Abstract
Combination of non-nicotine pharmacotherapies has been under-examined for cigarette smoking cessation. A randomized, double-blind, parallel-group double-dummy study evaluated two medications, bupropion (BUP) and naltrexone (NTX), in treatment-seeking cigarette smokers (N = 121) over a 7-week treatment intervention with 6-month follow-up. Smokers were randomized to either BUP (300 mg/day) + Placebo (PBO) or BUP (300 mg/day) + NTX (50 mg/day). The primary outcome was biochemically-verified (saliva cotinine, carbon monoxide) 7-day, point-prevalence abstinence. BUP+NTX was associated with significantly higher point-prevalence abstinence rates after 7-weeks of treatment (BUP+NTX, 54.1%; BUP+PBO, 33.3%), p = 0.0210, but not at 6-month follow-up (BUP+NTX, 27.9%; BUP+PBO, 15.0%), p = 0.09. Continuous abstinence rates did not differ, p = 0.0740 (BUP+NTX, 26.2%; BUP+PBO, 13.3%). Those receiving BUP+NTX reported reduced nicotine withdrawal, p = 0.0364. The BUP+NTX combination was associated with elevated rates of some side effects, but with no significant difference in retention between the groups.
INTRODUCTION
Recent efforts to achieve higher smoking cessation rates have turned to combination pharmacotherapies (CP) for nicotine dependence.(1-3) Initial efforts at developing CPs focused on existing nicotine replacement therapies (NRTs), most commonly coupling the nicotine patch and an ad libitum system (i.e., nicotine gum, lozenge, inhaler, or nasal spray). NRT+NRT combinations held the potential for higher delivery of nicotine and provision of both stable and acute dosing for craving and withdrawal symptom relief; however reported effects have been clinically modest.(4) With the advent of non-nicotine pharmacotherapies (NNPs), such as bupropion and varenicline, CP research expanded to include NRT+NNP treatments.(5) Multiple trials have combined bupropion (BUP) and the nicotine patch. The benefits of adding NRT to BUP appear to be limited.(6) More recently, research has examined the combination of NRT with varenicline.(7-10) Of the three controlled clinical trials, two reported no benefit of adding the nicotine patch to varenicline for long-term abstinence(8, 10) while one substantially larger study did find evidence favoring the CP.(9)
The potential for improving abstinence rates through non-nicotine CPs, with different mechanisms of action is plausible but not well studied.(11-13) Two studies compared the combination of BUP and varenicline to varenicline alone, with encouraging findings for the CP, specifically among heavy smokers.(12, 13) The combination of BUP and naltrexone (NTX) was initially proposed for smoking cessation in weight-concerned(14) and overweight or obese smokers,(15) based on the weight-suppressant effects of NTX. While preliminary studies yielded equivocal results, recent FDA approval of this CP for the treatment of obesity provides a basis for further investigation of its therapeutic potential for smoking cessation.(16)
An alternative pharmacologic rationale for BUP+NTX as a CP for smoking cessation is based on the different and possibly complementary mechanisms of action. BUP is posited to act through inhibiting reuptake of noradrenaline and dopamine,(17) and may antagonize nicotine by occupying some cholinergic receptors.(18) NTX is a mu-opioid antagonist that may inhibit some mesolimbic dopamine activity(19) and alter cholinergic receptors’ function and expression in the brain.(20) Therefore, these two drugs may act through both the same and different transmitter systems.
BUP effectively improves several nicotine withdrawal symptoms including decreases in positive affect, increases in negative affect, irritability, concentration impairment, and craving.(21-23) NTX may work to abate craving and urges to smoke.(24, 25) BUP appears to dampen the rewarding effects of smoking slips (22) and NTX may also block the reinforcing effects of smoking, thus serving as relapse-prevention agents.(24) NTX may be particularly helpful in attenuating the rewarding effects of slips that occur in high stress situations,(26) as well as attenuating reactivity to smoking stimuli.(19) Thus, BUP may palliate most withdrawal complaints and craving, with NTX offering further improvement in craving as well as protection from slips to smoking.
Combining BUP and NTX may offset side effects associated with each medication thus enhancing medication compliance; one of the proposed benefits of the CP. BUP most frequently produces the side effects of insomnia and dry mouth. The side effect profile of NTX may include headache, sedation, distractibility, and dysphoria marked by irritability and negative affect. While some of these effects are consistent with nicotine withdrawal, studies of naltrexone in non-dependent adults(27) have reported dysphoric effects marked by heightened negative affect, irritability, and distractibility. An obvious approach to counteract dysphoric effects in NTX therapy is to use adjunctive antidepressant therapy. Combination of an antidepressant (fluoxetine and paroxetine, respectively) with NTX has led to improved retention in treatment of opioid–dependent individuals.(28) Finally, BUP as a component of the CP provides antidepressant effects that may enhance efficacy while the agonist-like and antagonist effects of BUP+NTX may counter side effects of each.
The current controlled clinical trial evaluated the additive effects of combining two NNPs using a randomized, double-blind, double-dummy design in a sample of 121 treatment-seeking cigarette smokers diagnosed with nicotine dependence. Two medication treatment groups were assessed over a 7-week treatment phase: BUP (300 mg/day) + placebo (PBO) versus BUP (300 mg/day) + NTX (50 mg/day). The goal was to obtain preliminary data on the predicted benefits of BUP+NTX compared with BUP+PBO on smoking cessation efficacy, retention, and treatment tolerability. The primary outcome measure was 7-day, biochemically confirmed, point-prevalence abstinence analyzed under an intention-to-treat policy where missing values were coded to indicate relapse to smoking.
RESULTS
Sample Description
The demographic and smoking history characteristics of participants at randomization are presented in Table 1. No significant differences were observed between medication groups.
Table 1.
