
CD4 nadir B (SE) |
p- value |
ART type B (SE) |
p- value |
| Average drinks per week |
30.10 (10.65) |
.005 | 67.86 (74.60) |
.363 | −26.51 (75.09) |
.724 | 241.68 (504.75) |
.632 | −1.08 (2.85) |
.704 | −141.06 (1035.72) |
.892 |
| Drinking days |
68.08 (24.93) |
.006 | 72.41 (68.32) |
.289 | −41.30 (65.84) |
.531 | 223.47 (483.00) |
.644 | −1.15 (2.85) |
.688 | 1.78 (916.71) |
.998 |
| Heavy drinking days |
106.25 (24.82) |
< .001 | 56.89 (75.51) |
.451 | −9.78 (76.14) |
.898 | 62.10 (486.41) |
.898 | −.832 (2.86) |
.771 | −412.16 (993.04) |
.678 |
| Drinks per drinking day |
153.43 (102.20) |
.133 | 52.96 (83.21) |
.525 | −8.70 (83.61) |
.917 | 203.89 (534.99) |
.703 | −0.96 (3.00) |
.747 | −430.00 (1194.33) |
.719 |
| Days since last drink |
-126.01 (24.15) |
< .001 | 78.53 (70.97) |
.269 | −17.82 (71.07) |
.802 | 788.06 (607.89) |
.195 | −1.64 (3.09) |
.596 | −322.89 (918.66) |
.725 |
Abbreviations: ART = antiretroviral therapy; PI = protease inhibitor.
Notes: Beta coefficients represent change in sCD14 (ng/ml) for a 1-unit change in the predictor. Categorical variables were coded as follows: smoking status, non-smoker = 0 and smoker =1; ART type, protease inhibitor-sparing ART = 0, protease inhibitor-containing ART = 1. Repeating Model Set 2 analyses with current CD4 count instead of CD4 nadir produced comparable results.
Figure 1.
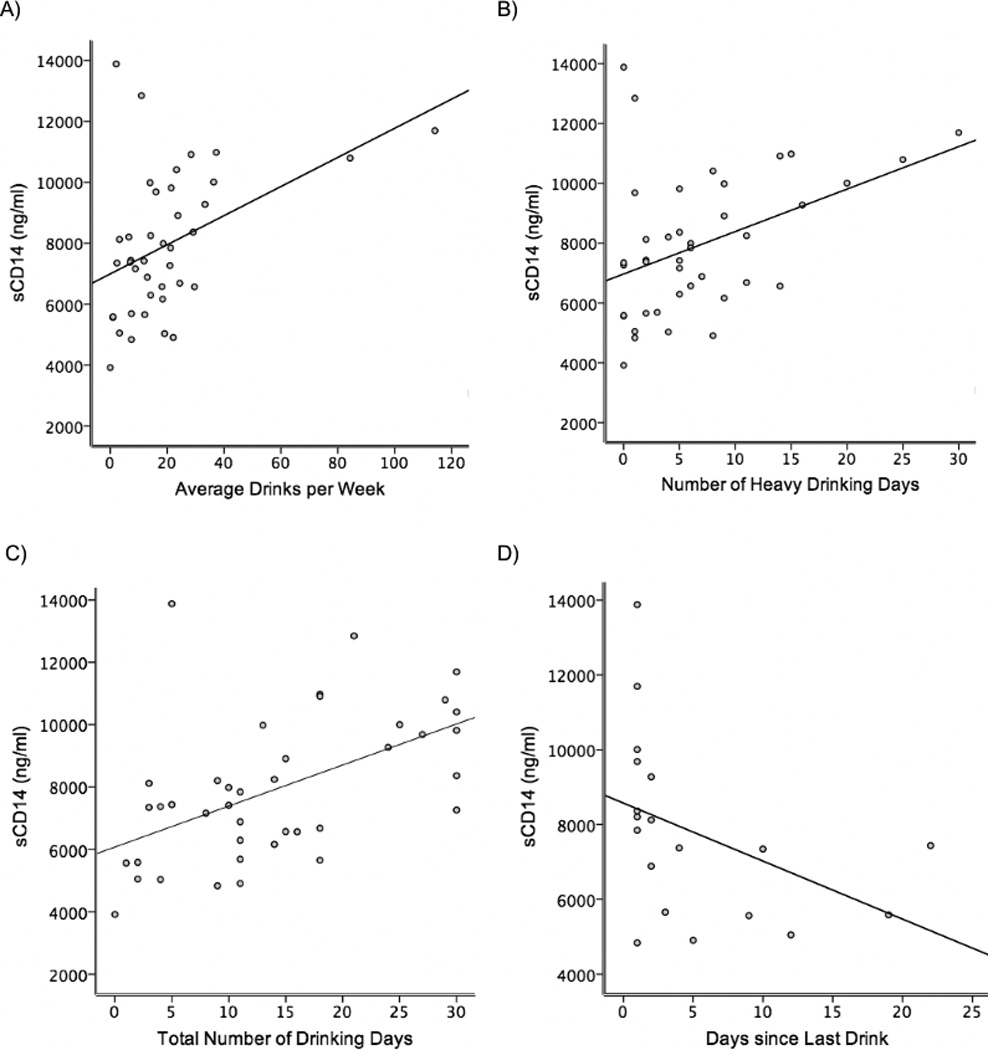
Associations of sCD14 with A) average drinks per week (baseline and three-month follow-up shown); B) heavy drinking days (baseline and follow-up shown); C) total drinking days (baseline and follow-up shown); D) days since last drink at follow-up.