Baseline Characteristics of Participants by Treatment Group
| Bupropion + Placebo n = 60 |
Bupropion + Naltrexone n = 61 |
|
|---|---|---|
| Age (years)a | 39.5 (11.2) | 40.2 (11.3) |
| Gender (% female) | 50.2 | 50.4 |
| Race (% White) | 93.3 | 85.3 |
| Marital status (% married) | 44.1 | 42.0 |
| Employed (%)b | 61.0 | 67.0 |
| Cigarettes/day | 18 (6.0) | 19.3 (5.4) |
| Years smoked | 17.0 (11.7) | 16.9 (11.9) |
| FTNDc | 5.8 (1.2) | 5.7 (1.2) |
| Quit attemptsd | 5.3 (5.6) | 4.7 (6.7) |
| Motivation level (0 – 10) | 9.1 (1.1) | 9.1 (1.0) |
| Total saliva cotinine (ng/mL)d | 330.4 (170.5) | 310.4 (167.7) |
| Carbon monoxide | 23.8 (12.8) | 28.2 (13.3) |
Note. Continuous measures were analyzed using t-tests. Categorical measures analyzed with chi-square tests. No significant differences were observed.
Continuous variables reported as means and standard deviations.
Employed indicates % of subjects who reported full or part-time employment.
FTND = Fagerstrom Test of Nicotine Dependence (36).
Quit attempts and baseline cotinine were positively skewed and were transformed as follows prior to analysis: log10(quit attempts + 1) and log10(total urine cotinine).
Mean reported quit attempts and total urine cotinine were transformed back into original scale for ease of interpretation.
Retention
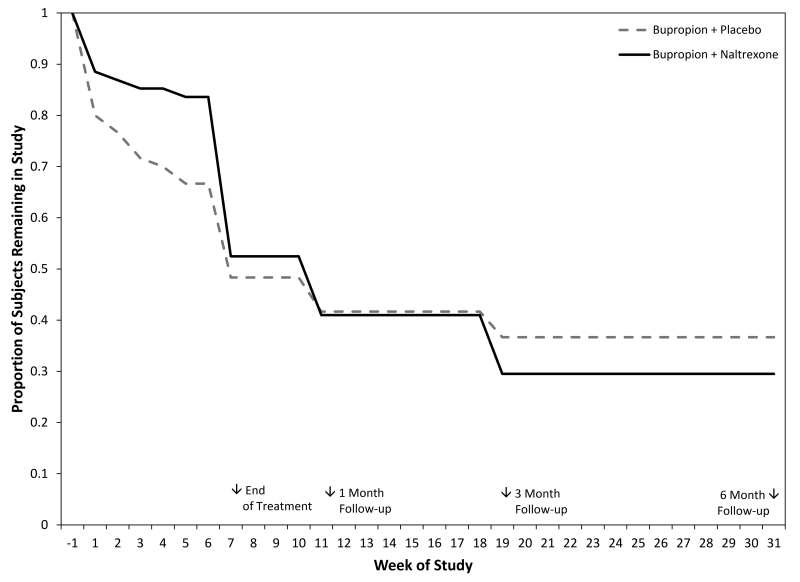
Survival analysis indicated no difference in dropout rates between medication groups (see Figure 3), Log Rank Statistic, χ2(1) = 0.2676, p = 0.6049, with 51.3% of participants completing 7 weeks of treatment. The two medication groups did not differ in the number of weeks of treatment completed (M = 3.9, SD = 2.2).
Figure 3.
Participant retention figure. Proportion of subject retained in the study over treatment and follow-up periods.
Side Effects
The “ever” occurrence of 24 side effects were compared between treatment groups. Higher rates of side effects were reported in the BUP+NTX relative to BUP+PBO for Lightheadedness, χ2(1) = 3.70 , p = 0.0543, (9.8% vs. 1.7%); Nausea, χ2(1) = 3.82 , p = 0.0507, (13.1% vs. 3.3%); Stomach Aches χ2(1) = 7.94 , p = 0.0048, (16.4% vs. 1.7%); Shakiness χ2(1) = 4.46 , p = 0.0347, (11.7% vs. 6.7%); and Muscle Aches χ2(1) = 4.71, p = 0.0299, (11.5% vs. 1.7%). In the intention-to-treatment sample of 121 subjects, the following health conditions were noted: hypertension (n = 6), diabetes (n = 1), asthma (n = 3), GERD (n = 7), and high cholesterol (n = 9). By category of prescribed medication, results were as follows: anti-hypertensive (n = 8), anti-acid reflux (n = 7), asthma inhaler (n = 3), anti-cholesterol (n = 8), and n = hormone birth control (n = 7).
Relatively few subjects discontinued participation in this trial due to medication adverse events (BUP+PBO = 4; BUP+NTX = 3). At total of 6 subjects had their dosage of bupropion reduced to 150 mg per day due to side effects, while 2 subjects (one from each condition) discontinued naltrexone. Only one serious AE occurred in this trial. A subject receiving BUP+PBO developed hematospermia.(29) He discontinued medication and continued in the trial during which time his hematospermia completely remitted.
Vital Signs
Weight, diastolic blood pressure, systolic blood pressure, and heart rate were evaluated weekly during the treatment periods. Baseline values were included as covariates.
Body Weight
No effects of Medication, Time, or their interaction were observed.
Heart Rate
Heart rate tended to decline slightly during the treatment phase, F(6, 333) = 2.42, p = 0.0264.
Systolic Blood Pressure
Over the treatment phase, average systolic blood pressure for those in the BUP+NTX condition (M = 126.29, SE = 0.98) was higher than for those in the BUP+PBO condition (M = 123.34, SE = 1.10), F(1, 108) = 4.00, p = 0.0480.
Diastolic Blood Pressure
No effects of Medication, Time, or their interaction were observed.
Abstinence Rates
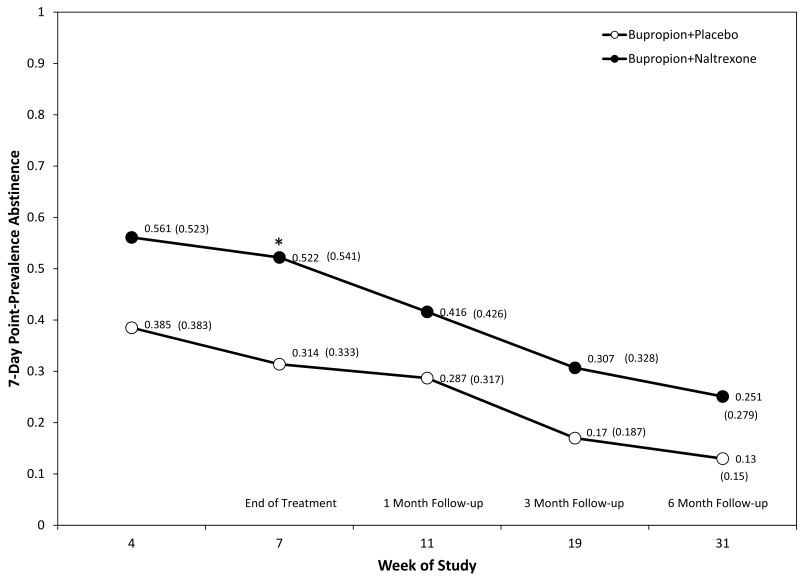
The primary outcome of this study was the point-prevalence abstinence rate (based on saliva cotinine tests and no reported smoking in the 7 days prior to the visit with a CO ≤ 8 PPM). In this intention-to-treat analysis, missing values were imputed to indicate smoking relapse. Figure 4. presents the results of this analysis starting at treatment week 5 and running through the 6-month follow-up phase. It shows both observed abstinence rates (shown in parentheses) and those estimated (derived the logistic statistical model) from the repeated-measures logistic regression.
Figure 4.
Biochemically-confirmed (saliva cotinine), 7-day, point-prevalence abstinence rates significantly differed between medication groups with BUP+NTX subjects. Estimated (derived the logistic statistical model) and observed abstinence rates (presented in parentheses) are displayed at each time point.
Across the study period, point-prevalence abstinence rates for each treatment group at each assessment time point are displayed in Figure 4, and were higher in the combination BUP + NTX group compared to the BUP + PBO group Medication, χ2(1) = 4.41, p = 0.0357, and Time effects, χ2(4) = 26.52, p < .0001, were observed with abstinence rates declining over the course of the study. Differences between medication groups at each time point were evaluated, revealing a significant difference in abstinence rates favoring the CP treatment condition at the end of treatment (Week 7), z(476) = 2.31, p = 0.0210 (see Figure 4). Survival analysis indicated continuous abstinence rates did not significantly differ, BUP+NTX (26.2%) compared to BUP+PBO (13.3%), Log-Rank Statistic, χ2(1) = 3.1911, p = 0.0740.
Subjective Measures
Minnesota Nicotine Withdrawal Scale
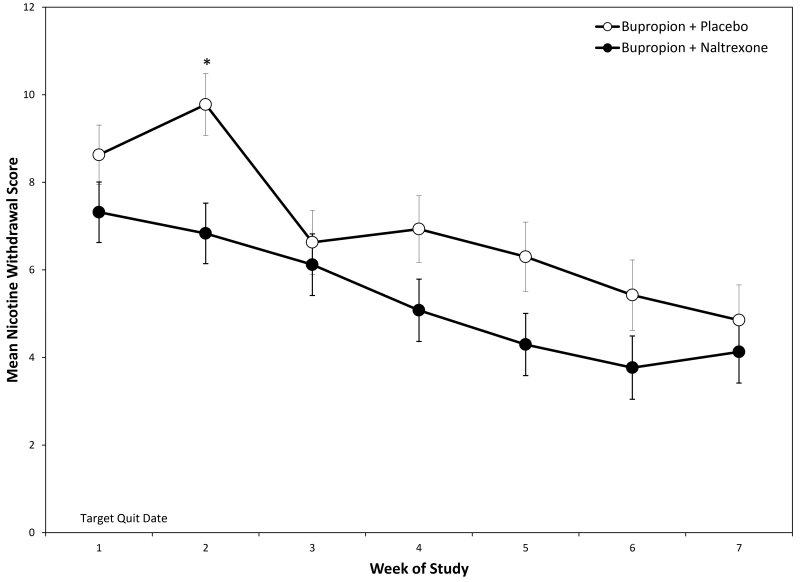
Total nicotine withdrawal scores (MNWS) showed an effect of Medication, F(1, 118) = 4.48, p = 0.0364, Time, F(6, 457) = 7.59, p <0.0001, and their interaction, F(6, 457) = 2.64, p = .0159 (see Figure 5). MNWS scores differed at Week 2 (1-week post-quit), t(268) = −2.97, p = 0.0032.
Figure 5.
Nicotine withdrawal symptoms as measured by the MWSC, varied significantly as a function of Medication, Time, and their interaction.
Questionnaire of Smoking Urges
Anticipated Pleasure from Smoking, F(6, 472) = 29.16, p < .0001, and Anticipated Withdrawal Relief, F(6, 437) = 19.17, p < .0001 declined across the treatment phase in both groups. No Medication or Medication by Time effects were observed.
Positive and Negative Affect Scales
No effects were observed for Positive Affect. Negative Affect declined across the treatment phase, F(6, 434) = 4.28, p = .0003. No Medication or Medication by Time effects were observed.
Integrity of Study Blind
Subjects receiving BUP+NTX were significantly more likely to accurately judge their medication assignment than BUP+PBO subjects at Week 1, χ2(1) = 10.15, p = 0.0014, and Week 7. χ2(1) = 11.08 , p = 0.0009. The BUP+PBO judged their medication assignment at chance levels of accuracy (Week 1 = 53.6%; Week 7 = 53.6%), while those in the BUP+NTX condition ascertained their medication assignment accurately far above chance. (Week 1 = 84.6%, Week 7 = 85.7%). At Week 7, the most common reasons given for medication judgments in BUP+NTX treated subjects were presence of side effects (38.7%) and ease of not smoking (40.8%).