ART type was a significant predictor of LPS, Wald χ2(1)=5.56, p=.018. Being on a PI-based regimen was associated with higher LPS levels than a PI-sparing regimen. Because a PI-containing regimen typically is not first-line treatment, we considered that illness history might differ for these groups. Post-hoc t-tests showed that current CD4, nadir CD4, and age did not differ, but duration of infection was significantly longer in those receiving PI-based ART (16.7 years) compared to PI-sparing ART (7.6 years), t(18)=2.41, p=.027.
Biomarker change by group
At follow-up, 11/21 participants (Reducers) had reduced intake by an average of 79%, or 19.2 drinks per week. The remaining 10/21 (Maintainers) continued heavy drinking. A paired t-test showed that average sCD14 decreased significantly in Reducers from baseline (8046 ng/ml) to follow-up (7102 ng/ml), t(10) =2.33, p =.042, Cohen’s d =.37 (Figure 2). Change within Maintainers was not significant, t(8) =1.09, p =.308 (baseline, 8578 ng/ml; follow-up, 8165 ng/ml). Between-group tests were not significant (p’s >.20).
Figure 2.
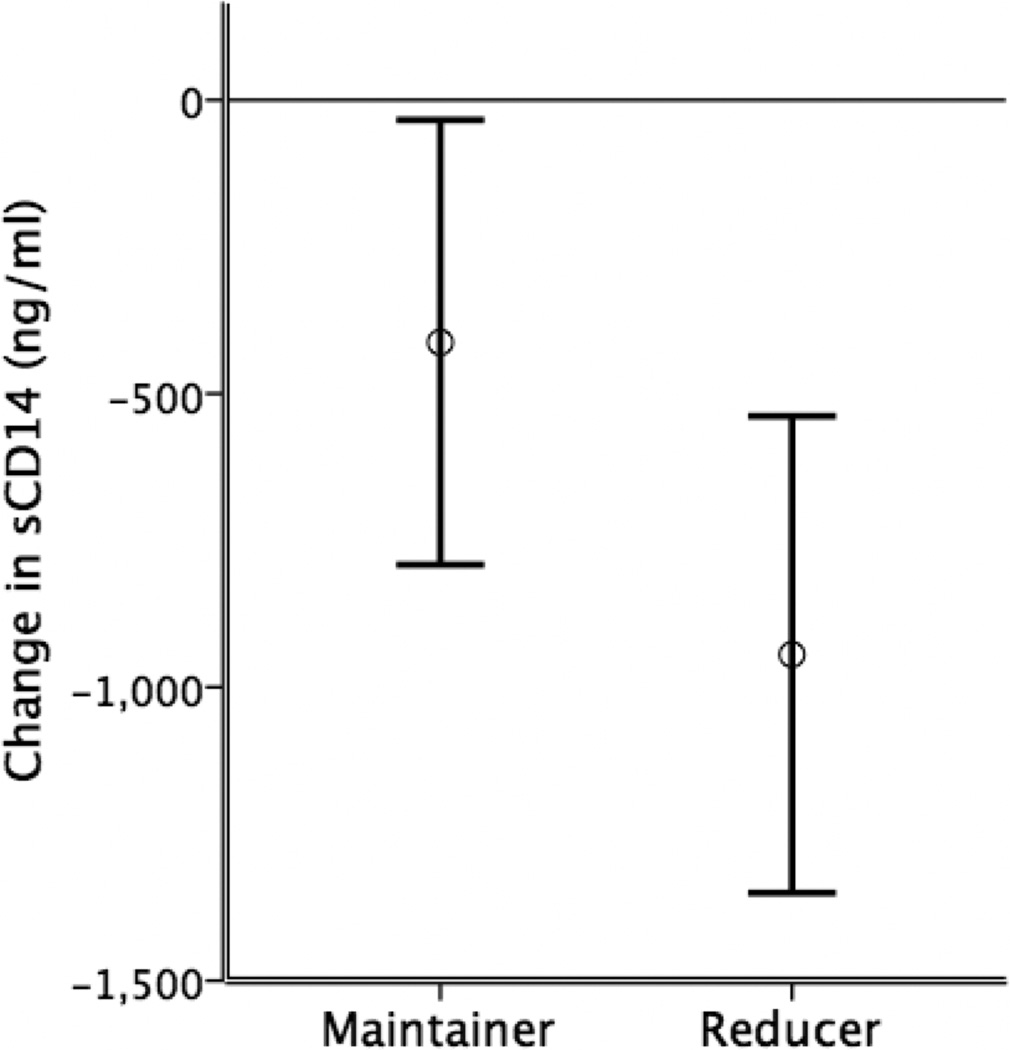
Change in sCD14 levels for Maintainer and Reducer groups at three-month follow-up. Bars represent mean ± standard error. The Reducer group showed a significant decrease in sCD14 [t(10) = 2.33, p = .04], whereas change in the Maintainer group was not significant.