Adherence
Those receiving BUP+PBO (M = 7:52 a.m., SE = 17 minutes), reported taking their morning doses earlier (BUP+PBO, M = 8:30 a.m., SE = 22 minutes), t(102) = 2.43, p = 0.0167. No differences were observed for the evening doses (BUP+PBO, M = 7:34 p.m., SE = 22 minutes BUP+NTX, M = 7:05 p.m., SE = 23 minutes). No differences in the mean interval between doses was observed for medication assignment (BUP+PBO, M = 11.08 hours, SE = 13 minutes; BUP+NTX, M = 11.3, SE = 11 minutes). No differences were found in the average number of doses reported taken for morning doses out of a possible 49 (BUP+PBO, M = 29.4, SE = 2.2; BUP+NTX, M = 30.1, SE = 2.6), or evening doses out of a possible 46 (BUP+PBO, M = 27.9, SE = 2.1; BUP+NTX, M = 28.7, SE = 2.5). Adherence rates for the total 95 doses did not differ by medication assignment, χ2(1) = 0.66, p = 0.4167, (BUP+PBO, 64.7%; BUP+NTX, 69.6%). The rate of subjects taking 80% or more of the 95 total possible dosages did not differ by medication, χ2(1) = 2.0538, p = 0.15 (BUP+PBO, 45.1%; BUP+NTX, 59.6%).
DISCUSSION
Key Observations
In this first randomized, controlled double-dummy parallel groups study, we found that treatment-seeking smokers receiving the combination of BUP+NTX were more likely to be abstinent from tobacco during treatment those receiving BUP+PBO. The combination of BUP+NTX was tolerated as well as BUP+PBO as indexed by retention rates and medication adherence levels; however subjects receiving BUP+NTX treatment reported more side effects. BUP+NTX was superior to BUP+PBO at reducing nicotine withdrawal during the critical one week following the target quit date.
Smoking Cessation
At the end of the 7-week treatment period, those receiving BUP+NTX achieved a point-prevalence abstinence rate of 54.1% compared to 33.3% for those treated with BUP+PBO. BUP+NTX treated subjects exhibited numerically greater rates of continuous abstinence compared to BUP+PBO, 26.2% versus 13.3%, respectively, though the effect was not statistically significant. Our results differ from the two previous studies of BUP+NTX that showed equivocal findings.(15, 30) This study involved a sample 4-6 times larger than the earlier studies, and employed a dosage of NTX one-half to one-third higher.
Safety and Tolerability
Rates of subject attrition did not differ between treatment conditions, although some side effects occurred more frequently in the BUP+NTX group, in particular, reports of lightheadedness and nausea. Elevated rates of nausea have been reported in the two smoking cessation studies of BUP+NTX, (15, 30) as well as in studies of Contrave (BUP+NTX) for weight loss(16). Nausea is a common side effect in those treated with NTX alone or with NRT in quitting smokers.(31, 32) Lightheadedness or dizziness have been documented in a prior treatment study of NTX alone(31) and in Contrave trials.(16)
Nicotine Withdrawal
Nicotine withdrawal plays a critical role in relapse to cigarette smoking.(33) Nicotine withdrawal is generally worse in the initial weeks following cessation, after which most symptoms return to baseline levels.(34) In this study, BUP+NTX was superior to BUP+PBO in suppressing the nicotine withdrawal syndrome after one week of abstinence. Bupropion is known to effectively decrease several nicotine withdrawal symptoms, including increased negative affect, decreased positive affect, irritability, concentration impairment, and craving.(21-23) Naltrexone is posited to facilitate smoking cessation by reduction in craving for tobacco. It is unclear why the addition of NTX to BUP led to the pattern of withdrawal symptom relief seen in this trial, as previous trials have not generally observed this.
Tobacco Craving
NTX or NTX plus the nicotine patch have been shown to decrease tobacco craving compared to placebo or nicotine patch alone, respectively.(24, 25, 31, 32) Specifically previous studies have found effects on both of the QSU’s subscales. In this study, a pattern favoring BUP+NTX was observed for Factor 2 (i.e., Anticipated Withdrawal Relief), but was not significant. It may be that the dose of naltrexone used in this study was insufficient to provide maximal craving relief. In fact, O’Malley and colleagues(32) found that in subjects treated with NRT, and a dose of 25, 50 mg or 100 of NTX, that NTX subjects receiving the highest dose NTX showed the greatest reductions in craving.
Weight
Two studies found some evidence that BUP+NTX suppressed post-cessation weight gain.(15, 30) In one trial, over-weight or obese smokers were specifically evaluated.(15) In the present study, weight gain was low in the total sample, with no differences between groups. One factor that might have accounted for limited weight gain and no differences between groups is that bupropion has a weight suppressant effect following smoking cessation.(23)
Limitations
This study has some limitations. Study sample size was relatively small, but had sufficient power to examine whether or not the combination medication should be further investigated. Nevertheless, attrition rates in this study were unusually high compared to the published literature. Ideally, medication taking would be measured directly, (i.e., direct observation) or through a strong indirect method (e.g., adulterating medication with a tracer such as riboflavin). Instead, diary card records were employed, an indirect subjective approach to the measurement of medication taking vulnerable to error and bias. We failed to identify the psychological mechanism of the observed treatment effects; more frequent assessment or use of ecological momentary assessment of withdrawal, craving, and affect would likely have improved this study. The study sample was 89.3% White, limiting race-based comparisons to other reports with greater racial diversity. An essential bulwark against experimental bias is the study blind since it minimizes the effects of investigator and subject expectations. In our study, those receiving BUP+NTX judged their medication assignment substantially above chance, whereas subjects receiving BUP+PBO judged their assignment at chance levels.
Conclusions and Future Directions
This study is clinically significant in that it demonstrates the efficacy of combining BUP and NTX on the primary outcomes of smoking cessation and nicotine withdrawal. Strengths include being a rigorous randomized, controlled trial with adequate sample size and resultant statistical power. Results are strengthened by evidence of good self-reported adherence to the study medication regimens. Our assessment of blindness integrity is rare in medication trials, yet aids in evaluating results and informing future designs.
New treatments to assist the growing population of smokers refractory to current interventions are needed. Proceeding to examine mechanisms of action of combined BUP and NTX will inform the optimal dosing strategy for this combination pharmacotherapy. To the extent that these initial findings are confirmed in a larger trial, future studies might consider the newer sustained-release formulation of bupropion and naltrexone in a single combination pill (e.g., Contrave), which offers advantages in terms of medication compliance and convenience. Additional evaluation of different ratios BUP and NTX should be explored (e.g., Contrave employs a ratio of 90 mg of bupropion to 8 mg of naltrexone). Additionally, future studies might consider whether specific populations of smokers are more likely to benefit from this combination approach.
METHODS
Participants
The study protocol and all related materials were reviewed and approved by the University of Minnesota Committee for the Protection of Human Subjects (Study Number: 0603M83886) and registered with ClinicalTrials.gov (NCT00419731). Participants were recruited through advertisements in local media, and underwent a telephone interview to establish initial eligibility from April 2007 to April 2010. Male and female subjects meeting the following eligibility criteria were randomized: (a) age between 18 and 65; (b) smoked at least 10 cigarettes daily for at least 1 year; (c) English speaking; (d) females of childbearing potential using effective contraception and provided a urine sample for a pregnancy screen; (e) willingness to reduce alcohol consumption during study to 2 or fewer standard drinks/day; (f) no contraindications to use of BUP (i.e., MAOIs, seizure disorders or other clinical history predisposing one to seizures); (g) no contraindications of NTX (i.e., opioid use in past 30 days; hepatocellular injury); (h) good physical health as established by a review of a health history form by our study nurse practitioner; and (i) no active, untreated major mental illness.
Procedures
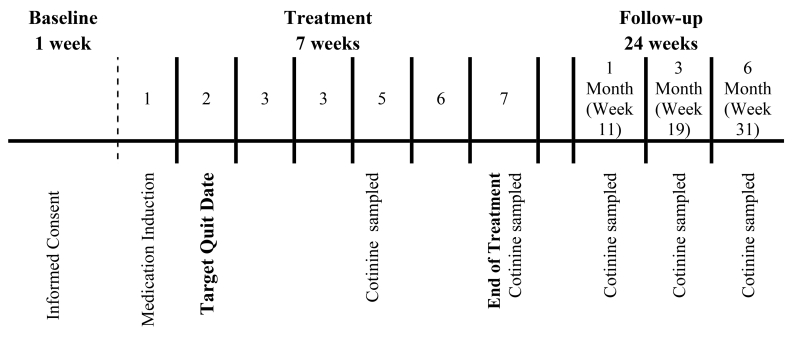
The research was conducted at the Tobacco Research Programs, a component of the Department of Psychiatry at the University of Minnesota. The study occurred over the course of 33 weeks and involved three consecutive phases: (a) baseline, (b) treatment, and (c) follow-up (see Figure 1).
Figure 1.
Study design showing the three phases of the trial: baseline, treatment, and follow-up. The targeted quit date occurred during Week 1 of treatment, following a 7-day medication induction phase. At Weeks 5, 7 (end of treatment), 1-month follow-up, 3-month follow-up, and 6-month follow-up, saliva cotinine samples were collected from those reporting abstinence from tobacco in the preceding week and having a CO ≤ 8 P.P.M. Participants discontinued medication at the end of the treatment phase (Week 7). The initial counseling session prior to TQD lasted 20 minutes. All subsequent sessions included counseling lasting approximately 10 minutes.
Baseline
Callers meeting initial telephone screen criteria received an appointment for an orientation meeting, consent process and a pre-treatment evaluation, which included a medical history and physical examination. Assessment involved laboratory evaluation of liver, kidney, and thyroid function, heart rate and blood pressures, and weight. In addition, tests were conducted for pregnancy (urine). The Fagerstrom Test of Nicotine Dependence (FTND) was used as a measure of nicotine dependence.(35) Study eligibility was determined after 2 visits, after which subjects were randomized to treatment and given study medications.
Treatment
At Day 8, after 7 days of medication induction, participants had their Target Quit Date (TQD). At this and the following 6 weekly visits that bracketed the treatment phase, subjects provided carbon monoxide samples, completed subjective measures, underwent assessment of vital signs, returned daily diary cards of smoking and medication taking, and received a one-week supply of study medication. At weeks 5 and 7, abstinent subjects provided a saliva sample for assessment of cotinine levels.
Follow-up
Participants returned to clinic for evaluation 1, 3, and 6 months weeks post-treatment. Assessment procedures were the same as in the treatment phase except that saliva samples were collected at each visit from eligible subjects.
Interventions
Pharmacotherapy
The study medications were dispensed in a placebo-controlled, double-dummy design. Two different medications were used (i.e., bupropion sustained release or SR [150 mg, twice each day], and placebo or naltrexone [25 mg, twice each day]). Naltrexone pills were jacketed to look identical to the placebo pills. The dose of 300 mg of bupropion was based FDA guidelines(36) while the dose of naltrexone was chosen as being intermediary in the range of doses employed in the literature (24, 31, 37, 38). Bupropion SR treatment began 7 days prior to TQD to achieve functional plasma levels. In order to maintain blindness in the study, naltrexone therapy began at the same time, even though NTX did not require a 7-day induction phase. Participants were instructed to take two pills by mouth each morning and one pill each night for 3 days. For these first three days, subjects took 150 mg of bupropion SR in the morning and a second pill that was either placebo or naltrexone (25 mg); each evening, they took a pill that was either placebo or naltrexone (25 mg). Beginning at day 4 of the induction phase and throughout of the remainder of the treatment phase, each participant took two 2 pills in the AM (i.e., 1 bupropion SR pill [150 mg] and 1 pill containing either naltrexone [25 mg or placebo] and 2 pills in the PM, (i.e., 1 bupropion SR pill (150 mg) and 1 pill containing either naltrexone (25 mg) or placebo), 8 to 12 hours after the AM pills.
Behavioral Counseling
Brief behavioral counseling was provided to all subjects. It involved a structured presentation on the benefits of quitting, a review of coping skills to deal with craving and withdrawal, and support and encouragement.(39) The initial counseling session prior to TQD lasted 20 minutes. All subsequent sessions included counseling lasting approximately 10 minutes.
Measures
Primary outcome measures
Smoking was recorded on daily diaries that captured information about the time at which each cigarette was smoked. The primary outcome variable was 7-day, biochemically-verified point-prevalence abstinence across the treatment and follow-up phases.(40) At each clinic visit, subjects provided expired carbon monoxide (CO) samples in parts-per-million (PPM); samples were considered positive if CO levels exceeded 8 PPM.(41) At weeks 5 and 7 during treatment and at every follow up visit, those reporting abstinence from smoking in the preceding 7 days and who provided a CO ≤ 8 PPM, were also asked to provide a saliva sample for analysis of cotinine concentrations. Saliva samples were analyzed by Salimetrics, Inc. (State College, PA), and were considered positive for tobacco use if cotinine levels exceeded 15 ng/mL.(41)
An Adverse Events Form (AEF) was used to assess the duration, severity, and frequency of the most common side effects reported in clinical trials of bupropion and naltrexone.(31, 42) The AEF included a section to evaluate the extent to which the event was judged to be related to the study drugs (i.e., unrelated, unlikely, possible, probable).