Discussion
This pilot study linked greater quantity and frequency of drinking in PLWH to elevation in plasma sCD14, a marker of monocyte activation. Conversely, longer abstinence from alcohol predicted lower sCD14: abstaining from alcohol for one week predicted sCD14 decrease of approximately 10%. In addition, participants who reduced drinking by ≥50% at follow-up showed a significant decrease in sCD14. This preliminary evidence suggests that alcohol use contributes to systemic immune activation in PLWH. Because sCD14 independently predicts all-cause mortality and cognitive impairment in PLWH, associations of alcohol use with sCD14 are noteworthy (Marcotte et al., 2013; Sandler et al., 2011). With increased life expectancy for PLWH on ART (Nakagawa, May, & Phillips, 2013), the presence of chronic immune dysfunction despite virologic suppression could have serious implications for long-term health.
These pilot results require replication in a full-scale study. Although having an all-male sample removed variability due to sex differences in alcohol pharmacokinetics (Erol & Karpyak, 2015), generalizability is limited by this factor and by the sample’s high current CD4 and nadir CD4. Null findings for LPS and EndoCAb in the current study may be attributable to limited power. Although liver damage did not appear to be a major contributor, it is possible that other health conditions not assessed in this study may have affected biomarker levels. Differences from previous studies, which included PLWH with detectable viral loads (Ancuta et al., 2008; Cioe et al., 2015), may have arisen because the current investigation assessed PLWH who were all heavy drinkers with virologic suppression. sCD14 is not specific to LPS and is induced by other TLR ligands (Shive, Jiang, Anthony, & Lederman, 2015). Interestingly, studies have shown that alcohol modulates LPS activation of TLR4 on monocytes (Mandrekar, Bala, Catalano, Kodys, & Szabo, 2009) and that pro-inflammatory effects of alcohol in the central nervous system are mediated by TLR4 on glia, independent of LPS (Fernandez-Lizarbe, Pascual, & Guerri, 2009).
For PLWH with virologic suppression, there currently are no established methods to reduce chronic immune activation, a key predictor of cognitive impairment and mortality. Findings suggest that reducing drinking may have beneficial effect on immune status. Finally, sCD14 may have potential as a biomarker to monitor alcohol-related immune consequences in PLWH.
Acknowledgments
Funding: This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development under grant K24HD080539 (PI: Ramratnam); by the Retrovirology Core of the Lifespan Tufts Brown CFAR under grant P30AI042853; by the National Institute on Alcohol Abuse and Alcoholism grants under grants P01AA019072 (PI: Monti), K05AA019681 (PI: Monti), and T32AA007459 (PI: Monti); and by the National Institute of Nursing Research under grant K23NR014951 (PI: Cioe).
Footnotes
Disclosure Statement: The authors have no conflicts of interest to disclose.