Secondary outcome measures
The Minnesota Nicotine Withdrawal Scale (MNWS) was used to assess symptoms of nicotine withdrawal from the Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition (DSM-IV).(43, 44) Urges and craving to smoke were assessed via the brief version of the Questionnaire of Smoking Urges (QSU).(45, 46) The QSU consists of two scales derived from factor analysis: Factor 1 (smoking for positive reinforcement, Anticipated Pleasure from Smoking), and Factor 2 (smoking for negative reinforcement, Anticipated Withdrawal Relief). The Positive and Negative Affect Scale (PANAS)(47) yielded two subscale scores measuring Positive Affect (e.g., alertness, enthusiasm) and Negative Affect (e.g., sadness, distress). The integrity of the study blind was assessed at treatment weeks 1 and 7 by having participants judge their medication assignment and provide reasons for those judgments.
Statistical Analysis
Sample size
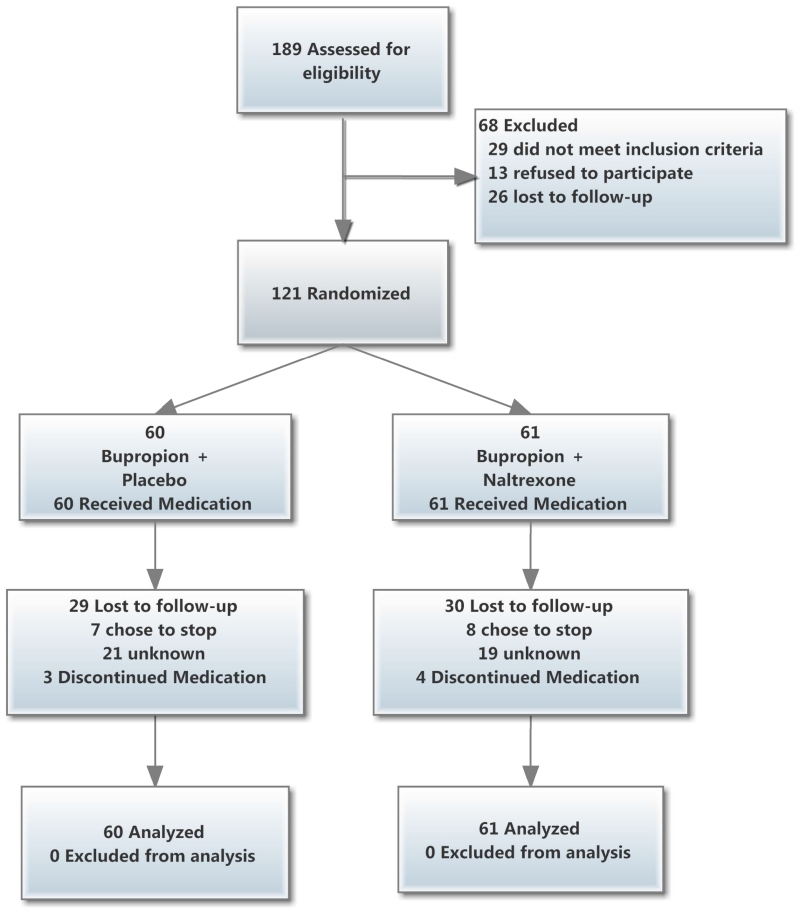
The primary outcome was 7-day, biochemically-confirmed, point-prevalence smoking abstinence. This analysis was conducted under an intention-to-treat policy: Missing values were coded to indicate relapse to smoking. Continuous abstinence indicated negative tests of point-prevalence abstinence at each of 5 time points (Weeks 5 and 7 of Treatment, and Months 1, 3, and 6 of Follow-up). Sample sizes calculations were made using a specialized macro for repeated measures designs.(48) In order to estimate treatment effects, point-prevalence abstinence rates were pooled from 6 placebo-controlled trials of bupropion.(21, 49-53) Total available sample sizes at each time point in the study are displayed in Figure 2.
Figure 2.
Study participant flow diagram (CONSORT).
Assumptions
All analyses were conducted using the Statistical Analysis System Version 9.4.(54) Unless otherwise stated, values of p < .05 were considered statistically significant, based on two-tailed tests. Due to participant attrition and occasional missing data, the number of subjects or data points available for statistical analysis varied, except for the primary outcome where missing values were imputed to indicate smoking (see Figure 2). All confidence intervals are constructed with α = .05.
Repeated measures
Repeated measures analyses were conducted in the framework of generalized linear mixed models (GLMM)(55) with the MIXED procedure for linear outcomes and GENMOD procedure for nonlinear outcomes of daily smoking abstinence, with specification of appropriate error distributions (i.e., binomial) and link functions (i.e., logistic). In all analyses, the effects of Medication (i.e., BUP+PBO or BUP+NTX), Time (i.e., week), and their interaction were tested. Average baseline scores for all measures (except for abstinence status) were included in treatment phase analyses as covariates.
STUDY HIGHLIGHTS.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
The potential for improving cigarette smoking abstinence rates through the combination of non-nicotine pharmacotherapies (NNPs), with different mechanisms of action, has been under-examined. Two medications, bupropion (BUP) and naltrexone (NTX) have been evaluated as a smoking cessation combination pharmacotherapy in two small trials with equivocal results.
WHAT QUESTION DID THIS STUDY ADDRESS?
Whether BUP+NTX compared with BUP+PBO was superior at promoting smoking cessation rates?
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE?
BUP+NTX is a potentially effective smoking cessation treatment.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Our findings may lead to increased clinical pharmacological research into NNPs for smoking cessation.
ACKNOWLEDGEMENTS
All authors were supported by funding from the National Institute on Drug Abuse of the National Institutes of Health. Dr. Mooney was supported in part by National, Institute of Drug Abuse (NIDA) grant K01-DA-019446 and NIDA K99-DA-023548. We thank the participants for taking part in this study. We thank Joni Jensen who contributed to the study execution.
Footnotes
AUTHOR CONTRIBUTIONS
M.M., J.S., S.A., J.G., P.P., A.O., and D.H. wrote the manuscript; M.M., J.S., S.A., J.G., P.P., and D.H. designed the research; M.M. and A.O. performed the research; M.M. analyzed the data.
CONFLICT OF INTEREST
M.M., J.S., S.A., J.G., P.P., A.O., and D.H. declare no conflicts of interest.
ClinicalTrials.gov identifier: NCT00419731.
References
- (1).Bader P, McDonald P, Selby P. An algorithm for tailoring pharmacotherapy for smoking cessation: results from a Delphi panel of international experts. Tobacco Control. 2009;18:34–42. doi: 10.1136/tc.2008.025635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Bolt DM, Piper ME, Theobald WE, Baker TB. Why Two Smoking Cessation Agents Work Better Than One: Role of Craving Suppression. Journal of Consulting and Clinical Psychology. 2012;80:54–65. doi: 10.1037/a0026366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Ebbert JO, Hays JT, Hurt RD. Combination pharmacotherapy for stopping smoking: what advantages does it offer? Drugs. 2010;70:643–50. doi: 10.2165/11536100-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Stead LF, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews. 2012 doi: 10.1002/14651858.CD000146.pub4. [DOI] [PubMed] [Google Scholar]
- (5).Jorenby DE, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. New England Journal of Medicine. 1999;340:685–91. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- (6).Hughes JR, Stead LF, Hartmann-Boyce J, Cahill K, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;1:CD000031. doi: 10.1002/14651858.CD000031.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Ebbert JO, Burke MV, Hays JT, Hurt RD. Combination treatment with varenicline and nicotine replacement therapy. Nicotine & Tobacco Research. 2009;11:572–6. doi: 10.1093/ntr/ntp042. [DOI] [PubMed] [Google Scholar]
- (8).Hajek P, Smith KM, Dhanji AR, McRobbie H. Is a combination of varenicline and nicotine patch more effective in helping smokers quit than varenicline alone? A randomised controlled trial. Bmc Medicine. 2013;11 doi: 10.1186/1741-7015-11-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Koegelenberg CFN, et al. Efficacy of Varenicline Combined With Nicotine Replacement Therapy vs Varenicline Alone for Smoking Cessation A Randomized Clinical Trial. Jama-Journal of the American Medical Association. 2014;312:155–61. doi: 10.1001/jama.2014.7195. [DOI] [PubMed] [Google Scholar]
- (10).Ramon JM, Morchon S, Baena A, Masuet-Aumatell C. Combining varenicline and nicotine patches: a randomized controlled trial study in smoking cessation. Bmc Medicine. 2014;12 doi: 10.1186/s12916-014-0172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Ebbert JO, Croghan IT, Sood A, Schroeder DR, Hays JT, Hurt RD. Varenicline and bupropion sustained-release combination therapy for smoking cessation. Nicotine & Tobacco Research. 2009;11:234–9. doi: 10.1093/ntr/ntn031. [DOI] [PubMed] [Google Scholar]
- (12).Ebbert JO, et al. Combination Varenicline and Bupropion SR for Tobacco-Dependence Treatment in Cigarette Smokers A Randomized Trial. Jama-Journal of the American Medical Association. 2014;311:155–63. doi: 10.1001/jama.2013.283185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Rose JE, Behm FM. Combination Treatment With Varenicline and Bupropion in an Adaptive Smoking Cessation Paradigm. American Journal of Psychiatry. 2014;171:1199–205. doi: 10.1176/appi.ajp.2014.13050595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Toll BA, et al. Comparing gain- and loss-framed messages for smoking cessation with sustained-release bupropion: a randomized controlled trial. Psychol Addict Behav. 2007;21:534–44. doi: 10.1037/0893-164X.21.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Wilcox CS, et al. An open-label study of naltrexone and bupropion combination therapy for smoking cessation in overweight and obese subjects. Addictive behaviors. 2009 doi: 10.1016/j.addbeh.2009.10.017. [DOI] [PubMed] [Google Scholar]
- (16).Caixas A, Albert L, Capel I, Rigla M. Naltrexone sustained-release/bupropion sustained-release for the management of obesity: review of the data to date. Drug Design Development and Therapy. 2014;8:1419–27. doi: 10.2147/DDDT.S55587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Ascher JA, et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995;56:395–401. [PubMed] [Google Scholar]
- (18).Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther. 2000;295:321–7. [PubMed] [Google Scholar]
- (19).Hutchison KE, et al. Effects of naltrexone with nicotine replacement on smoking cue reactivity: preliminary results. Psychopharmacology (Berl) 1999;142:139–43. doi: 10.1007/s002130050872. [DOI] [PubMed] [Google Scholar]
- (20).Almeida LE, Pereira EF, Alkondon M, Fawcett WP, Randall WR, Albuquerque EX. The opioid antagonist naltrexone inhibits activity and alters expression of alpha7 and alpha4beta2 nicotinic receptors in hippocampal neurons: implications for smoking cessation programs. Neuropharmacology. 2000;39:2740–55. doi: 10.1016/s0028-3908(00)00157-x. [DOI] [PubMed] [Google Scholar]
- (21).Lerman C, et al. Pharmacogenetic investigation of smoking cessation treatment. Pharmacogenetics. 2002;12:627–34. doi: 10.1097/00008571-200211000-00007. [DOI] [PubMed] [Google Scholar]
- (22).Shiffman S, et al. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology. 2000;148:33–40. doi: 10.1007/s002130050022. [DOI] [PubMed] [Google Scholar]