References
- Ancuta P, Kamat A, Kunstman KJ, Kim EY, Autissier P, Wurcel A, Gabuzda D. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala S, Marcos M, Gattu A, Catalano D, Szabo G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One. 2014;9:e96864. doi: 10.1371/journal.pone.0096864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay GR. Endogenous endotoxin-core antibody (EndoCAb) as a marker of endotoxin exposure and a prognostic indicator: a review. Prog Clin Biol Res. 1995;392:263–272. [PubMed] [Google Scholar]
- Brenchley JM, Douek DC. Microbial translocation across the GI tract. Annu Rev Immunol. 2012;30:149–173. doi: 10.1146/annurev-immunol-020711-075001. 10.1146/annurev-immunol-020711-075001. Epub 2012 Jan 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Behavioral and clinical characteristics of persons receiving care for HIV infection--Medical Monitoring Project, United States, 2010. 2014 [PubMed]
- Cioe PA, Baker J, Kojic E, Onen N, Hammer J, Patel P, Kahler CW. Elevated soluble CD14 and lower D-dimer are associated with cigarette smoking and heavy episodic alcohol use in persons living with HIV (PLWH) J Acquir Immune Defic Syndr. 2015 doi: 10.1097/QAI.0000000000000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA. Defining risk drinking. Alcohol Res Health. 2011;34(2):144–156. doi:Fea-AR&H-53Fea-AR&H-53. [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Kitchen CM, Liu L, Guo H, Gascon R, Narvaez AB, Hecht FM. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104(4):942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- Dinh DM, Volpe GE, Duffalo C, Bhalchandra S, Tai AK, Kane AV, Ward HD. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis. 2015;211(1):19–27. doi: 10.1093/infdis/jiu409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erol A, Karpyak VM. Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug Alcohol Depend. 2015;156:1–13. doi: 10.1016/j.drugalcdep.2015.08.023. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. Guideline on the development of medicinal products for the treatment of alcohol dependence. 2010 Retrieved from http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/03/WC500074898.pdf.
- Falk DE, Litten RZ, Anton RF, Kranzler HR, Johnson BA. Cumulative proportion of responders analysis (CPRA) as a tool to assess treatment outcome in alcohol clinical trials. J Stud Alcohol Drugs. 2014;75(2):335–346. doi: 10.15288/jsad.2014.75.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol. 2009;183:4733–4744. doi: 10.4049/jimmunol.0803590. 10.4049/jimmunol.0803590. Epub 2009 Sep 14. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- Frank J, Witte K, Schrodl W, Schutt C. Chronic alcoholism causes deleterious conditioning of innate immunity. Alcohol Alcohol. 2004;39:386–392. doi: 10.1093/alcalc/agh083. [DOI] [PubMed] [Google Scholar]
- Hahn JA, Samet JH. Alcohol and HIV disease progression: weighing the evidence. Curr HIV/AIDS Rep. 2010;7(4):226–233. doi: 10.1007/s11904-010-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. (Released 2013). IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp; [Google Scholar]
- Kahler CW, Wray TB, Pantalone DW, Kruis RD, Mastroleo NR, Monti PM, Mayer KH. Daily Associations Between Alcohol Use and Unprotected Anal Sex Among Heavy Drinking HIV-Positive Men Who Have Sex with Men. AIDS Behav. 2014 doi: 10.1007/s10461-014-0896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazbariene B, Krikstaponiene A, Monceviciute-Eringiene E. Disturbance of human immunohomeostasis by environmental pollution and alcohol consumption. Acta Microbiol Immunol Hung. 2006;53(2):209–218. doi: 10.1556/AMicr.53.2006.2.7. [DOI] [PubMed] [Google Scholar]
- Kitchens RL, Thompson PA, Viriyakosol S, O'Keefe GE, Munford RS. Plasma CD14 decreases monocyte responses to LPS by transferring cell-bound LPS to plasma lipoproteins. J Clin Invest. 2001;108:485–493. doi: 10.1172/JCI13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq S, Cani PD, Neyrinck AM, Starkel P, Jamar F, Mikolajczak M, de Timary P. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav Immun. 2012;26:911–918. doi: 10.1016/j.bbi.2012.04.001. 10.1016/j.bbi.2012.04.001. Epub 2012 Apr 10. [DOI] [PubMed] [Google Scholar]
- Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clinical Microbiology Reviews. 2013;26(1):2–18. doi: 10.1128/CMR.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J Immunol. 2009;183:1320–1327. doi: 10.4049/jimmunol.0803206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte TD, Deutsch R, Michael BD, Franklin D, Cookson DR, Bharti AR, Letendre SL. A concise panel of biomarkers identifies neurocognitive functioning changes in HIV-infected individuals. J Neuroimmune Pharmacol. 2013;8(5):1123–1135. doi: 10.1007/s11481-013-9504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa F, May M, Phillips A. Life expectancy living with HIV: recent estimates and future implications. Curr Opin Infect Dis. 2013;26(1):17–25. doi: 10.1097/QCO.0b013e32835ba6b1. [DOI] [PubMed] [Google Scholar]
- Park BS, Lee JO. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med. 2013;45:e66. doi: 10.1038/emm.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Group ISS. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shive CL, Jiang W, Anthony DD, Lederman MM. Soluble CD14 is a nonspecific marker of monocyte activation. AIDS. 2015;29(10):1263–1265. doi: 10.1097/QAD.0000000000000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Followback: A technique for assessing self-reported alcohol consumption. In: Allen JP, Columbus M, editors. Assessing Alcohol Problems: A Guide for Clinicians and Researchers. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 1992. pp. 55–73. [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16:1321–1329. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–1060. [PubMed] [Google Scholar]