- (23).Mooney ME, Sofuoglu M. Bupropion for the treatment of nicotine withdrawal and craving. Expert Rev Neurother. 2006;6:965–81. doi: 10.1586/14737175.6.7.965. [DOI] [PubMed] [Google Scholar]
- (24).Krishnan-Sarin S, Meandzija B, O’Malley S. Naltrexone and nicotine patch smoking cessation: a preliminary study. Nicotine Tob Res. 2003;5:851–7. doi: 10.1080/14622200310001614601. [DOI] [PubMed] [Google Scholar]
- (25).Wong GY, Wolter TD, Croghan GA, Croghan IT, Offord KP, Hurt RD. A randomized trial of naltrexone for smoking cessation. Addiction. 1999;94:1227–37. doi: 10.1046/j.1360-0443.1999.948122713.x. [DOI] [PubMed] [Google Scholar]
- (26).Hutchison KE, Collins F, Tassey J, Rosenberg E. Stress, naltrexone, and the reinforcement value of nicotine. Experimental & Clinical Psychopharmacology. 1996;4:431–7. [Google Scholar]
- (27).Hollister LE, Johnson K, Boukhabza D, Gillespie HK. Aversive effects of naltrexone in subjects not dependent on opiates. Drug Alcohol Depend. 1981;8:37–41. doi: 10.1016/0376-8716(81)90084-3. [DOI] [PubMed] [Google Scholar]
- (28).Landabaso MA, et al. A randomized trial of adding fluoxetine to a naltrexone treatment programme for heroin addicts. Addiction. 1998;93 doi: 10.1046/j.1360-0443.1998.9357399.x. [DOI] [PubMed] [Google Scholar]
- (29).Stefanovic KB, Gregg PC, Soung M. Evaluation and Treatment of Hematospermia. American Family Physician. 2009;80:1421–7. [PubMed] [Google Scholar]
- (30).Toll BA, Leary V, Wu R, Salovey P, Meandzija B, O’Malley SS. A preliminary investigation of naltrexone augmentation of bupropion to stop smoking with less weight gain. Addictive behaviors. 2008;33 doi: 10.1016/j.addbeh.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).King A, de Wit H, Riley RC, Cao D, Niaura R, Hatsukami D. Efficacy of naltrexone in smoking cessation: a preliminary study and an examination of sex differences. Nicotine Tob Res. 2006;8:671–82. doi: 10.1080/14622200600789767. [DOI] [PubMed] [Google Scholar]
- (32).O’Malley SS, et al. A controlled trial of naltrexone augmentation of nicotine replacement therapy for smoking cessation. Arch Intern Med. 2006;166 doi: 10.1001/archinte.166.6.667. [DOI] [PubMed] [Google Scholar]
- (33).Piasecki TM. Relapse to smoking. Clinical Psychology Review. 2006;26:196–215. doi: 10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- (34).Shiffman S, et al. Natural history of nicotine withdrawal. Addiction. 2006;101:1822–32. doi: 10.1111/j.1360-0443.2006.01635.x. [DOI] [PubMed] [Google Scholar]
- (35).Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- (36).PDR . Physicians’ desk reference. Medical Economics Company; Monvale, NJ: 2015. [Google Scholar]
- (37).Ahmadi J, Ashkani H, Ahmadi M, Ahmadi N. Twenty-four week maintenance treatment of cigarette smoking with nicotine gum, clonidine and naltrexone. J Subst Abuse Treat. 2003;24:251–5. doi: 10.1016/s0740-5472(03)00027-8. [DOI] [PubMed] [Google Scholar]
- (38).Covey LS, Glassman AH, Stetner F. Naltrexone effects on short-term and long-term smoking cessation. J Addict Dis. 1999;18 doi: 10.1300/J069v18n01_04. [DOI] [PubMed] [Google Scholar]
- (39).Fiore MC, et al. Treating tobacco use and dependence clinical practice guideline. U.S. Department of Health and Human Services; Washington D.C.: 2000. [Google Scholar]
- (40).Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- (41).Benowitz NL, et al. Biochemical verification of tobacco use and cessation. Nicotine and Tobacco Research. 2002;4:149–59. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- (42).Aubin HJ. Tolerability and safety of sustained-release bupropion in the management of smoking cessation. Drugs. 2002;62:45–52. doi: 10.2165/00003495-200262002-00005. [DOI] [PubMed] [Google Scholar]
- (43).American Psychiatric Association . Diagnostic and statistical manual of mental disorders : DSM-IV. 4th edn. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- (44).Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- (45).Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- (46).Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86:1467–76. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- (47).Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- (48).Rochon J. Application of GEE procedures for sample size calculations in repeated measures experiments. Stat Med. 1998;17:1643–58. doi: 10.1002/(sici)1097-0258(19980730)17:14<1643::aid-sim869>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- (49).Ahluwalia JS, Harris KJ, Catley D, Okuyemi KS, Mayo MS. Sustained-release bupropion smoking cessation in African Americans - A randomized controlled trial. Jama-Journal of the American Medical Association. 2002;288:468–74. doi: 10.1001/jama.288.4.468. [DOI] [PubMed] [Google Scholar]
- (50).Hall SM, Humfleet GL, Reus VI, Munoz RF, Hartz DT, Maude-Griffin R. Psychological intervention and antidepressant treatment in smoking cessation. Archives of General Psychiatry. 2002;59:930–6. doi: 10.1001/archpsyc.59.10.930. [DOI] [PubMed] [Google Scholar]
- (51).Hurt RD, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337:1195–202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- (52).Tashkin D, et al. Smoking cessation in patients with chronic obstructive pulmonary disease: a double-blind, placebo-controlled, randomised trial. Lancet. 2001;357:1571–5. doi: 10.1016/s0140-6736(00)04724-3. [DOI] [PubMed] [Google Scholar]
- (53).Tonnesen P, et al. A multicentre, randomized, double-blind, placebo-controlled, 1-year study of bupropion SR for smoking cessation. Journal of Internal Medicine. 2003;254:184–92. doi: 10.1046/j.1365-2796.2003.01185.x. [DOI] [PubMed] [Google Scholar]
- (54).SAS Institute Inc. The SAS System for Windows. 9.4. edn. SAS Institute Inc.; Cary, NC: 2014. [Google Scholar]
- (55).Hall SM, et al. Statistical analysis of randomized trials in tobacco treatment: longitudinal designs with dichotomous outcome. Nicotine Tob Res. 2001;3:193–202. doi: 10.1080/14622200110050411. [DOI] [PubMed] [Google Scholar]